Abstract
Background/purpose
Acidic diet is one major cause of dentine hypersensitivity. The objective of this study was to determine the effects of different tropical fruit juices on dentine permeability and their erosive ability to remove the smear layer in extracted human teeth.
Materials and methods
Thirty-six noncarious human premolars were used, and the dentine was exposed at the tip of the buccal cusp by cutting a cavity (diameter 3 mm, depth 3 mm). Permeability of the dentine was tested under different conditions: with a smear layer and 5 minutes after the application of freshly squeezed green mango, lime, tamarind, and starfruit juices. The smear layer was created before each treatment by gently cutting the dentine with a diamond bur. In the final treatment, the dentine was etched with 37% phosphoric acid for 30 seconds. The erosive ability of these fruit juices to remove the smear layer was also examined using a scanning electron microscope.
Results
Results revealed that application of green mango, tamarind, lime, and starfruit juices for 5 minutes significantly increased dentine permeability by 128.2%, 73.4%, 80.6%, and 70.4%, respectively (P < 0.05, Friedman repeated measures analysis of variance on ranks). The corresponding value of 37% phosphoric acid was 125.1%. Scanning electron microscopy data showed that green mango and lime juices had very strong erosive ability to remove the smear layer, similar to 37% phosphoric acid.
Conclusion
We conclude that tropical fruit juices, especially green mango and lime, increase dentine permeability and have a strong erosive ability to remove the smear layer, which causes dentine hypersensitivity.
Keywords: dentine hypersensitivity, erosion, hydraulic conductance, smear layer, tropical fruit juices
Introduction
Dentine hypersensitivity is a common oral health condition in the adult population,1 with a prevalence ranging between 4% and 57% in general population.2, 3 In patients with dentine hypersensitivity, the dentine is exposed due to either loss of enamel or gingival recession.4
The transduction mechanism of pain in dentine is different from that in other organs of the body because there are no nerve terminals on the exposed dentine surface. The intradental nerves that are responsible for pain in teeth are mostly located in the pulp.5 At present, most evidence supports the so-called hydrodynamic theory explaining the mechanism of dentine sensitivity.6, 7, 8 This mechanism involves a rapid movement of fluid in the dentinal tubules produced by pain-producing stimuli, which is strong enough to excite the nerve terminals in the underlying pulp.6, 7, 8
However, patients who have their cervical dentine exposed either due to a loss of enamel or due to gingival recession may not develop dentine hypersensitivity, because the dentine is covered by a smear layer that occludes the exposed dentinal tubules.9 These patients feel no pain or only mild pain when their teeth are exposed to stimuli such as cold water or air blasts. The term “dentine sensitivity” was used to represent these cases. The term “dentine hypersensitivity” was used when these patients described a significant increase in their dentine sensitivity.9 One mechanism that could develop dentine hypersensitivity is exposure of patients' dentine to acidic diets.10, 11, 12 Absi et al10 demonstrated that hypersensitive dentine had more open dentinal tubules per unit area and a larger diameter of the dentinal tubules. Many natural fruit juices are acidic, which could remove the protective smear layer and increase dentine permeability.13, 14, 15
Ajcharanukul et al16 developed a fluid filtration technique to compare the permeability of dentine; they applied different treatments on the same dentine of the crown in extracted teeth. On the basis of their study, we evaluated the effect of different treatments on the same exposed dentine.
This study aimed to determine the effects of different tropical fruit juices, including green mango, lime, tamarind, and starfruit juices, on dentine permeability using this method. The morphology of the exposed dentine after treatment with these juices was also investigated to evaluate their erosive ability to remove the smear layer.
Materials and methods
The experiments were carried out on 36 extracted premolars. All teeth were free of caries and extracted for orthodontic purposes. After extraction, the teeth were stored in 0.9% normal saline solution with amoxycillin (500 mg/L) at 4°C and used within 2 weeks. The experimental protocol was approved by the Institutional Review Board, Faculty of Dentistry/Faculty of Pharmacy, Mahidol University, Bangkok, Thailand (COE. No. MU-DT/PY-IRB 2012/017.2908).
