Abstract
The influence of different levels of heat exposure on the functions of ovarian and adrenal gland were investigated in pre-puberty female rats. Three-week old female rats were treated with control (26°C) or three higher temperatures (38, 40 and 42°C) for 2hr/day. After 9 days of treatment, blood samples were collected for measurement of luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol-17β, corticosterone, cholesterol and triglyceride. Adrenal glands, ovaries and liver were collected for analyzing gene expressions. Body and liver weight were significantly low in the 42°C heating group. Circulating LH and triglyceride in the 42°C heating group were significantly lower, and estradiol-17β, corticosterone and cholesterol were significantly higher than those of the control group. The gene expression of 3β-HSD and P450c21 in the adrenal gland; 3β-HSD, receptors of LH, FSH and estrogen in the ovary were significantly low in heated rats. The liver gene expressions of caspase 3 and NK-κB were significantly high in 42°C heated rats, suggesting that the ability of liver metabolic function reduced in the 42°C heated rats. These results demonstrated that the high temperature is responsible for suppression of ovarian function by decreasing the expression of steroidogenic enzymes, estrogen and gonadotropin receptors in the ovary. Increase in circulating estradiol-17β in the heated rats may be due to accumulate this hormone in circulation by potential changes in liver metabolism during the heat stress.
Keywords: corticosterone, estradiol-17β, heat stress, rat, steroidogenesis
Climate change due to global warming is a great concern with significant financial burden to animal production in most areas of the world. Over the past decades, effect of heat stress on the reproductive and immune system is well-represented in the literature [3, 10, 14, 19, 31]. It is the serious problem that high temperature suppresses gonadal function in both female and male domestic animals, so called “summer sterility”. Although this phenomenon is the heat stress in animals, the endocrine mechanism is not clear. As the body’s main regulator of stress, the hypothalamic-pituitary-adrenal axis (HPA axis) may be particularly important in this regard [13, 30]. Corticosterone and cortisol, the endogenous main glucocorticoid, modulates homeostatic processes under basal and stress conditions, under the regulation of hypothalamic corticotropin-releasing hormone (CRH) from the hypothalamus and adrenocorticotropic hormone (ACTH) from the anterior pituitary gland [22].
The major role of the ovary is to produce oocytes for fertilization and to secrete reproductive hormones including estrogen, progesterone and inhibin that play a role in the estrous cycle and fertility. The ovarian function is controlled by gonadotropin-releasing hormone (GnRH) secreted from hypothalamus which sends the messages to the anterior pituitary gland to stimulate secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH). FSH and LH act on the ovary and stimulate the expressions of steroidogenic enzymes in granulosa cells and theca cells to produce estrogen [20].
Previous papers reported the significant interaction between the HPA axis and the hypothalamic-pituitary-gonadal (HPG) axis [21]. The higher concentrations of cortisol suppressed LH secretion in heat stressed cows [7], rat primary pituitary cells cultured with high levels of corticosterone (10–100 Mm) resulted inhibitory effects on LH secretion at in vitro experiment [15]. Inconsistently, previous articles reported that unchanged or increased LH concentrations in peripheral blood in heat stressed cows [8, 9, 23]. These discrepancies may be associated with differences in sampling frequency, which varied from interval of sampling time and depend on whether stress is acute or chronic.
The present study was conducted to evaluate ovarian and adrenal gland function under the hyperthermia environment in pre-pubertal female rats. The effect of different levels of heat exposure on the secretion of gonadotropins and steroid hormones, and the expression of steroidogenic enzymes in the adrenal gland, ovary and liver, and receptors of LH, FSH and estradiol-17β in the ovary were investigated.
MATERIALS AND METHODS
Animals
Twenty one days old female rats of the Wistar Imamichi strain (Imamich Institute for Animal Reproduction, Kasumigaura, Ibaraki, Japan), were used in this study. They were kept in the animal room of Laboratory of Veterinary Physiology at Tokyo University of Agriculture and Technology, maintained 26°C for control group, 38, 40 and 42°C for heat-treated groups using Biomulti incubator (Nippon medical & Chemical instrument Co., Ltd., Tokyo, Japan) under controlled lighting schedule (lights on 05:00–19:00 hr) and humidity (50 ± 10%). Food and water were available ad libitum. All animals were weighed on postnatal day 21 (PND21), 25 (PND25) and 30 (PND30). All procedures were carried out in accordance with the guidelines established by Tokyo University of Agriculture and Technology, for use of laboratory animals (No. 23-1).
Experimental designs
The control group was maintained at 26°C, and the three different groups exposed to higher temperatures (38, 40 and 42°C, respectively) from PND21 for 2 hr (12:00–14:00 hr) per day (N=4). After 9-days of treatment, the animals were decapitated 30 min after the last heat exposure. All blood samples were collected into plastic tubes containing heparin (15 IU/ml blood) as an anticoagulant to prevent clotting. The blood samples were stored on ice and centrifuged at 1,700 g for 15 min at 4°C immediately after the experiment was completed. The resulting plasma was stored at −20°C until assayed for plasma hormones, and cholesterol and triglyceride. Adrenal glands and ovaries were collected for analyzing basal expression of steroidogenic enzymes and LH, FSH and estradiol-17β receptors by real time PCR. Another group of animals exposed in 26 and 42°C in same schedule were used for biological examination. Day of vaginal opening was observed, and vaginal smears were examined daily after vaginal opening until 80 days of age.
Measurement of LH, FSH and corticosterone
Plasma concentrations of LH and FSH were determined by homologous double-antibody rat radioimmunoassay (RIA) methods. Iodinated preparations were rat LH-I-7 and rat FSH-I-7, and the antisera were anti-rat LH-S-11 and anti-rat FSH-S-11, respectively. Results were expressed in terms of National Institute of Diabetes (NIDDK) rat LH-RP-3 and FSH-RP-2. The intra- and interassay coefficients of variation were 5.5 and 14.3% for LH, and 6.7 and 13.8% for FSH.
