Abstract
Background/purpose
Yakult is a well-known probiotic beverage consisting of a single live bacterial species, Lactobacillus casei Shirota. However, the potential cariogenic/cariostatic effects of Yakult intake among children have not been studied yet. Hence, this study aimed to investigate the clinical effects of short-term Yakult intake on oral biofilm acidogenicity, cariogenic bacterial counts, and caries risk in children.
Materials and methods
Eighteen children, 7–11 years of age, consumed standard Yakult daily for 7 days. Prior to and after intervention, functional oral biofilm acidogenicity characterized by the Stephan curve, Lactobacillus and Streptococcus mutans counts, and caries risk were determined.
Results
Probiotic intervention demonstrated significant increase in minimum pH from 4.88 to 5.14 (P = 0.02), 18.2% reduction in area under the Stephen curve [area under the curve (AUC)], and 29.3% decrease in pH recovery time, although these two differences were not statistically significant. No difference was observed in S. mutans and Lactobacillus counts or caries risk after intervention (all P > 0.05). However, on subgroup analysis using “reduction of AUC” to separate “responders” from the “nonresponders”, the significant cariostatic effects on oral biofilm acidogenicity, among “responders”, were revealed by an increase in minimum pH (P = 0.005) and a reduction in pH recovery time (P = 0.003).
Conclusion
There may be a potential cariostatic effect of short-term Yakult intake in reducing functional biofilm acidogenicity in children with certain oral biofilm and risk profile. Further studies may be needed to validate this probiotic effect. Quality risk assessment may be critical prior to prescribing/recommending Yakult as an adjunct caries-preventive treatment for children.
Keywords: caries, Lactobacillus casei Shirota, probiotics
Introduction
Probiotics are defined as living microorganisms that, when administered in adequate amounts, confer a health benefit on the host.1 Clinical studies support the use of specific Lactobacillus and Bifidobacterium bacterial strains for the management of rotavirus and Clostridium difficile diarrhea, bladder cancer, allergic hypersensitivity, lactase deficiency, and surgical infections.2, 3, 4 There has also been increasing interest in the potential usage of probiotics for prevention of dental caries.5, 6 A double-blind, randomized, placebo-controlled clinical trial demonstrated that a 6-week intake of lozenges, containing Lactobacillus brevis CD2, effectively reduced plaque acidogenicity and salivary Streptococcus mutans concentration in high caries risk (CR) school children.7 Preliminary clinical data suggest that Lactobacillus rhamnosus and Lactobacillus salivarius may offer protection against dental caries in young children.8 In a double-blind placebo-controlled trial conducted in 2- to 3-year-old children, daily intake of three probiotic Streptococcus strains significantly reduced 1-year caries increments by 4-fold.9 Therefore, probiotics may be considered an adjunct to the current caries control measures such as tap water fluoridation and restriction of dietary sugar intake.10
Yakult, a well-known probiotic product with more than 50 years of market history in Japan and Taiwan, is a dairy beverage consisting of a suspension of a single live bacterial species, Lactobacillus casei Shirota, in artificially sweetened skimmed milk. Yakult has been shown to be safe with no adverse effects in both healthy and immunocompromised children when administered daily for up to 55 months,11 and L. casei Shirota has been classified as a “generally recognized as safe” additive by the United States Food and Drug Administration.11 In addition, the inclusion of sweeteners in Yakult improves palatability and compliance when administered to children.12 Yakult intake has been correlated with relief of chronic idiopathic constipation symptoms13 and increase in natural killer cell counts among habitual smokers.14 Furthermore, enteral feeding with Yakult containing a coculture of L. casei Shirota and Bifidobacterium breve strain significantly reduced postoperative infections among biliary cancer patients.15 Although some potential oral benefits associated with Yakult are still under investigation,4 putative effects on caries, especially among children, remain largely unknown. Although there seem to be several risk factors related to Yakult (such as acidity, sugars, and the acid-producing bacteria L. casei Shirota), there is no study or evidence demonstrating whether intake of Yakult has potential cariogenic or cariostatic effect among children.
