Abstract
Background. The results of previous studies on the association between polymorphisms of CYP1A1 and CYP1B1 and prostate cancer (PCa) susceptibility are inconsistent. The aim of the present study was to conduct a meta-analysis in order to better estimate this association. Methods. A systematic search was carried out on PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI) databases for relevant articles published up to 15 August 2018. Pooled odds ratios (ORs) and 95% confidence intervals were obtained using fixed-effect or random-effect models. Results. A significant association was found between the CYP1A1 rs1048943 polymorphism and PCa in the overall population (B [the minor allele] vs. A [the major allele]: OR = 1.20, 95% confidence interval (CI) = 1.04–1.39, P=0.014; AB vs. AA: OR = 1.24, 95% CI = 1.02–1.51, P=0.029; BB + AB vs. AA: OR = 1.25, 95% CI = 1.04–1.50, P=0.018) and Asian population (B vs. A: OR = 1.32, 95% CI = 1.11–1.56, P=0.001; BB vs. AA: OR = 1.81, 95% CI = 1.20–2.72, P=0.005; AB vs. AA: OR = 1.30, 95% CI = 1.03–1.64, P=0.029; BB + AB vs. AA: OR = 1.38, 95% CI = 1.11–1.73, P=0.004; BB vs. AA + AB: OR = 1.58, 95% CI = 1.08–2.01, P=0.019), but not in the Caucasian population. Moreover, we found that the rs4646903 polymorphism was associated with a significant increase in the risk of PCa in the Asian population (AB vs. AA: OR = 1.43, 95% CI = 1.13–1.80, P=0.003) and Caucasian population (BB vs. AA: OR = 2.12, 95% CI = 1.29–3.49, P=0.003). Conclusion. This meta-analysis revealed a clear association between rs1048943 and rs4646903 polymorphisms of the CYP1A1 gene but not between CYP1B1 rs10012, rs162549, rs1800440, and rs2551188 polymorphisms and the risk of PCa.
Keywords: CYP1A1, CYP1B1, meta-analysis, polymorphisms, prostate cancer
Introduction
Prostate cancer (PCa) is the most common malignant tumor of the urinary system in men in the western world, the second most frequent cancer and the fifth leading cause of tumor-related deaths worldwide [1,2]. Globally, it is estimated that there will be approximately 1.3 million newly diagnosed cases of PCa and 359000 associated deaths in 2018 [1], making the disease a major health problem in men. The occurrence and development of PCa is influenced by both environmental and genetic factors. Risk factors associated with PCa include age, lifestyle, familial heredity, and hormonal status, but the exact etiology is unclear [3]. It has been shown that exposure to aromatic hydrocarbons is associated with a high risk of PCa [4], and racial differences and familial aggregation are amongst the genetic factors that influence the incidence and development of the disease. Therefore, an increasing number of studies are attempting to explain the occurrence, development, and prognosis of PCa through analysis of variations in the genes involved in hormone metabolism.
A single nucleotide polymorphism (SNP) is a single nucleotide variation at the genomic level, which can appear in coding or non-coding sequences. In recent years, third-generation genetic markers have attracted a lot of attention for their potential role in predicting cancer susceptibility. A number of genes including those encoding the androgen receptor (AR), prostate-specific antigen (PSA), 5a-reductase type II (SRD5A2), and cytochrome P450 (CYP) have been confirmed as PCa susceptibility genes [4]. The CYPs are a large superfamily of conserved proteins found in animals, plants, and microorganisms. Cytochrome P450 1A1 (CYP1A1) and 1B1 (CYP1B1) are pivotal members of the CYP1 family. Classified as a phase I enzyme, CYP1A1 is involved in aryl hydrocarbon hydroxylase activity, while CYP1B1 participates in hydroxylation of estrogens. He and Feng [5] reported that CYP1A1 is involved in the metabolism of estrogen and also activates procarcinogens. Furthermore, it has been shown that the CYP1A1 polymorphism is associated with PCa development [6]. Tokizane et al. [7] found that hypomethylation of the promoter/enhancer region leads to overexpression of CYP1B1 in PCa cells, and metabolites from CYP1B1 catalysis have been demonstrated to induce PCa in an animal model [8]. As an androgen-dependent organ, the prostate contains several enzymes, including CYP1A1 and CYP1B1, which participate in the metabolism of steroid hormones. It is evident from these reports that CYP1A1 and CYP1B1 play crucial roles in the development of PCa and are potential diagnostic and predictive markers, as well as therapeutic targets.
Previous meta-analyses investigating the relationship between CYP1A1 and CYP1B1 polymorphisms and the risk of PCa reported inconsistent results. Cui et al. [9] did not find the CYP1B1 rs1056836 polymorphism to be associated with PCa, while another study found the CYP1A1 rs1048943, but not rs4646903, polymorphism to be associated with a high risk of PCa [10]. Another study on an Indian cohort reported the CYP1A1 rs1048943 polymorphism to be associated with a lower risk of PCa, while rs4646903 was related to a high PCa risk [11]. Although these findings strongly suggest that CYP1A1 and CYP1B1 polymorphisms may be closely related to the susceptibility to PCa, they do not provide an across-the-board estimate of the association. In the past few years, attempts to define the exact relationship have been stymied because of differences in study designs, genotyping methods, and the ethnicity and genetics of the study populations. Therefore, the present meta-analysis, which includes previous and some recently published studies, was undertaken to gain a more precise estimation of the association of polymorphisms with PCa, and to evaluate the possible risk factors.
Materials and methods
Literature search strategy
The PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI) databases were systematically searched independently by two authors (W.Z. and H.L.) on 15 August 2018 to retrieve eligible articles. The search terms included: ‘cytochrome P450 1A1’, ‘cytochrome P450 1B1’, ‘CYP1A1’, ‘CYP1B1’, ‘prostate cancer’, ‘PCa’, ‘PC’, ‘gene’, ‘polymorphism’, ‘allele’ and ‘variation’. In addition, reference lists of the retrieved papers were manually reviewed to obtain additional studies that met the inclusion criteria.
Study selection criteria
Studies selected for the meta-analysis had to meet the following inclusion criteria: (i) the study investigated the association between CYP1A1, CYP1B1 polymorphism, and risk of PCa, (ii) the design was a case–control study, (iii) sufficient data were reported to determine the pooled odds ratios (ORs) and 95% confidence intervals (95% CIs), and (iv) the full text was published. Meta-analyses, letters, single-case reports, duplicate studies, animal model studies, and studies without available data were excluded.
Data extraction
Two authors (W.Z. and X.W.) independently extracted the available data from included papers, and any disagreements were resolved by the third investigator (J.L.). The following information was extracted and recorded: author, year of publication, ethnicity of subjects (Asian, African, Caucasian, or mixed), the name of gene, the names of SNPs, genotyping method (PCR-restriction fragment length polymorphism [PCR-RFLP], Taqman, allele-specific PCR [AS-PCR] or Genechips), source of controls (population or hospital), total numbers of cases and controls, and the Newcastle–Ottawa Scale (NOS). Studies which investigated more than one kind of SNP were counted as individual data for the present meta-analysis. The quality of all included studies was assessed independently by the three researchers using the validated NOS, and disagreements were resolved through discussion with another researcher (H.Z.). Studies with scores of >6 were considered as high-quality studies, and those with scores of ≤6 as low-quality studies.
