Abstract
Background/purpose
The pathogenesis of rheumatoid arthritis (RA)-related temporomandibular joint (TMJ) disorder remains unclear. Studies have reported the change of the TMJ after complete Freund's adjuvant (CFA) injection, which is consistent with osteoarthritis. However, few studies have reported that the tissue response of the TMJ in collagen-induced arthritis (CIA) can mimic RA. The present study was aimed to investigate the TMJ response in rat models by CFA-induced arthritis and CIA to verify the proper RA-related TMJ arthritis rat model.
Materials and methods
In total, 24 rats were randomly divided into four groups: (1) control group; (2) type I collagen injection group; (3) CFA-induced arthritis group; and (4) CIA group. Drugs were injected on Day 0, and the rats were sacrificed on Days 7 and 35. Next, TMJ tissue was collected for hematoxylin and eosin staining, and inflammatory gene (IL-1β and MMP3) expression was investigated.
Results
Compared with the control group, the type I collagen injection group confirmed the negative inflammatory response through hematoxylin and eosin staining and IL-1βand MMP3 expression. Although CFA-induced arthritis and CIA groups showed inflammatory response (P < 0.05) compared with the control group, histological changes were different. The 7-day CFA-induced arthritis group showed adaptive changes and partly recovered after 35 days of induction. In contrast, 7- and 35-day CIA groups underwent a degenerative process.
Conclusion
Considering the study limitations, the CIA method is a proper method to study the mechanism of RA-related TMJ arthritis.
Keywords: collagen-induced arthritis, rheumatoid arthritis, temporomandibular joint arthritis
Introduction
Temporomandibular joint (TMJ) arthritis induced by rheumatoid arthritis (RA) or osteoarthritis (OA) are common in clinical settings. Inflammatory response of patients may damage the joints and affect their normal function.1 TMJ arthritis may limit jaw movement or cause chewing difficulty, TMJ disc internal derangement, and even bone destruction. Some of the studies reported that OA is the most common type of arthritis in human body that can also affect the TMJ function.2, 3, 4 There were 42.6% of patients with TMJ disorder presenting with radiographic evidence of TMJ-OA changes.5 The destructive process of TMJ can be observed in OA patients. Their bony surfaces of condyle and fossa become altered because of inflammatory response; radiographic images may show flattening of the condyle.6, 7 Furthermore, osseous changes of condyle also occur in response to TMJ-OA and lead to disc displacement, frequently with longstanding disc displacement without reduction.8 On the other hand, Lin et al9 reported a higher prevalence of TMJ abnormalities in patients with RA, as determined through physical (85.7%) and radiological (74.5%) examinations. Studies indicated that RA is derived from a complex interaction between genes and environment, leading to a breakdown of immune tolerance, synovial inflammation, and autoantibody production. Distinct mechanisms may induce joint swelling and tenderness and ultimately bone destruction and joint deformity.10, 11, 12 With damage to the joint tissues, severe osseous changes can occur in the TMJ-RA patients. TMJ-RA can cause painful symptoms of the joint and cause destruction of the temporomandibular articular surfaces.13, 14, 15
TMJ inflammation is a key factor of OA and RA; however, the detailed degeneration process in the inflamed TMJ remains unclear. In studies regarding TMJ-OA, complete Freund's adjuvant (CFA)-induced arthritis has been widely used in physiological, biochemical, and histopathological studies.16, 17, 18 CFA is a solution of antigen emulsified in mineral oil and consists of inactivated and dried mycobacteria. It has been injected into the intra-articular space of the joint. Edema and pain are produced within 12 hours. After that, articular cartilage destruction and bone erosion may persist for several weeks. In CFA-induced arthritis model, higher level of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, can be detected in the serum and synovial fluid. Proinflammatory cytokine expression in the inflamed synovial membrane of arthritis leads to an imbalance in bone and articular cartilage formation.19
However, the pathogenesis of CFA-induced TMJ arthritis in an animal model is more similar to OA than to RA. OA and RA are different types of arthritis.20 The development of OA is driven by inflammatory processes and finally leads to joint degeneration. RA is an autoimmune disease which will lead the body's immune system to attack its own tissues. These two diseases share some similar characteristics, but each has different symptoms and requires different treatment modalities.21 Therefore, the animal model of CFA-induced TMJ arthritis is not appropriate for research in TMJ-RA. Among the induced method of RA animal models, Bolon et al22 used different arthritis-inducing methods, such as adjuvant-induced arthritis, collagen-induced arthritis (CIA), and streptococcal cell wall-induced arthritis, to evaluate the appropriateness of these methods for inducing RA in knee joint. Results indicated that the immunological change of CIA was similar to that of RA. CIA has been widely used in experimental model of RA by the injection of heterologous collagen emulsified in CFA.23, 24 Collagen is highly conserved between different species, and the pathogenesis of CIA is due to heterologous collagen eliciting cross-reactive autoimmune responses to endogenous collagen and then both CD4 T-cells activation and the production of anticollagen antibody, leading to CIA.25 The role of anticollagen antibodies is as direct mediators of tissue pathology in the spontaneous autoimmune disease. The anticollagen antibody reaction which will lead to complement activation and cause inflammatory response.26 However, most of the RA studies were focused on the knee joint, but few studies have discussed animal models of RA-related TMJ arthritis. This means that the experience of CIA in knee joint of RA animal model may help to clarify the RA-related TMJ arthritis. Based on the above researches, the aim of this study was to investigate the response of the TMJ tissue by CFA-induced arthritis and CIA. The experiments were designed to clarify: (1) Did CIA have the same inflammatory response as CFA-induced arthritis? (2) Did CIA have the same TMJ change as CFA-induced arthritis? (3) Was CIA more appropriate than CFA-induced arthritis in RA-related TMJ arthritis research?