Early childhood caries is a serious chronic oral health problem with an alarmingly high prevalence among children in both developed and developing countries, despite implementation of established caries management measures.16, 17, 18, 19 With the popularity, generally accepted safety, and palatability of Yakult, it may be worthwhile to characterize the unknown probiotic effects of this L. casei Shirota-containing probiotic beverage on CR indicators in children. Therefore, this study is aimed to investigate among schoolchildren the potential cariostatic/cariogenic effects of short-term Yakult intake on their oral biofilm acidogenicity, S. mutans and Lactobacillus counts, and CR.
Materials and methods
Clinical study
Ethical approval for this study was granted by the Institutional Review Board of Chang Gung Memorial Hospital (reference number 99-1697B). The clinical study was conducted in accordance with the ethical principles of the Declaration of Helsinki, and is registered at Clinicaltrials.in.th (identification number TCTR20150922002). With written parental permission and child’s assent, participants were recruited from the Children’s Dental Clinic of Kaohsiung Chang Gung Memorial Hospital based on the following inclusion criteria:
-
1.
Schoolchildren of at least 7 years of age
-
2.
Cooperative disposition
-
3.
Absence of any systemic disease
-
4.
No habitual consumption of Yakult
-
5.
No restorations on maxillary canines or premolars
Both at baseline and after 1-week Yakult consumption, caries risk assessment (CRA; using Cariogram20), oral bacterial counts, and functional biofilm pH characterization (plotting of Stephan curves) were performed as detailed below. Study participants abstained from oral hygiene procedures 2 days prior, and food intake 2 hours prior, to all measurements. Because L. casei Shirota is not a first colonizer, gargling might increase the colonization of the probiotic bacteria; hence, participants were instructed to gargle with one bottle (100 mL) standard Yakult (Yakult Co. Ltd., Taipei, Taiwan), for 1 minute prior to swallowing the contents, daily after the evening meal for 7 consecutive days.
Based on the study design, this project is a single-group pilot clinical study (without control and randomization). Dependent variables include three parameters representing biofilm acidogenicity (3BA) derived from the “Stephan curve” [including lowest pH reached, pH recovery time, and the area under curve (AUC) below the critical pH 5.5], caries risk (CR), salivary S. mutans, Lactobacillus, and buffering capacity (BC). The independent variables include consumption of Yakult (before/after), and response to Yakult consumption based on AUC change (respondent/nonrespondent). All participants were recruited from outpatient pool as a convenient sample and were informed via doctors.
Oral examination and salivary tests
Oral examinations were conducted by an experienced pediatric dentist (C-Y.S.H.), using mirrors and explorers under focused flashlights in a conventional dental chair. Caries rate was assessed using deft and defs indices based on the World Health Organization diagnostic criteria.21 Oral hygiene status was evaluated for six index teeth (1E, 1B, 2D, 3E, 3B, and 4D) according to the Silness-Löe Plaque Index.22 Salivary S. mutans and Lactobacillus counts, and buffer capacity were semiquantified using Dentocult S. mutans Strip mutans, Dentocult Lactobacillus, and Dentobuff test kits (Orion Diagnostica, Espoo, Finland), respectively.
Plaque pH (acidogenicity) characterization
Plaque pH measurements were performed using a Beetrode NMPH-3 (World Precision Instruments Inc., Connecticut, Sarasota, FL, USA) 0.1-mm-diameter palladium touch microelectrode connected to a portable Orion PerpHect Model 370 unit (Thermo Fisher Scientific, Beverly, Essex, MA, USA). To create a reference salt bridge, participants immersed a finger in 3M KCl solution containing a DRIREF-5 4.7-mm-diameter porous glass reference electrode (World Precision Instruments Inc., Connecticut, Sarasota, FL, USA) connected to the pH meter unit. Electrodes were sterilized in 2.5% w/v glutaraldehyde and recalibrated against pH 4.0 and 7.0 standard buffers between each reading. Oral biofilm pH was measured at the distal surfaces of the maxillary canines, as previously described.23 Participants were instructed to rinse with a 10% sucrose solution for 1 minute. pH measurements were taken immediately prior to and after rinsing, then at 2 minutes, 5 minutes, and subsequently at 5-minute intervals henceforth until a stabilized pH was reached. A Stephan curve24 was generated for each participant at each visit, with three parameters derived from the curve, namely, the lowest pH reached, pH recovery time, and the AUC below the critical pH 5.5.