Tooth preparation
All teeth were sectioned 1–2 mm below the cementoenamel junction using a diamond disc. The coronal pulp was removed, and a cavity (3 mm in width and 3 mm in depth) was prepared on the buccal cusp using a diamond bur in a high-speed handpiece under water spray.
Preparation of freshly squeezed tropical fruit juices
Tropical fruits, including green mango (Manaifera indica Linn), lime (Citrus aurantifolia), tamarind (Tamaridus indica Linn), and starfruit (Averrhoa carambola Linn), were purchased from a local market. The juices were freshly squeezed just before application.
Measurement of pH
The initial pH of each freshly squeezed acidic fruit was determined using an Orion 2 star benchtop pH meter (Thermo Fisher Scientific Inc., Beverly, MA, USA) at room temperature. Each fruit was tested using four different samples.
The total acidity of each freshly squeezed acidic fruit was determined by placing 20 mL of each fruit juice in a glass beaker and titrating with 0.1M sodium hydroxide solution until the pH reached 7.0. Each solution was stirred continuously as the sodium hydroxide was added. The volume of sodium hydroxide required to increase the pH of the sample to neutrality was noted, and this was repeated four times for each fruit.
Dentine permeability measurement
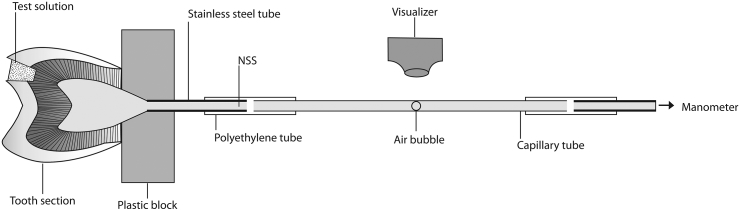
Permeability of dentine was determined by measuring its hydraulic conductance using the fluid filtration method.16 In brief, after tooth preparation, the cut dentine surface of the crown was glued with cyanoacrylate adhesive (Alteco Inc., Osaka, Japan) to a plastic block, which had been sealed to a stainless-steel tube (G18). The tube was connected to a glass capillary with an internal diameter of 300 μm (DADE, Miami, FL, USA) and to a mercury manometer via the polyethylene tube. The pulp chamber, tube, and capillary were filled with normal saline solution. Fluid flow through dentine was detected by observing the movement of a small air bubble introduced into the capillary (Figure 1).
Figure 1.
Diagram of the experimental setup for measurement of fluid flow through dentine in vitro (not to scale). NSS = normal saline solution.
Fluid flow through dentine was recorded before and after juice treatment with a positive pressure of 100 mmHg. Hydraulic conductance values of dentine were calculated.
Application of freshly squeezed tropical fruit juices and experimental design
Four fruit juices were applied to each crown (green mango, lime, tamarind, and starfruit) and lastly crowns were treated with 37% phosphoric acid. The order in which the juices were applied was randomly assigned. In the 24 specimens, each juice would have an equal chance.
After cavity preparation, each tooth was etched with 37% phosphoric acid for 15 seconds. The smear layer was recreated again by gently cutting the dentine with a fine-grain diamond bur (No. RA 4223; Intensive, Viganello-Lugano, Switzerland) in a slow-speed handpiece under a spray of water. The baseline fluid flow through dentine was recorded with a positive pressure of 100 mmHg. Distilled water (negative control) was filled into a cavity (with the smear layer) for 5 minutes, with the pressure of the pulp set at 11 mmHg above atmospheric pressure,7 and the fluid flow through dentine was recorded with a positive pressure of 100 mmHg again. Then, the first juice was filled into a cavity for 5 minutes, with the pressure of the pulp set at 11 mmHg above atmospheric pressure. The cavity was washed with distilled water and the fluid flow through dentine was recorded again. After the first juice treatment, the smear layer of the dentine at the floor of the cavity was recreated again, and exactly the same procedure was repeated for all other fruit juices (randomly assigned). In the last application for all teeth, fluid flow was recorded before and after treatment with 37% phosphoric acid for 30 seconds.
