Abstract
Background/purpose
Increasing studies have indicated the involvement of Porphyromonas gingivalis in atherosclerosis. T helper 17 (Th17)/Treg balance is critical during atherosclerosis. However, whether P. gingivalis oral infection is associated with Th17/Treg imbalance is unclear. The aim of the present study was to investigate the effect of P. gingivalis on Th17/Treg balance during atherosclerosis.
Materials and methods
ApoE–/– and C57BL/6 mice were inoculated orally with P. gingivalis ATCC 33277 for 9 weeks. The alveolar bone loss was assessed by microcomputerized tomography. The area of atherosclerosis plaque was identified by oil red O staining. Plaque stability was analyzed by CD68 and αSMA immunohistochemistry staining and Masson staining. The frequency of Th17 and Treg in spleen was detected by flow cytometry. The mRNA expression of Th17- and Treg-related factors was determined by quantitative polymerase chain reaction. Interleukin (IL)-6, a critical factor in modulating T-cell differentiation, was determined from spleen cells and mouse dendritic cells by enzyme-linked immunosorbent assay.
Results
Long-term P. gingivalis oral infection induced alveolar bone resorption. In ApoE–/– mice, P. gingivalis enhanced atherosclerotic lesion formation and plaque instability accompanied with a decreased Treg frequency and an increased Th17 cell frequency. In addition, mRNA expression of retinoic acid receptor-related orphan receptor γt and IL-17 was increased, and that of transforming growth factor (TGF) β and IL-10 was decreased in P. gingivalis-infected ApoE–/– mice. Furthermore, secretion of IL-6 was elevated in the spleen of P. gingivalis-infected ApoE–/– mice, as well as in mouse dendritic cells after P. gingivalis infection.
Conclusion
P. gingivalis oral infection may promote Th17/Treg imbalance by influencing T-cell differentiation during the process of atherosclerosis, with a larger lesion area and decreasing plaque instability.
Keywords: atherosclerosis, plaque instability, Porphyromonas gingivalis, T helper 17/Treg balance
Introduction
Chronic periodontitis is a common chronic oral infectious disease characterized by gingival bleeding, alveolar bone destruction, and tooth mobility.1 The anaerobic bacteria Porphyromonas gingivalis is one of the main pathogens of periodontitis.2 Studies have indicated that in addition to local inflammation at the initial site of infection, P. gingivalis has the ability to disseminate from ulcerative periodontal pockets to circulation, and interact with the heart, liver, and other tissues.3 Therefore, P. gingivalis plays an important role in periodontitis-associated systemic disease, such as atherosclerosis.
Kozarov et al4 reported that P. gingivalis was examined in human atherosclerosis lesions, indicating the oral pathogen spread to vessel via general circulation. In vivo studies suggested that P. gingivalis infection promotes atherosclerotic plaque formation.3, 5 Nowadays, P. gingivalis infection is assumed to be a risk factor for cardiovascular disease.6 P. gingivalis and its virulence factors may promote the development of atheromatous plaque through different pathways.7, 8 Owing to the homology between GroEL in bacteria and heat shock protein 60 in mammals, the cross reactivity between P. gingivalis GroEL and endothelial cell heat shock protein 60 would activate the autoimmune response mediated by CD4+ T cells, which contributes to the physiopathology of atherosclerosis.9 P. gingivalis plays a prominent role in modulating immune reaction in its relationship with atherosclerosis.6, 10
T lymphocytes, especially CD4+ T cells are shown to play an important role in promoting atherosclerosis.11 The immune balance between T helper (Th) cells and Tregs are considered to be critical for controlling systemic inflammation.12 Th17/Treg balance is important in the pathogenesis of atherosclerosis.13 Th17 cells are a newly discovered T-cell subset and are implicated to have a critical role in this progression of atherosclerosis. Th17 cells express retinoic acid-related orphan receptor γt and mainly produce proinflammatory cytokine interleukin (IL)-17A, which participates in the induction of inflammation of atherosclerotic plaque.14 Tregs are characterized by the expression of the forkhead box P3 (FoxP3). Tregs may suppress inflammation by contacting with other effective cells or by secreting anti-inflammatory IL-10 and transforming growth factor β.15 The balance between Th17 cells and Tregs may be critical in regulating immune homeostasis. It has been reported that Th17/Treg imbalance existed in acute coronary syndrome patients.16
Studies have shown that Treg is a protective CD4+ T subset in the development of periodontitis. Th17 cell-related cytokines are involved in periodontal lesions.17 Wang et al18 reported that P. gingivalis decreased the percentage of Tregs and increased Th17 cells in cervical lymph nodes and gingival tissues in experimental periodontitis. Our previous clinical study indicated that the percentage of Tregs in P. gingivalis-infected atherosclerotic patients was decreased.19 Hagiwara et al20 found that sublingual immunization with GroEL vaccine could increase CD4+FoxP3+ cells in submandibular glands and prevent P. gingivalis-accelerated atherosclerosis. As observed in these studies,18, 19 P. gingivalis infection could induce T-cell reaction in periodontal tissue and adjacent lymph nodes; however, little is known about whether oral infection of P. gingivalis could induce T-cell-mediating response in atherosclerosis. In the current study, we studied the process of P. gingivalis-associated atherosclerosis by inducing long-term oral infection with P. gingivalis 33277 in mice to investigate the relationship between P. gingivalis infection and Th17/Treg balance in atherosclerosis.
Materials and methods
Mice
Four-week-old male C57BL/6 mice (n = 12) and ApoE–/– mice (apolipoprotein E knockout mice in C57BL/6 background; n = 12) were purchased from Model Animal Research Center of Nanjing University, Nanjing, China. Mice were maintained in specific pathogen-free conditions with a regular diet and cared for according to the ethics committees of Nanjing University; this study was conducted in accordance with the standards of the Helsinki Declaration.
