Abstract
Background/purpose
Plasma rich in growth factors (PRGFs), which is prepared from autologous blood from patients, has been reported with regards to bone regeneration for dental implants. Human dental follicle cells (hDFCs) have the capacity to commit to multiple cell types such as the osteoblastic lineage. The aim of this study is to evaluate the effects of PRGFs for mineralization in hDFCs.
Materials and methods
PRGFs was prepared from whole blood centrifuged at 460g for 8 minutes. hDFCs isolated from the dental follicle with collagenase/dispase were cultured with growth medium or osteogenic induction medium (OIM) containing PRGFs or fetal bovine serum. Concentrations of the growth factors were examined using an enzyme-linked immunosorbent assay kit. A cell migration assay was used for two-dimensional movement. Gene expressions were examined with real-time polymerase chain reaction using a DyNAmo SYBR Green quantitative polymerase chain reaction kit.
Results
The platelet concentration in PRGF Fraction 2 was 2.14-fold higher than in whole blood. White blood cells were not detected in PRGFs. Transforming growth factor-β levels were higher than insulin-like growth factor-1, platelet-derived growth factor-AB and -BB, and vascular endothelial growth factors in PRGF Fraction 2. Proliferation and migration by hDFCs increased in OIM supplemented with PRGFs in a dose-dependent manner and were higher in hDFCs cultured in OIM plus 10% PRGFs compared with OIM plus 10% fetal bovine serum. PRGFs upregulated the gene expression of type I collagen, osteomodulin, alkaline phosphatase, bone morphogenic protein-4, and transforming growth factor-β in hDFCs.
Conclusion
PRGFs may promote bone regeneration due to it including high levels of growth factors.
Keywords: PRGF, growth factors, dental follicle cells, osteogenic differentiation
Introduction
Platelet concentration products, which are autologous constituents of inductive factors obtained from blood, contain high concentrations of platelets with various growth factors.1 Platelet-rich plasma (PRP) has been used in bone augmentation for dental implants.2, 3 However, PRP formulations have different biological activities, depending on the various protocols used to obtain them.4, 5 The system of plasma rich in growth factors (PRGFs) is a method for concentrating platelets6, 7 and is advantageous as it requires only one centrifugation step and is leukocyte-free, thus avoiding high levels of proinflammatory cytokines.6 PRGFs allows delivery to the site of injury of a cocktail of proteins and growth factors that promotes wound healing and regeneration of tissue and bone.8, 9 Numerous studies have been published describing the benefits of PRGFs in vivo. However, the majority of studies about the use of PRGFs only describe the final outcome at the tissue level and do not investigate the mechanism of PRGFs. Therefore, investigation of the role of PRGFs in bone and/or tissue regeneration should involve a study of the biological properties and molecular functions using a cell culture system.
The dental follicle, an ectomesenchymal tissue that surrounds the developing tooth germ, contains stem cells and lineage committed progenitor cells or osteoblast/cementoblast precursor cells.10 Human dental follicle cells (hDFCs) also have the capacity to commit to multiple cell types, not only to cells of the osteoblastic lineage, but also to cells of adipogenic and neurogenic lineages.11, 12 We previously reported that hDFCs can differentiate into osteogenic lineage cells in osteogenic induction medium (OIM) without dexamethasone,13 which has various biological effects such as anti-inflammatory properties. In addition, hDFCs express stem cell markers and growth factor receptors, and highly express LIM homeobox 8, which is associated with development of the palatal mesenchyme and tooth germ.14 In addition, hDFCs are easily accessible for cell culture and have a higher proliferation capacity.15 According to these findings, we suggested that hDFCs may be useful for therapy and in basic research of the maxillofacial region bone.
In this study, we measured the concentration of growth factors in PRGFs and examined the effect of the soluble factors in PRGFs on proliferation and gene expression in hDFCs treated with PRGF supernatant compared with fetal bovine serum (FBS). We evaluated the efficacy of PRGFs as a substitute for engineered bone tissue in the maxillofacial region.
Materials and methods
Preparation of different blood products
For blood product preparation, whole blood from four young healthy donors (two men and two women, average age 29.5 years) was collected from the external jugular vein after informed consent was obtained. Whole blood was immediately placed into 5-mL sterile extraction tubes containing 0.5 mL of 3.8% sodium citrate as an anticoagulant. The whole blood was divided into two aliquots.