After the experiments, each tooth was sectioned longitudinally through a cavity, and the remaining dentine thickness was measured between the floor of the cavity and the closest pulpal horn with callipers.
Scanning electron microscopy
After cavity preparation and recreation of the smear layer, 12 specimens were divided into six groups. In Group 1, no treatment was performed. In Groups 2, 3, 4, and 5 the cavity was filled for 5 minutes with green mango, lime, tamarind, and starfruit juices, respectively. In Group 6, the dentine was filled with 37% phosphoric acid for 30 seconds.
All teeth were processed and examined using a scanning electron microscope (JSM-5410 LV; JEOL, Tokyo, Japan).
Statistical analysis
The mean hydraulic conductance and standard deviation (SD) at the baseline and after treatment were compared using paired t test. A P value < 0.05 was considered significant.
Increases in the percentages of hydraulic conductance are summarized as box plots. Comparisons among the different types of freshly squeezed acidic fruits were made using Friedman repeated measures analysis of variance on ranks. Student–Newman–Keuls test was used to make comparisons between the medians, and P < 0.05 was considered significant.
Results
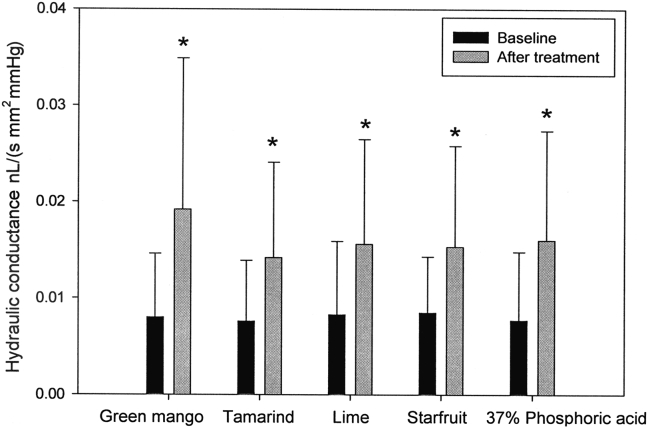
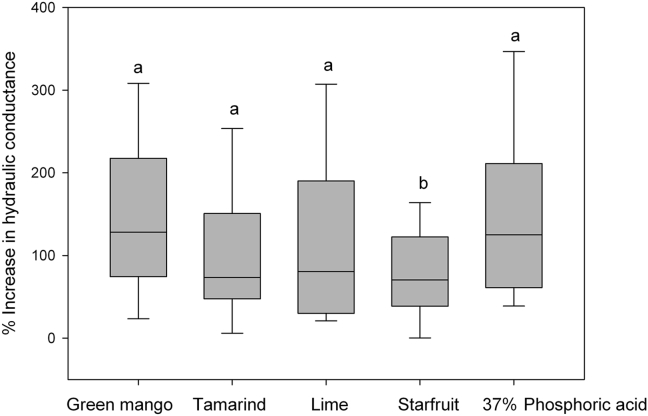
The mean hydraulic conductance of the smear layer and SD before treatment with green mango juice, tamarind juice, lime juice, starfruit juice, and 37% phosphoric acid were 0.008 ± 0.007 nL/(s mm2 mmHg), 0.008 ± 0.006 nL/(s mm2 mmHg), 0.008 ± 0.008 nL/(s mm2 mmHg), 0.009 ± 0.006 nL/(s mm2 mmHg), and 0.008 ± 0.007 nL/(s mm2 mmHg), respectively. The corresponding values after treatment were 0.019 ± 0.016 nL/(s mm2 mmHg), 0.014 ± 0.010 nL/(s mm2 mmHg), 0.016 ± 0.011 nL/(s mm2 mmHg), 0.015 ± 0.011 nL/(s mm2 mmHg), and 0.016 ± 0.011 nL/(s mm2 mmHg), respectively. The values were considered statistically significant (P < 0.001, paired t test; Figure 2). The percentages increased from the baseline after treatment, which were 128.2% (green mango), 73.4% (tamarind), 80.6% (lime), 70.4% (starfruit), and 125.1% (37% phosphoric acid; Figure 3).