Statistical analysis
We utilized Stata 12.0 software (Stata Corp LP, College Station, TX) to conduct the meta-analysis and estimate heterogeneity between the included studies. ORs and 95% confidence intervals (CIs) were used to assess any associations between CYP1A1 and CYP1B1 polymorphisms and the risk of PCa. The pooled ORs were calculated in BB vs. AA (A: the major allele, B: the minor allele), BB + AB vs. AA, BB vs. AB + AA, B vs. A, and AB vs. AA genetic models using either the fixed-effects model or the random-effect model. In case of significant heterogeneity (Q-test: I2 ≥ 50% or P≤0.05) between studies, a random-effect model was used for analysis; otherwise, a fixed-effect model would be chosen [12]. Subgroup analyses were performed based on ethnicity (Caucasian, Asian, African, or mixed). Finally, sensitivity analysis was performed to examine whether the independent studies included in the meta-analysis affected the pooled results, and publication bias was evaluated by Egger’s test [13].
Results
Characteristics of included studies
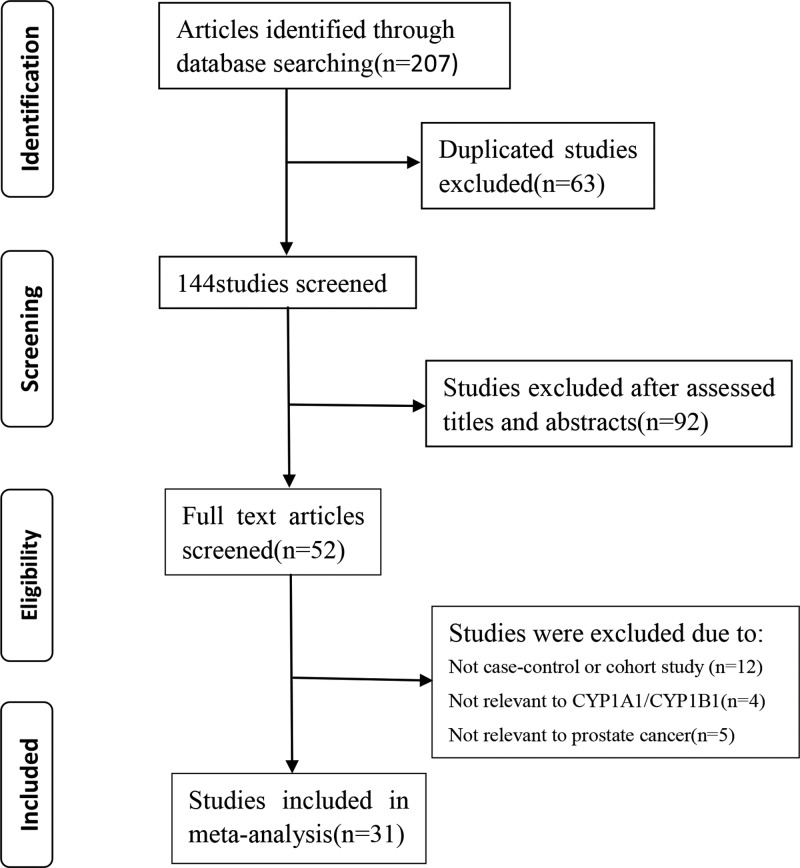
A flow diagram of the study selection is shown in Figure 1. We retrieved 207 studies from the PubMed, Embase, Cochrane Library, and CNKI databases. Initial screening excluded 63 duplicated articles, and 92 papers that did not fit the inclusion criteria were excluded after careful review of the titles and abstracts. Out of the 52 studies selected for full publication review, 21 were excluded (12 studies were not case–control studies and 9 were not related to CYP1A1/CYP1B1 or PCa). Finally, 31 articles [11,14–43] including 12745 cases and 12471 controls that met the inclusion criteria were included in the final analysis. The main characteristics of these studies are summarized in Table 1. Genotype frequency was available in all included studies. A total of 16 Caucasian, 12 Asian, 1 African, and 2 mixed populations were represented in the 31 selected studies. Of note, three SNPs in CYP1A1 and twelve in CYP1B1 were excluded from the final analysis due to lack of sufficient research.
Figure 1. The flow diagram of this meta-analysis.
Table 1. Characteristics of the studies included in the meta-analysis.
| Author | Year | Ethnicity | Gene | SNP | Genotyping method | Source of control | Cases/controls | NOS |
|---|---|---|---|---|---|---|---|---|
| Tanaka et al. | 2002 | Asian | CYP1B1 | rs1056827, rs2551188, rs10012, rs1056836, rs1056837 | PCR-RFLP | Hospital | 117/200 | 8 |
| Murata et al. | 2001 | Asian | CYP1A1 | rs1048943 | PCR-RFLP | Hospital | 115/200 | 7 |
| Suzuki et al. | 2003 | Asian | CYP1A1 | rs4646903, rs1048943 | PCR-RFLP | Hospital | 81/105 | 8 |
| Mandić et al. | 2014 | Caucasian | CYP1A1 | rs4646903 | Taqman | Hospital | 120/120 | 7 |
| Fukatsu et al. | 2004 | Asian | CYP1B1 | rs1056836 | PCR-RFLP | Population | 147/266 | 7 |
| Acevedo et al. | 2003 | Caucasian | CYP1A1 | rs4646903 | PCR-RFLP | Population | 102/102 | 6 |
| Aktas et al. | 2004 | Caucasian | CYP1A1 | rs4986883 | AS-PCR | Hospital | 100/107 | 7 |
| Beer et al. | 2002 | Caucasian | CYP1A1 | rs1048943 | PCR-RFLP | Population | 117/183 | 7 |
| Beuten et al. | 2008 | Caucasian | CYP1B1 | rs2567206, rs2551188, rs2617266, rs10012, rs1056836, rs1800440 | Taqman | Population | 649/738 | 8 |
| Brureau et al. | 2016 | Mixed | CYP1B1 | rs1056836 | PCR-RFLP | Population | 660/709 | 7 |
| Cáceres et al. | 2005 | Caucasian | CYP1A1 | rs4646903 | PCR-RFLP | Population | 103/132 | 6 |
| Vijayalakshmi et al. | 2005 | Asian | CYP1A1 | rs4646903, rs1048943 | PCR-RFLP | Population | 50/50 | 7 |
| Catsburg et al. | 2012 | Caucasian | CYP1B1 | rs1056836 | Taqman | Population | 1343/800 | 9 |
| Cicek et al. | 2005 | Caucasian | CYP1B1 | rs1056827, rs1056836 | PCR-RFLP | Population | 439/479 | 8 |
| Cusseno et al. | 2007 | Caucasian | CYP1B1 | rs1056836 | Taqman | Population | 1101/882 | 6 |
| Lima et al. | 2008 | Caucasian | CYP1A1 | rs4646903 | PCR-RFLP | Hospital | 125/100 | 7 |
| Holt et al. | 2012 | Caucasian | CYP1A1/CYP1B1 | rs162549, rs2855658, rs1056836, rs1456432, rs4886605 | PCR-RFLP | Population | 1304/1266 | 8 |
| Gu et al. | 2018 | Asian | CYP1B1 | rs9341266, rs162549, rs10916, rs162562, rs2551188, rs9341250, rs1056827, rs1056836 | Taqman | Population | 1015/1052 | 7 |
| Kachakova et al. | 2016 | Caucasian | CYP1B1 | rs1056836, rs1056837, rs1800440, rs1056827, | PCR-RFLP | Hospital | 246/261 | 6 |
| Kato et al. | 2018 | Caucasian | CYP1B1 | rs2551188, rs2567206, rs2567207, rs162556, rs10175368, rs163090, rs162330, rs162331 | Taqman | Population | 400/405 | 8 |
| Kumar et al. | 2010 | Asian | CYP1A1 | rs4646903, rs1048943 | PCR-RFLP | Population | 70/61 | 6 |
| Mittal et al. | 2007 | Asian | CYP1A1 | rs4646903 | PCR-RFLP | Population | 130/140 | 6 |
| Nock et al. | 2006 | Mixed | CYP1A1 | rs1048943 | PCR-RFLP | Population | 439/479 | 6 |
| Price et al. | 2016 | Caucasian | CYP1B1 | rs1800440 | Taqman | Population | 1506/1380 | 9 |
| Quiñones et al. | 2006 | Caucasian | CYP1A1 | rs4646903 | PCR-RFLP | Population | 60/117 | 6 |
| Rodrigues et al. | 2010 | Caucasian | CYP1A1/CYP1B1 | rs1048943, rs1056827 | AS-PCR | Population | 154/154 | 6 |
| Sobti et al. | 2006 | Asian | CYP1B1 | rs1056836 | PCR-RFLP | Population | 100/100 | 7 |
| Souiden et al. | 2012 | African | CYP1A1 | rs4646903 | PCR-RFLP | Population | 138/138 | 6 |
| Tang et al. | 2017 | Asian | CYP1B1 | rs10012, rs1800440 | Taqman | Population | 1506/1380 | 8 |
| Yang et al. | 2006 | Asian | CYP1A1 | rs4646903, rs1048943 | AS-PCR | Hospital | 225/250 | 6 |
| Guan et al. | 2005 | Asian | CYP1A1 | rs4646903, rs1048943 | Genechips | Hospital | 83/115 | 6 |
Meta-analysis
The results of the meta-analysis are shown in Table 2. Meanwhile, the forest plots for positive results are shown in Figure 2.