Material and methods
Rat TMJ injection technology
All animal protocols were performed in accordance with the protocol of the Institutional Animal Care and Use Committee of National Yang-Ming University. The animals were housed in a temperature controlled environment under a 12-hour light/dark cycle. In this study, 8-week-old female Sprague-Dawley rats weighing 250–320 g were used. Furthermore, 50 μL of alcian blue dye was injected into the superior space of the TMJ to demonstrate the TMJ injection technology. Next, the rats were sacrificed to evaluate the accuracy of TMJ injection technology.
Drug-induced TMJ arthritis model
In total, 24 rats were randomly divided into four groups. The first group was the control group, which did not receive any drug injection. The second group was the collagen I group, which received 50 μL of type I collagen (sc-29009; Santa Cruz; Dallas, USA), to determine whether type I collagen alone leads to rat arthritis. The third group was the CFA-induced arthritis group; the induced method of this group was followed by other previous studies.16, 17, 26 This group was injected with 50 μL of CFA (F5881; Sigma; St. Louis, USA). The fourth group was the CIA group, which received 50 μL of type I collagen and CFA (1:1). The injection dose was as followed in previous studies in knee joint and TMJ.16, 22 The drugs were injected on Day 0, and the rats were sacrificed on Days 7 and 35. After the rats were sacrificed on Day 7, the TMJ tissue was collected to investigate the inflammatory RNA (IL-1β and MMP3) expression to ensure whether the TMJ inflammatory response had occurred. In addition, changes in the TMJ tissue of rats sacrificed on Days 7 and 35 were investigated through hematoxylin and eosin (H&E) staining.
Tissue preparation and histopathological staining
Half of the rat TMJ tissue samples were collected for H&E staining, and the other half was separated as the disc, condyle, and synovial tissue for RNA extraction. RNA was isolated using Trizol (Invitrogen, Carlsbad, CA, USA) and purified using Quick-RNATM MiniPrep Kit (Zymo Research, Irvine, CA, USA). The RNA purity was determined through spectrophotometry by measuring the absorbance of an aliquot at 260 nm and 280 nm. The total mRNA was reverse-transcribed to cDNA using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA, USA).
Semi-quantitative polymerase chain reaction
Conventional polymerase chain reaction (PCR) was used to investigate IL-1β and MMP3 expression in the disc, condyle, and synovial tissues. β-actin was used as the internal control. The PCR mixture (20 μL) contained a concentration of 150nM for each primer, 10 μL of 2 × PCR buffer (Ampliqon III, Odense, Denmark), and 1 μL of purified DNA. Denaturing (95°C), annealing (60°C), and extension (72°C) times were 1 minute for each. The specific product was separated on a 2% agarose gel and detected under UV illumination after staining with ethidium bromide (Table 1). The procedure for ratio image generation was conducted using the ImageJ 1.48v software ( National Institutes of Health, Bethesda, MD, USA).
Table 1.