The first aliquot was used to obtain PRGFs that was prepared from whole blood, which was centrifuged in accordance with Anitua's protocol.6, 7, 8 Briefly, tubes were centrifuged at 460g for 8 minutes. The plasma fraction (1 mL over the buffy coat) was collected as Fraction 2 (F2), whereas Fraction 1 (F1) was the layer above F2. PRGF F2 was incubated with 10% calcium chloride solution at 37°C for 1 hour to trigger platelet activation and growth factor release. Activated PRGF F2 was centrifuged at 3000g for 15 minutes, and then the supernatant was isolated (PRGF F2 supernatant).
The second aliquot was used to obtain serum. Whole blood was left for 30 minutes at room temperature and then centrifuged at 1000g for 15 minutes.
The numbers of blood cells in PRGFs and serum were immediately measured, and then the samples were stored at −80°C until use.
PRGF extraction was performed according to the guidelines established by the Ethics Committee of Nihon University School of Dentistry at Matsudo (Recognition number: EC14-13-029-1).
Number of blood cells
The numbers of platelets and white blood cells (WBCs) were counted using XE-2100 (Sysmex, Hyogo, Japan).
Enzyme-linked immunosorbent assay
Concentrations of growth factors including insulin-like growth factor (IGF)-1, transforming growth factor (TGF)-β, platelet-derived growth factor (PDGF)-AB and -BB, and vascular endothelial growth factor (VEGF) were measured using enzyme-linked immunosorbent assay kits (Quantikine enzyme-linked immunosorbent assay kits; R&D Systems, Mckinley, MN, USA) according to the manufacturer's instructions.
Cell cultures
hDFCs were obtained using a previously reported method.11 Briefly, normal human impacted third molars were surgically removed and collected from a patient aged 14 years, after obtaining informed consent. Dental follicle tissues were minced with sterilized scalpels and digested in a solution of 0.1-U/mL collagenase type I and 1-U/mL dispase (Roche, Mannheim, Germany) for 1 hour at 37°C. Attached hDFCs were cultured in 100-mm dishes using mesenchymal stem cells (MSC) growth medium (GM; Lonza, Walkersville, MD, USA) in a CO2 incubator (Sanyo, Osaka, Japan) in the presence of 5% CO2 in air at 37°C.
For induction of osteogenic differentiation, hDFCs from the fifth to sixth passage were seeded at 3.1 × 103 cells/cm2 in GM. After 24 hours (Day 0), medium was replaced with MSC OIM (Lonza) consisting of osteogenic basal medium containing ascorbate, and β-glycerophosphate supplemented with FBS (OIM-FBS) or PRGFs F2 supernatant (OIM-PRGFs). The PRGFs that were added to OIM was a mixture of equal volumes of each PRGF sample from the four donors.
Experiments using hDFCs were performed in accordance with the guidelines established by the Ethics Committee of Nihon University School of Dentistry at Matsudo (Recognition number: EC10-036).
Cell numbers
hDFCs were seeded in a 24-well plate at a density of 1 × 104 cells/well in GM. After 24 hours, medium was replaced with GM, OIM-FBS, or OIM-PRGFs. Cell numbers were counted with a Z1 Counter Particle Counter (Beckman Coulter, Miami, FL, USA).
Cell migration assay
hDFCs were seeded in a 24-well plate at a density of 1 × 104 cells/well in GM and were cultured in GM for 4 days until 80% confluent. A scratch was then made on the monolayer using a Cell Scratcher (AGC Thecno Glass, Tokyo, Japan), leaving two separated cell monolayers with a cell-free gap that was approximately 2-mm wide. Then, the medium was replaced with GM, OIM-FBS, or OIM-PRGFs. After 72 hours, photographs of hDFCs were taken using a DP12 Microscope Digital Camera (Olympus, Tokyo, Japan). The areas of migratory cells were measured using Image J (National Institutes of Health, Bethesda, MD, USA). Results were expressed as percent of the migratory cell area per mm2.
Total RNA isolation
Total RNA from hDFCs was isolated using miRNeasy Mini Kits (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. RNA was stored at −80°C until use.
Real-time polymerase chain reaction
Complementary DNA was synthesized using a GeneAmp RNA polymerase chain reaction (PCR) kit (Life Technologies, Carlsbad, CA, USA). Real-time PCR was performed using a DyNAmo SYBR Green qPCR kit (Finnzymes, Espoo, Finland). The PCR mixture, containing 20-pmol forward and reverse primers and 2-μL complementary DNA, was subjected to amplification with a DNA Engine Opticon 1 (BioRad, Hercules, CA, USA), and was preheated at 95°C for 10 minutes, followed by 40 cycles of 94°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. Gene expression levels were calculated using the ΔΔCT method with normalization to glyceraldehyde 3-phosphate dehydrogenase.16 The primers are shown in Table 1.