Plasma concentrations of corticosterone was measured by double-antibody RIA system using 125I-labeled radioligand as described previously [17]. The antisera against corticosterone (GDN337) were kindly provided by Dr, G.D. Niswender (Colorado State University, Fort Collins, CO, U.S.A.). The intra- and interassy coefficient of variation were 8.9 and 14.6%.
Measurement of estradiol-17β
Plasma concentrations of estradiol-17β was determined by enzyme-linked immunosorbent assay using commercial kits (Item No.582251, Cayman Chemical Co., Ann Arbor, MI, U.S.A.).
Measurement of cholesterol and triglyceride
Plasma concentrations of cholesterol and triglyceride was determined by enzyme reaction using LabAssayTM Choleserol kit (Wako, Osaka, Japan) and LabAssayTM Triglyceride kit (Wako).
RNA extraction and qRT-PCR analysis
Total RNA was extracted from ovaries, adrenal glands and liver using Isogen II reagent kit (Nippon Gene, Tokyo, Japan). cDNA was generated using Primescript reverse transcriptase (Takara Bio, Kusatsu, Japan). The oligonucleotide primer for qRT-PCR analysis were designed using the Primer shown in Table 1. The PCR reaction were using Ex TaqR hot Start Version containing SYBR-Green I (Takara Bio). The temperature profile for the reactions was 95°C for 5 sec, 60°C for 30 sec, and 40 cycles consisting of 95°C for 5 sec, 60°C for 30 sec. The expression level of each target mRNA relative to β-actin mRNA was determined using the 2−ΔΔCT method [26]. Relative expression was calculated by normalization to the mean of the 26°C control group.
Table 1. Nucleotitide sequences of the primers used for real-time PCR.
| Genes name | Forward | Reverse |
|---|---|---|
| StAR | 5′-GGGAGAGTGGAACCCAAATGT-3′ | 5′-CATGGGTGATGACTGTGTCTTTTC-3′ |
| P450scc | 5′-GGAGGAGATCGTGGAC-3 | 5′-TGGAGGCATGTTGAGC-3′ |
| 3βHSD | 5′-GGTGCAGGAAAGAA-3′ | 5′-TGACATCAATGACAGC-3′ |
| P450c21 | 5′-CCTCTCCATGGGGGAT-3′ | 5′-CCAGCTGATAGTGACC-3′ |
| CYP17α | 5′-TTTTGGCCCAAGTCAA-3′ | 5′-AAGGTTGGCAGCTCTCATGT-3′ |
| ERα | 5′-CATCGATAAGAACCGGAGGA-3′ | 5′-AAGGTTGGCAGCTCTCATGT-3′ |
| FSHR | 5′-CTCATCTTTCCCTCCA-3′ | 5′-GGACTCATGCATCCCT-3′ |
| LHR | 5′-GCATTCAATGGGACGACTCT-3′ | 5′-GTAGGAAGACAGGGCGATGA-3′ |
| NF-κB | 5′-CCGAGAAGGACCAATTGAAA-3′ | 5′-AGAAGCCCTTTGCAAGTTCA-3′ |
| Caspase3 | 5′-TCATCATGCCCACTTCGTAA-3′ | 5′-GCTACGATCCACCAGCATTT-3′ |
| GAPDH | 5′-CCTGCCCAAGATTGTTGAGT-3′ | 5′-TGTACCGATCGATGTCTGGA-3′ |
| β-Actin | 5′-AGCCATGTACGTAGCCATCC-3′ | 5′-CTCTCAGCTGTGGTGGTGAA-3′ |
Statistical analysis
The results shown in tables and figures are expressed as mean ± SEM. Statistical analyses were performed using the GraphPad Prism 6 (Graphpad Software, Inc., La Jolla, CA, U.S.A.). Differences among experiment groups were evaluated using one-way analysis of variance (ANOVA) followed by Student’s t-test. P<0.05 was considered as significant differences.
RESULTS
Body weights and organs weights
Body weights and liver weights under different temperature treatments were shown in Figs. 1 and 2. The body and liver weights on PND30 were significantly low in the 42°C heat treated rats as compared with the 26°C control group. There were no significant different in the weights of ovaries, adrenal glands, spleens, kidneys and thymus among the 26°C control group and the heat treated groups (data not shown).
Fig. 1.
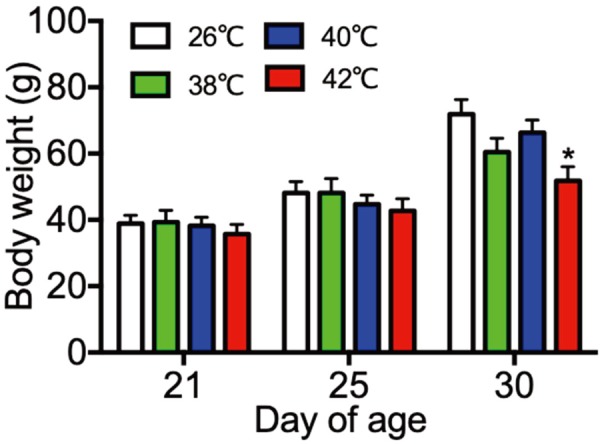
Body weight of control (26°C) and heat treated immature female rats (38, 40, 42°C) at 21, 25 and 30 day of age. Each bar represents mean ± S.E.M. of 4 rats. Asterisks indicate significant difference compared to the control. *P<0.05.
Fig. 2.