Bacteria and oral infection
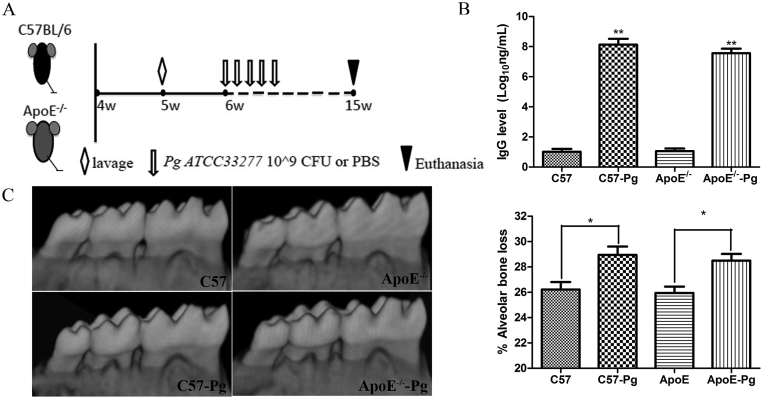
P. gingivalis strain ATCC 33277 was cultured anaerobically on blood agar plates and seeded in trypticase soy broth supplemented with yeast extract, hemin, and menadione, as we described previously.21 The colony-forming unit (CFU) was standardized as OD660nm of 0.8 = 109 CFU/mL. The model of experimental periodontitis was developed following the method of Hayashi et al22 with slight modification. At 5 weeks, the mice were given a high-fat diet and treated with antibiotics orally for 5 days. Thereafter, C57BL/6 and ApoE–/– mice were divided into four groups (n = 6 per group; Figure 1A): (1) C57BL/6; (2) C57BL/6 + P. gingivalis; (3) ApoE–/–; and (4) ApoE–/– + P. gingivalis. P. gingivalis 33277 in 100 μL 2% carboxymethylcellulose with phosphate-buffered saline (PBS; 108 CFU) was applied to the buccal surface of mandible five times a week for 9 weeks in Groups 2 and 4. Mice in the other two groups (Groups 1 and 3) received vehicle (100 μL 2% carboxymethylcellulose with PBS). Mice were euthanized 5 days after the final oral challenge.
Figure 1.
P. gingivalis-infected periodontitis schedule and induced alveolar bone resorption. (A) Five-week-old male C57BL/6 mice (n = 12) and ApoE–/– mice were treated with antibiotics orally (⋄) for 5 days. Thereafter, oral infection ( ↓) of P. gingivalis or vehicle was given to the buccal surface of mandible five times a week for 9 weeks. Mice were euthanized ( ∇) 5 days after the final oral challenge. (B) Plasma IgG antibody levels in both P. gingivalis-infected mice and sham-infected mice at 15 weeks were determined by ELISA. Concentration of plasma IgG antibody levels in P. gingivalis-infected mice was increased after infection. (C) Evidence of P. gingivalis-induced periodontitis shown by alveolar bone loss on 3-D tomography analysis of mandibular molars. * P < 0.05. ** P < 0.001. CFU = colony-forming unit; 3-D = three-dimensional; ELISA = enzyme-linked immunosorbent assay; IgG = immunoglobulin G; PBS = phosphate-buffered saline; Pg = Porphyromonas gingivalis.
Tissue preparation
All mice were euthanized by exsanguination under anesthesia with i.p. injections of chloral hydrate. Muscle relaxation was observed to decide the adequate amount of anesthesia. Spleens were collected immediately and immersed in cold PBS containing antibiotics for flow cytometry and real-time polymerase chain reaction (RT-PCR) analysis. Mandibles were harvested and fixed in 10% formalin immediately for microcomputerized tomography assay. The heart and the aorta were dissected out and stored at 4°C until processing.
Anti-P. gingivalis immunoglobulin G antibody in plasma
Anti-P. gingivalis immunoglobulin G (IgG) antibody in plasma was determined by enzyme-linked immunosorbent assay (ELISA) following the method of Bainbridge et al.23 Briefly, P. gingivalis ATCC33277 was cultured anaerobically in trypticase soy broth containing yeast extract, hemin, and menadione. In order to prepare the antibody, P. gingivalis was treated with 0.5% formalin overnight and diluted to an OD600 of 0.3. The antibody was deposited in the wells of microtiter plates. Plasma was diluted at 1:100 before detection. Plasma was added into microtiter plates and reacted with the bacteria for 2 hours at room temperature. Alkaline phosphatase-labeled goat antimouse IgG was added. The OD405 values were tested after adding NaOH into each well. For the analyses, plasma from eight mice was used to obtain standard titration curves.
Measurement of alveolar bone resorption
Mandible was scanned using microcomputerized tomography (Skyscan, AArselaar). Three-dimensional views were reconstructed with CTan version 1.5.0 software (Skyscan, AArselaar). Alveolar bone loss was measured by calculating bone volume fraction according to Park et al.24 Briefly, roofs of the furcations and the root apexes were defined as references to identify the borders of the region of interest. Contours were drawn beginning immediately below the roofs of the furcations in the coronal plane and moving in the apical direction. Two-dimensional contours were chosen at regular intervals. Two-dimensional regions of interest were defined as the distance from the cementoenamel junction to a fixed base at the palatal surfaces of the roots of the first and second molar teeth. The remaining two-dimensional bone regions of interest were defined as the distance from the crest of ridge to a fixed base. Three-dimensional version was reconstructed to quantify the volume of bone. Bone volume fraction was the remaining bone volume in volume of interest/volume of interest.