Table 1.
Primers used in Real – time PCR.
| Gene | Primers | bp |
|---|---|---|
| Col I α1 | F: 5′- ggc caa gag gaa ggc caa gt- 3′ | 251 |
| R: 5′- tgg tcg gtg ggt gac tct ga- 3′ | ||
| OMD | F: 5′- cat ctt ctt ctg ctt ccc tca- 3′ | 123 |
| R: 5′- gtc aaa gtg ccc ttc tgc tc- 3′ | ||
| ALP | F: 5′- gtc atc atg ttc ctg gga ga- 3′ | 123 |
| R: 5′- gaa ggg gaa ctt gtc cat ct- 3′ | ||
| BMP-2 | F: 5′- cat gtg gac gct ctt tca at - 3′ | 113 |
| R: 5′- gaa gca gca acg cta gaa ga - 3′ | ||
| BMP-4 | F: 5′- gct agg agc cat tcc gta gt - 3′ | 193 |
| R: 5′- cct agc agg act tgg cat aa - 3′ | ||
| TGF-β | F:5′-gactcgccagagtggttat-3′ | 125 |
| R: 5′-agtgtgttatccctgctgtc-3′ | ||
| GAPDH | F: 5′- atc acc atc ttc cag gag- 3′ | 315 |
| R: 5′- atg gac tgt ggt cat gag- 3′ |
Col I α1; type I collagen α chain 1, OMD; osteomodulin, ALP; Alkaline Phosphatase, BMP; bone morphogenetic protein, TGF-β; Transforming growth factor β, GAPDH; glyceraldehydes-3-phosphate dehydrogenase, F = forward; R = Reverse.
Statistical analysis
Data are expressed as means ± standard deviation of the three samples. Significance of differences between culture samples was determined using Student t test and a value of P < 0.05 was considered to be statistically significant.
Results
Concentrations of platelets and WBCs
The platelet concentration in PRGFs F2 was 2.14 ± 0.10-fold higher than in whole blood, whereas that in PRGF F1 was 0.59 ± 0.19-fold of that in whole blood (Table 2). WBCs were not detected in PRGFs from any donors. The number of platelets and WBCs in whole blood and PRGFs varied among individual donors.
Table 2.
Numbers of blood cells.
| Platelets (×104 cells/μL) |
WBCs (cells/μL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| WB | Serum | F1 (fold) | F2 (fold) | WB | Serum | F1 | F2 | |
| Donor 1 | 19.7 | 0.0 | 16.2 (0.8) | 39.9 (2.0) | 3870.0 | 0.0 | 0.0 | 0.0 |
| Donor 2 | 25.1 | 0.0 | 12.3 (0.5) | 55.8 (2.2) | 3410.0 | 0.0 | 0.0 | 0.0 |
| Donor 3 | 14.4 | 0.0 | 10.6 (0.7) | 32.4 (2.3) | 3850.0 | 0.0 | 0.0 | 0.0 |
| Donor 4 | 26.2 | 0.0 | 8.7 (0.3) | 54.0 (2.1) | 5510.0 | 0.0 | 0.0 | 0.0 |
F1 = plasma rich in growth factors F1 fraction; F2 = plasma rich in growth factors F2 fraction; Fold = number F1 or F2/number WB; WB = whole blood; WBCs = white blood cells.
Concentrations of growth factors
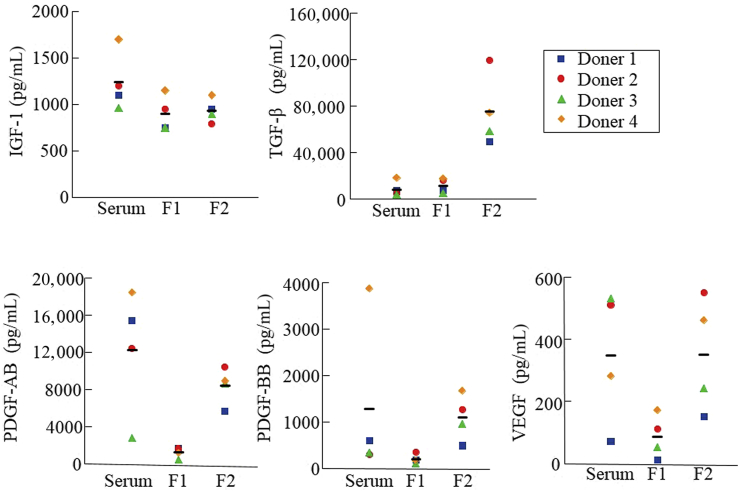
The concentration of TGF-β was higher in PRGF F2 compared with PRGF F1 and serum, although the levels of each growth factor varied among individual donors (Figure 1). The concentrations of IGF-1, PDGF-AB, PDGF-BB, and VEGF were similar between PRGF F2 and serum, and were low in PRGF F1 compared with other blood products (Figure 1).