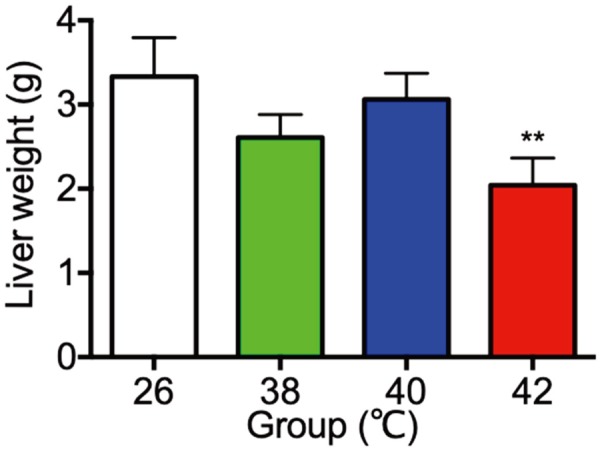
The liver weight of control (26°C) and heat treated immature female rats (38, 40, 42°C) at 30 day of age. Each bar represents mean ± S.E.M. of 4 rats. Asterisks indicate significant difference compared to the control. **P<0.01.
Plasma concentrations of LH, FSH, estradiol-17β, corticosterone and cholesterol
Plasma concentrations of LH, FSH, estradiol-17β, corticosterone and cholesterol on PND30 were shown in Fig. 3. Plasma concentrations of LH in the 42°C heat treated rats were significanty lower than the 26°C control rats (Fig. 3a), whereas plasma concentrations of FSH were no difference among four groups (Fig. 3b). Plasma concentrations of estradiol-17β and corticosterone were significantly higher in the 42°C heat treated rats than the 26°C control rats (Fig. 3c, 3d). Moreover, plasma concentrations of cholesterol were significantly higher in the 38, 40 and 42°C heat treated rats than the 26°C control rats (Fig. 3e).
Fig. 3.
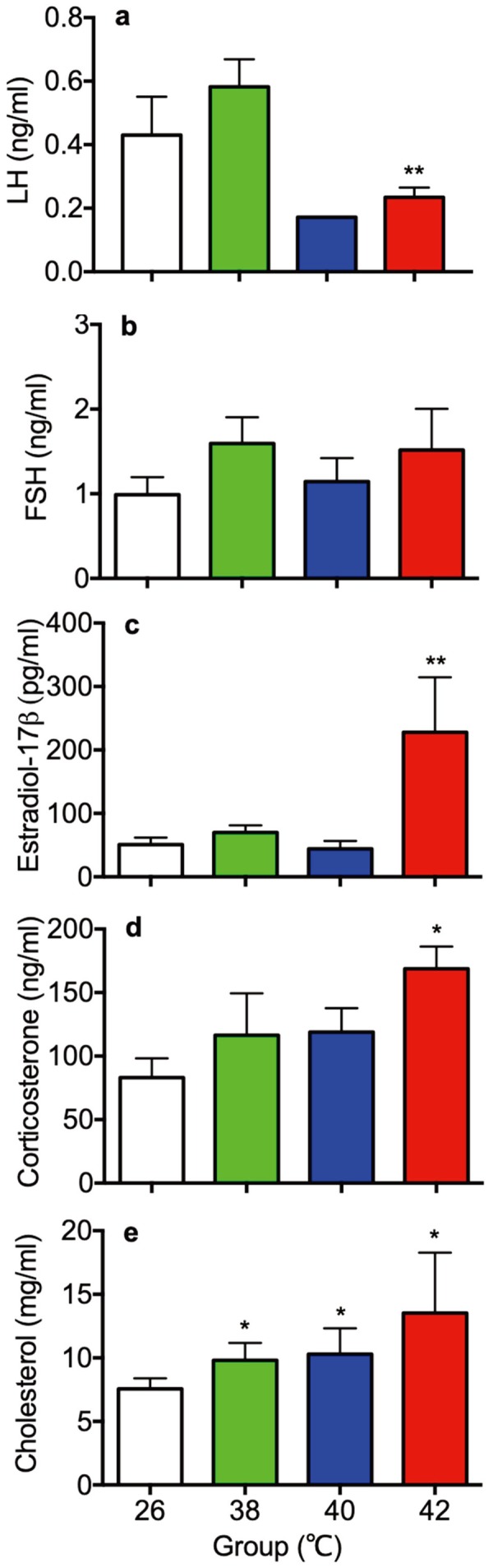
Plasma concentrations of luteinizing hormone (LH; a), follicle-stimulating hormone (FSH; b), estradiol-17β (c), corticosterone (d) and cholesterol (e) of control (26°C) and heat treated immature female rats (38, 40, 42°C) at 30 day of age. Each bar represents mean ± S.E.M. of 4 rats except 40°C treated rats in the panel a (n=2). Asterisks indicate significant difference compared to the control. *P<0.05; **P<0.01.
Gene expression of StAR and steroidogenic enzymes in adrenal glands
Adrenal gland gene expression of StAR and steroidogenic enzymes including P450scc, 3β-HSD and P450c21 were shown in Fig. 4. Expression of StAR in the 38 and 40°C heat treated rats were significantly lower than the 26°C control rats (Fig. 4a). Expression of P450scc in the 42°C heat treated rats were significantly higher than the 26°C control rats (Fig. 4b). Expression of 3β-HSD in the 42°C heat treated rats were significantly lower than the 26°C control rats (Fig. 4c). Expression of P450c21 in the 38, 40°C and 42°C heat treated rats were significantly lower than the 26°C control rats (Fig. 4d).
Fig. 4.