Table 2. Meta-analysis of CYP1 gene polymorphisms and PCa.
| Polymorphisms | Comparisons | Number of studies | Test of association | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Z | P-value | Model | P-value | I2 (%) | |||
| CYP1A1 | ||||||||
| rs1048943 | ||||||||
| Overall | B vs. A | 9 | 1.20 (1.04, 1.39) | 2.46 | 0.014 | F | 0.157 | 32.6 |
| BB vs. AA | 9 | 1.37 (0.97, 1.93) | 1.8 | 0.072 | F | 0.122 | 38.6 | |
| AB vs. AA | 9 | 1.24 (1.02, 1.51) | 2.15 | 0.029 | F | 0.11 | 38.8 | |
| BB + AB vs. AA | 9 | 1.25 (1.04, 1.50) | 2.37 | 0.018 | F | 0.132 | 35.8 | |
| BB vs. AA + AB | 9 | 1.21 (0.92, 1.61) | 1.35 | 0.178 | F | 0.135 | 36.9 | |
| Asian | B vs. A | 6 | 1.32 (1.11, 1.56) | 3.23 | 0.001 | F | 0.235 | 26.7 |
| BB vs. AA | 5 | 1.81 (1.20, 2.72) | 2.84 | 0.005 | F | 0.649 | 0 | |
| AB vs. AA | 6 | 1.30 (1.03, 1.64) | 2.19 | 0.029 | F | 0.151 | 38.3 | |
| BB + AB vs. AA | 6 | 1.38 (1.11, 1.73) | 2.87 | 0.004 | F | 0.129 | 41.5 | |
| BB vs. AA + AB | 6 | 1.58 (1.08, 2.01) | 2.38 | 0.019 | F | 0.699 | 0 | |
| Caucasian | B vs. A | 2 | 0.86 (0.60, 1.23) | 0.83 | 0.405 | F | 0.984 | 0 |
| BB vs. AA | 2 | 0.66 (0.31, 1.37) | 1.12 | 0.262 | R | 0.114 | 60 | |
| AB vs. AA | 2 | 1.16 (0.71, 1.89) | 0.59 | 0.557 | R | 0.038 | 76.8 | |
| BB + AB vs. AA | 2 | 0.97 (0.63, 1.49) | 0.15 | 0.883 | F | 0.33 | 0 | |
| BB vs. AA + AB | 2 | 0.63 (0.32, 1.22) | 1.36 | 0.172 | R | 0.1 | 63.1 | |
| rs4646903 | ||||||||
| Overall | B vs. A | 12 | 1.18 (1.04, 1.33) | 2.62 | 0.009 | R | 0.001 | 65.7 |
| BB vs. AA | 11 | 1.16 (0.88, 1.53) | 1.07 | 0.286 | F | 0.111 | 35.9 | |
| AB vs. AA | 12 | 1.41 (1.19, 1.68) | 3.99 | <0.001 | R | 0.007 | 57.5 | |
| BB + AB vs. AA | 12 | 1.36 (1.15, 1.59) | 3.69 | <0.001 | R | 0.001 | 65 | |
| BB vs. AA + AB | 12 | 0.95 (0.74, 1.23) | 0.36 | 0.72 | F | 0.458 | 0 | |
| Asian | B vs. A | 6 | 1.07 (0.92, 1.26) | 0.88 | 0.377 | R | 0.038 | 57.6 |
| BB vs. AA | 6 | 0.88 (0.63, 1.24) | 0.7 | 0.482 | F | 0.791 | 0 | |
| AB vs. AA | 6 | 1.43 (1.13, 1.80) | 2.99 | 0.003 | F | 0.169 | 35.6 | |
| BB + AB vs. AA | 6 | 1.30 (1.04, 1.61) | 2.31 | 0.021 | R | 0.068 | 51.3 | |
| BB vs. AA + AB | 6 | 0.79 (0.58, 1.07) | 1.52 | 0.128 | F | 0.901 | 0 | |
| Caucasian | B vs. A | 5 | 1.43 (1.16, 1.75) | 3.41 | 0.001 | R | 0.007 | 71.9 |
| BB vs. AA | 4 | 2.12 (1.29, 3.49) | 2.97 | 0.003 | F | 0.2 | 35.4 | |
| AB vs. AA | 5 | 1.54 (1.17, 2.02) | 3.08 | 0.002 | R | 0.004 | 74 | |
| BB + AB vs. AA | 5 | 1.57 (1.21, 2.04) | 3.38 | 0.001 | R | 0.001 | 77.5 | |
| BB vs. AA + AB | 5 | 1.49 (0.93, 2.37) | 1.67 | 0.095 | F | 0.366 | 5.4 | |
| CYP1B1 | ||||||||
| rs1056836 | ||||||||
| Overall | B vs. A | 12 | 1.02 (0.96, 1.07) | 0.61 | 0.54 | R | <0.001 | 73.4 |
| BB vs. AA | 12 | 1.04 (0.93, 1.17) | 0.65 | 0.514 | R | <0.001 | 70.5 | |
| AB vs. AA | 12 | 1.03 (0.96, 1.12) | 0.84 | 0.401 | F | 0.077 | 39.5 | |
| BB + AB vs. AA | 12 | 1.03 (0.96, 1.11) | 0.77 | 0.441 | R | 0.002 | 63.1 | |
| BB vs. AA + AB | 12 | 1.01 (0.91, 1.12) | 0.1 | 0.916 | R | 0.002 | 62.2 | |
| Asian | B vs. A | 4 | 0.99 (0.87, 1.14) | 0.09 | 0.932 | R | <0.001 | 84.6 |
| BB vs. AA | 4 | 1.20 (0.78, 1.85) | 0.85 | 0.398 | R | 0.004 | 77.1 | |
| AB vs. AA | 4 | 0.96 (0.81, 1.12) | 0.54 | 0.587 | R | 0.026 | 67.7 | |
| BB + AB vs. AA | 4 | 0.97 (0.83, 1.14) | 0.36 | 0.722 | R | 0.002 | 79.4 | |
| BB vs. AA + AB | 4 | 1.17 (0.76, 1.79) | 0.71 | 0.477 | R | 0.012 | 72.8 | |
| Caucasian | B vs. A | 7 | 1.02 (0.96, 1.09) | 0.78 | 0.433 | R | 0.002 | 72 |
| BB vs. AA | 7 | 1.03 (0.91, 1.16) | 0.45 | 0.649 | R | 0.001 | 74.8 | |
| AB vs. AA | 7 | 1.07 (0.97, 1.17) | 1.39 | 0.163 | R | 0.314 | 15.2 | |
| BB + AB vs. AA | 7 | 1.05 (0.97, 1.15) | 1.19 | 0.234 | R | 0.03 | 57 | |
| BB vs. AA + AB | 7 | 1.00 (0.90, 1.11) | 0.06 | 0.951 | R | 0.007 | 66.1 | |
| rs10012 | ||||||||
| Overall | G vs. C | 3 | 1.05 (0.91, 1.21) | 0.66 | 0.508 | F | 0.545 | 0 |
| rs162549 | ||||||||
| Overall | T vs. A | 2 | 1.07 (0.96, 1.19) | 1.21 | 0.226 | F | 0.515 | 0 |
| rs1056827 | ||||||||
| Overall | T vs. G | 5 | 1.14 (1.03, 1.26) | 2.47 | 0.014 | R | <0.001 | 84 |
| rs1056837 | ||||||||