Caries risk assessment
The caries risk of each participant was assessed using Cariogram as described previously.25, 26 Questionnaire data, caries experience, oral hygiene status, and biological parameters (salivary S. mutans, Lactobacillus, and BC) were entered into the Cariogram software. For each participant, the Cariogram output of “actual chance to avoid new caries” was subtracted from 100% to obtain the percentage chance of developing caries. Participants were subsequently categorized into caries risk groupings of very low risk (0–20%), low risk (21–40%), moderate risk (41–60%), high risk (61–80%), and very high-risk (81–100%).
Statistical analyses
Statistical analyses were performed using the SPSS statistical package, version 20.0 (International Business Machines, Corp., Armonk, NY, USA). The Shapiro–Wilk test was used to test for normality, and Levene’s test was used to test for homogeneity of variance, prior to selection of appropriate parametric/nonparametric tests. The overall data were analyzed using two-tailed paired-sample t tests regarding CR and 3BA derived from the Stephan curve, with the subgroup analysis using Wilcoxon signed-rank test to assess the CR and 3BA outcome variables and the chi-square test for S. mutans, Lactobacillus, and BC dependent variables. For all analyses, P < 0.05 was considered statistically significant.
Results
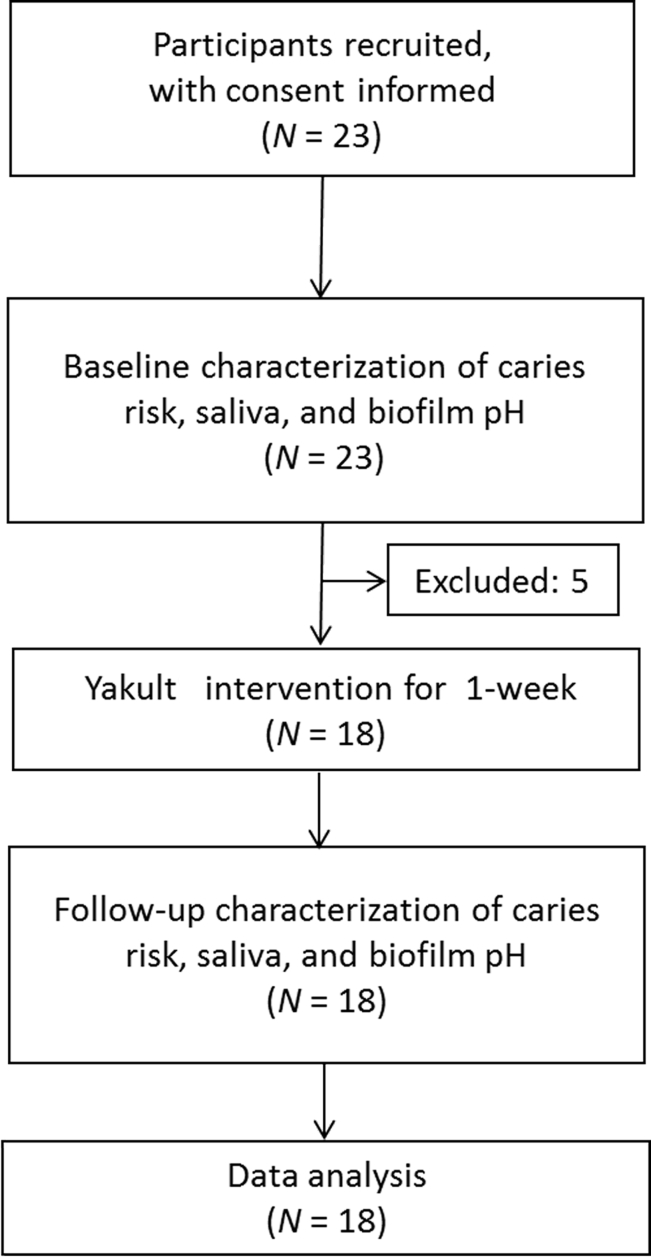
Twenty-three children were recruited, but five of them did not complete the study. Eighteen children, seven boys and 11 girls, aged between 7 and 11 years (mean, 9.17 ± 1.15 years) participated throughout the entire clinical study (Figure 1). The basic information of the 18 study participants is shown in Table S1. The questionnaire revealed no existing oral or caries-related diseases among participants. Of the 18 participants, 14 children (77.8%) consumed 4–5 meals/d, and all used fluoride toothpaste. The caries risk of study participants was assessed using Cariogram and categorized based on percentage risk. At baseline, the mean caries risk of all participants was 40.4% ± 23.8%. Based on the caries risk categorization, 44.4% of participants had low or very low caries risk, 38.9% had moderate caries risk, and 16.7% had high or very high caries risk at baseline.