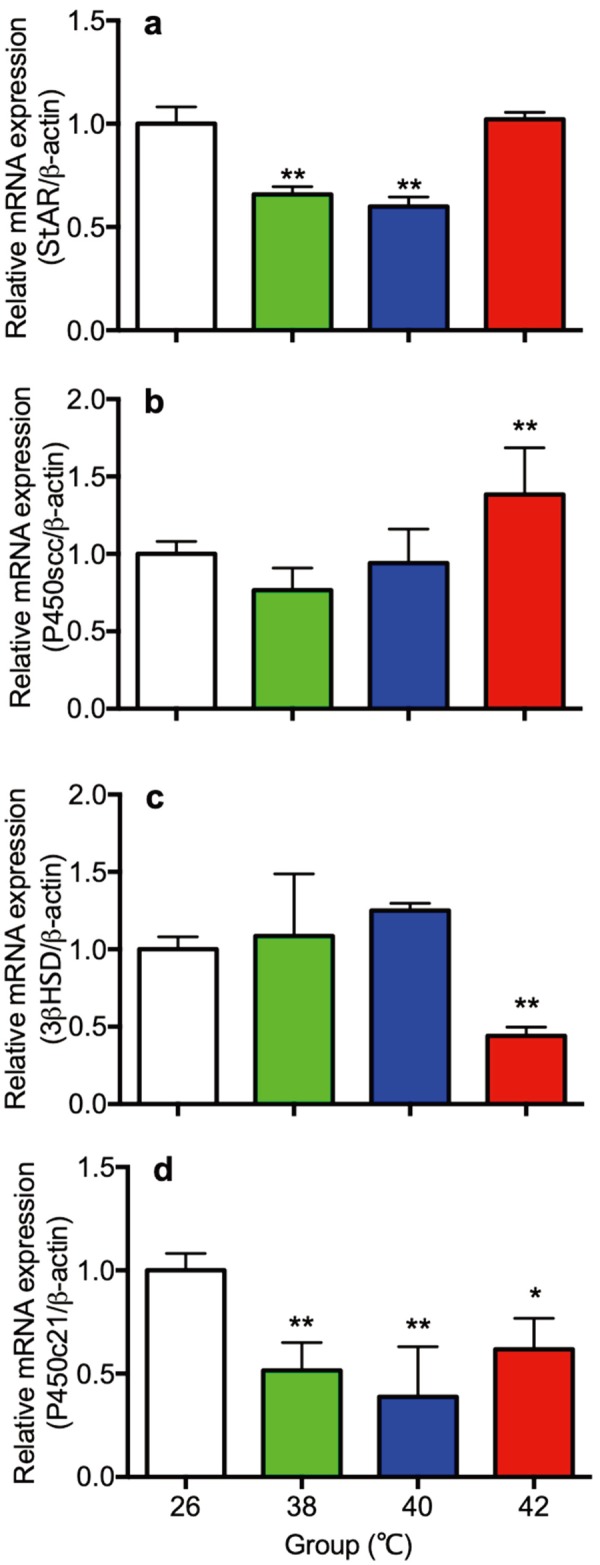
The gene expression of StAR (a) steroidogenic enzymes P450scc (b), 3β-HSD (c) and P450c21 (d) in the adrenal gland of control (26°C) and heat treated immature female rats (38, 40, 42°C) at 30 day of age. Each bar represents mean ± S.E.M. of 4 rats. Asterisks indicate significant difference compared to the control (*P<0.05 and **P<0.01).
Gene expression of StAR and steroidogenic enzymes in ovaries
Gene expression of StAR, 3β-HSD and CYP17α were shown in Fig. 5. Expression of StAR in the 38°C heat treated rats (Fig. 5a), 3β-HSD in the 40 and 42°C heat treated rats (Fig. 5b), and CYP17α in the 38°C heat treated rats (Fig. 5c) were significantly lower than the 26°C control rats.
Fig. 5.
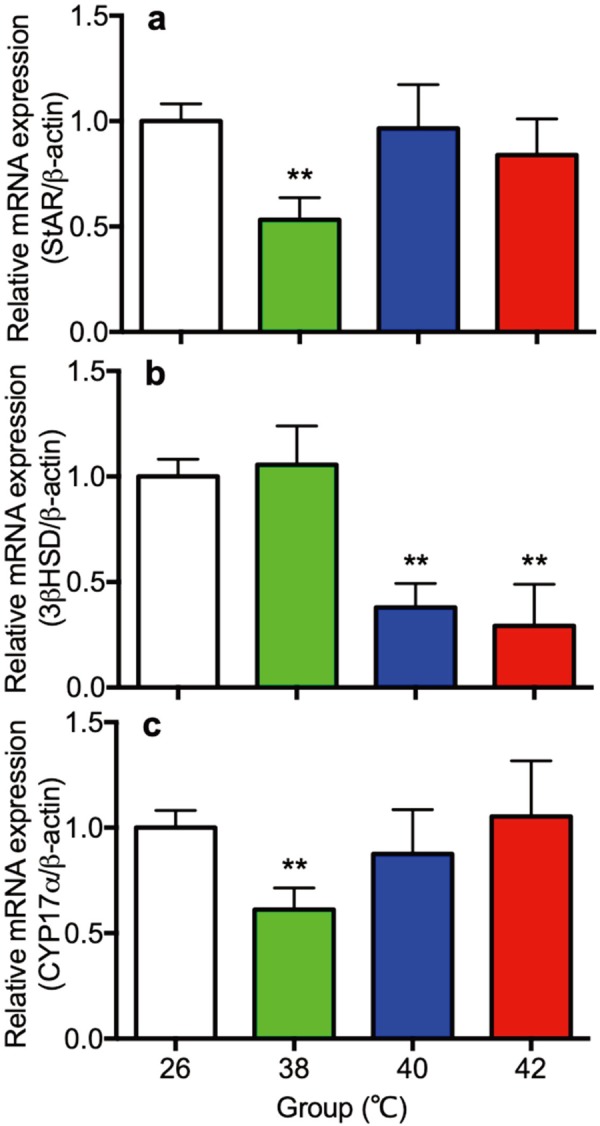
The gene expression of StAR (a) steroidogenic enzymes 3β-HSD (b) and CYP17α (c) in the ovary of control (26°C) and heat treated immature female rats (38, 40, 42°C) at 30 day of age. Each bar represents mean ± S.E.M. of 4 rats. Asterisks indicate significant difference compared to the control (*P<0.05 and **P<0.01).