Primers used in this study.
| Gene | Primer Sequences | Product size (bp) | Cycles |
|---|---|---|---|
| IL-1β | Forward: 5′- CAAAAATGCCTCGTGCTGTCT -3′ | 130 | 32 |
| Reverse: 5′- CAGGGATTTTGTCGTTGCTTG -3′ | |||
| MMP3 | Forward: 5′- TCCCTGAAACCGTCCAGAAG -3′ | 173 | 32 |
| Reverse: 5′- ATCCACCTTTGTGCCAATGC -3′ | |||
| β-actin | Forward: 5′- CTATGAGGGTTACGCGCTCC-3′ | 141 | 28 |
| Reverse: 5′- ATGTCACGCACGATTTCCCT-3′ |
bp = base pair.
Statistical analysis
Statistical analysis was performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA). All data were presented as the mean ± standard error of mean. The nonparametric Kruskal–Wallis was used for the data analysis. Results with P < 0.05 were considered significant.
Results
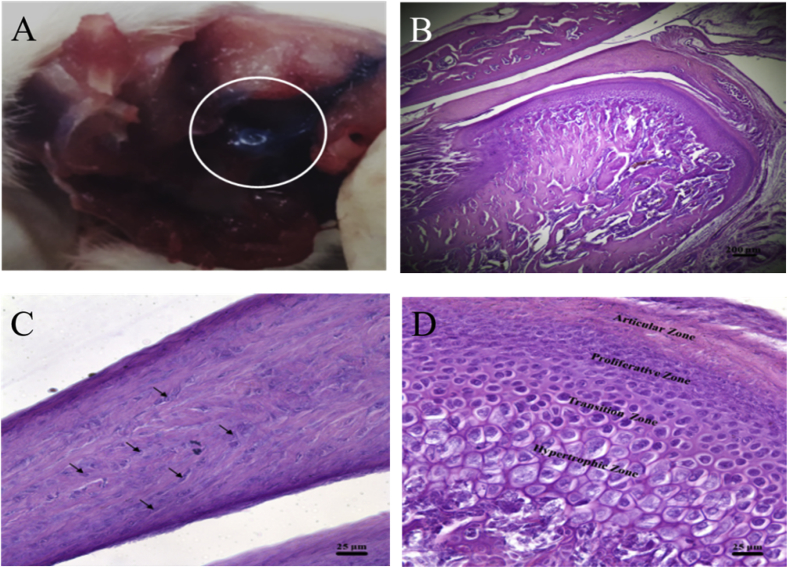
Alcian blue was injected into the TMJ upper space to demonstrate the TMJ injection technology. The TMJ disc was found to be blue after the removal of the surrounding connective tissue of the TMJ. These results confirmed that the TMJ injection technology accurately delivered the vehicle into the TMJ upper space (Figure 1A). The TMJ histology was investigated through H&E staining. The sagittal view of the TMJ was divided into three parts: glenoid fossa, TMJ disc, and TMJ condyle (Figure 1B). Under a magnified microscopic view, some chondrocyte cells were observed inside the TMJ disc (Figure 1C, black arrow). The control group showed a complete structure of the TMJ condyle subchondral bone, which was divided into four zones, namely articular, proliferative, transition, and hypertrophic zones (Figure 1D).
Figure 1.
Effects of the injection technology on the histology of the control group. (A) Observation of the temporomandibular joint (TMJ) disc after removal of the surrounding connective tissues showed the blue disc which was stained by alcian blue. (B) Sagittal view of the TMJ divided into three parts: glenoid fossa, the TMJ disc, and the TMJ condyle. Scale bar: 200 μm. (C) Enlarged view of the TMJ disc showed clear fibrocartilage cells (black arrow). Scale bar: 25 μm. (D) Enlarged view of the TMJ condyle subchondral bone separated into articular, proliferative, transition, and hypertrophic zones. Scale bar: 25 μm.