Figure 2.
Mean values of the hydraulic conductance in 24 teeth treated with different freshly squeezed acidic fruits and 37% phosphoric acid. Data were recorded before (black column) and after (gray column) treatment with each tropical fruit juice. Each error bar represents 1 SD. Significantly higher values (P < 0.001) were found after treatment with each fruit juice compared to that before treatment. * Significant differences of paired t test (P < 0.001). SD = standard deviation.
Figure 3.
Percentage increase of the hydraulic conductance of dentine after being exposed to tropical fruit juices for 5 minutes and 37% phosphoric acid for 30 seconds. Each box shows the median and the 10th percentile, 25th percentile, 75th percentile, and 90th percentile. The same lowercase letter indicates that there was no significant difference in the median values among the treatment groups (P > 0.05).
There was a significant difference in the percentage increase in hydraulic conductance between mango and starfruit, tamarind and starfruit, lime and starfruit, and phosphoric acid and starfruit (P < 0.05). The mean remaining dentine thickness and SD was 1.82 ± 0.43 mm (n = 24). Acidity values of the juices are represented as mean (SD) in Table 1. The pH values of green mango and lime were similar, while tamarind was found to be less acidic. Lime juice showed the highest total acidity, followed by green mango, starfruit, and tamarind juices.
Table 1.
Mean values and standard deviations of acidity of freshly squeezed tropical fruit juices and 37% phosphoric acid immediately after preparation of juice but before application to dentine samples.
| Substances | pH (SD) | Total acidity (M) (SD) |
|---|---|---|
| Green mango | 2.54 (0.01) | 0.755 (0.01) |
| Tamarind | 4.16 (0.03) | 0.365 (0.02) |
| Lime | 2.39 (0.02) | 0.94 (0.05) |
| Starfruit | 3.07 (0.01) | 0.40 (0.01) |
| 37% Phosphoric acid | 1.69 (0.00) | 5.88 (0.00) |
SD = standard deviation.
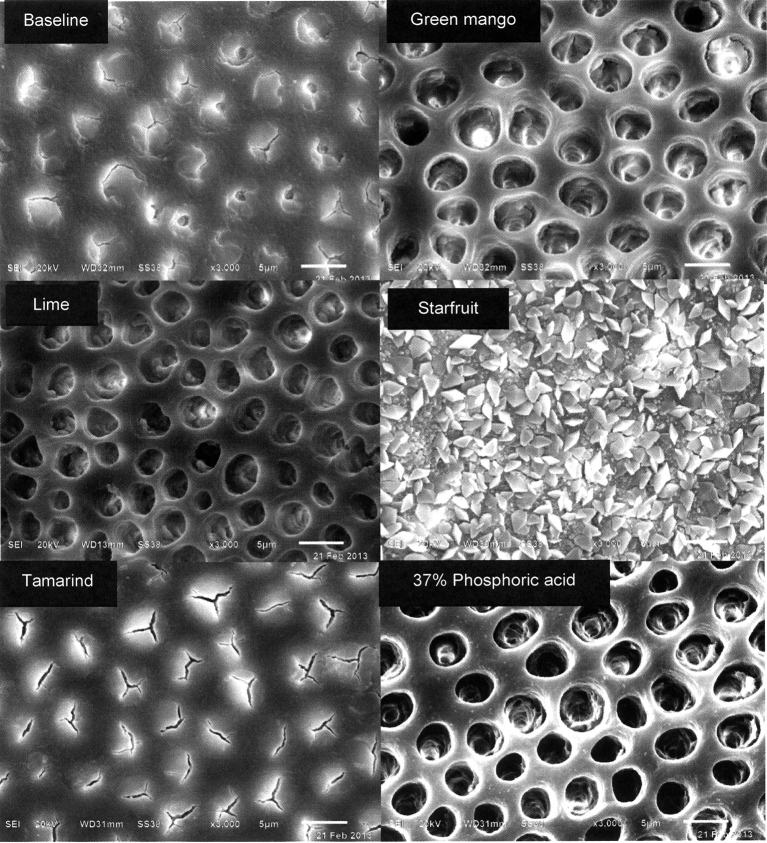
Figure 4 shows the scanning electron micrographs of the exposed dentine after the smear layer was created with bur, and after treatment with fruit juices and 37% phosphoric acid. Green mango and lime juices demonstrated a very strong erosive ability to remove the smear layer, similar to that resulting from 37% phosphoric acid. Tamarind and starfruit juices had less erosive ability, and starfruit juice caused formation of crystals covering the dentine surface (Figure 4).
