Abstract
Differentiation syndrome (DS) is a life-threatening complication arising during retinoid treatment of acute promyelocytic leukemia (APL). Administration of all-trans retinoic acid leads to significant changes in gene expression, among the most induced of which is transglutaminase 2, which is not normally expressed in neutrophil granulocytes. To evaluate the pathophysiological function of transglutaminase 2 in the context of immunological function and disease outcomes, such as excessive superoxide anion, cytokine, and chemokine production in differentiated NB4 cells, we used an NB4 transglutaminase knock-out cell line and a transglutaminase inhibitor, NC9, which inhibits both transamidase- and guanosine triphosphate-binding activities, to clarify the contribution of transglutaminase to the development of potentially lethal DS during all-trans retinoic acid treatment of APL. We found that such treatment not only enhanced cell-surface expression of CD11b and CD11c but also induced high-affinity states; atypical transglutaminase 2 expression in NB4 cells activated the nuclear factor kappa (κ)-light-chain-enhancer of the activated B-cell pathway, driving pathogenic processes with an inflammatory cascade through the expression of numerous cytokines, including tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and monocyte chemoattractant protein 1. NC9 decreased the amount of transglutaminase 2, p65/RelA, and p50 in differentiated NB4 cells and their nuclei, leading to attenuated inflammatory cytokine synthesis. NC9 significantly inhibits transglutaminase 2 nuclear translocation but accelerates its proteasomal breakdown. This study demonstrates that transglutaminase 2 expression induced by all-trans retinoic acid treatment reprograms inflammatory signaling networks governed by nuclear factor κ-light-chain-enhancer of activated B-cell activation, resulting in overexpression of TNF-α and IL-1β in differentiating APL cells, suggesting that atypically expressed transglutaminase 2 is a promising target for leukemia treatment.
Introduction
Acute promyelocytic leukemia (APL), an acute myeloid leukemia (AML) subtype, is identified by clonal proliferation of promyelocytic precursor cells with reduced ability to differentiate into mature neutrophil granulocytes.1–6 Expression of PML/RARα in APL suppresses differentiation along the neutrophil lineage.7–9 In clinical settings, the target is primarily the PML/RARα chimeric protein and its degradation, initiated by all-trans retinoic acid (ATRA) or arsenic trioxide.10–12 ATRA-induced differentiation therapy leads to differentiation syndrome (DS), which can be fatal in 2.5-30% of cases. DS is characterized by large numbers of inflammatory differentiating leukemic cells in the bloodstream, releasing chemokines and cytokines in a so-called cytokine storm, which shifts endothelial cell function from normal toward inflammatory processes. DS is also characterized by manifestation of unexplained fever, respiratory distress, pleural and pericardial effusions, pulmonary edema, episodic hypotension, and vascular capillary leakage, which may lead to acute renal failure.13,14 Although glucocorticoid treatment leads to recovery in most patients within 12 hours (h) and resolution of symptoms within 24 h, the condition is fatal in 1-5% of patients. Dexamethasone treatment will not inhibit the induction of chemokines in differentiating APL cells.15,16
ATRA-induced differentiation can be modeled to a certain extent using NB4 APL cells.17–19 The differentiation process involves modulation of thousands of genes to produce functional neutrophil granulocytes. The most highly up-regulated gene in ATRA-activated maturation of NB4 cells is tissue transglutaminase (TG2). TG2 expression silencing in NB4 cells has revealed functional TG2 participation in modulation of gene expression, reactive oxygen species (ROS) generation, cytokine expression, adhesion, and migration, and phagocytic capacity of differentiated neutrophil granulocytes.20,21
TG2 is a Ca2+-dependent protein cross-linking enzyme that also adds amines to proteins and is capable of deamidating γ-carboxamide groups of particular protein-bound glutamines.22,23 In addition, TG2 has several enzymatic activities that do not require Ca2+; it can hydrolyze guanosine triphosphate (GTP) and adenosine triphosphate (ATP), can mediate signal transduction via G-protein-coupled receptors, and has protein kinase and protein disulfide isomerase activities. Recent evidence shows that TG2 in the GTP-bound/closed (signaling) conformation drives cancer cell survival.24,25
To provide firm evidence for the critical involvement of TG2 in ATRA-induced differentiation of promyelocytic leukemia cells to inflammatory neutrophils, we generated TG2-deleted NB4 cells and applied a cell-penetrable, irreversible TG2 inhibitor to observe how TG2 influences the development of inflammatory states. Our results demonstrate that ATRA-induced atypical TG2 expression enhances NF-κB gene expression, nuclear translocation, and transcriptional activation of NF-κB target genes, leading to unregulated production of inflammatory cytokines and chemokines.