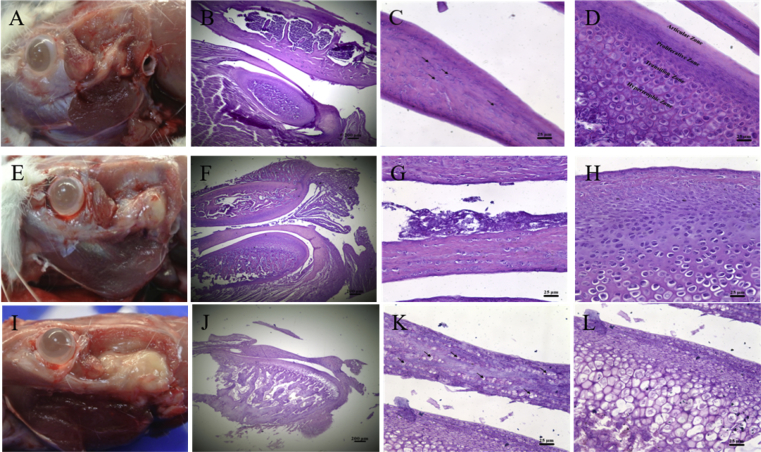
To develop a TMJ-CIA rat model, mechanism of which is similar to RA, type I collagen was injected to determine whether it alone causes TMJ immune responses; this was subsequently compared with the CFA-induced arthritis and CIA groups. Histological data of both the collagen type I rats that were sacrificed on Day 7 and the control rats revealed that their conditions were identical (Figure 2A). A sagittal view of the TMJ revealed regular disc thickness and chondrocyte cells (Figure 2B, C). The TMJ condyle subchondral bone was clearly observable in the four zones (Figure 2D). However, a bright view revealed inflamed cysts in the CFA-induced arthritis group rats sacrificed on Day 7 (Figure 2E). The subchondral bone of the CFA-induced arthritis group rats sacrificed on Day 7 showed an adaptive changes (Figure 2F). The thickness of the TMJ disc also did not change in the CFA-induced arthritis group (Figure 2G). The adaptive transition zone of the bone change was observed using an enlarged view of the TMJ condyle subchondral bone (Figure 2H). The CIA group rats that were sacrificed on Day 7 showed more severely inflamed cysts (Figure 2I) and degradation than did the CFA-induced arthritis group (Figure 2J). Although the TMJ disc thickness of this group was maintained, the chondrocyte cells had disappeared (Figure 2K, black arrow). The TMJ condyle subchondral bone was unclearly stratified and severely degraded in the hypertrophic zone (Figure 2L).
Figure 2.
Histological changes in the drug-induced groups after 7 days. (A) Bright view of the type I collagen group showing the temporomandibular joint (TMJ) structure. (B) Sagittal view of the TMJ of the type I collagen group. (C) Enlarged view of the TMJ disc in the type I collagen group showing the regular thickness and some chondrocyte cells (black arrows). (D) Enlarged view of the TMJ condyle subchondral bone separated into four layers in type I collagen group. (E) Bright view of the complete Freund's adjuvant (CFA)-induced arthritis group showing inflamed cysts. (F) Sagittal view of the TMJ in the CFA-induced arthritis group. (G) Enlarged view of the TMJ disc in the CFA-induced arthritis group. (H) Enlarged view of the TMJ condyle subchondral bone in the CFA-induced arthritis group for investigation of bone changes in the adaptive transition zone. (I) Bright view of the collagen-induced arthritis (CIA) group showing severely inflamed cysts. (J) Sagittal view of the TMJ in the CIA group. (K) Enlarged view of the TMJ disc in the CIA group. Although the TMJ disc thickness was maintained, the chondrocyte cells disappeared (black arrows). (L) Enlarged view of the TMJ condyle subchondral bone in the CIA group. The TMJ condyle subchondral bone appeared unclearly stratified and severely degraded in the hypertrophic zone. Scale bar for (B), (F), and (J) is 200 μm. Scale bar for (C), (D), (G), (H), (K) and (L) is 25 μm.