Figure 1.
The levels of growth factors in serum and plasma rich in growth factor (PRGF) fractions. Serum and PRGFs were prepared from four healthy donors. Growth factor concentrations are expressed as the means ± standard deviation. F1 indicates PRGF fraction 1 and F2 indicates PRGF fraction 2. Bar indicates the mean value. IGF = insulin-like growth factor; PDGF = platelet-derived growth factor; TGF = transforming growth factor; VEGF = vascular endothelial growth factor.
Next, the PRGF F2 supernatant was isolated by centrifugation of PRGF F2 clots in order to investigate the biological effects of soluble factors in PRGF F2. The concentration of IGF-1 was slightly increased in the supernatant compared with that in PRGF F2, whereas the concentration of TGF-β was markedly decreased in the supernatant (Table 3). The concentrations of PDGF-AB, PDGF-BB, and VEGF were decreased about 50% in the supernatant (Table 3).
Table 3.
Levels of growth factors.
| Growth factors (pg/mL) | F2 | F2 supernatant | Average F2 supernatant/average F2 (fold) |
|---|---|---|---|
| IGF-I | 935.0 ± 128.7 | 1087.5 ± 209.0 | 1.2 |
| TGF-β | 75,000.0 ± 31,102.0 | 10,850.0 ± 2820.8 | 0.1 |
| PDGF-AB | 8000.0 ± 1990.0 | 5050.0 ± 1497.8 | 0.6 |
| PDGF-BB | 1025.0 ± 499.4 | 397.5 ± 233.9 | 0.4 |
| VEGF | 340.0 ± 186.4 | 142.5 ± 110.3 | 0.4 |
F2 = plasma rich in growth factors F2 fraction; F2 supernatant = property of F2 supernatant was described in material and methods; IGF = insulin like growth factor; PDGF = platelet derived growth factor; TGF = transforming growth factor; VEGF = vascular endothelial growth factor.
Cell proliferation
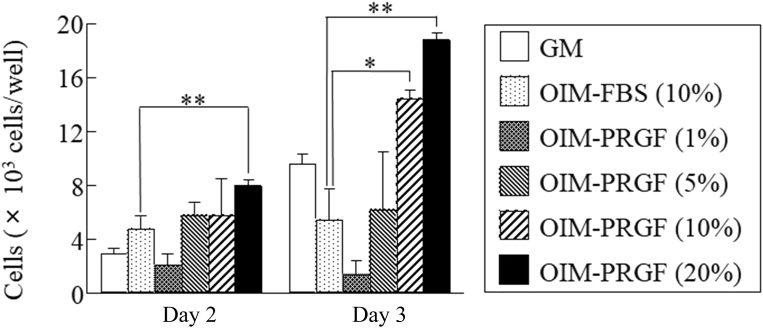
The cell proliferation of hDFCs was increased in OIM supplemented with PRGFs in a dose-dependent manner (Figure 2). On Culture Day 3, the proliferation was significantly higher in hDFCs cultured in OIM-PRGFs (10% and 20%) compared with cells cultured in OIM-FBS (10%; Figure 2). The proliferation ability was similar between OIM-PRGFs (5%) and OIM-FBS (10%; Figure 2).
Figure 2.
Effect of plasma rich in growth factors (PRGFs) on human dental follicle cell proliferation. Human dental follicle cells were cultured in growth medium (GM), osteogenic induction medium (OIM) supplemented with 10% fetal bovine serum (FBS), or OIM supplemented with the indicated PRGF concentrations for 2 or 3 days. Values represent the means ± standard deviation. * P < 0.05. ** P < 0.01.