| Overall | T vs. C | 2 | 0.90 (0.74, 1.11) | 0.96 | 0.338 | R | 0.136 | 54.9 |
| rs1800440 | ||||||||
| Overall | G vs. A | 4 | 0.95 (0.85, 1.06) | 0.89 | 0.374 | F | 0.477 | 0 |
| rs2551188 | ||||||||
| Overall | T vs. C | 4 | 0.96 (0.87, 1.07) | 0.72 | 0.474 | F | 0.873 | 0 |
| rs2567206 | ||||||||
| Overall | T vs. C | 2 | 1.21 (1.01, 1.45) | 2.03 | 0.042 | R | 0.077 | 68 |
Abbreviations: F, fixed-effect model; R, random-effect model; vs., versus.
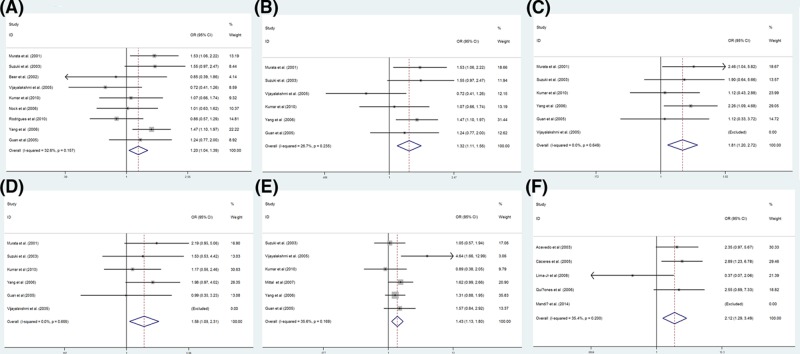
Figure 2. Forest plot for the association between gene polymorphisms and PCa.
Forest plot for rs1048943 polymorphism in overall population (B vs. A, (A)) and Asian population (B vs. A, (B); BB vs. AA, (C); BB vs. AA+AB, (D)), for rs4646903 polymorphism in Asian population (AB vs. AA, (E)) and Caucasian population (BB vs. AA, (F)). Abbreviation: vs., versus.
Analysis of the CYP1A1 rs1048943 polymorphism
Overall, analysis of the association between the rs1048943 polymorphism and PCa from nine independent studies revealed no significant heterogeneity by a fixed-effect model. The results, however, showed a significant association between the rs1048943 polymorphism and PCa after comparing the B allele vs. A allele, AB vs. AA and BB + AB vs. AA (OR = 1.20, 95% CI = 1.04–1.39, P=0.014; OR = 1.24, 95% CI = 1.02–1.51, P=0.029; OR = 1.25, 95% CI = 1.04–1.50, P=0.018; respectively). Meanwhile, no association was evident when examining BB vs. AA and BB vs. AA + AB (OR = 1.37, 95% CI = 0.97–1.93, P=0.072; OR = 1.21, 95% CI = 0.92–1.61, P=0.178; respectively).
The Q-test revealed no significant heterogeneity in the Asian populations when analyzed from six independent studies. Therefore, we conducted an analysis using the fixed-effect model which showed a significant association between the rs1048943 polymorphism and PCa when comparing the B allele vs. A allele, BB vs. AA, AB vs. AA, BB + AB vs. AA, and BB vs. AA + AB (OR = 1.32, 95% CI = 1.11–1.56, P=0.001; OR = 1.81, 95% CI = 1.20–2.72, P=0.005; OR = 1.30, 95% CI = 1.03–1.64, P=0.029; OR = 1.38, 95% CI = 1.11–1.73, P=0.004; OR = 1.58, 95% CI = 1.08–2.01, P=0.019; respectively).
In the Caucasian population, significant between-study heterogeneity was found when comparing BB vs. AA, AB vs. AA, BB vs. AA + AB by the random-effect model. There was no significant heterogeneity for B allele vs. A allele or BB + AB vs. AA models, and the results showed no association of the rs1048943 polymorphism with PCa susceptibility (B vs. A, OR = 0.86, 95% CI = 0.60–1.23, P=0.405; BB + AB vs. AA, OR = 0.97, 95% CI = 0.63–1.49, P=0.883).
Analysis of the CYP1A1 gene rs4646903 polymorphism
Overall, significant between-study heterogeneity was detected for most of the genetic models, except for the BB vs. AA and BB vs. AA + AB models. Fixed-effect model analysis of these two genetic models indicated no significant association (BB vs. AA, OR = 1.16, 95% CI = 0.88–1.53, P=0.286; BB vs. AA + AB, OR = 0.95, 95% CI = 0.74–1.23, P=0.72).
In the Asian populations, there was significant between-study heterogeneity in the B allele vs. A allele and BB + AB vs. AA models, and the results showed an association between rs4646903 and PCa when comparing AB vs. AA (OR = 1.43, 95% CI = 1.13–1.80, P=0.003), which was not evident when comparing BB vs. AA (OR = 0.88, 95% CI = 0.63–1.24, P=0.482) or BB vs. AA + AB (OR = 0.79, 95% CI = 0.58–1.07, P=0.128).
In the Caucasian populations, significant between-study heterogeneity was found in the B allele vs. A allele, AB vs. AA, and BB + AB vs. AA models. There was a significant association between rs4646903 and PCa when comparing BB vs. AA (OR = 2.12, 95% CI = 1.29–3.49, P=0.003). Comparing BB vs. AA + AB, however, indicated no association (OR = 1.49, 95% CI = 0.93–2.37, P=0.095).
Analysis of the CYP1B1 rs1056836 polymorphism
Except for AB vs. AA in the overall population, significant between-study heterogeneity was found in all other genetic models. However, the association between the CYP1B1 gene rs1056836 polymorphism and risk of PCa was not significant (AB vs. AA, OR = 1.03, 95% CI = 0.96–1.12, P=0.401).