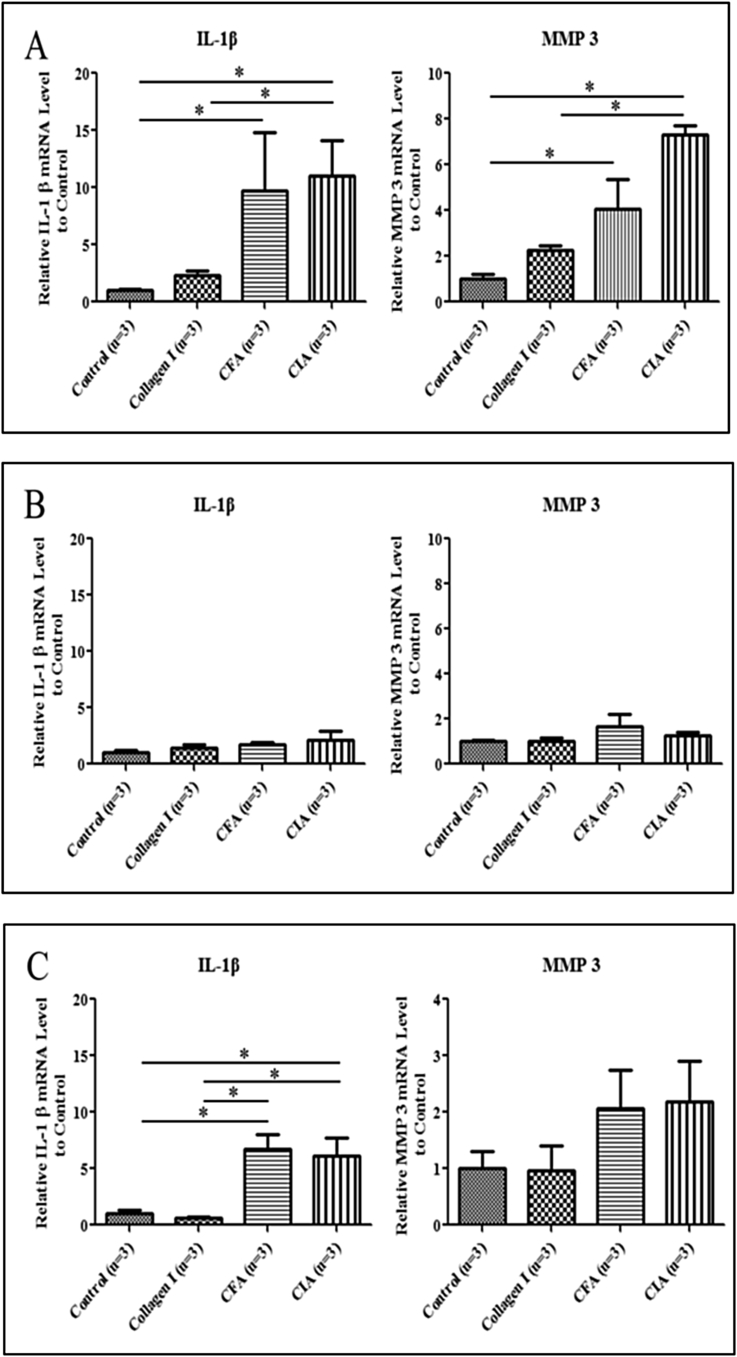
After drug injection for 7 days, both IL-1β and MMP3 expression of disc increased in the CFA-induced arthritis and CIA groups compared with the control (P < 0.05). As well as it showed significant differences in CIA groups compared with the type I collagen groups (Figure 3A). However, the expression of these inflammatory genes was lower in the condyle (Figure 3B). IL-1β expression of synovial tissue showed significant differences in CFA-induced arthritis and CIA groups compared with control group and type I collagen groups (P < 0.05). Although MMP3 expression of synovial tissue did not show difference, it showed the increasing trend in CFA-induced arthritis and CIA groups (Figure 3C).
Figure 3.
IL-1β and MMP3 expression in the temporomandibular joint (TMJ) tissue after drug injection for 7 days. (A) Gene expression in the TMJ disc. IL-1β and MMP3 expression was higher in the CFA-induced arthritis group and CIA groups than in the control group and type I collagen groups. (B) Gene expression in the TMJ condyle. No significant difference was observed among the four groups. (C) Gene expression in the TMJ synovial tissue. IL-1β and MMP3 expression was higher in the CFA-induced arthritis and CIA groups than in the control and type I collagen groups. (* P < 0.05). CFA = complete Freund's adjuvant; CIA = collagen-induced arthritis.
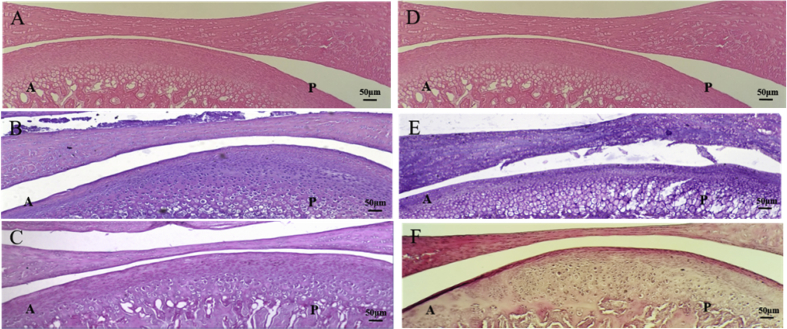
In this study, the CFA-induced arthritis and CIA induction period was 35 days. Compared with the control group (Figure 4A), the 7-day CFA-induced arthritis group showed an adaptive transition zone of bone change in the TMJ condyle (Figure 4B). However, this zone partly recovered after 35 days of CFA induction. The TMJ disc in the 35-day CFA induction group became thinner than that in the control and 7-days CFA induction groups (Figure 4C). By contrast, compared with the control group (Figure 4D), the 7-day CIA group showed unclear stratification and severe degradation in the hypertrophic zone (Figure 4E). Moreover, the TMJ disc became thinner in the 35-day CIA group than in the control and 7-day groups. The TMJ condyle subchondral bone of the 35-day CIA group not only appeared unclearly stratified and severely degraded in the hypertrophic zone but also showed irregular cell distribution and abnormal calcification (Figure 4F). These results suggest markedly different histologically changes between the CFA-induced arthritis and CIA groups. These results may be due to different pathogeneses leading to different TMJ condyle subchondral bone responses.