Cell migration
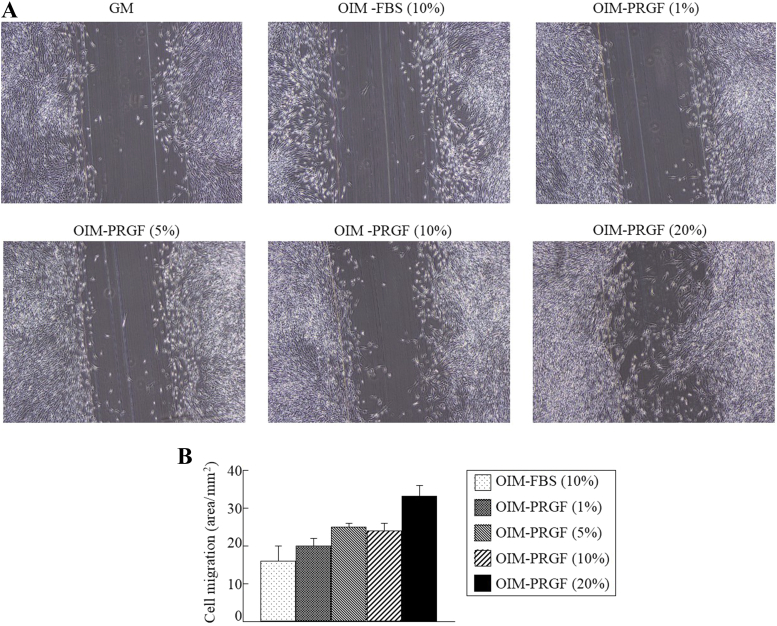
A scratch mark was drawn in a cell culture dish to create a distinct border from which cell migration can be observed. The cells successfully bridged the gap when supplemented with the blood products, especially in the case of OIM-PRGFs (20%) on Culture Day 3 (Figures 3A and 3B). Cell migration was increased in the culture with OIM-PRGFs (10%) compared with OIM-FBS (10%), although no statistical differences were found between OIM-PRGFs and OIM-FBS (Figure 3B).
Figure 3.
Effect of plasma rich in growth factors (PRGFs) on migration of human dental follicle cells (hDFCs). hDFCs were cultured in growth medium (GM), osteogenic induction medium (OIM) supplemented with 10% fetal bovine serum (FBS), or OIM supplemented with the indicated PRGF concentrations for 3 days. (A) Phase-contrast microscopy shows hDFC migration; (B) quantitation of cell migration using Image J software (National Institutes of Health, Bethesda, MD, USA). Values represent the means ± standard deviation.
Osteogenic gene expression
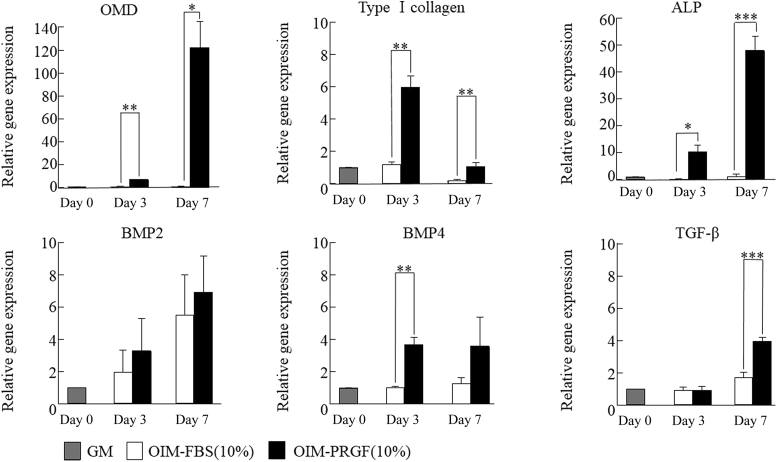
Figure 4 showed gene expression associated with osteoblast differentiation in hDFCs cultured in OIM-FBS (10%) or OIM-PRGFs (10%). The expressions of type I collagen α1, osteomodulin (OMD), and alkaline phosphatase (ALP) were significantly higher in hDFCs cultured in OIM-PRGFs compared with cells cultured in OIM-FBS on Culture Day 3 and Day 7. hDFCs cultured in OIM-PRGFs highly expressed bone morphogenetic protein (BMP)-4 on Culture Day 3 and TGF-β on Culture Day 7. BMP-2 expression was higher in OIM-PRGFs cultures compared with OIM-FBS cultures, but the difference was not significant.
Figure 4.
Gene expression of type I collagen α1, osteomodulin (OMD), alkaline phosphatase (ALP), bone morphogenetc proteins (BMPs), and transforming growth factor (TGF)-β in human dental follicle cells. Human dental follicle cells were cultured in growth medium (GM), osteogenic induction medium (OIM) supplemented with 10% fetal bovine serum (FBS), or OIM supplemented with the indicated PRGFs concentrations for 3 or 7 days. The gene expression was examined with real-time polymerase chain reaction. Values represent the means ± standard deviation of the results from three independent experiments. * P < 0.05. ** P < 0.01. *** P < 0.05 compared with Culture Day 0.