Analysis of plaques
The heart was embedded in tissue optimal cutting temperature compound. Cryosections (10 μm) of the aortic root containing the aortic valves were made. Hematoxylin and eosin staining was performed to observe the structural characteristic of aortic root. Atherosclerotic lesions were measured by lipid deposition stained with oil red O. In brief, five sections of aortic root, separated by 100 μm, per sample were dipped in 60% isopropanol and stained in oil red O for 30 minutes. Then, sections were washed in water and counterstained with hematoxylin. To calculate lesions, pictures were analyzed by computer image analysis software. The average area of the five sections for each sample was used for analysis as described by Nakajima et al.25
Immunohistochemistry was performed using rat antimouse CD68 and αSMA. Images were captured. Stained area was quantified blindly by computer-aided morphometry software (Image Pro Plus, Media Cybernetics, MD, USA). The area of CD68- or αSMA-positive staining in total plaque area was calculated to identify macrophages and smooth muscle cells. Masson staining was used to measure fibrosis in the aortic root. The percentage of positive staining area in total plaque area was calculated to identify the collagen content.
Flow cytometric analysis of Treg and Th17 cells in the spleen
Splenocytes were isolated from spleens. For Treg cell staining and detection, antimouse CD4 fluorescent monoclonal antibody labeled with fluorescein isothiocyanate (eBioscience, San Jose, CA, USA) and antimouse CD25 labeled with allophycocyanin (eBioscience) were used as surface markers. After washing with PBS, cells were fixed, permeabilized, and labeled with phycoerythrin-conjugated antimouse FoxP3 antibody (eBioscience). For Th17 cell analysis, cells were incubated with Phorbol-12-myristate-13-acetate (PMA) (50 ng/mL), ionomycin (1 μg/mL), and monesin (1.7 μg/mL; Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 6 hours to activate T cells and stimulate the accumulation of intracellular IL-17. Surface staining was performed with fluorescein isothiocyanate-conjugated antimouse CD4 antibody. Cytofix/Cytoperm reagent (BD Biosciences, San Jose, CA, USA) was used to fix and permeabilize cells. Cells were stained with phycoerythrin-conjugated antimouse IL-17A antibody (eBioscience). Stained cells were analyzed by flow cytometry on an FACS Calibur flow cytometer (BD Biosciences). The frequency of CD4+CD25+FoxP3+Tregs and CD4+IL17+Th17 cells was analyzed using FlowJo software (BD Biosciences).
RT-PCR analysis of transcriptional factors
Total RNA of splenocytes and aorta was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). The cDNA was obtained with PrimeScript RT reagent kit with a gDNA eraser (Takara, Dalian, China). RT-PCR was performed with iTaq Universal SYBR green Supermix (Bio-Rad, Hercules, CA, USA). Primers for transcription factors and cytokines were used as listed in Table 1. All PCR reactions were performed with the Applied Biosystems (ABI, CA, USA). Briefly, reactions contained 2* SYBR Green PCR Master Mix (Bio-Rad), an upstream primer, a downstream primer, and reverse-transcribed RNA. Amplification was carried out as follows: 95°C for 30 seconds, followed by 40 cycles at 95°C for 5 seconds, and 60°C for 30 seconds. Each gene was tested in triplicate.
Table 1.
Primer sequences used for RT-PCR.
| Specific primer set | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| FoxP3 | GTTTAGTGCTGTGGCACTGT | GGTTCAAGGAAGAAGAGGTGT |
| RORγt | GCGGAGCAGACACACTTACA | TTGGCAAACTCCACCACATA |
| TGFβ1 | TGACGTCACTGGAGTTGTACGG | GGTTCATGTCATGGATGGTGC |
| IL-2 | CACTGACACTTGTGCTCCTT | GAAAGTCCACCACAGTTGCT |
| IL-10 | GGTTGCCAAGCCTTATCGGA | ACCTGCTCCACTGCCTTGCT |
| IL-17A | CCTCAGACTACCTCAACCG | CTCCCTCTTCAGGACCAG |
| IL-6 | CCACTTCACAAGTCGGAGGCTTA | GCAAGTGCATCATCGTGTTCATAC |
| GAPDH | AGCAATGCCTCCTGCACCACCAAC | CCGGAGGGGCCATCCACAGTCT |
FoxP3 = forkhead box P3; IL = interleukin; RORγt = receptor-related orphan receptors γt; RT-PCR = real-time polymerase chain reaction; TGFβ1 = transforming growth factor β1; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
Cell culture
To analyze the T-cell response during atherosclerosis, cells from the spleen were cultured with anti-CD3 (1 μg/mL) and soluble anti-CD28 (2 μg/mL) for 96 hours. The culture supernatant was collected to determine cytokine levels.
For culturing mouse dendritic cells, 5–6-week-old ApoE–/– mice were killed by cervical dislocation and disinfected in 75% ethanol for 5 minutes. The tibias and femurs were removed to isolate bone marrow cells. Cells were cultured in RPMI 1640 complete media containing 20 ng/mL granulocyte-macrophage colony stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN, USA) and 1 ng/mL IL-4. The unattached cells and cell debris were removed 12 hours later, and then the fresh medium was supplemented with GM-CSF and IL-4. On Day 9, cells were collected and resuspended in PBS. Dendritic cells were defined using anti-CD11c (eBioscience). Dendritic cells were seeded in 12-well dishes and infected with P. gingivalis at multiplicity of infection (MOI) 25 and MOI 100. Supernatants were collected after 24 hours and stored at −80°C.
ELISA of cytokines in plasma
The levels of mouse IL-6 (Dakewe, Shanghai, China), IL-17A (eBioscience), and mouse IL-10 (eBioscience) in the supernatant were detected with ELISA kits following the manufacturer's instructions. Every sample was measured in duplicate. Concentrations of the cytokines were calculated according to the standard curve.