Figure 4.
Histological changes in the drug-induced groups on Days 7 and 35. (A and D) Sagittal view of the TMJ in the control group. (B) Sagittal view of the TMJ after 7 days of CFA induction. The TMJ condyle showed adaptive changes in the transition zone. (C) Sagittal view of the TMJ after 35 days of CFA induction. The TMJ condyle transition zone partly recovered and the TMJ disc became thinner than that observed in the control and 7-day CFA induction groups. (E) Sagittal view of the TMJ after 7 days of CIA induction. The condyle subchondral bone of TMJ was unclearly stratified and severely degraded in the hypertrophic zone. (F) Sagittal view of the TMJ after 35 days of CIA induction. The TMJ disc became thinner in the 35-day CFA induction group than that in the control and 7-day CIA induction groups. The TMJ condyle subchondral bone not only appeared unclearly stratified and severely degraded in the hypertrophic zone but also showed irregular cell distribution and abnormal calcification. Scale bar is 50μm. A = anterior; CFA = complete Freund's adjuvant; CIA = collagen-induced arthritis; TMJ = temporomandibular joint; P = posterior.
Discussion
The present study investigated the inflammatory response and histological changes of the TMJ tissue by CFA-induced arthritis and collagen-induced arthritis in rat models. The experiments were designed to clarify: (1) Did CIA have the same inflammatory response as CFA-induced arthritis? (2) Did CIA have the same TMJ change as CFA-induced arthritis? (3) Was CIA more appropriate than CFA-induced arthritis in RA-related TMJ arthritis research?
In the first question, CFA-induced arthritis and CIA animal models were induced to simulate OA and RA-like TMJ arthritis, respectively. After drug injection, the animal models were investigated at Day 7 and Day 35 to determine the short- and long-term changes in the TMJ disc, synovial tissue, and condyle. Drug injection and sacrifice time points were considered by other studies16; thus, the data in this study can also be compared with previous studies. Our results indicated that both the induction methods of CFA-induced arthritis and CIA can cause inflammation and appeared to have different histological changes in TMJ. Only the type I collagen injection group confirmed the negative inflammatory response through H&E staining and IL-1β and MMP3 expression. From the previous studies, CFA-induced arthritis also showed the positive inflammatory response and became adaptive changes of the TMJ tissue.16, 17, 18 A previous study has shown that after 35 days of CFA injection, rat TMJ disc showed degenerative changes, including deformation, thickening, increases in wet and net weights, increased cellularity, increased iNOS and IL-1β expression, and significant increases of collagen type I and aggrecan contents, in addition to infiltration of mononucleated cells and abundant adipose tissue in the synovium.16 Other studies also showed hypertrophic response in TMJ and observed phenotypic changes in the condylar cartilage.17, 18 These results are consistent with the data of CFA-induced arthritis in our study. However, it is noteworthy that even changes in the condylar fibrocartilage can be observed in histology, but there are similar results among these four groups in gene expression. Therefore, further study is needed to determine how the TMJ condylar changes in the TMJ arthritis.
Regarding the second question, this study also injected type I collagen + CFA in the CIA group to induce TMJ arthritis; the results revealed different morphological changes compared with other groups. The condyle of the CIA group was thinnest among all the groups at Day 7; the subchondral bone appeared unclearly stratified and severely degraded in the hypertrophic zone but also showed an irregular cell distribution and abnormal calcification at Day 35. Iy contrast, the 7- and 35-day CFA induction groups showed adaptive changes of the TMJ subchondral bone. However, a study reported changes in the condylar fibrocartilage after CFA induction for 7 days.17 In addition, in another study that used CFA to induce arthritis for 35 days, the condylar fibrocartilage maintained its shape but thicker than the normal group.16 These results are in agreement with our results of CFA-induced arthritis group. In recent years, more and more TMJ studies have focused on the effect of subchondral bone on TMJ arthritis. These studies reported that increased turnover of subchondral bone plays a role in the initiation or progression of TMJ arthritis.16, 17, 27 The results also indicated that the subchondral bone manifested a severe change in histological observation compared with the disc during inflammation. These results may explain why the TMJ disc remained observable in severe degeneration or deformation of TMJ disorder.