Gene expression of receptors of LH (LHR), FSH (FSHR) and estrogen (ERα) in ovaries
Gene expression of LHR, FSHR and ERα in ovaries were shown in Fig. 6. Expression of LHR, FSHR and ERα in ovaries in the 38, 40 and 42°C heat treated rats (Fig. 6a–c) were significantly lower than the 26°C control rats.
Fig. 6.
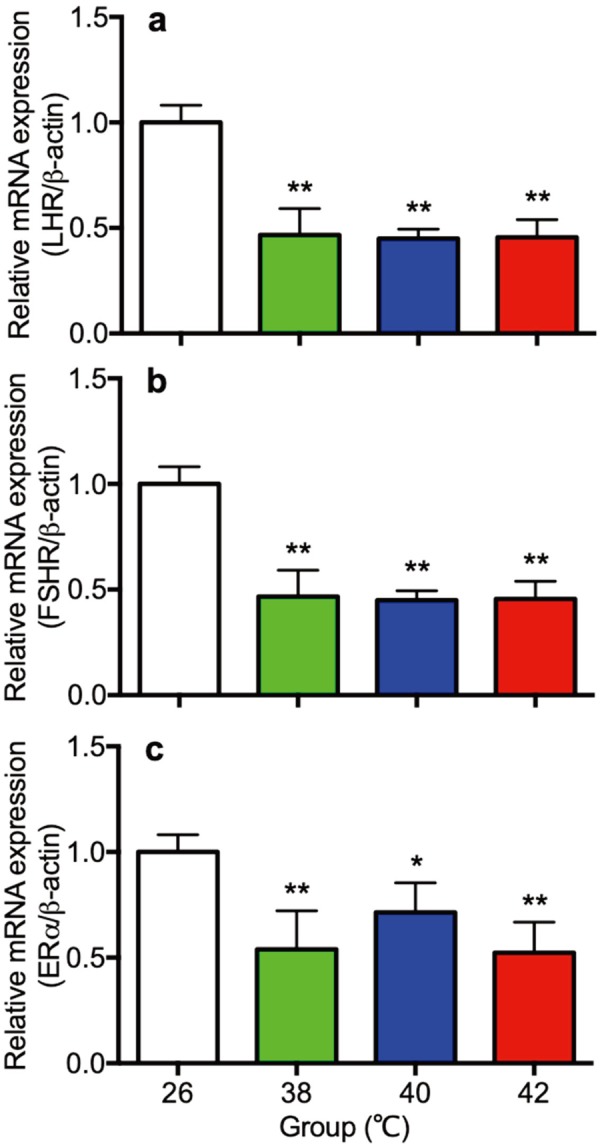
The gene expression of receptor of LH (LHR: a), FSH (FSHR: b) and estrogen (ER: c) in the ovary of control (26°C) and heat treated immature female rats (38, 40, 42°C) at 30 day of age. Each bar represents mean ± S.E.M. of 4 rats. Asterisks indicate significant difference compared to the control (*P<0.05 and **P<0.01).
Gene expression of caspase 3 and NK-κB in liver
Gene expression of Caspase 3 and NK-κB in liver were shown in Fig. 7. Expression of caspase 3 in the 42°C (Fig. 7a), and NK-κB in the 40°C and the 42°C heat treated rats (Fig. 7b) in the liver were significantly higher than the 26°C control rats.
Fig. 7.
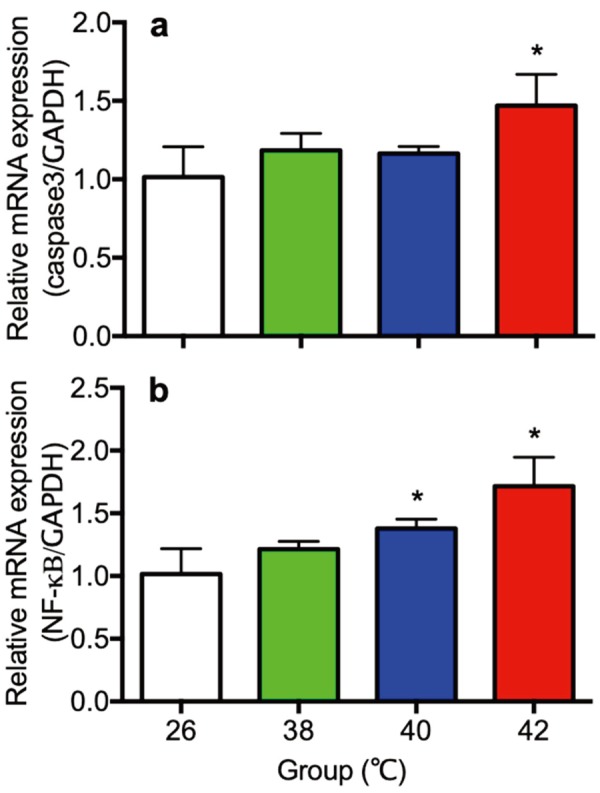
The gene expression of Caspase 3 (a) and NK-κB (b) in liver of control (26°C) and heat treated immature female rats (38, 40, 42°C) at 30 day of age. Each bar represents mean ± S.E.M. of 4 rats. Asterisks indicate significant difference compared to the control (*P<0.05).