Statistical analysis
Data were analyzed with statistical software SPSS19.0. The Shapiro–Wilk test was performed to determine normal distribution. All data followed a normal distribution, so the results were presented as mean ± standard deviation. The t test was used for values between two groups. A P value < 0.05 was considered to be statistically significant. One-way analysis of variance was used for values among three or four groups, and Dunnett t test was used for every two groups after Bonferroni correction.
Results
P. gingivalis oral infection induced alveolar bone resorption
Plasma antibody to P. gingivalis is an indicator of periodontal risk. Plasma IgG level in P. gingivalis-infected C57BL/6 mice and ApoE–/– mice were 8.12 ± 0.50 and 7.57 ± 0.58, respectively, which are obviously higher than those in uninfected mice (0.99 ± 0.30 and 1.05 ± 0.24, respectively). No difference was observed between wild-type and ApoE–/– mice (Figure 1B).
To confirm that P. gingivalis oral infection resulted in periodontitis, we measured bone loss in both wild-type and ApoE–/– mice. As expected, long-term P. gingivalis infection induced alveolar bone resorption for both wild-type and ApoE–/– mice. Moreover, P. gingivalis infection-induced resorption between was not different between wild-type and ApoE–/– mice (Figure 1C).
P. gingivalis oral infection promoted atherosclerosis formation with decreased plaque stability
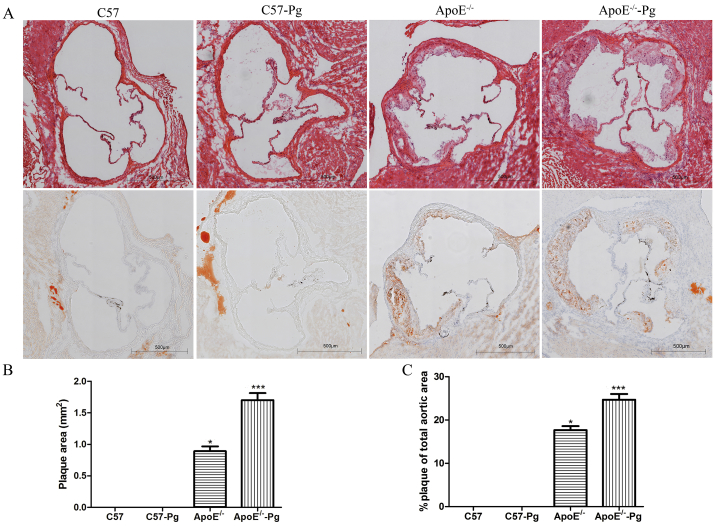
To explore the effect of P. gingivalis on the progression of atherosclerosis, the plaque area at aortic sinus was examined by histomorphological analysis. Hematoxylin staining showed that the intima was continuous, and smooth muscle cells were distributed uniformly in wild-type mice, whereas lipid and inflammatory cells were apparent in ApoE–/– mice. P. gingivalis-infected ApoE–/– mice had significantly larger lesion areas compared with uninfected ApoE–/– mice (Figure 2).
Figure 2.
Plaque formation in the aortic root. (A) Representative aortas from P. gingivalis-infected and sham-infected mice were stained with HE and oil red O (40× magnification). (B and C) Atherosclerosis of aortic section was expressed in terms of plaque area and percentage of the total area (n = 6 mice per group). Lesion areas of P. gingivalis-infected ApoE–/– mice were larger than those of uninfected ApoE–/– mice. * P < 0.0125 versus C57, Bonferroni correction, α = 0.05/4. ** P < 0.0125 versus ApoE–/–, Bonferroni correction, α = 0.05/4. Pg = Porphyromonas gingivalis.
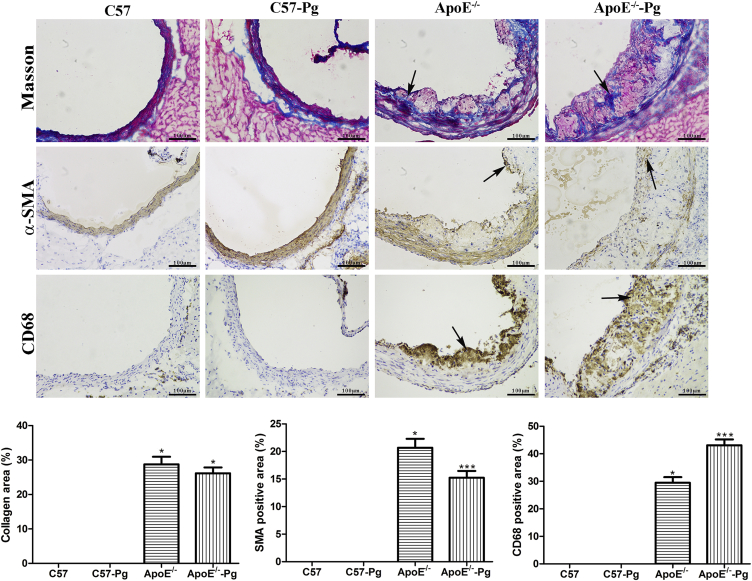
Atherosclerotic plaque components including smooth muscle cells, macrophage, and collagen content in ApoE–/– mice were analyzed to evaluate plaque instability (Figure 3). Relative smooth muscle cell content stained with αSMA in plaques was decreased in P. gingivalis-infected ApoE–/– group compared with that in uninfected ApoE–/– group. Moreover, macrophage accumulation stained with CD68 was significantly increased in the P. gingivalis-infected ApoE–/– group. However, no significant changes were found in the collagen content stained by Masson staining. Taking together all these observations, P. gingivalis promotes atherosclerosis instability with a larger area, decreasing smooth muscle cell content and increasing inflammation reaction.
Figure 3.