In the third question, previous studies showed that CIA has been widely used in the experimental model of RA by injection of heterologous collagen emulsified in CFA. During CIA, heterologous collagen elicits cross-reactive autoimmune responses to endogenous collagen, and then, the inflammatory response and the production of anticollagen antibody finally attacks the joint. Anticollagen antibodies play a role as direct mediators of tissue pathology in the spontaneous autoimmune disease. This anti-collagen antibody reaction which will lead to complement activation and cause inflammatory response. However, previous RA studies were focused on the knee joint rather than TMJ.25, 26 The major cartilage type of articular cartilage in knee joint is hyaline cartilage which is rich in type II collagen. This is probably the reason that most studies used type II collagen for CIA induction. Considering the different cartilage component between the knee joint and TMJ, we have tried to use type I collagen which is the main component of fibrocartilage in TMJ tissue for CIA induction. To determine that only type I collagen injection did not cause inflammatory response, this study also compared the type I collagen injection group. Compared with the control group, the type I collagen injection group confirmed the negative inflammatory response through H&E staining and IL-1β and MMP3 expression. On the other hand, even though CFA-induced arthritis and CIA groups showed inflammatory response, the histological change was different. The 7-day CFA-induced arthritis group showed adaptive changes in the condyle transition zone and partly recovered after 35 days induction, and the TMJ disc became thinner than that observed in the control and 7-day CFA induction groups. In contrast, the 7-day CIA group appeared unclearly stratified and severely degraded in the hypertrophic zone of TMJ condyle. The TMJ disc became thinner in the 35-day CFA induction group than that in the control and 7-day CIA induction groups. The TMJ condyle subchondral bone not only appeared unclearly stratified and severely degraded in the hypertrophic zone but also showed irregular cell distribution and abnormal calcification. These results indicated that the method of CIA can lead to TMJ arthritis, and the histological changes varied between the CFA-induced arthritis and CIA groups in the present study. Considering the mechanism of these two arthritis induction methods, we concluded that the CIA method is a more appropriate method than CFA-induced arthritis in RA-related TMJ arthritis research.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the authorship and publication of this article.
Acknowledgments
This research project was supported by a grant from the Ministry of Science and Technology, Taiwan. We thank Dr. Hsiao-Yi Lin from Cheng Hsin General Hospital, Taipei, Taiwan who kindly provided professional information.
References
- 1.Akerman S., Kopp S., Nilner M., Petersson A., Rohlin M. Relationship between clinical and radiologic findings of the temporomandibular joint in rheumatoid arthritis. Oral Surg Oral Med Oral Pathol. 1988;66:639–643. doi: 10.1016/0030-4220(88)90308-8. [DOI] [PubMed] [Google Scholar]
- 2.Poole A.R. Osteoarthritis as a whole joint disease. HSS J. 2012;8:4–6. doi: 10.1007/s11420-011-9248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiao K., Niu L.N., Wang M.Q. Subchondral bone loss following orthodontically induced cartilage degradation in the mandibular condyles of rats. Bone. 2011;48:362–371. doi: 10.1016/j.bone.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Dijkgraaf L.C., Liem R.S., de Bont L.G. Ultrastructural characteristics of the synovial membrane in osteoarthritic temporomandibular joints. J Oral Maxillofac Surg. 1997;55:1269–1279. doi: 10.1016/s0278-2391(97)90183-x. [DOI] [PubMed] [Google Scholar]
- 5.Wiese M., Svensson P., Bakke M. Associations between temporomandibular joint symptoms, signs, and clinical diagnosis using the RDC/TMD and radiographic findings in temporomandibular joint tomograms. J Orofac Pain. 2008;1:239–251. [PubMed] [Google Scholar]