Figure 1.
Flowchart of the participants.
After 1 week of Yakult consumption, the mean lowest pH displayed a significant 5.2% increase (P = 0.02: Table 1). Nonsignificant decreases in mean pH recovery time (29.3%) and AUC (18.2%) were also observed compared to baseline data (Table 1). The subgroup analysis using “reduction of AUC” to separate “responders” from the “nonresponders”, AUC revealed that responders (N = 12, Table 1) experienced significant 74.1% decrease in AUC (P = 0.002), substantial reduction in recovery time by 74.0% (P = 0.003), and increase of lowest pH by 0.41 (P = 0.005). These results indicated significantly decreased functional biofilm acidogenicity after 1-week consumption of Yakult. However, nonresponders (N = 6) underwent negative changes in all three parameters, indicating the increase in functional biofilm acidogenicity (Table 1). The data of Yakult effect on plaque acidity in all participants are shown in Table S2.
Table 1.
Effects of probiotic beverage on plaque acidity.
| N | Mean (SD) |
P | ||
|---|---|---|---|---|
| Before | After | |||
| Area under the curve | 18a | 5.97 (4.86) | 4.88 (6.44) | 0.396 |
| 12b | 5.67 (5.32) | 1.47 (3.58) | 0.002* | |
| 6b | 6.55 (4.18) | 11.71 (5.38) | 0.028* | |
| Lowest pH | 18a | 4.88 (0.27) | 5.14 (0.49) | 0.020* |
| 12b | 4.93 (0.29) | 5.34 (0.46) | 0.005* | |
| 6b | 4.80 (0.22) | 4.74 (0.27) | 0.6 | |
| Recovery time | 18a | 16.18 (9.75) | 11.44 (13.04) | 0.121 |
| 12b | 14.42 (9.22) | 3.75 (4.65) | 0.003* | |
| 6b | 19.83 (10.67) | 27 (9.84) | 0.075 | |
| CRA (%) | 18a | 40.42 (23.17) | 40.31 (21.76) | 0.976 |
| 12b | 39.79 (27.82) | 37.50 (26.31) | 0.126 | |
| 6b | 41.67 (15.06) | 45.92 (11.21) | 0.465 | |
CRA = caries risk assessment by Cariogram; SD = standard deviation.
*Statistically significant at P < 0.05.
Results from two-tailed paired t test.
Results from Wilcoxon signed-rank test.
Overall, participants had higher S. mutans scores than Lactobacillus scores, both prior to and after Yakult intervention. Twelve (before) or 13 participants (after) had high S. mutans scores ranging from 2 to 3, compared to only four participants with similarly high Lactobacillus scores (Table 2). S. mutans and Lactobacillus counts were not significantly changed in children after 1-week Yakult consumption (Table 2). The data of Yakult effect on oral bacterial counts prior to and after intake in all participants are shown in Table S3.
Table 2.