Atherosclerotic plaque components in the aortic root. Aortic sinus was stained with αSMA for smooth muscle cells and CD68 for macrophage to perform immunohistochemistry in mice at 15 weeks. Fibrous area was stained by Masson staining (200× magnification). Quantitative analysis of CD68-, αSMA-, and Masson-positive staining in the total plaque area was performed. Data are presented as means ± standard deviation. P. gingivalis infection decreased smooth muscle cell content and increased macrophage content in the plaque of ApoE–/– mice (n = 6 mice per group). * P < 0.0125 versus C57, Bonferroni correction, α = 0.05/4. ** P < 0.0125 versus ApoE–/–, Bonferroni correction, α = 0.05/4. Pg = Porphyromonas gingivalis; aSMA = alpha-smooth muscle actin.
P. gingivalis promoted Th17/Treg imbalance in the development of atherosclerosis
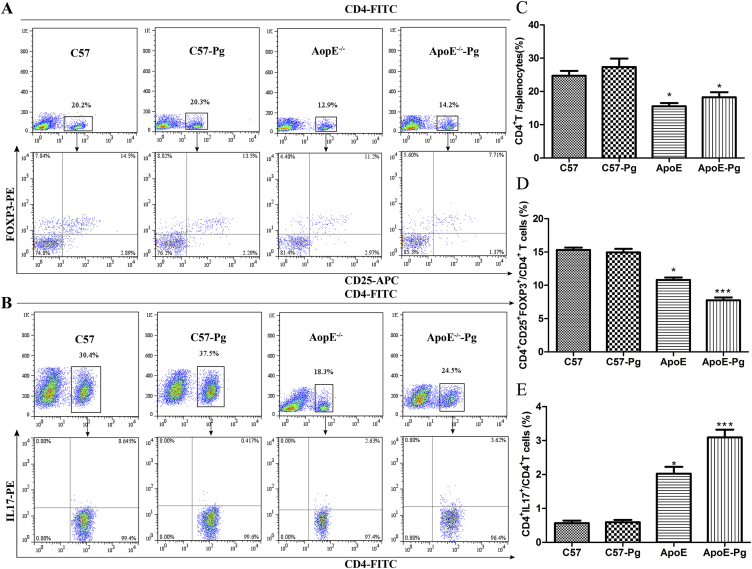
The percentages of Th17 cells and Tregs in splenic cells were analyzed. No significant difference was found in CD4+ T cells between sham-infected mice and P. gingivalis-infected mice. Moreover, there was no statistically significant difference between sham-infected wild-type mice and infected wild-type mice. However, the proportion of CD4+CD25+FoxP3+ cells decreased in ApoE–/– mice. Moreover, P. gingivalis-infected ApoE–/– mice had a lower proportion of Tregs compared with sham-infected ApoE–/– mice (Figures 4A and 4D). By contrast, the percentages of CD4+IL17+ T cells were elevated in ApoE–/– mice, especially in P. gingivalis-challenged ApoE–/– mice (Figures 4B and 4E).
Figure 4.
Flow cytometric analysis of Th17 and Treg in splenocytes of C57BL/6 and ApoE–/– mice. Representative profiles for detecting (A) CD25+FOXP3+ T cells and (B) IL-17+ T cells by flow cytometry within the CD4+ gate. (C) The number of CD4+T cells in P. gingivalis-infected and sham-infected C57 and ApoE–/– mice. Percentages of (D) CD4+IL17+Th17 cells and (E) CD4+CD25+FoxP3+T cells in P. gingivalis-infected and sham-infected C57 and ApoE–/– mice (n = 6 mice per group). * P < 0.0125 versus C57, Bonferroni correction, α = 0.05/4. ** P < 0.0125 versus ApoE–/–, Bonferroni correction, α = 0.05/4. APC = allophycocyanin; FITC = fluorescein isothiocyanate; FoxP3 = forkhead box P3; IL = interleukin; PE = phycoerythrin; Pg = Porphyromonas gingivalis.
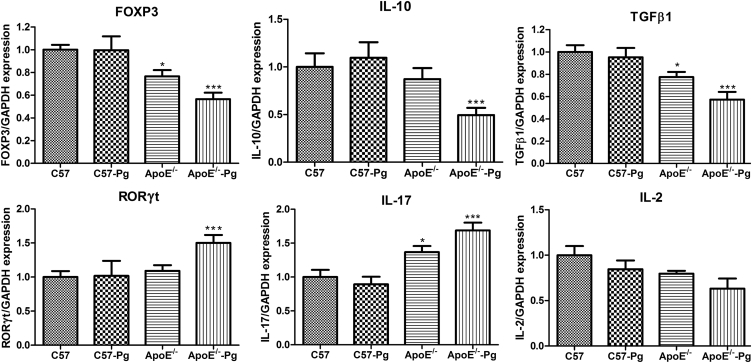
The mRNA expression of Treg-related factors, such as FoxP3, IL-10, and transforming growth factor β1, was decreased during the development of atherosclerosis. P. gingivalis infection further promoted the decrease of these factors in ApoE–/– mice. In addition, levels of Th17-related molecules, such as retinoic acid-related orphan receptor γt and IL-17, were elevated. No significant difference was found in IL-2 expression (Figure 5).
Figure 5.