Figure 4.
Scanning electron micrographs of exposed human dentine with a smear layer and after being treated with different tropical fruit juices and 37% phosphoric acid.
Discussion
These experiments demonstrated that tropical fruit juices increased dentine permeability from 70% to 128%. Green mango and lime juices had very strong erosive ability to remove the smear layer, similar to 37% phosphoric acid. People consuming these tropical fruits and juices could develop dentine hypersensitivity.
Previous studies by Pashley et al17 suggest that the permeability of dentine mostly depends on surface resistance (86.4%). Other contributors were the intratubular resistance (7.5%) and pulpal resistance including odontoblasts (6.3%). This indicates that our method, cleaning the exposed dentine surface and recreating the standard smear layer with the same bur, is useful when comparing the effects of different fruit juices, since it uses the same dentine with the same pulpal and intratubular resistance. This method is more reliable than using different teeth for each group.
Some very interesting questions remain to be answered for understanding the mechanism of dentine sensitivity. Recently, transient receptor potential channels were found in odontoblasts.18 Transient receptor potential melastatine family member 8 is a cold receptor, which suggests that odontoblasts may play a role in dentine sensitivity. However, further studies are needed to confirm the odontoblast as a pain sensor. Current evidence supports the hydrodynamic theory. Vongsavan and Matthews7 demonstrated a direct relationship between discharge of the intradental nerves and fluid flow through dentine in cats. A similar relationship between dentine sensitivity and fluid flow through dentine has also been reported in humans.8
Currently, it is well accepted that hypersensitive dentine has a higher number of open dentinal tubules per unit area, with a larger diameter of the dentinal tubule.9, 10, 11, 12 Acidic diets that have the erosive ability not only to remove the smear layer, but also to decalcify dentine are major factors causing this problem.9 The results of our experiments agreed with those of Prati et al15 that dietary acidic substances such as fruit juices significantly increase dentine permeability by opening dentinal tubules, leading to dentine hypersensitivity. Scanning electron microscopy data revealed that tropical fruit juice had an erosive ability to remove the smear layer similar to green apple, lemon, and orange.13, 14
Acidic fruit juices of our study consist of many organic acids, including citric acid, tartaric acid, mangiferonic acid, malic acid, ascorbic acid, and oxalic acid, which contribute to the low pH value.19 Larsen and Nyvad19 also reported that erosion was minimal in beverages having pH above 4.2 but became more evident as pH decreased below 4.0. In this study, a single exposure of dentine to green mango (pH 2.54), tamarind (pH 4.16), lime (pH 2.39), or starfruit (pH 3.07) juice, or to 37% phosphoric acid (pH 1.69) for a relatively short period of time (5 minutes) was sufficient to remove the smear layer. Initial pH is easily obtained and is the most used index to indicate the acidity of a product, but the pH value gives no indication of the overall acidic content of the fruit juice.20 Whereas titratable acidity (or neutralizable acidity), which is a measure of the total acid content in fruit juices, is a more important indicator than actual pH value in determining erosive potential of beverages.21
Titratable acidity, pH, and acid type of fruit juices might be important factors responsible for the removal of the smear layer and creation of different patterns on the exposed dentine surface.22, 23, 24 Mango and lime juices removed both the smear layer and smear plugs, whereas tamarind and starfruit juices could only partially dissolve the smear layer. Other components in fruits may also play an important role in the mechanism. Starfruit juice containing a high concentration of oxalic acid could form the crystals covering the dentine surface.25 Oxalate nephropathy after ingesting starfruit was also reported in humans.26
The Canadian Advisory Board in Dentin Hypersensitivity recommends brushing of teeth before ingestion of acidic meals, although it appears uncommon for people to brush their teeth before meals.4 Brushing teeth with toothpaste significantly lowers the hydraulic conductance of dentine after acidic drinks.15 Recently, Kiettipirodom et al27 reported that topical application of cow's milk could reduce dentine permeability, similar to a desensitizing toothpaste. This method of rinsing the teeth with cow's milk after an acidic meal would be convenient and practical for people.