Effect of probiotic beverage on oral bacterial counts before and after intake.
| N |
Streptococcus mutans score (N) |
% of participants |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Decrease | Same | Increase | |||
| Before | 18 | 4 | 2 | 4 | 8 | ||||
| After | 18 | 4 | 1 | 6 | 7 | ||||
| All participants | 18 | 22.22% | 61.11% | 16.67% | 0.864 | ||||
| N |
Lactobacillus score (N) |
% of participants |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Decrease | Same | Increase | |||
| Before | 18 | 9 | 5 | 3 | 1 | ||||
| After | 18 | 10 | 4 | 1 | 3 | ||||
| All participants | 18 | 5.56% | 83.33% | 11.11% | 0.564 | ||||
S. mutans scores: (0: <104 CFU/mL; 1: <105 CFU/mL; 2: 105–106 CFU/mL; 3: >106 CFU/mL).
Lactobacillus scores: (0: ≤103 CFU/mL; 1: 104 CFU/mL; 2: 105 CFU/mL; 3: ≥106 CFU/mL).
S. mutans and Lactobacillus counts were not significantly changed after 1-week probiotic beverage consumption (P > 0.05).
CFU = colony forming units.
Discussion
The present study demonstrated that the probiotic effects of Yakult intake in children may vary significantly depending on individuals’ biofilm ecology prior to treatment. After a 7-day consumption of Yakult, an acidic sweet drink, significant cariostatic effects in children with certain biofilm profile were demonstrated, whereas children with “low” caries risk may experience potential cariogenic/detrimental effects after consuming the probiotic drink.
Recently, the cariostatic effects of regular long-term (10 months) Lactobacillus intake in high-caries preschool children has been demonstrated,27 in which the percentage of new individuals who developed cavitated lesions (9.7%) in the probiotic group (consuming milk supplemented with L. rhamnosus SP1) was shown to be substantially lower than 24.3% of the control group (consuming standard milk). Another clinical study found that a 12-week intake of two strains of Lactobacillus reuteri (D 17938 and ATCC PTA 5289) considerably reduced the prevalence of high Candida counts in frail elderly patients.28 Furthermore, in vitro studies have proven the effectual inhibition of S. mutans growth and biofilm formation by L. casei Shirota,29 and other L. casei strains.30, 31 Interestingly, a previous study found that L. casei Shirota and B. breve strain Yakult, introduced into hepatectomy patients in formulation with prebiotic galacto-oligosaccharides, had successfully colonized patients and persisted 14 days after probiotic treatment,15 suggesting that combined probiotic and prebiotic formulations (“synbiotics”) may promote probiotic establishment and retention in vivo.
Theoretically, consumption of Yakult can be cariogenic because of its acidity, sweet contents (fructose/glucose), and acid-producing bacteria; however, our study discovered that the cariostatic effects of Yakult surprisingly outweighed its cariogenic risk in children with certain biofilm ecology and/or caries risk. Hence, being sweet and palatable, Yakult may be a promising caries-preventive agent especially for children with high caries risk and those “addicted” to sugar-containing food. As such, further clinical investigations on the specific cariogenic/cariostatic effects of Yakult are of significant importance.
Considering that standard Yakult contains 17% w/w carbohydrates, including the cariogenic fermentable sugars, namely, sucrose, glucose, fructose, and maltodextrin,32 L. casei Shirota/Yakult may have a decreased cariostatic effect on oral biofilms when administered in such a sweet and acidic beverage. Alternative vehicles for probiotic administration have been proposed, including cheese, tablets, yoghurt, and straws,9, 33, 34, 35, 36 and may be more effective for L. casei Shirota as an adjunct caries-preventive treatment. Furthermore, certain patients with established oral biofilms may inhibit colonization of probiotic species and thus hinder probiotic activity. In children aged 6–12 years, rinsing the oral cavity with a chlorhexidine-containing antimicrobial solution prior to probiotic intake was correlated with increased oral L. rhamnosus GG colonization and reduced counts of S. mutans for up to 5 weeks after treatment.34 A similar procedure may potentially improve L. casei Shirota uptake and persistence in children.
Although the exact mechanism(s) of probiotics in caries prevention remain unknown, there have been a few speculations, including the coaggregation of S. mutans,37, 38 bacteriocidic effects on S. mutans,37 reduced production of insoluble extracellular polysaccharides in biofilm formation,39 and reduction of salivary counts of S. mutans.35 Nevertheless, probiotic effect has been demonstrated without reduction of S. mutans level in biofilm,40 similar to our findings. Our recent laboratory study found the decreased acid production of S. mutans via reduction of gtfB, gtfC, and ldh expressions in the presence of L. casei Shirota without significant change in S. mutans counts.41 Therefore, it is substantiated in our study that bacterial counts in saliva or biofilm might not be a reliable outcome parameter to assess the probiotic effect.