The mRNA expression of Th17- and Treg-related factors in the spleen. Expression levels of FoxP3, RORγt, TGFβ, IL-10, IL-17, and IL-2 mRNA were determined by RT-PCR and normalized to GAPDH. Fold changes relative to each baseline group are shown (n = 6 mice per group). * P < 0.0125 versus C57, Bonferroni correction, α = 0.05/4. ** P < 0.0125 versus ApoE–/–, Bonferroni correction, α = 0.05/4. FoxP3 = forkhead box P3; IL = interleukin; Pg = Porphyromonas gingivalis; RORγt = retinoic acid receptor-related orphan receptor γt; RT-PCR = real-time polymerase chain reaction; TGFβ = transforming growth factor β; Th = T helper; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
P. gingivalis might alter Th17 and Treg differentiation cytokine production in ApoE–/– mice
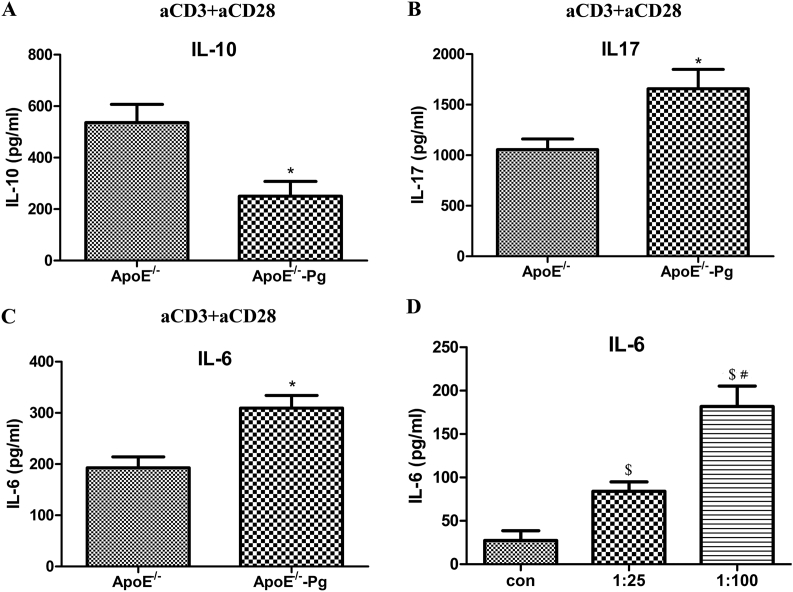
To investigate the role of P. gingivalis in inducing the release of T-cell differentiation cytokines, spleen T cells were isolated and stimulated with anti-CD3 and anti-CD28 antibodies. Production of IL-17 was increased and that of IL-10 decreased after infection in ApoE–/– mice (Figures 6A and 6B).
Figure 6.
Treg and Th17 function-associated cytokines in the spleen and moDCs. Splenic T cells of ApoE–/– mice and ApoE–/––P. gingivalis mice were isolated and stimulated with anti-CD3 and anti-CD28 antibodies for 72 hours. The supernatants were collected. (A) IL-10 (B) and IL-17 in supernatants of T cells were determined by ELISA. (C) IL-6 in supernatants of splenic cells was assessed by ELISA (n = 6 mice per group).(D) Bone marrow cells were isolated from 5–6-week-old ApoE–/– mice, cells were cultured with 20 ng/mL granulocyte-macrophage colony stimulating factor (GM-CSF) and 1 ng/mL IL-4 for 9 days to obtain DCs. DCs were infected with P. gingivalis at multiplicity of infection (MOI) 0, 25 and 100 for 24 hours. Supernatants were collected to detect the production of IL-6. * P < 0.05 vs. ApoE–/– (Figures 6C). $ P < 0.0167 versus con, Bonferroni correction, α = 0.05/3 (Figure 6D). # P < 0.0167 versus 1:25, Bonferroni correction, α = 0.05/3. DC = dendritic cells; ELISA = enzyme-linked immunosorbent assay; IL = interleukin; moDC = mouse dendritic cells; Pg = Porphyromonas gingivalis.
IL-6 is a critical factor for regulatory T-cell differentiation. In order to determine whether antigen-presenting cells in P. gingivalis-infected ApoE–/– mice show a similar IL-6 response, the expression of IL-6 in spleen cells was detected. As shown in Figure 6C, IL-6 was increased in ApoE–/––P. gingivalis mice. Signals from mouse dendritic cells can induce differentiation of T cells into particular subsets. Mouse dendritic cells from 5- to 6-week-old ApoE–/– mice were stimulated with P. gingivalis at different MOI; IL-6 secretion showed a dose-dependent increase (Figure 6D).
Discussion
P. gingivalis is a Gram-negative rod-shaped anaerobic bacterium in oral cavity.26 To assess the effect of oral infection with P. gingivalis on atherosclerosis, in this study ApoE–/– mice were treated with P. gingivalis to mimic the pathology of periodontitis combined with atherosclerosis. Alveolar bone resorption and elevated titers of plasma anti-P. gingivalis antibodies were detected in infected mice. These results indicate that P. gingivalis infection not only leads to periodontal tissue destruction locally, but also causes a systemic immune response. In our study, P. gingivalis infection did not induce the formation of atherosclerotic lesions in wild-type mice. Atherosclerotic plaque was formed spontaneously in hypercholesterolemic mice. Nevertheless, the development of atherosclerosis in P. gingivalis-infected ApoE–/– mice was accelerated with a larger plaque area. These findings are consistent with those of the recent studies reported by Lalla et al27 and Maekawa et al.28 P. gingivalis infection is a synergistic factor for pre-existing risk factors, such as hyperlipidemia, for the development of atherosclerosis. Besides the plaque area, instability of plaque was assessed in the study. Unstable atherosclerotic plaque is generally characterized by active inflammation (monocyte/macrophage), paucity of smooth muscle cells, and inadequate collagen proteins.29 We found that P. gingivalis increased high-fat diet-induced atherosclerosis lesion instability, as indicated by increased contents of foam cells and decreased smooth muscle cells in the lesion. T-cell-mediated immune reaction is important in plaque instability.30 Therefore, P. gingivalis may further induce plaque instability by promoting Th17/Treg imbalance.