- 6.Paniagua B., Cevidanes L., Walker D., Zhu H., Guo R., Styner M. Clinical application of SPHARM-PDM to quantify temporomandibular joint osteoarthritis. Comput Med Imaging Graph. 2011;35:345–352. doi: 10.1016/j.compmedimag.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernal R., Velásquez E., Gamonal J., Garcia-Sanz J.A., Silva A., Sanz M. Expression of proinflammatory cytokines in osteoarthritis of the temporomandibular joint. Arch Oral Biol. 2008;53:910–915. doi: 10.1016/j.archoralbio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Nah K.S. Condylar bony changes in patients with temporomandibular disorders: a CBCT study. Imaging Sci Dent. 2012;42:249–253. doi: 10.5624/isd.2012.42.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y.C., Hsu M.L., Yang J.S., Liang T.H., Chou S.L., Lin H.Y. Temporomandibular joint disorders in patients with rheumatoid arthritis. J Chin Med Assoc. 2007;70:527–534. doi: 10.1016/S1726-4901(08)70055-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee D.M., Weinblatt M.E. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 11.Wong S.H., Lord J.M. Factors underlying chronic inflammation in rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 2004;52:379–388. [PubMed] [Google Scholar]
- 12.Danoff J.R., Moss G., Liabaud B., Geller J.A. Total knee arthroplasty considerations in rheumatoid arthritis. Autoimmune Dis. 2013;2013:185340. doi: 10.1155/2013/185340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helenius L.M., Tervahartiala P., Helenius I. Clinical, radiographic and MRI findings of the temporomandibular joint in patients with different rheumatic diseases. Int J Oral Maxillofac Surg. 2006;35:983–989. doi: 10.1016/j.ijom.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Sodhi A., Naik S., Pai A., Anuradha A. Rheumatoid arthritis affecting temporomandibular joint. Contemp Clin Dent. 2015;6:124–127. doi: 10.4103/0976-237X.149308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gynther G.W., Tronje G., Holmlund A.B. Radiographic changes in the temporomandibular joint in patients with generalized osteoarthritis and rheumatoid arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:613–618. doi: 10.1016/s1079-2104(96)80058-8. [DOI] [PubMed] [Google Scholar]
- 16.Wang X.D., Kou X.X., Mao J.J., Gan Y.H., Zhou Y.H. Sustained inflammation induces degeneration of the temporomandibular joint. J Dent Res. 2012;91:499–505. doi: 10.1177/0022034512441946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L., Guo H., Li C., Xu J., Fang W., Long X. A time-dependent degeneration manner of condyle in rat CFA-induced inflamed TMJ. Am J Transl Res. 2016;8:556–567. [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroki Y., Honda K., Kijima N. In vivo morphometric analysis of inflammatory condylar changes in rat temporomandibular joint. Oral Dis. 2011;17:499–507. doi: 10.1111/j.1601-0825.2010.01782.x. [DOI] [PubMed] [Google Scholar]
- 19.Chung J.I., Barua S., Choi B.H., Min B.H., Han H.C., Baik E.J. Anti-inflammatory effect of low intensity ultrasound (LIUS) on complete Freund's adjuvant-induced arthritis synovium. Osteoarthritis Cartilage. 2012;20:314–322. doi: 10.1016/j.joca.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Abhijeet D., Shirish D.S. Clinical and CT scan evaluation of temporomandibular joints with osteoarthritis and rheumatoid arthritis. J Indian Acad Oral Med Radiol. 2010;22:1–5. [Google Scholar]
- 21.Pap T., Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis–two unequal siblings. Nat Rev Rheumatol. 2015;11:606–615. doi: 10.1038/nrrheum.2015.95. [DOI] [PubMed] [Google Scholar]
- 22.Bolon B., Stolina M., King C. Rodent preclinical models for developing novel antiarthritic molecules: comparative biology and preferred methods for evaluating efficacy. J Biomed Biotechnol. 2011;2011:569068. doi: 10.1155/2011/569068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shakya A.K., Nandakumar K.S. Applications of polymeric adjuvants in studying autoimmune responses and vaccination against infectious diseases. J R Soc Interface. 2013;10:20120536. doi: 10.1098/rsif.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams R.O. Collagen-induced arthritis as a model for rheumatoid arthritis. Methods Mol Med. 2004;98:207–216. doi: 10.1385/1-59259-771-8:207. [DOI] [PubMed] [Google Scholar]
- 25.Luross J.A., Williams N.A. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology. 2001;103:407–416. doi: 10.1046/j.1365-2567.2001.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell I.K., Kinkel S.A., Drake S.F. Autoimmune regulator controls T cell help for pathogenetic autoantibody production in collagen-induced arthritis. Arthritis Rheum. 2009;60:1683–1693. doi: 10.1002/art.24501. [DOI] [PubMed] [Google Scholar]
- 27.Harper R.P., Kerins C.A., McIntosh J.E., Spears R., Bellinger L.L. Modulation of the inflammatory response in the rat TMJ with increasing doses of complete Freund's adjuvant. Osteoarthritis Cartilage. 2001;9:619–624. doi: 10.1053/joca.2001.0461. [DOI] [PubMed] [Google Scholar]