Based on the findings of subgroup analysis, this study highlighted the importance of preintervention patient selection via a valid CRA program. Although Cariogram has demonstrated good validity among Swedish schoolchildren,26 its performance on Asian children appeared less satisfactory and inferior to the National University of Singapore Caries Risk Assessment (NUS-CRA) program.42 It is substantiated in this study that Cariogram was not sensitive enough to show the probiotic/beneficial effect of Yakult among the respondents, as shown in Table 2 that the risk % of the respondents did not significantly change (P > 0.05). Therefore, to maximize the cariostatic benefit while preventing the cariogenic effect of Yakult among Asian children, it may be prudent to carefully select participants using an appropriate CRA system, such as NUS-CRA community-screening model published earlier,25 prior to recommendation and/or administration of Yakult, before an ideal/perfect CRA program is developed.
As the first study to investigate the cariogenic/cariostatic effect of Yakult among children, this study has multiple limitations. First, it is not a randomized clinical trial with the control group receiving placebo. Therefore, the results need to be interpreted cautiously, taking into consideration the potential Hawthorne effect and potentially weak external validity. Second, the sample size is rather small and may be underpowered. However, the significant effects shown in the respondent group indicates that the potential effect may warrant further investigation. Nevertheless, to assess the validity of the result, the post hoc power calculation was carried out. With a sample size of 18, the detectible difference between before and after treatment will be 70% of the standard deviation of the difference. The estimated standard deviation for the difference in lowest pH, before and after treatment, is about 0.4 unit. Hence, with 80% power, the detectible difference is about 0.28 (which is slightly more than what we observed 0.25). Assuming the mean difference is 0.25, the power of detecting the difference is about 70%, which is reasonably acceptable. Third, the clinical effects may be further attenuated by potential noncompliance of children such as insufficient rinsing duration and daily intake of the probiotic beverage or inability to refrain from brushing prior to the clinical measurements. However, these noncompliant factors are likely to reduce the cariostatic effects observed in our study. Therefore, the actual cariostatic effect of Yakult may be underestimated in this study. Future studies may enlist parental oversight in ensuring compliance by the children and increase the observation period in a randomized clinical trial. Fourth, the duration of intervention time may be too short for the probiotic effect to take place. In the future, longer intervention time with randomized controlled design and stratified caries risk groups may be considered.
In conclusion, there may be a potential cariostatic effect of short-term Yakult intake in reducing functional biofilm acidogenicity in children with certain oral biofilm and risk profile. Future randomized clinical trials may be needed to validate the potential cariostatic effect observed in our study. Quality risk assessment may be critical prior to prescribing/recommending Yakult as an adjunct caries-preventive treatment for children.
Conflicts of interest
The authors declare that they have no conflicts of interest relevant to this article.