Inflammation plays an important role during all developmental stages of atherosclerosis.11, 31 In recent years, the immune balance between different subsets of T cells, rather than their percentages, is increasingly being accepted to play a central role in atherosclerosis.32 In vivo and clinical studies have suggested that functional imbalance of Th17/Tregs in atherosclerosis was characterized by decreased Treg levels and increased expression of Th17-related mediators.13, 33, 34 In order to determine whether P. gingivalis can induce systemic T-cell response in atherosclerosis, Th17/Treg balance in the spleen was detected in an animal model. We found that P. gingivalis further increased the percentages of Th17 cells and decreased Treg percentages in ApoE–/– mice. The expression of transcriptional factor and cytokines showed the same tendency. No change was found in wild-type mice. The results indicated that P. gingivalis may promote Th17/Treg imbalance by upregulating Th17 cells and downregulating Tregs to accelerate atherosclerosis. It is consistent with the findings of our previous study19 in which the percentage of Tregs was decreased in P. gingivalis-infected atherosclerotic patients. However, this finding was not consistent with that of Cai et al,35 in which intravenously injected P. gingivalis 381 in ApoE–/– mice with on a diet altered T-cell response by enhancing the Th17 cell response in accelerating atherosclerosis, without any changes in FoxP3+ cell frequencies and FoxP3 mRNA expression. The conflict with the results of Cai et al35 may be due to multiple reasons such as a different P. gingivalis strain, different stages of atherosclerosis, and different methods for bacteria inoculation such as orally and intravenously. Stages of atherosclerotic plaque are associated with Treg frequencies, FoxP3 expression, and plaque formation.36 In our study, mice were fed a high-fat diet, the area of atherosclerotic plaque was larger, and the stability of plaque was decreased. Furthermore, intravenous injection-induced bacteremia may not reflect the effect of P. gingivalis on atherosclerosis development in individuals.37, 38, 39 P. gingivalis is a component of normal oral microbiome; a small number of P. gingivalis can be found in the oral cavity of healthy individuals.38, 39 P. gingivalis 33277 studied in this study was a low-virulence strain and can be examined in health periodontal tissue. In the present study, ApoE–/– mice were inoculated with this low-virulence strain for a long term to imitate the slow process of periodontitis. It is more relevant to explore the effect of P. gingivalis on atherosclerosis development in normal individuals.
Antigen presenting cells may directly affect T-cell subsets by secreting cytokines. With the regulation of specific cytokines, naive T cells differentiate into different subsets of CD4+ T cells.32 The dynamic balance between Th17 and Treg cells is controlled by the cytokine environment.40 IL-2 and IL-6 are two determining modulators of Th17 and Treg balance by activating the expression of transcriptional factors.41 In this study, although no significant difference was observed in the expression of IL-2, IL-6 was upregulated in infected mice. Secretion of IL-6 in a dendritic cell, an antigen presenting cell, was elevated after P. gingivalis infection. Our data implied that the upregulation of IL-6 might be in favor of Th17 cell production and might attenuate Treg production in local microenvironments. Further studies are required to observe the precious process of P. gingivalis on T-cell-mediated response in atherosclerosis.
In conclusion, P. gingivalis plays an important role in the progression of atherosclerosis. P. gingivalis may promote atherosclerosis with increased plaque instability by regulating Th17/Treg imbalance in atherosclerosis.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81271155 and 514721115 to W.S., and 81300852 to L.M.), and Key Project supported by Medical Science and Technology Development Foundation, Nanjing Department of Health (ZKX13050 to W.S., and YKK15165 to J.W.).
References
- 1.Armitage G.C., Research, Science and Therapy Committee of the American Academy of Periodontology Diagnosis of periodontal diseases. J Periodontol. 2003;74:1237–1247. doi: 10.1902/jop.2003.74.8.1237. [DOI] [PubMed] [Google Scholar]
- 2.Ezzo P.J., Cutler C.W. Microorganisms as risk indicators for periodontal disease. Periodontol 2000. 2003;32:24–35. doi: 10.1046/j.0906-6713.2003.03203.x. [DOI] [PubMed] [Google Scholar]
- 3.Velsko I.M., Chukkapalli S.S., Rivera M.F. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One. 2014;9:e97811. doi: 10.1371/journal.pone.0097811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozarov E.V., Dorn B.R., Shelburne C.E., Dunn W.A., Jr., Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. 2005;25:e17–e18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 5.Li L., Messas E., Batista E.L., Jr., Levine R.A., Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105:861–867. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- 6.Huck O., Saadi-Thiers K., Tenenbaum H. Evaluating periodontal risk for patients at risk of or suffering from atherosclerosis: recent biological hypotheses and therapeutic consequences. Arch Cardiovasc Dis. 2011;104:352–358. doi: 10.1016/j.acvd.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Chou H.H., Yumoto H., Davey M. Porphyromonas gingivalis fimbria-dependent activation of inflammatory genes in human aortic endothelial cells. Infect Immun. 2005;73:5367–5378. doi: 10.1128/IAI.73.9.5367-5378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deleon-Pennell K.Y., de Castro Bras L.E., Lindsey M.L. Circulating Porphyromonas gingivalis lipopolysaccharide resets cardiac homeostasis in mice through a matrix metalloproteinase-9-dependent mechanism. Physiol Rep. 2013;1:e00079. doi: 10.1002/phy2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamb D.J., El-Sankary W., Ferns G.A. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. 2003;167:177–185. doi: 10.1016/s0021-9150(02)00301-5. [DOI] [PubMed] [Google Scholar]