Plasma concentrations of triglyceride
Plasma concentrations of triglyceride in the 26°C control rats and the 42°C heat treated rats were shown in Fig. 8. Plasma concentrations of triglyceride in the 42°C heat treated rats were significant low as compared with the 26°C control rats.
Fig. 8.
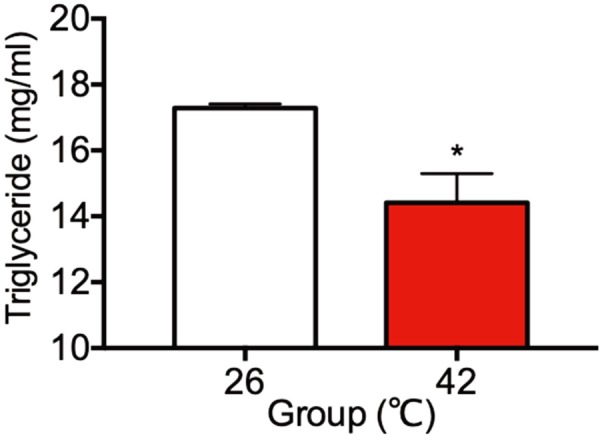
Plasma concentrations of triglyceride in the 26°C control rats and the 42°C heat treated rats at 30 day of age. Each bar represents mean ± S.E.M. of 4 rats. Asterisks indicate significant difference compared to the control (*P<0.05).
Vaginal opening and estrous cycle
Day of vaginal opening and estrous cycle after vaginal opening were shown in Table 2. Day of vaginal opening in the 42°C heat treated rats were significant delayed as compared with the 26°C control rats. Day of age at starting 4-day estrous cycle in the 42°C heat treated rats were significant delayed as compared with the 26°C control rats.
Table 2. Vaginal opening and estrous cycle.
| Group | Vaginal opening | Estrous cycle |
|---|---|---|
| 26°C | 34.4 ± 0.2 | 38.8 ± 0.9 |
| 42°C | 37.0 ± 0.0a) | 48.8 ± 0.7a) |
Result are expressed as mean ± S.E.M. (n=5). a) Significant difference between 26°C group and 42°C group.
DISCUSSION
The global warming is a worldwide serious problem in the livestock production. The suppression of gonadal function occurred in both female and male animals during summer season. This phenomenon is the heat stress, and heat stress affects fertility, immunity, and endocrine function as observed in the dairy cow [1, 11, 24, 28]. However, the mechanism of heat stress is still unclear. To clarify the mechanism of heat stress on the ovarian and adrenal function, pre-pubertal female rats were treated with three different levels of heat in the present study. The present study clearly demonstrated that high temperature caused liver damage and lipid metabolic in the immature female rat.
In the present study, immature female rats exposed to heat stress during the pre-pubertal period (PND 21–30) had reduction of circulating LH, and ovarian gene expression of LH, FSH and estrogen receptors. The lower LHR and FSHR gene expression may be due to decreased secretion of LH and FSH from pituitary to the theca cells and granulosa cells, this in turn could suppress ovarian follicular development and estradiol-17β secretion. The present study demonstrated that day of vaginal opening and start of estrous cycle delayed in the 42°C heat treated rats. These results suggest that the heat stress in the present study suppressed ovarian follicular maturation in the pre-pubertal female rat. Previous studies reported that heat stress induced low concentration of estradiol-17β in follicular fluids in cows [4, 32]. The decline of circulating LH and the gene expression of StAR and steroidogenic enzymes 3βHSD and CYP17, seen in the present study could support decrease secretion of estradiol-17β in the ovary. Unexpectedly, however, the plasma concentration of estradiol-17β dramatically increased in 42°C heat treated rats in the present study. Concentrations of estradiol-17β in circulation in the 42°C heat treated rats were much higher than those of the day of proestrous in cyclic female rats (127.7 ± 12.1; n=4). These results may be due to decreased liver metabolic function in the 42°C heat treated rats. The present study clearly demonstrated that liver weight significantly decreased in the 42°C heated rats, and expression of caspase 3 in the 42°C and NK-κB in the 40°C and the 42°C heat treated rat in the liver were significantly increased. In addition, plasma concentrations of triglyceride in the 42°C heat treated rats significant decreased. These results strongly suggested that the ability of liver metabolic function reduced in heat treated rats. Therefore, estradiol-17β were accumulated in circulation, this in turn suppression of LH secretion by negative feedback effect of estradiol-17β on the hypothalamus-pituitary axis. In the preliminary experiment, observation of liver histopathology of these same rats suggest structural abnormalities in the 42°C heated rats (data not shown). Similar result was found by another group in heat treated rats [2], in which the hepatic cells around the hepatic vein developed hypertrophy to a great extent, and vacuolated degeneration under the hot environment. On the other hand, there is a discrepancy that the day of vaginal opening delayed in the 42°C heated rats, whereas circulating estradiol-17β are higher than adult female rats. The exact mechanism is not clear at the present time, the amount of α-fetoprotein in circulation may be related, because the liver function decreased in the 42°C heated rats. Further studies will be required to demonstrate this discrepancy.
The HPA axis was also activated by heat stress. Plasma concentrations of corticosterone were significantly increased in 42°C heat treated group in the present study. These results consistent with previous findings in pigs [33]. The mechanisms involved in the change of corticosterone might be the expression levels of StAR and steroidogenic enzymes in adrenal gland. The present study showed significantly increased P450scc in 42°C heated rats, and significantly decreased expression of StAR in 38°C and 40°C, 3β-HSD in 42°C, and P450c21 in all heat treated rats. These results indicate that heat treatment increases adrenal response capacity of corticosterone by enhancing the expression levels of steroidogenic enzyme P450scc in pre-pubertal female rats.