Acknowledgments
This study was financially supported by the Kaohsiung Chang Gung Memorial Hospital, Taiwan (grant #CMRPG890991). This research is also partially supported by the Singapore Ministry of Health's National Medical Research Council under its “Clinician Scientist - Individual Research Grant” Scheme, NMRC/CIRG/1341/2012 (grant #R-221-000-059-511). We would like to thank Dr Dan ZHENG for revising the manuscript and completing the proofreading.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jds.2016.09.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Hill C., Guarner F., Reid G. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 2.Fuller R. Probiotics in human medicine. Gut. 1991;32:439–442. doi: 10.1136/gut.32.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid G., Jass J., Sebulsky M.T., McCormick J.K. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–672. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saxelin M., Tynkkynen S., Mattila-Sandholm T., De Vos W.M. Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotechnol. 2005;16:204–211. doi: 10.1016/j.copbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Devine D.A., Marsh P.D. Prospects for the development of probiotics and prebiotics for oral applications. J Oral Microbiol. 2009;1:1949. doi: 10.3402/jom.v1i0.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen D.M. Probiotics and oral health. J Dent Hyg. 2013;87:5–9. [PubMed] [Google Scholar]
- 7.Campus G., Cocco F., Carta G. Effect of a daily dose of Lactobacillus brevis CD2 lozenges in high caries risk schoolchildren. Clin Oral Investig. 2014;18:555–561. doi: 10.1007/s00784-013-0980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nase L., Hatakka K., Savilahti E. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35:412–420. doi: 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- 9.Hedayati-Hajikand T., Lundberg U., Eldh C., Twetman S. Effect of probiotic chewing tablets on early childhood caries—a randomized controlled trial. BMC Oral Health. 2015;15:112. doi: 10.1186/s12903-015-0096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moynihan P.J., Kelly S.A.M. Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. J Dent Res. 2014;93:8–18. doi: 10.1177/0022034513508954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keefe DM. Agency response letter GRAS Notice No. Grn 000429. Available at: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm335746.htm. [Date accessed: May 6, 2016].
- 12.Mennella J.A., Beauchamp G.K. Optimizing oral medications for children. Clin Ther. 2008;30:2120–2132. doi: 10.1016/j.clinthera.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koebnick C., Wagner I., Leitzmann P., Stern U., Zunft H.J.F. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol. 2003;17:655–659. doi: 10.1155/2003/654907. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto K., Takeshita T., Nanno M., Tokudome S., Nakayama K. Modulation of natural killer cell activity by supplementation of fermented milk containing Lactobacillus casei in habitual smokers. Prev Med. 2005;40:589–594. doi: 10.1016/j.ypmed.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa H., Nagino M., Kamiya S. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg. 2005;390:104–113. doi: 10.1007/s00423-004-0536-1. [DOI] [PubMed] [Google Scholar]
- 16.Marcenes W., Kassebaum N.J., Bernabé E. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. 2013;92:592–597. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services . US Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; Rockville, MD: 2000. Oral health in America: a report of the surgeon general—executive summary. [Google Scholar]
- 18.Gao X.L., Hsu C.Y., Loh T., Koh D., Hwamg H.B., Xu Y. Dental caries prevalence and distribution among preschoolers in Singapore. Community Dent Health. 2009;26:12–17. [PubMed] [Google Scholar]
- 19.Mattila M.L., Rautava P., Sillanpää M., Paunio P. Caries in five-year-old children and associations with family-related factors. J Dent Res. 2000;79:875–881. doi: 10.1177/00220345000790031501. [DOI] [PubMed] [Google Scholar]
- 20.Bratthall D., Petersson G.H. Cariogram–a multifactorial risk assessment model for a multifactorial disease. Community Dent Oral Epidemiol. 2005;33:256–264. doi: 10.1111/j.1600-0528.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . World Health Organization; Geneva, Switzerland: 1997. Oral health surveys: basic methods. [Google Scholar]