Analysis of the CYP1B1 rs10012, rs162549, rs1056827, rs1056837, rs1800440, rs2551188, and rs2567206 polymorphisms
The meta-analysis for associations between the rs10012, rs162549, rs1056827, rs1056837, rs1800440, rs2551188, and rs2567206 polymorphisms and PCa risk in the overall population included three, two, five, two, four, four, and four independent studies, respectively. Due to significant between-study heterogeneity indicated by the Q-test, rs1056827, rs1056837, and rs2567206 were analyzed using the random-effect model. The fixed model was used to analyze the rs10012, rs162549, rs1800440, and rs2551188 polymorphisms. Overall, no significant association was detected between these SNPs and PCa risk (rs10012, G vs. C, OR = 1.05, 95% CI = 0.91–1.21, P=0.508; rs162549, T vs. A, OR = 1.07, 95% CI = 0.96–1.19, P=0.226; rs1800440, G vs. A, OR = 0.95, 95% CI = 0.85–1.06, P=0.374; rs2551188, T vs. C, OR = 0.96, 95% CI = 0.87–1.07, P=0.474).
Evaluation of publication bias
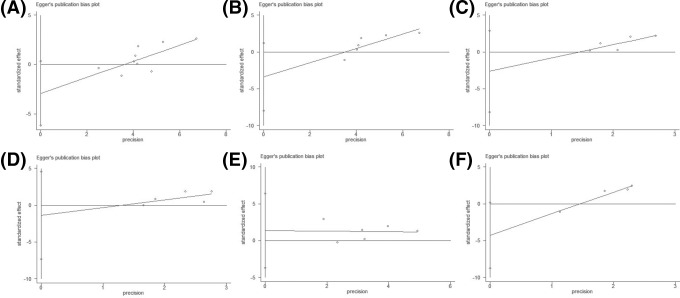
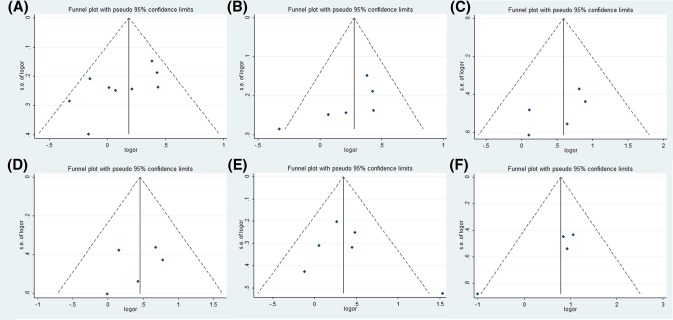
Egger’s linear regression test was used to evaluate any publication bias by funnel plot asymmetry (Table 3). The intercept provides an assessment of asymmetry—more deviation from zero indicates more asymmetry. Egger’s linear regression test provided evidence of publication bias for the rs1048943 polymorphism in the overall population (AB vs. AA: t = −2.95, P=0.021; BB + AB vs. AA: t = −3.53, P=0.010) and in the Asian populations (BB + AB vs. AA: t = −3.15, P=0.035). Egger’s publication bias plots and Funnel plots for positive results are shown in Figures 3 and 4, respectively. No publication bias was found in other analyses.
Table 3. Egger’s linear regression test to measure the funnel plot asymmetric.
| Polymorphisms | Y axle intercept: a (95% CI) | ||||
|---|---|---|---|---|---|
| B vs. A | BB vs. AA | AB vs. AA | BB + AB vs. AA | BB vs. AA + AB | |
| rs1048943 (Overall) | −2.93 (−6.19, 0.33) | 0.41 (−3.32, 4.14) | −2.75 (−4.95, −0.54)* | −2.89 (−4.82, −0.96)* | 0.533 (−2.45, 3.51) |
| rs1048943 (Asian) | −3.39 (−7.96, 1.18) | −2.63 (−8.19, 2.92) | −2.52 (−5.26, 0.22) | −2.75 (−5.18, −0.33)* | −1.38 (−7.33, 4.56) |
| rs4646903 (Asian) | 2.35 (−2.77, 7.47) | 0.74 (−1.30, 2.79) | 1.378 (−3.65, 6.40) | 1.69 (−4.03, 7.42) | 0.59 (−1.04, 2.21) |
| rs4646903 (Caucasian) | −7.21 (−17.40, 2.98) | −4.27 (−8.73, 0.19) | −1.64 (−31.65, 28.37) | −3.49 (−30.55, 23.57) | −3.46 (−6.38, −0.54)* |
P<0.05
Figure 3. Egger’s publication bias analysis.
Egger’s publication bias plot for rs1048943 polymorphism in overall population (B vs. A, (A)) and Asian population (B vs. A, (B); BB vs. AA, (C); BB vs. AA+AB, (D)), for rs4646903 polymorphism in Asian population (AB vs. AA, (E)), and Caucasian population (BB vs. AA, (F)). Abbreviation: vs., versus.
Figure 4. Funnel plots for statistically significant meta-analysis.
Funnel plots for rs1048943 polymorphism in overall population (B vs. A, (A)) and Asian population (B vs. A, (B); BB vs. AA, (C); BB vs. AA+AB, (D)), for rs4646903 polymorphism in Asian population (AB vs. AA, (E)), and Caucasian population (BB vs. AA, (F)). Abbreviation: vs., versus.
Discussion
In recent years, genetic susceptibility to cancer has been a hotspot of research in the scientific community. Emerging evidence has demonstrated the potential association between gene polymorphisms and cancer risk, particularly in the CYP1 family including CYP1A1 and CYP1B1. For instance, CYP1A1 polymorphisms are known to be associated with susceptibility to a wide variety of cancers including lung [44], bladder [45], pancreatic [46], and breast cancers [47]. Similarly, the association of CYP1B1 polymorphisms and the risk of several types of cancer has been explored [48–50]. Currently, the link between CYP1 family gene polymorphisms and PCa susceptibility is attracting widespread attention. Numerous studies have focussed on the relationship between CYP proteins and their SNPs, as well as their possible effects on the development of PCa. Both CYP1A1 and CYP1B1 are involved in the metabolism of numerous carcinogens and steroidal hormones including estrogens [51,52]. Furthermore, Cavalieri et al. [53] reported that metabolites of chemical carcinogens generated by CYP1B1 catalysis can induce PCa in animal models. The CYP1A1*2A (rs4646903) polymorphism involves a thymidine to cytosine substitution at position 3801 of the 3′-non-coding region downstream of the polyadenylation site [54]. Although some studies have shown that the rs4646903 variant significantly increases the activity of mutant enzymes, the results are conflicting [55]. The CYP1A1*2B (rs1048943) polymorphism is the second most common CYP1A1 polymorphism, which involves an adenine to guanine transition at position 2455 of codon 462 at exon 7 [56]. It has been reported that the rs1048943 polymorphism induces an increase in CYP1A1 at the mRNA level [57], and Kisselev et al. [58] reported that this variant significantly increases the catalytic activity for all hydroxylation sites toward substrates including 17β-estradiol (E2) and estrone (E1), most significantly for 2-hydroxylation. The CYP1B1*3 (rs1056836) polymorphism is located in the third exon, and can result in a leucine to valine substitution [59]. This variant has been associated with higher hydroxylation activity, which may consequently increase the risk of various forms of cancer including PCa [51]. These results suggest that polymorphisms of CYP1A1 and CYP1B1 might play a role in promoting tumorigenesis by changing the activity of hydroxylase in the process of estrogen hydroxylation to 2-hydroxyestrogen (2-OH HE) and 4-OH HEs. However, the conclusions of meta-analyses of the association between CYP1A1 and CYP1B1 polymorphisms and PCa risk have been inconsistent [6,9,10,60], and so we endeavored to conduct a systematic meta-analysis to precisely estimate this association, and provide a more comprehensive and reliable conclusion.