Previous study clearly demonstrated that the acute restraint stress rapidly increased the activity of the hypothalamus-pituitary-adrenal axis, whereas hypothalamus-pituitary-gonadal axis activity decreased in male rats [25]. Restraint stress induced a significant elevation in plasma adrenocorticotropic hormone (ACTH), prolactin, corticosterone and progesterone, and decreased FSH, LH, testosterone and immunoreactive (ir-) inhibin in the male rat. Previous papers demonstrated that the stress suppressed the secretion of both LH and FSH, and this effect is mediated by the inhibition of Kisspeptin and GnRH secretion from the hypothalamus [18]. Previous studies also suggested that the stress-induced inhibition of Kisspeptin and GnRH may be primary mediated by endogenous CRH and opioid peptides. [18, 27, 29]. Growing evidence indicated that gonadotropin inhibitory hormone (GnIH) also play pivotal roles in the stress-induced disruption of the hypothalamus-pituitary-gonadal axis [5, 12].
Previous studies reported that heat stress increased plasma levels of cholesterol and decreased body weight (or/and feed intake) in human [16] and rats [6]. Consistent with that evidence, the present study also showed elevated plasma cholesterol in all heat-treated rats and decreased body weight in 42°C treated rats. Although food intake was not measured in the present study, the decreased body weight in 42°C heat exposed rats indicates that the feed intake might decreased in this experiment, which may also relate to the increased plasma level of cholesterol in heated rats because lower feed intake leads to a net increase in endogenous production of cholesterol, whereas higher intake from food has the opposite effect. Higher concentrations of corticosterone in 42°C heated rats may relate to the increased cholesterol, since cholesterol is a precursor molecule for several biochemical, including corticosterone.
In summary, the results of the present study may thus indicate that (1) the high temperature is responsible for suppression of ovarian function by decreasing the expression of steroidogenic enzymes, estrogen and gonadotropin receptors in the ovaries; (2) the heat treatment increase concentrations of estradiol-17β in plasma may be due to accumulate this hormone in circulation by potential changes in liver metabolism during the heat stress; (3) the high temperature is also responsible for suppression the hypothalamus-pituitary axis by central mechanisms. It is, therefore, possible that the present study adds new knowledge about effects of heat on stress responses and the reproductive system in pre-pubertal animals.
Acknowledgments
We are grateful to the National Hormone and Pituitary Program, NIDDK, National Institutes of Health, and Dr. A. F. Parlow for rat LH and FSHRIA kits. We are also grateful to Dr. G. D. Niswender (Animal Reproduction and Biotechnology Laboratory, Colorado State University, Fort Collins, CO, U.S.A.) for providing anti-corticosterone (GDN 377).
REFERENCES
- 1.al-Katanani Y. M., Webb D. W., Hansen P. J.1999. Factors affecting seasonal variation in 90-day nonreturn rate to first service in lactating Holstein cows in a hot climate. J. Dairy Sci. 82: 2611–2616. doi: 10.3168/jds.S0022-0302(99)75516-5 [DOI] [PubMed] [Google Scholar]
- 2.Ando M., Katagiri K., Yamamoto S., Wakamatsu K., Kawahara I., Asanuma S., Usuda M., Sasaki K.1997. Age-related effects of heat stress on protective enzymes for peroxides and microsomal monooxygenase in rat liver. Environ. Health Perspect. 105: 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black J. L., Mullan B. P., Lorschy M. L., Giles L. R.1993. Lactation in the sow during heat stress. Livest. Prod. Sci. 35: 153–170. doi: 10.1016/0301-6226(93)90188-N [DOI] [Google Scholar]
- 4.Bridges P. J., Brusie M. A., Fortune J. E.2005. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest. Anim. Endocrinol. 29: 508–522. doi: 10.1016/j.domaniend.2005.02.017 [DOI] [PubMed] [Google Scholar]
- 5.Clarke I. J., Bartolini D., Conductier G., Henry B. A.2016. Stress increases gnadotropin inhibitory hormone cell activity and input to GnRH cells in ewes. Endocrinology 157: 4339–4350. doi: 10.1210/en.2016-1513 [DOI] [PubMed] [Google Scholar]
- 6.Djordjević J., Cvijić G., Davidović V.2003. Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiol. Res. 52: 67–72. [PubMed] [Google Scholar]
- 7.Gangwar P. C., Branton C., Evans D. L.1965. Reproductive and physiological response of Holstein heifers to controlled and natural climatic conditions. J. Dairy Sci. 48: 222–227. doi: 10.3168/jds.S0022-0302(65)88200-5 [DOI] [PubMed] [Google Scholar]
- 8.Gauthier D.1986. The influence of season and shade on oestrous behaviour, timing of preovulatory LH surge and the pattern of progesterone secretion in FFPN and Creole heifers in a tropical climate. Reprod. Nutr. Dev. 26: 767–775. doi: 10.1051/rnd:19860502 [DOI] [PubMed] [Google Scholar]
- 9.Gwazdauskas F. C., Thatcher W. W., Kiddy C. A., Paape M. J., Wilcox C. J.1981. Hormonal patterns during heat stress following PGF(2)alpha-tham salt induced luteal regression in heifers. Theriogenology 16: 271–285. doi: 10.1016/0093-691X(81)90012-1 [DOI] [PubMed] [Google Scholar]