Discussion
PRGFs was used following tooth extraction and around dental implants to improve healing of the peri-implant bone and was reported to accelerate bone regeneration in clinical studies.6, 7, 8, 17, 18 It has been reported that PRGFs exert positive effects on periodontal ligament fibroblasts and alveolar bone osteoblasts, which could be positive for periodontal regeneration.6, 7 However, recent advances in stem cell research have revealed the existence of various types of adult tissue stem cells that contribute to the functional maintenance of organs and to cell renewal, tissue remodeling, and repair.19, 20 hDFCs include mesenchymal stem cells that have the capacity to form various types of cells committed osteoblastic/cementoblastic,10 adipogenic,11 and neurogenic lineages.12 In addition, hDFCs express the genes associated with development of the palatal mesenchyme and tooth germ,14 and have a higher proliferation capacity.15 Human dental follicles, which are a source of hDFCs, can be very easily obtained during various types of surgical operations, such as the extraction of impacted teeth. Therefore, hDFC have great potential for utilization in regenerative cell therapy and in basic research of the maxillofacial region bone. In this study, we examined the effect of PRGFs on proliferation and gene expression in hDFCs, and evaluated the efficacy of PRGFs as a substitute for engineered bone tissue in the maxillofacial region.
First, we examined the properties of the PRGFs obtained from four donors in this study. The platelet concentration in PRGF F2 appeared to be linked with that in each donor's whole blood. A previous report also showed that the platelet concentration in blood bank PRP was correlated with the platelet count in the donor whole blood.21 However, WBCs were not detected in PRGFs (Table 2) from any donors as in previous reports.6 WBCs, such as macrophages and lymphocytes, secrete inflammatory cytokines and reactive oxygen species22 and may induce inflammatory disorders and ultimately bone loss.23 A previous study suggested that the absence of WBCs in PRGFs drastically reduces the amount of these proinflammatory and profibrotic agents at the site of injury, probably contributing to more balanced bone tissue healing.6
The concentrations of growth factors associated with bone generation were measured (Figure 1). The concentrations of growth factors were related to the concentration of platelets in PRGF F2 from different donors. The level of TGF-β in PRGF F2 was higher than that in serum and PRGF F1, and was higher compared with levels of other growth factors. TGF-β is a stimulator of both chondrogenic and osteogenic MSC differentiation, suggesting that this factor plays a critical role in early- and mid-phase processes in the endochondral bone healing pathway.24 Other growth factor levels in PRGF F2 were higher than those in PRGF F1, although they were similar to those in serum. PDGF is suggested to be a crucial initiator of bone healing as PDGF can initiate callus formation through chemotaxis and mitogenesis of MSCs.25, 26 IGF is expressed in the bone matrix and regulates proliferation and maturation of chondrocytes to induce hypertrophyy.27 VEGF is an important factor in angiogenesis that is necessary for the transition from soft to hard callus.28 The present study revealed that the PRGFs had a stimulating effect on the initial cell growth and migration of hDFCs (Figure 2, Figure 3). Thus, PRGFs may induce chemotaxis and mitogenesis of progenitor cells in the osteoblast lineage and accelerate bone healing.
Gene expression of type I collagen α1, OMD, BMP-2, BMP-4, and TGF-β was examined in hDFCs treated with 10% PRGF F2 supernatant or 10% FBS. The expression of type I collagen α1 and OMD was significantly upregulated in hDFCs with OIM-PRGFs compared with OIM-FBS. Type I collagen is a main matrix protein in bone, and its production is increased by several factors such as TGF-β. OMD (also called osteoadherin) is a small leucine-rich proteoglycan that is specifically located in mineralized tissues, possibly reflecting a role in collagen fibrillogenesis.29 Upregulation of matrix protein of type I collagen and OMD by PRGFs may be useful for bone healing. The expression of ALP, which is an early marker of osteogenic differentiation, was also upregulated by PRGFs. Therefore, PRGFs may promote the deposition of the mineralized bone matrix.
BMPs and TGF-β are stored in the bone matrix and play important roles in bone modeling and remodeling. BMP-2 and -4 have been suggested to be required for cells to differentiate into functional osteoblasts.30 TGF-β stimulates the proliferation of osteoblast progenitors.31 The expression of BMP-2 and -4 were increased in hDFCs with OIM-PRGFs compared with OIM-FBS, although only BMP-4 on Culture Day 3 was significantly different. The expression of TGF-β was significantly elevated in hDFCs cultured with OIM-PRGFs on Day 7. Previous studies have reported that osteoblast-like cells or periodontal ligament cells upregulate TGF-β production when cultured with xenogenic bone substitutes or enamel matrix derivatives.32, 33 Therefore, upregulation of TGF-β expression on Day 7 may be related to the elevation of type I collagen and OMD expression in hDFCs cultured in OIM-bPRGFs. These findings suggested that PRGFs may be useful for bone regeneration through upregulation of matrix proteins and growth factors associated with bone remodeling.