The present study provides a systematic analysis of all available case–control studies on the CYP1A1 rs1048943 and rs4646903; and CYP1B1 rs1056836, rs10012, rs162549, rs1056827, rs1056837, rs1800440, rs2551188, and rs2567206 polymorphisms and the risk of PCa. Previously, no significant associations have been found between the CYP1B1 rs10012, rs162549, rs1800440, and rs2551188 polymorphisms and PCa risk in the overall population. A possible explanation is that PCa is a multigenic disease, and the effect of a single polymorphism may therefore be limited. On the other hand, our study demonstrated that the rs1048943 polymorphism was associated with PCa susceptibility in the overall population. Results were similar in the Asian populations; however, no effect was detectable in men of Caucasian descent. Furthermore, our meta-analysis provided evidence that the rs4646903 polymorphism in the AB genotype compared with AA, and BB genotype compared with AA were considered as risk factors for PCa in Asian and Caucasian populations, respectively. However, the CYP1A1 rs4646903 polymorphism was not associated with an increased risk of PCa overall. The effect of polymorphisms on PCa susceptibility is influenced by ethnicity. Data from the National Central for Biotechnology Information (NCBI) show that the B allele (the minor allele) of rs4646903 is present in 34.0% of the Asian population, 10.7% of the Caucasian population, and 23.4% of the African-American population. However, African Americans are reported to have the highest incidence of PCa, which not only emphasizes the racial background of the disease [61], but also confirms the interaction between genetic and environmental factors in PCa. The interaction of genetic and lifestyle factors including dietary fat, obesity, and sexual factors could explain these ethnic differences to some extent. Undoubtedly, a larger study would provide more insight into the association of the rs1048943 and rs4646903 polymorphisms with PCa susceptibility in different ethnicities, especially African Americans.
A meta-analysis performed by Zhang et al. [60] showed that the CYP1B1 rs1056836, rs1800440, and rs1056827 polymorphisms were associated with susceptibility to PCa, which contradicts the results of Cui et al. [9] who found no association between the CYP1B1 rs1056836 polymorphism and PCa risk in the overall and Caucasian populations. No significant association with PCa susceptibility was detected for rs1800440 in our study; however, we understand that with much larger populations and more updated studies, our results may be more reliable. In agreement with our results, Ding et al. [6] found that the CYP1A1 rs4646903 polymorphism was responsible for increasing the risk of developing PCa. Li et al. [10], on the other hand, reported that the rs1048943—and not rs4646903—polymorphism was associated with PCa susceptibility, in line with the results of the present analysis.
Between-study heterogeneity is one of the pivotal issues affecting the results of the present meta-analysis. Subgroup analysis based on ethnic groups revealed a decrease in the heterogeneity amongst studies. These results indicate that ethnic subgroups contribute significantly to the high heterogeneity observed in our analysis. Due to the limited studies of hospital-based controls and genotyping methods other than PCR-RFLP, we were unable to conduct subgroup analysis based on the source of the controls and genotyping methods. However, their impact on heterogeneity should not be ignored. It is well known that using different detection methods to determine genotypes increases the heterogeneity between studies. Therefore, additional studies with different sources of control groups and genetic methods are still necessary in order to assess the impact of these polymorphisms on PCa risk. Studies that include hospital-based control groups and genotyping methods other than PCR-RFLP such as AS-PCR, Taqman, and Genechips are particularly important. Publication bias was observed in some comparisons of the present meta-analysis, which may be because the present study only included English and Chinese publications, possibly resulting in selective bias.
There are several potential limitations of this meta-analysis that should be considered. First, patients with benign prostatic hyperplasia (BPH) were enrolled as controls in our study, which may have an effect on the final results. Second, accurate determination of the association between the CYP1B1 rs1056836 polymorphism and the risk of PCa was not possible due to significant heterogeneity in the overall, Caucasian, and Asian populations. Third, our results were based on unadjusted estimates. A more reliable estimate could have been achieved if individual data such as age and other environmental factors were available.
Conclusion
To summarize, this meta-analysis suggests that the CYP1A1 rs1048943 and rs4646903 polymorphisms are associated with PCa risk, especially in the Asian and Caucasian populations, respectively. No significant associations were detected for the CYP1B1 rs10012, rs162549, rs1800440, or rs2551188 polymorphisms. Larger studies are necessary to verify our findings and elucidate the exact association between CYP1A1 and CYP1B1 polymorphisms and susceptibility to PCa.
Abbreviations
- AS-PCR
allele-specific PCR
- CI
confidence interval
- CNKI
China National Knowledge Infrastructure
- CYP
cytochrome P450
- CYP1A1
cytochrome P450 1A1
- CYP1B1
cytochrome P450 1B1
- NOS
Newcastle–Ottawa Scale
- OR
odds ratio
- PCa
prostate cancer
- PCR-RFLP
PCR–restriction fragment length polymorphism
- SNP
single nucleotide polymorphism
Author contribution
Conception and design: W.Y. and S.W. Collection and extraction of data: W.Z. and H.L. Data analysis and statistical practice: W.Z., X.W., and H.Z. Manuscript writing and final approval: W.Z. and J.L.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81573776].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Bray F., Ferlay J., Soerjomataram I.. et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Center M.M., Jemal A., Lortet-Tieulent J.. et al. (2012) International variation in prostate cancer incidence and mortality rates. Eur. Urol. 61, 1079–1092 10.1016/j.eururo.2012.02.054 [DOI] [PubMed] [Google Scholar]
- 3.Stein Q.P. and Flanagan J.D. (2010) Genetic and familial factors influencing breast, colon, prostate and lung cancers. South Dakota Med. Spec No, 16–22 [PubMed] [Google Scholar]
- 4.Gsur A., Feik E. and Madersbacher S. (2004) Genetic polymorphisms and prostate cancer risk. World J. Urol. 21, 414–423 10.1007/s00345-003-0378-4 [DOI] [PubMed] [Google Scholar]
- 5.He X. and Feng S. (2015) Role of metabolic enzymes P450 (CYP) on activating procarcinogen and their polymorphisms on the risk of cancers. Curr. Drug Metab. 16, 850–863 10.2174/138920021610151210164501 [DOI] [PubMed] [Google Scholar]