- 10.Hansen P. J.2009. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364: 3341–3350. doi: 10.1098/rstb.2009.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingraham R. H., Gillette D. D., Wagner W. D.1974. Relationship of temperature and humidity to conception rate of Holstein cows in subtropical climate. J. Dairy Sci. 57: 476–481. doi: 10.3168/jds.S0022-0302(74)84917-9 [DOI] [PubMed] [Google Scholar]
- 12.Iwasa T., Matsuzaki T., Yano K., Mayila Y., Irahara M.2018. The roles of kisspeptin and gonadotropin inhibitory hormone in stress-induced reproductive disorders. Endocr. J. 65: 133–140. doi: 10.1507/endocrj.EJ18-0026 [DOI] [PubMed] [Google Scholar]
- 13.Joseph D. N., Whirledge S.2017. Stress and the HPA axis: Balancing homeostasis and fertility. Int. J. Mol. Sci. 18: 2224. doi: 10.3390/ijms18102224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan E. R.2003. Effects of heat stress on reproduction. J. Dairy Sci. 86: E104–E114. doi: 10.3168/jds.S0022-0302(03)74043-0 [DOI] [Google Scholar]
- 15.Kamel F., Kubajak C. L.1987. Modulation of gonadotropin secretion by corticosterone: interaction with gonadal steroids and mechanism of action. Endocrinology 121: 561–568. doi: 10.1210/endo-121-2-561 [DOI] [PubMed] [Google Scholar]
- 16.Keatinge W. R., Coleshaw S. R., Easton J. C., Cotter F., Mattock M. B., Chelliah R.1986. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am. J. Med. 81: 795–800. doi: 10.1016/0002-9343(86)90348-7 [DOI] [PubMed] [Google Scholar]
- 17.Kanesaka T., Taya K., Sasamoto S.1992. Radioimmunoassay of corticosterone using 123I-labeled radioligand. J. Reprod. Dev. 38: 85–89. doi: 10.1262/jrd.38.6_j85 [DOI] [Google Scholar]
- 18.Luo L., Wang Y., Deng D., Cheng Y., Zhou H.2016. Effects of light stress on the expression of Kisspeptin/GnRH in rat hypothalamus. Int. J. Clin. Exp. Med. 9: 16964–16970. [Google Scholar]
- 19.Marai I. F. M., El-Darawany A. A., Fadiel A., Abdel-Hafez M. A. M.2007. Physiological traits as affected by heat stress in sheep-A review. Small Rumin. Res. 71: 1–12. doi: 10.1016/j.smallrumres.2006.10.003 [DOI] [Google Scholar]
- 20.Meethal S.V., Liu T., Chan H.W., Ginsbrg E., Wilson A.C., Gray D.N., Bowen R.L., Vonderhaar B.K., Atwood C.S.2009. Identification of a regulatory loop for the synthesis of neurosteroids: a steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors. J. Neurochem. 110: 1014–1027. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyola M. G., Handa R. J.2017. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress 20: 476–494. doi: 10.1080/10253890.2017.1369523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pariante C. M., Lightman S. L.2008. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 31: 464–468. doi: 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 23.Roman-Ponce H., Thatcher W. W., Wilcox C. J.1981. Hormonal interelationships and physiological responses of lactating dairy cows to a shade management system in a subtropical environment. Theriogenology 16: 139–154. doi: 10.1016/0093-691X(81)90097-2 [DOI] [PubMed] [Google Scholar]
- 24.Ray D. E., Halbach T. J., Armstrong D. V.1992. Season and lactation number effects on milk production and reproduction of dairy cattle in Arizona. J. Dairy Sci. 75: 2976–2983. doi: 10.3168/jds.S0022-0302(92)78061-8 [DOI] [PubMed] [Google Scholar]
- 25.Ren L., Li X., Weng Q., Trisomboon H., Yamamoto T., Pan L., Watanabe G., Taya K.2010. Effects of acute restraint stress on sperm motility and secretion of pituitary, adrenocortical and gonadal hormones in adult male rats. J. Vet. Med. Sci. 72: 1501–1506. doi: 10.1292/jvms.10-0113 [DOI] [PubMed] [Google Scholar]
- 26.Schmittgen T. D., Livak K. J.2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3: 1101–1108. doi: 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 27.Taya K., Sasamoto S.1989. Inhibitory effects of corticotrophin-releasing factor and β-endorphin on LH and FSH secretion in the lactating rat. J. Endocrinol. 120: 509–515. doi: 10.1677/joe.0.1200509 [DOI] [PubMed] [Google Scholar]
- 28.Thompson J. A., Magee D. D., Tomaszewski M. A., Wilks D. L., Fourdraine R. H.1996. Management of summer infertility in Texas Holstein dairy cattle. Theriogenology 46: 547–558. doi: 10.1016/0093-691X(96)00176-8 [DOI] [PubMed] [Google Scholar]
- 29.Tohei A., Tomabechi T., Mamada M., Akai M., Watanabe G., Taya K.2001. Immunoneutralization of endogenous corticotropin releasing hormone (CRH) blocks the suppression of luteinizing hormone (LH) secretion induced by adrenalectomy and restained stress. J. Reprod. Dev. 47: 211–216. doi: 10.1262/jrd.47.211 [DOI] [Google Scholar]
- 30.Webster Marketon J. I., Glaser R.2008. Stress hormones and immune function. Cell. Immunol. 252: 16–26. doi: 10.1016/j.cellimm.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 31.Wettemann R. P., Bazer F. W.1985. Influence of environmental temperature on prolificacy of pigs. J. Reprod. Fertil. Suppl. 33: 199–208. [PubMed] [Google Scholar]
- 32.Wolfenson D., Lew B. J., Thatcher W. W., Graber Y., Meidan R.1997. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 47: 9–19. doi: 10.1016/S0378-4320(96)01638-7 [DOI] [PubMed] [Google Scholar]
- 33.Yu J., Yin P., Liu F., Cheng G., Guo K., Lu A., Zhu X., Luan W., Xu J.2010. Effect of heat stress on the porcine small intestine: a morphological and gene expression study. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 156: 119–128. doi: 10.1016/j.cbpa.2010.01.008 [DOI] [PubMed] [Google Scholar]