The PRGF F2 supernatant was prepared for addition to hDFC culture medium according to a previous report6 and the concentration of growth factors between PRGF F2 and PRGF F2 supernatant was compared (Table 3). The IGF level was almost the same between PRGF F2 and PRGF F2 supernatant. The levels of other growth factors were lower in the supernatant, and the TGF-β level was especially lower. In a previous report, IGF was not able to bind fibrin(ogen) directly, but TGF-β showed a high binding affinity to fibrin(ogen). By contrast, PDGF showed binding to fibrin(ogen), but its release from the matrix was relatively rapid.34 We suggested that our data regarding growth factor levels in PRGF F2 and its supernatant reflect the information from this report.
We examined the effects of PRGF F2 supernatant on proliferation, migration, and regulation of bone-associated genes in cultures of hDFCs in this study. However, the levels and the ratio of growth factors were different between PRGF F2 and PRGF F2 supernatant. In addition, the biological effects of PRGF for regeneration involved soluble factors released from platelets such as growth factors and scaffolding by fibrin. PRGF clots are three-dimensional fibrin matrices that can provide transient space for the key tissue-forming cells. At the same time, the clots act as a biomolecule delivery system and are transplanted into lost or damaged bone such as the cavity following tooth extraction in clinical and in vivo studies. New in vitro experimental models are necessary to elucidate all the biological functions of PRGFs including scaffolds and release of growth factors.
This study showed that the soluble factors in PRGFs increased proliferation, migration, and gene expression that is associated with osteogenic induction using hDFC cultures. PRGFs are a source of autogenous growth factors that help with bone regeneration and may be convenient for clinical application.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by a Grant-in Aid for Scientific Research (23592947 and 26463026) from the Japan Society for the Promotion of Science.
References
- 1.Fréchette J.P., Martineau I., Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84:434–439. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 2.Marx R.E., Carlson E.R., Eichstaedt R.M., Schimmele S.R., Strauss J.E., Georgeff K.R. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 3.Inchingolo F., Tatullo M., Marrelli M. Regenerative surgery performed with platelet-rich plasma used in sinus lift elevation before dental implant surgery: an useful aid in healing and regeneration of bone tissue. Eur Rev Med Pharmacol Sci. 2012;16:1222–1226. [PubMed] [Google Scholar]
- 4.Choi B.H., Im C.J., Huh J.Y., Suh J.J., Lee S.H. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg. 2004;33:56–59. doi: 10.1054/ijom.2003.0466. [DOI] [PubMed] [Google Scholar]
- 5.Gerard D., Carlson E.R., Gotcher J.E., Jacobs M. Effects of platelet-rich plasma on the healing of autologous bone grafted mandibular defects in dogs. J Oral Maxillofac Surg. 2006;64:443–451. doi: 10.1016/j.joms.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Anitua E., Tejero R., Zalduendo M.M., Orive G. Plasma rich in growth factors promotes bone tissue regeneration by stimulating proliferation, migration, and autocrine secretion in primary human osteoblasts. J Periodontol. 2013;84:1180–1190. doi: 10.1902/jop.2012.120292. [DOI] [PubMed] [Google Scholar]
- 7.Anitua E., Troya M., Orive G. An autologous platelet-rich plasma stimulates periodontal ligament regeneration. J Periodontol. 2013;84:1556–1566. doi: 10.1902/jop.2013.120556. [DOI] [PubMed] [Google Scholar]
- 8.Paknejad M., Shayesteh Y.S., Yaghobee S., Shariat S., Dehghan M., Motahari P. Evaluation of the effect of plasma rich in growth factors (PRGF) on bone regeneration. J Dent (Tehran) 2012;9:59–67. [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshioka T., Kanamori A., Washio T. The effects of plasma rich in growth factors (PRGF-Endoret) on healing of medial collateral ligament of the knee. Knee Surg Sports Traumatol Arthrosc. 2013;21:1763–1769. doi: 10.1007/s00167-012-2002-x. [DOI] [PubMed] [Google Scholar]