- 6.Ding G., Xu W., Liu H.. et al. (2013) CYP1A1 MspI polymorphism is associated with prostate cancer susceptibility: evidence from a meta-analysis. Mol. Biol. Rep. 40, 3483–3491 10.1007/s11033-012-2423-0 [DOI] [PubMed] [Google Scholar]
- 7.Tokizane T., Shiina H., Igawa M.. et al. (2005) Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clin. Cancer Res. 11, 5793–5801 10.1158/1078-0432.CCR-04-2545 [DOI] [PubMed] [Google Scholar]
- 8.Williams J., Martin F., Muir G.. et al. (2000) Metabolic activation of carcinogens and expression of various cytochromes P450 in human prostate tissue. Carcinogenesis 21, 1683–1689 10.1093/carcin/21.9.1683 [DOI] [PubMed] [Google Scholar]
- 9.Cui L., Dillehay K., Chen W.. et al. (2012) Association of the CYP1B1 Leu432Val polymorphism with the risk of prostate cancer: a meta-analysis. Mol. Biol. Rep. 39, 7465–7471 10.1007/s11033-012-1579-y [DOI] [PubMed] [Google Scholar]
- 10.Li H., Xiao D., Hu L.. et al. (2012) Association of CYP1A1 polymorphisms with prostate cancer risk: an updated meta-analysis. Mol. Biol. Rep. 39, 10273–10284 10.1007/s11033-012-1904-5 [DOI] [PubMed] [Google Scholar]
- 11.Vijayalakshmi K., Vettriselvi V., Krishnan M.. et al. (2005) Cytochrome p4501A1 gene variants as susceptibility marker for prostate cancer. Cancer Biomarkers 1, 251–258 10.3233/CBM-2005-14-508 [DOI] [PubMed] [Google Scholar]
- 12.Higgins J.P., Thompson S.G., Deeks J.J.. et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egger M., Smith G.D., Schneider M.. et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acevedo C., Opazo J.L., Huidobro C.. et al. (2003) Positive correlation between single or combined genotypes of CYP1A1 and GSTM1 in relation to prostate cancer in Chilean people. Prostate 57, 111–117 10.1002/pros.10274 [DOI] [PubMed] [Google Scholar]
- 15.Aktas D., Hascicek M., Sozen S.. et al. (2004) CYP1A1 and GSTM1 polymorphic genotypes in patients with prostate cancer in a Turkish population. Cancer Genet. Cytogenet. 154, 81–85 10.1016/j.cancergencyto.2004.01.023 [DOI] [PubMed] [Google Scholar]
- 16.Beer T., Evans A., Hough K.. et al. (2002) Polymorphisms of GSTP1 and related genes and prostate cancer risk. Prostate Cancer Prostatic Dis. 5, 22 10.1038/sj.pcan.4500549 [DOI] [PubMed] [Google Scholar]
- 17.Beuten J., Gelfond J.A., Byrne J.J.. et al. (2008) CYP1B1 variants are associated with prostate cancer in non-Hispanic and Hispanic Caucasians. Carcinogenesis 29, 1751–1757 10.1093/carcin/bgm300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brureau L., Moningo D., Emeville E.. et al. (2016) Polymorphisms of estrogen metabolism-related genes and prostate cancer risk in two populations of African ancestry. PLoS ONE 11, e0153609 10.1371/journal.pone.0153609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catsburg C., Joshi A.D., Corral R.. et al. (2012) Polymorphisms in carcinogen metabolism enzymes, fish intake, and risk of prostate cancer. Carcinogenesis 33, 1352–1359 10.1093/carcin/bgs175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cicek M.S., Liu X., Casey G.. et al. (2005) Role of androgen metabolism genes CYP1B1, PSA/KLK3, and CYP11α in prostate cancer risk and aggressiveness. Cancer Epidemiol. Prev. Biomarkers 14, 2173–2177 10.1158/1055-9965.EPI-05-0215 [DOI] [PubMed] [Google Scholar]
- 21.Cussenot O., Azzouzi A.R., Nicolaiew N.. et al. (2007) Combination of polymorphisms from genes related to estrogen metabolism and risk of prostate cancers: the hidden face of estrogens. J. Clin. Oncol. 25, 3596–3602 10.1200/JCO.2007.11.0908 [DOI] [PubMed] [Google Scholar]
- 22.Fukatsu T., Hirokawa Y., Araki T.. et al. (2004) Genetic polymorphisms of hormone-related genes and prostate cancer risk in the Japanese population. Anticancer Res. 24, 2431–2438 [PubMed] [Google Scholar]
- 23.Gu C.-Y., Li G.-X., Zhu Y.. et al. (2018) A single nucleotide polymorphism in CYP1B1 leads to differential prostate cancer risk and telomere length. J. Cancer 9, 269 10.7150/jca.21774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan T., Li M. and Na Y. (2005) Polymorphism of metabolic gene and genetic susceptibility to prostate cancer. Zhonghua Wai Ke Za Zhi 43, 1467–1470 [PubMed] [Google Scholar]
- 25.Holt S.K., Kwon E.M., Fu R.. et al. (2013) Association of variants in estrogen‐related pathway genes with prostate cancer risk. Prostate 73, 1–10 10.1002/pros.22534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iturrieta J., Acevedo C., Huidobro C.. et al. (2005) Relationship among metabolizing genes, smoking and alcohol used as modifier factors on prostate cancer risk: exploring some gene–gene and gene–environment interactions. Eur. J. Epidemiol. 20, 79–88 10.1007/s10654-004-1632-9 [DOI] [PubMed] [Google Scholar]
- 27.Kachakova D., Mitkova A., Popov E.. et al. (2016) Polymorphisms in androgen metabolism genes AR, CYP1B1, CYP19, and SRD5A2 and prostate cancer risk and aggressiveness in Bulgarian patients. Turkish J. Med. Sci. 46, 626–640 10.3906/sag-1501-124 [DOI] [PubMed] [Google Scholar]
- 28.Kato T., Hashimoto Y., Wong R.K.. et al. (2018) Influence of lifestyle choices on risks of CYP 1B1 polymorphisms for prostate cancer. J. Cell. Mol. Med. 22, 4676–4687 10.1111/jcmm.13696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar V., Yadav C.S., Singh S.. et al. (2010) CYP 1A1 polymorphism and organochlorine pesticides levels in the etiology of prostate cancer. Chemosphere 81, 464–468 10.1016/j.chemosphere.2010.07.067 [DOI] [PubMed] [Google Scholar]
- 30.Lima M., Oliveira M., Granja F.. et al. (2008) Lack of association of GSTT1, GSTM1, GSTO1, GSTP1 and CYP1A1 polymorphisms for susceptibility and outcome in Brazilian prostate cancer patients. Folia Biol. 54, 102–108 [PubMed] [Google Scholar]
- 31.Mandić S., Horvat V., Marczi S.. et al. (2014) Association study of cytochrome P450 1A1* 2A polymorphism with prostate cancer risk and aggressiveness in Croatians. Coll. Antropol. 38, 141–146 [PubMed] [Google Scholar]
- 32.Mittal R.D. and Srivastava D.L. (2007) Cytochrome P4501A1 and microsomal epoxide hydrolase gene polymorphisms: gene–environment interaction and risk of prostate cancer. DNA Cell Biol. 26, 791–798 10.1089/dna.2007.0630 [DOI] [PubMed] [Google Scholar]
- 33.Murata M., Watanabe M., Yamanaka M.. et al. (2001) Genetic polymorphisms in cytochrome P450 (CYP) 1A1, CYP1A2, CYP2E1, glutathione S-transferase (GST) M1 and GSTT1 and susceptibility to prostate cancer in the Japanese population. Cancer Lett. 165, 171–177 10.1016/S0304-3835(01)00398-6 [DOI] [PubMed] [Google Scholar]
- 34.Nock N.L., Liu X., Cicek M.S.. et al. (2006) Polymorphisms in polycyclic aromatic hydrocarbon metabolism and conjugation genes, interactions with smoking and prostate cancer risk. Cancer Epidemiol. Prev. Biomarkers 15, 756–761 10.1158/1055-9965.EPI-05-0826 [DOI] [PubMed] [Google Scholar]