- 10.Hussain M., Stover C.M., Dupont A. P. gingivalis in periodontal disease and atherosclerosis—scenes of action for antimicrobial peptides and complement. Front Immunol. 2015;6:45. doi: 10.3389/fimmu.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson G.K., Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2009;29:1714–1717. doi: 10.1161/ATVBAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S., Ono M., Setoguchi R. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 13.Xie J.J., Wang J., Tang T.T. The Th17/Treg functional imbalance during atherogenesis in ApoE(-/-) mice. Cytokine. 2010;49:185–193. doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Taleb S., Tedgui A., Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol. 2015;35:258–264. doi: 10.1161/ATVBAHA.114.303567. [DOI] [PubMed] [Google Scholar]
- 15.Liu H., Hu B., Xu D., Liew F.Y. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J Immunol. 2003;171:5012–5017. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 16.Cheng X., Yu X., Ding Y.J. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Gaffen S.L., Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Wang J., Jin Y., Gao H., Lin X. Oral administration of all-trans retinoic acid suppresses experimental periodontitis by modulating the Th17/Treg imbalance. J Periodontol. 2014;85:740–750. doi: 10.1902/jop.2013.130132. [DOI] [PubMed] [Google Scholar]
- 19.Yang J., Wu J., Liu Y. Porphyromonas gingivalis infection reduces regulatory T cells in infected atherosclerosis patients. PLoS One. 2014;9:e86599. doi: 10.1371/journal.pone.0086599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagiwara M., Kurita-Ochiai T., Kobayashi R., Hashizume-Takizawa T., Yamazaki K., Yamamoto M. Sublingual vaccine with GroEL attenuates atherosclerosis. J Dent Res. 2014;93:382–387. doi: 10.1177/0022034514523784. [DOI] [PubMed] [Google Scholar]
- 21.Sun W., Wu J., Lin L., Huang Y., Chen Q., Ji Y. Porphyromonas gingivalis stimulates the release of nitric oxide by inducing expression of inducible nitric oxide synthases and inhibiting endothelial nitric oxide synthases. J Periodontal Res. 2010;45:381–388. doi: 10.1111/j.1600-0765.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi C., Madrigal A.G., Liu X. Pathogen-mediated inflammatory atherosclerosis is mediated in part via Toll-like receptor 2-induced inflammatory responses. J Innate Immun. 2010;2:334–343. doi: 10.1159/000314686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bainbridge B., Verma R.K., Eastman C. Role of Porphyromonas gingivalis phosphoserine phosphatase enzyme SerB in inflammation, immune response, and induction of alveolar bone resorption in rats. Infect Immun. 2010;78:4560–4569. doi: 10.1128/IAI.00703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park C.H., Abramson Z.R., Taba M., Jr. Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. J Periodontol. 2007;78:273–281. doi: 10.1902/jop.2007.060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima K., Yamashita T., Kita T. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1963–1972. doi: 10.1161/ATVBAHA.111.229443. [DOI] [PubMed] [Google Scholar]
- 26.How K.Y., Song K.P., Chan K.G. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016;7:53. doi: 10.3389/fmicb.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalla E., Lamster I.B., Hofmann M.A. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003;23:1405–1411. doi: 10.1161/01.ATV.0000082462.26258.FE. [DOI] [PubMed] [Google Scholar]
- 28.Maekawa T., Takahashi N., Tabeta K. Chronic oral infection with Porphyromonas gingivalis accelerates atheroma formation by shifting the lipid profile. PLoS One. 2011;6:e20240. doi: 10.1371/journal.pone.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo P., Zhou Q., Zuo Z., Wang X., Chen L., Ma G. Effects of the factor Xa inhibitor, fondaparinux, on the stability of atherosclerotic lesions in apolipoprotein E-deficient mice. Circ J. 2015;79:2499–2508. doi: 10.1253/circj.CJ-15-0285. [DOI] [PubMed] [Google Scholar]
- 30.Gao Q., Jiang Y., Ma T. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PubMed] [Google Scholar]
- 31.Klingenberg R., Matter C.M., Luscher T.F. [Immune cells in atherosclerosis—good or bad?] Praxis (Bern 1994) 2016;105:437–444. doi: 10.1024/1661-8157/a002319. [DOI] [PubMed] [Google Scholar]
- 32.Hirahara K., Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol. 2016;28:163–171. doi: 10.1093/intimm/dxw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q., Wang Y., Yu F. Peripheral Th17/Treg imbalance in patients with atherosclerotic cerebral infarction. Int J Clin Exp Pathol. 2013;6:1015–1027. [PMC free article] [PubMed] [Google Scholar]
- 34.Potekhina A.V., Pylaeva E., Provatorov S. Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis. Atherosclerosis. 2015;238:17–21. doi: 10.1016/j.atherosclerosis.2014.10.088. [DOI] [PubMed] [Google Scholar]
- 35.Cai Y., Kobayashi R., Hashizume-Takizawa T., Kurita-Ochiai T. Porphyromonas gingivalis infection enhances Th17 responses for development of atherosclerosis. Arch Oral Biol. 2014;59:1183–1191. doi: 10.1016/j.archoralbio.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Han S.F., Liu P., Zhang W. The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin Immunol. 2007;124:90–97. doi: 10.1016/j.clim.2007.03.546. [DOI] [PubMed] [Google Scholar]
- 37.Wilson W., Taubert K.A., Gewitz M. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 38.Abiko Y., Sato T., Mayanagi G., Takahashi N. Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J Periodontal Res. 2010;45:389–395. doi: 10.1111/j.1600-0765.2009.01250.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X., Liu X., Li J., Aprecio R.M., Zhang W., Li Y. Real-time PCR quantification of six periodontal pathogens in saliva samples from healthy young adults. Clin Oral Investig. 2015;19:937–946. doi: 10.1007/s00784-014-1316-0. [DOI] [PubMed] [Google Scholar]
- 40.Noack M., Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L., Ivanov I.I., Spolski R. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]