It was concluded that tropical fruit juices, especially green mango and lime juices, increased dentine permeability and had a strong erosive ability to remove the smear layer, which may lead to dentine hypersensitivity. It is suggested that a preventive strategy for managing patients with hypersensitive dentine is avoiding consumption of these tropical juices.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by Faculty of Dentistry, Mahidol University.
References
- 1.Holland G.R., Narhi M.N., Addy M., Gangarosa L., Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–813. doi: 10.1111/j.1600-051x.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 2.Rees J.S., Addy M. A cross-sectional study of dentine hypersensitivity. J Clin Periodontol. 2002;29:997–1003. doi: 10.1034/j.1600-051x.2002.291104.x. [DOI] [PubMed] [Google Scholar]
- 3.Irwin C.R., McCuster P. Prevalence of dentine hypersensitivity in general dental population. J Ir Dent Assoc. 1997;43:7–9. [PubMed] [Google Scholar]
- 4.Canadian Advisory Board on Dentin Hypersensitivity Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–226. [PubMed] [Google Scholar]
- 5.Byers M., Kish S.J. Delineation of somatic nerve endings in rat teeth by radioautography of axon-transported protein. J Dent Res. 1976;55:419–425. doi: 10.1177/00220345760550032001. [DOI] [PubMed] [Google Scholar]
- 6.Brännström M. A hydrodynamic mechanism in the transmission of pain producing stimuli through the dentine. In: Anderson D.J., editor. Sensory Mechanism in Dentine. Pergamon; Oxford: 1963. pp. 73–79. [Google Scholar]
- 7.Vongsavan N., Matthews B. The relationship between the discharge of intradental nerves and the rate of fluid flow through dentine in the cat. Arch Oral Biol. 2007;52:640–647. doi: 10.1016/j.archoralbio.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Charoenlarp P., Wanachantararak S., Vongsavan N., Matthews B. Pain and the rate of dentinal fluid flow produced by hydrostatic pressure stimulation of exposed dentine in man. Arch Oral Biol. 2007;52:625–631. doi: 10.1016/j.archoralbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Pashley D.H. How can sensitive dentine become hypersensitive and can it be reversed? J Dent. 2013;41(Suppl. 4):S49–S55. doi: 10.1016/S0300-5712(13)70006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Absi E.G., Addy M., Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14:280–284. doi: 10.1111/j.1600-051x.1987.tb01533.x. [DOI] [PubMed] [Google Scholar]
- 11.Yoshiyama M., Masada A., Uchida A., Ishida H. Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J Dent Res. 1989;68:1458–1502. doi: 10.1177/00220345890680110601. [DOI] [PubMed] [Google Scholar]
- 12.Orchardson R., Gillam D.G. Managing dentin hypersensitivity. J Am Dent Assoc. 2006;137:990–998. doi: 10.14219/jada.archive.2006.0321. [DOI] [PubMed] [Google Scholar]
- 13.Addy M., Absi E.G., Adam D. Dentine hypersensitivity. The effect in vitro of acids and dietary substances on root-planned and burred dentine. J Clin Periodontol. 1987;14:274–279. doi: 10.1111/j.1600-051x.1987.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 14.Corrêa F., Sampaio J., Rossa C., Orrico S. Influence of natural fruit juices in removing the smear layer from root surfaces—an in vitro study. J Can Dent Assoc. 2004;70:697–702. [PubMed] [Google Scholar]