- 35.Price D.K., Chau C.H., Till C.. et al. (2016) Association of androgen metabolism gene polymorphisms with prostate cancer risk and androgen concentrations: results from the Prostate Cancer Prevention Trial. Cancer 122, 2332–2340 10.1002/cncr.30071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quiñones L.A., Irarrázabal C.E., Rojas C.R.. et al. (2006) Joint effect among p53, CYP1A1, GSTM1 polymorphism combinations and smoking on prostate cancer risk: an exploratory genotype‐environment interaction study. Asian J. Androl. 8, 349–355 10.1111/j.1745-7262.2006.00135.x [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues I.S., Kuasne H., Losi-Guembarovski R.. et al. (2011) Evaluation of the influence of polymorphic variants CYP1A1* 2B, CYP1B1* 2, CYP3A4* 1B, GSTM1* 0, and GSTT1* 0 in prostate cancer. In Urologic Oncology: Seminars and Original Investigations, Elsevier; [DOI] [PubMed] [Google Scholar]
- 38.Sobti R., Onsory K., Al-Badran A.I.. et al. (2006) CYP17, SRD5A2, CYP1B1, and CYP2D6 gene polymorphisms with prostate cancer risk in North Indian population. DNA Cell Biol. 25, 287–294 10.1089/dna.2006.25.287 [DOI] [PubMed] [Google Scholar]
- 39.Souiden Y., Mahdouani M., Chaieb K.. et al. (2012) Lack of association of CYP1A1 polymorphism with prostate cancer susceptibility of Tunisian men. Genet. Testing Mol. Biomarkers 16, 661–666 10.1089/gtmb.2011.0212 [DOI] [PubMed] [Google Scholar]
- 40.Suzuki K., Matsui H., Nakazato H.. et al. (2003) Association of the genetic polymorphism in cytochrome P450 (CYP) 1A1 with risk of familial prostate cancer in a Japanese population: a case-control study. Cancer Lett. 195, 177–183 10.1016/S0304-3835(03)00182-4 [DOI] [PubMed] [Google Scholar]
- 41.Tanaka Y., Sasaki M., Kaneuchi M.. et al. (2002) Polymorphisms of the CYP1B1 gene have higher risk for prostate cancer. Biochem. Biophys. Res. Commun. 296, 820–826 10.1016/S0006-291X(02)02004-1 [DOI] [PubMed] [Google Scholar]
- 42.Tang L., Platek M.E., Yao S.. et al. (2017) Associations between polymorphisms in genes related to estrogen metabolism and function and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Carcinogenesis 39, 125–133 10.1093/carcin/bgx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J., Qian L.X., Wu H.F.. et al. (2006) Genetic polymorphisms in the cytochrome P450 1A1 and 2E1 genes, smoking, drinking and prostate cancer susceptibility: a case‐control study in a Han nationality population in Southern China. Int. J. Urol. 13, 773–780 10.1111/j.1442-2042.2006.01401.x [DOI] [PubMed] [Google Scholar]
- 44.Nie Q., Yang X.-n., An S.-j.. et al. (2011) CYP1A1* 2A polymorphism as a predictor of clinical outcome in advanced lung cancer patients treated with EGFR-TKI and its combined effects with EGFR intron 1 (CA) n polymorphism. Eur. J. Cancer 47, 1962–1970 10.1016/j.ejca.2011.04.018 [DOI] [PubMed] [Google Scholar]
- 45.Öztürk T., Kahraman Ö.T., Toptaş B.. et al. (2011) The effect of CYP1A1 and GSTM1 gene polymorphisms in bladder cancer development in a Turkish population. In Vivo 25, 663–668 [PubMed] [Google Scholar]
- 46.Liu G., Ghadirian P., Vesprini D.. et al. (2000) Polymorphisms in GSTM1, GSTT1 and CYP1A1 and risk of pancreatic adenocarcinoma. Br. J. Cancer 82, 1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C., Huang Y., Li Y.. et al. (2007) Cytochrome P450 1A1 (CYP1A1) T3801C and A2455G polymorphisms in breast cancer risk: a meta-analysis. J. Hum. Genet. 52, 423 10.1007/s10038-007-0131-8 [DOI] [PubMed] [Google Scholar]
- 48.Berber U., Yilmaz I., Yilmaz O.. et al. (2013) CYP1A1 (Ile 462 Val), CYP1B1 (Ala 119 Ser and Val 432 Leu), GSTM1 (null), and GSTT1 (null) polymorphisms and bladder cancer risk in a turkish population. Asian Pac. J. Cancer Prev. 14, 3925–3929 10.7314/APJCP.2013.14.6.3925 [DOI] [PubMed] [Google Scholar]
- 49.Ibrahim M., Rashed R., Hassan N.. et al. (2016) ssociation of cytochrome P450-1B1 gene polymorphisms with risk of breast cancer: an Egyptian study. Asian Pac. J. Cancer Prev. 17, 2861–2866 [PubMed] [Google Scholar]
- 50.Shah I.A., Mehta P., Lone M.M.. et al. (2015) Leu432Val polymorphism of CYP1B1 is not associated with squamous cell carcinoma of esophagus-a case-control study from Kashmir, India. Asian Pac. J. Cancer Prev. 16, 5337–5341 10.7314/APJCP.2015.16.13.5337 [DOI] [PubMed] [Google Scholar]
- 51.Hanna I.H., Dawling S., Roodi N.. et al. (2000) Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res. 60, 3440–3444 [PubMed] [Google Scholar]
- 52.Kumar V., Singh S., Yadav C.S.. et al. (2010) CYP1A1 and CYP3A4 polymorphic variations in Delhi population of Northern India. Environ. Toxicol. Pharmacol. 29, 126–130 10.1016/j.etap.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 53.Cavalieri E.L., Devanesan P., Bosland M.C.. et al. (2002) Catechol estrogen metabolites and conjugates in different regions of the prostate of Noble rats treated with 4-hydroxyestradiol: implications for estrogen-induced initiation of prostate cancer. Carcinogenesis 23, 329–333 10.1093/carcin/23.2.329 [DOI] [PubMed] [Google Scholar]
- 54.Bale A. (1987) Subchromosomal localization of the dioxin-inducible P1450 locus (CYP1) and description of two RFLPs detected with 3′-P1450 cDNA probe. Cytogenet. Cell Genet. 46, 574–575 [Google Scholar]
- 55.Androutsopoulos V.P., Tsatsakis A.M. and Spandidos D.A. (2009) Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer 9, 187 10.1186/1471-2407-9-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayashi S.-i., Watanabe J., Nakachi K.. et al. (1991) Genetic linkage of lung cancer-associated Msp I polymorphisms with amino acid replacement in the heme binding region of the human cytochrome P450IA1 gene. J. Biochem. 110, 407–411 10.1093/oxfordjournals.jbchem.a123594 [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z.-Y., Fasco M.J., Huang L.. et al. (1996) Characterization of purified human recombinant cytochrome P4501A1-Ile462 and-Val462: assessment of a role for the rare allele in carcinogenesis. Cancer Res. 56, 3926–3933 [PubMed] [Google Scholar]
- 58.Kisselev P., Schunck W.-H., Roots I.. et al. (2005) Association of CYP1A1 polymorphisms with differential metabolic activation of 17β-estradiol and estrone. Cancer Res. 65, 2972–2978 10.1158/0008-5472.CAN-04-3543 [DOI] [PubMed] [Google Scholar]
- 59.Kumar V., Banerjee B.D., Datta S.K.. et al. (2014) Association of CYP1A1, CYP1B1 and CYP17 gene polymorphisms and organochlorine pesticides with benign prostatic hyperplasia. Chemosphere 108, 40–45 10.1016/j.chemosphere.2014.02.081 [DOI] [PubMed] [Google Scholar]
- 60.Zhang H., Li L. and Xu Y. (2013) CYP1B1 polymorphisms and susceptibility to prostate cancer: a meta-analysis. PLoS ONE 8, e68634 10.1371/journal.pone.0068634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsing A.W. and Devesa S.S. (2001) Trends and patterns of prostate cancer: what do they suggest? Epidemiol. Rev. 23, 3–13 10.1093/oxfordjournals.epirev.a000792 [DOI] [PubMed] [Google Scholar]