- 10.Morsczeck C., Götz W., Schierholz J. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Yao S., Pan F., Prpic V., Wise G.E. Differentiation of stem cells in the dental follicle. J Dent Res. 2008;87:767–771. doi: 10.1177/154405910808700801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Völlner F., Ernst W., Driemel O., Morsczeck C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation. 2009;77:433–441. doi: 10.1016/j.diff.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K., Ogura N., Aonuma H. Bone morphogenetic protein 6 stimulates mineralization in human dental follicle cells without dexamethasone. Arch Oral Biol. 2013;58:690–698. doi: 10.1016/j.archoralbio.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Aonuma H., Ogura N., Takahashi K. Characteristics and osteogenic differentiation of stem/progenitor cells in the human dental follicle analyzed by gene expression profiling. Cell Tissue Res. 2012;350:317–331. doi: 10.1007/s00441-012-1477-6. [DOI] [PubMed] [Google Scholar]
- 15.Shoi K., Aoki K., Ohya K., Takagi Y., Shimokawa H. Characterization of pulp and follicle stem cells from impacted supernumerary maxillary incisors. Pediatr Dent. 2014;36:79–84. [PubMed] [Google Scholar]
- 16.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Del Fabbro M., Boggian C., Taschieri S. Immediate implant placement into fresh extraction sites with chronic periapical pathologic features combined with plasma rich in growth factors: preliminary results of single-cohort study. J Oral Maxillofac Surg. 2009;67:2476–2484. doi: 10.1016/j.joms.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 18.Anitua E., Carda C., Andia I. A novel drilling procedure and subsequent bone autograft preparation: a technical note. Int J Oral Maxillofac Implants. 2007;22:138–145. [PubMed] [Google Scholar]
- 19.Suemori H., Yasuchika K., Hasegawa K., Fujioka T., Tsuneyoshi N., Nakatsuji N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun. 2006;345:926–932. doi: 10.1016/j.bbrc.2006.04.135. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K., Tanabe K., Ohnuki M. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Weibrich G., Kleis W.K., Kunz-Kostomanolakis M., Loos A.H., Wagner W. Correlation of platelet concentration in platelet-rich plasma to the extraction method, age, sex, and platelet count of the donor. Int J Oral Maxillofac Implants. 2001;16:693–699. [PubMed] [Google Scholar]
- 22.Doherty T.A., Brydges S.D., Hoffman H.M. Autoinflammation: translating mechanism to therapy. J Leukoc Biol. 2011;90:37–47. doi: 10.1189/jlb.1110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anitua E., Zalduendo M., Troya M., Padilla S., Orive G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS One. 2015;10:e0121713. doi: 10.1371/journal.pone.0121713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng F., Boucher S., Koh S. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 25.Rasubala L., Yoshikawa H., Nagata K., Iijima T., Ohishi M. Platelet-derived growth factor and bone morphogenetic protein in the healing of mandibular fractures in rats. Br J Oral Maxillofac Surg. 2003;41:173–178. doi: 10.1016/s0266-4356(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 26.Heldin C.H., Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 27.Fisher M.C., Meyer C., Garber G., Dealy C.N. Role of IGFBP2, IGF-I and IGF-II in regulating long bone growth. Bone. 2005;37:741–750. doi: 10.1016/j.bone.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Gerber H.P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 29.Couble M.L., Bleicher F., Farges J.C. Immunodetection of osteoadherin in murine tooth extracellular matrices. Histochem Cell Biol. 2004;121:47–53. doi: 10.1007/s00418-003-0608-2. [DOI] [PubMed] [Google Scholar]
- 30.Pereira R.C., Rydziel S., Canalis E. Bone morphogenetic protein-4 regulates its own expression in cultured osteoblasts. J Cell Physiol. 2000;182:239–246. doi: 10.1002/(SICI)1097-4652(200002)182:2<239::AID-JCP13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 31.Mimura S., Kimura N., Hirata M. Growth factor-defined culture medium for human mesenchymal stem cells. Int J Dev Biol. 2011;55:181–187. doi: 10.1387/ijdb.103232sm. [DOI] [PubMed] [Google Scholar]
- 32.Trasatti C., Spears R., Gutmann J.L., Opperman L.A. Increased Tgf-beta1 production by rat osteoblasts in the presence of PepGen P-15 in vitro. J Endod. 2004;30:213–217. doi: 10.1097/00004770-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Okubo K., Kobayashi M., Takiguchi T. Participation of endogenous IGF-I and TGF-beta 1 with enamel matrix derivative-stimulated cell growth in human periodontal ligament cells. J Periodontal Res. 2003;38:1–9. doi: 10.1034/j.1600-0765.2003.01607.x. [DOI] [PubMed] [Google Scholar]
- 34.Martino M.M., Briquez P.S., Ranga A., Lutolf M.P., Hubbell J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]