- 15.Prati C., Montebugnoli I., Suppa P., Valdrè G., Mongiorgi R. Permeability and morphology of dentin after erosion induced by acidic drinks. J Periodontol. 2003;74:428–436. doi: 10.1902/jop.2003.74.4.428. [DOI] [PubMed] [Google Scholar]
- 16.Ajcharanukul O., Oranratmanee K., Thitikunakorn S. Effect of different osmotic stimuli on fluid flow before and after self-etching adhesive application. J Adhes Dent. 2010;12:103–108. doi: 10.3290/j.jad.a17526. [DOI] [PubMed] [Google Scholar]
- 17.Pashley D.H., Livingston M.J., Greenhill J.D. Regional resistance to fluid flow in human dentine in vitro sensitivity. Arch Oral Biol. 1978;23:807–810. doi: 10.1016/0003-9969(78)90159-0. [DOI] [PubMed] [Google Scholar]
- 18.El Karim I.A., Linden G.J., Curtis T.M. Human odontoblasts express functional thermo-sensitive TRP channels implications for dentin sensitivity. Pain. 2011;152:2211–2223. doi: 10.1016/j.pain.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Larsen M.J., Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999;33:81–87. doi: 10.1159/000016499. [DOI] [PubMed] [Google Scholar]
- 20.Singh S., Jindal R. Evaluating the buffering capacity of various soft drinks, fruit juices and tea. J Conserv Dent. 2010;13:129–131. doi: 10.4103/0972-0707.71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards M., Creanor S.L., Foye R.H., Gilmour W.H. Buffering capacities of soft drinks: the potential influence on dental erosion. J Oral Rehabil. 1999;26:923–927. doi: 10.1046/j.1365-2842.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- 22.Batitucci R.G., Zandim D.L., Rocha F.R.G., Pinheiro M.C., Fontanari L.A., Sampaio J.E.C. Effect of acid fruit juices combined with electric or sonic toothbrushing on root dentin permeability—an in vitro study. Braz Dent J. 2012;23:667–671. doi: 10.1590/s0103-64402012000600007. [DOI] [PubMed] [Google Scholar]
- 23.Lussi A., Kohler N., Zero D., Schaffner M., Megert B. A comparison of the erosive potential of different beverages in primary and permanent teeth using an in vitro model. Eur J Oral Sci. 2000;108:110–114. doi: 10.1034/j.1600-0722.2000.90741.x. [DOI] [PubMed] [Google Scholar]
- 24.West N.X., Hughes J.A., Addy M. Erosion of dentine and enamel in vitro by dietary acids: the effect of temperature, acid character, concentration and exposure time. J Oral Rehabil. 2000;27:875–880. doi: 10.1046/j.1365-2842.2000.00583.x. [DOI] [PubMed] [Google Scholar]
- 25.Patil A.G., Patil D.A., Phatak A.V., Chandra N. Physical and chemical characteristics of carambola (Averrhoa carambola L.) fruit at three stages of maturity. Int J Appl Biol Pharm. 2010;1:624–629. [Google Scholar]
- 26.Chen C.L., Fang H.C., Chou K.J., Wang J.S., Chung H.M. Acute oxalate nephropathy after ingestion of star fruit. Am J Kidney Dis. 2001;37:418–422. doi: 10.1053/ajkd.2001.21333. [DOI] [PubMed] [Google Scholar]
- 27.Kiettipirodom W., Saeko S., Melanon S. IADR 83rd General Session and Exhibition. International Association for Dental Research; Capetown, South Africa: 2014. Effect of desensitizing dentifrices and milk on hydraulic conductance of human dentin. Abstract 1117. [Google Scholar]