- 22.Silness J., Löe H. Periodontal disease in pregnancy: II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 23.Donly K.J., Wefel J.S., Campbell S.L. In vivo plaque acid production with an experimental dentifrice. J Dent Res. 1998;77:144. [Google Scholar]
- 24.Stephan R.M., Miller B.F. A quantitative method for evaluating physical and chemical agents which modify production of acids in bacterial plaques on human teeth. J Dent Res. 1943;22:45–51. [Google Scholar]
- 25.Gao X.L., Hsu C.Y.S., Xu Y., Hwarng H.B., Loh T., Koh D. Building caries risk assessment models for children. J Dent Res. 2010;89:637–643. doi: 10.1177/0022034510364489. [DOI] [PubMed] [Google Scholar]
- 26.Hänsel Petersson G., Twetman S., Bratthall D. Evaluation of a computer program for caries risk assessment in schoolchildren. Caries Res. 2002;36:327–340. doi: 10.1159/000065963. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez G., Ruiz B., Faleiros S. Probiotic compared with standard milk for high-caries children: a cluster randomized trial. J Dent Res. 2016;95:402–407. doi: 10.1177/0022034515623935. [DOI] [PubMed] [Google Scholar]
- 28.Kraft-Bodi E., Jorgensen M.R., Keller M.K., Kragelund C., Twetman S. Effect of probiotic bacteria on oral Candida in frail elderly. J Dent Res. 2015;94:181S–186S. doi: 10.1177/0022034515595950. [DOI] [PubMed] [Google Scholar]
- 29.Lin X., Chen X., Chen Y., Jiang W., Chen H. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 2015;21:e128–e134. doi: 10.1111/odi.12257. [DOI] [PubMed] [Google Scholar]
- 30.Teanpaisan R., Piwat S., Dahlén G. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett Appl Microbiol. 2011;5:452–459. doi: 10.1111/j.1472-765X.2011.03132.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee S.-H., Kim Y.-J. A comparative study of the effect of probiotics on cariogenic biofilm model for preventing dental caries. Arch Microbiol. 2014;196:601–609. doi: 10.1007/s00203-014-0998-7. [DOI] [PubMed] [Google Scholar]
- 32.Grenby T.H., Mistry M. Properties of maltodextrins and glucose syrups in experiments in vitro and in the diets of laboratory animals, relating to dental health. Br J Nutr. 2000;84:565–574. [PubMed] [Google Scholar]
- 33.Ahola A.J., Yli-Knuuttila H., Suomalainen T. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol. 2002;47:799–804. doi: 10.1016/s0003-9969(02)00112-7. [DOI] [PubMed] [Google Scholar]
- 34.Aminabadi N.A., Erfanparast L., Ebrahimi A., Oskouei S.G. Effect of chlorhexidine pretreatment on the stability of salivary lactobacilli probiotic in six- to twelve-year-old children: a randomized controlled trial. Caries Res. 2011;45:148–154. doi: 10.1159/000325741. [DOI] [PubMed] [Google Scholar]
- 35.Caglar E., Cildir S.K., Ergeneli S., Sandalli N., Twetman S. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand. 2006;64:314–318. doi: 10.1080/00016350600801709. [DOI] [PubMed] [Google Scholar]
- 36.Nishihara T., Suzuki N., Yoneda M., Hirofuji T. Effects of Lactobacillus salivarius-containing tablets on caries risk factors: a randomized open-label clinical trial. BMC Oral Health. 2014;14:110. doi: 10.1186/1472-6831-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keller M.K., Hasslöf P., Stecksén-Blicks C., Twetman S. Co-aggregation and growth inhibition of probiotic lactobacilli and clinical isolates of mutans streptococci: an in vitro study. Acta Odontol Scand. 2011;69:263–268. doi: 10.3109/00016357.2011.554863. [DOI] [PubMed] [Google Scholar]
- 38.Lang C., Böttner M., Holz C. Specific lactobacillus/mutans streptococcus co-aggregation. J Dent Res. 2010;89:175–179. doi: 10.1177/0022034509356246. [DOI] [PubMed] [Google Scholar]
- 39.Lodi C.S., Manarelli M.M., Sassaki K.T., Fraiz F.C., Delbem A.C.B., Martinhon C.C.R. Evaluation of fermented milk containing probiotic on dental enamel and biofilm: in situ study. Arch Oral Biol. 2010;55:29–33. doi: 10.1016/j.archoralbio.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Marttinen A.M., Haukioja A.L., Keskin M., Söderling E.M. Effects of Lactobacillus reuteri PTA 5289 and L. paracasei DSMZ16671 on the adhesion and biofilm formation of Streptococcus mutans. Curr Microbiol. 2013;67:193–199. doi: 10.1007/s00284-013-0352-3. [DOI] [PubMed] [Google Scholar]
- 41.Chen H.-Z. National University of Singapore; Singapore: 2013. Oral probiotic effect of Lactobacillus casei Shirota on Streptococcus mutans. PhD thesis. [Google Scholar]
- 42.Gao X., Wu I.D., Lo E.C.M., Chu C.H., Hsu C.Y.S., Wong M.C.M. Validity of caries risk assessment programmes in preschool children. J Dent. 2013;41:787–795. doi: 10.1016/j.jdent.2013.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.