Methods
Cell lines, treatments and measurements
The cell culture conditions of the NB4 APL cell line have been described previously.18
The NB4 TG2-KO cell line was generated from the wild-type cell line by TALEN which is described in detail in the Online Supplementary Appendix. NB4 cell lines were treated with 1 μM ATRA (Sigma-Aldrich) or 1 μM ATRA + 30 μM NC9 (30 mM stock solution) and collected at the indicated time points. Phorbolmyristate acetate (PMA) in 10 nM or tumor necrosis factor alpha (TNF-α) at a concentration of 2 ng/mL were used.
Western blot analysis (preparation of cell lysate and subcellular fractions), cell preparation, and staining methods for fluorescence-activated cell sorting (FACS) analysis and the nitro-blue-tetrazolium test (NBT) have been described previously.20,21
To evaluate NF-κB pathway activity, an NF-κB promoter-driven luciferase construct was used, stably integrated into the genomic DNA of NB4 cell lines (CLS-013-L8-QIAGEN). The assay was performed according to the manufacturer’s protocol. The transfected cells were selected by administration of puromycin (Sigma-Aldrich) at a final concentration of 10 μg/mL. Measurement of luciferase activity was performed using a Bright-Glo™ Luciferase Assay System (Promega). The data were validated by GraphPad Prism 7.0 using a parallel normalizing method based on cell numbers and protein concentration.
The preparation of the RNA samples has been published previously.20,21 Real-time Q-PCR reaction utilized the following probes (ABI, Applied Biosystems): TGM2, MCP-1, TNF-α, IL-1β, GP91PHOX, NCF2, GAPDH, and CYCLO-D. The analysis was performed using an ABI Prism 7900 (ABI, Applied Biosystems). Relative expression levels were normalized to the level of cyclophilin-D and glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Secreted cytokine concentration was measured using an ELISA Kit (BioLegend/RayBiotech) according to the manufacturer’s instructions.
NB4-WT cells were treated as described above for 11 days. At day 11, cells were treated with 5 μM MG132 for 3 h. Samples were harvested after 30 minutes, 1 h, 2 h, and 3 h, and handled as described previously.20,21
A more detailed description of the methods used are available in the Online Supplementary Appendix.
Results
TG2 accelerates phagocytotic and antimicrobial functions of differentiating NB4 cell lines
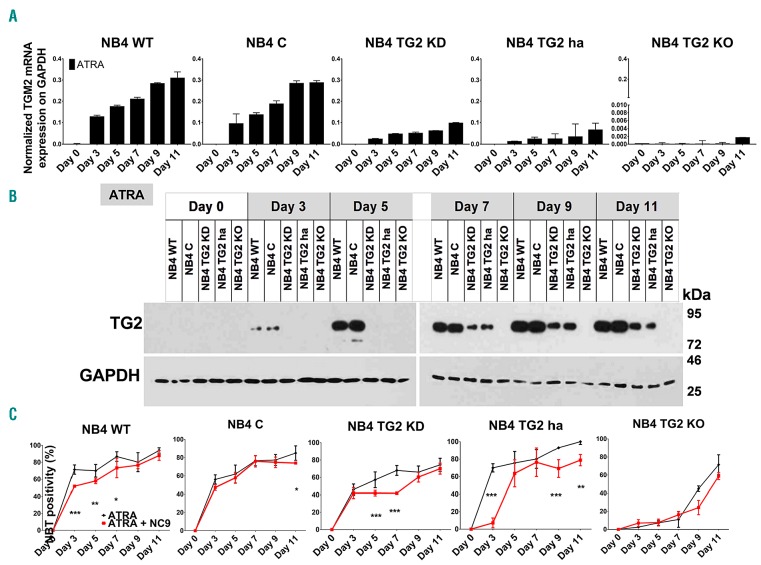
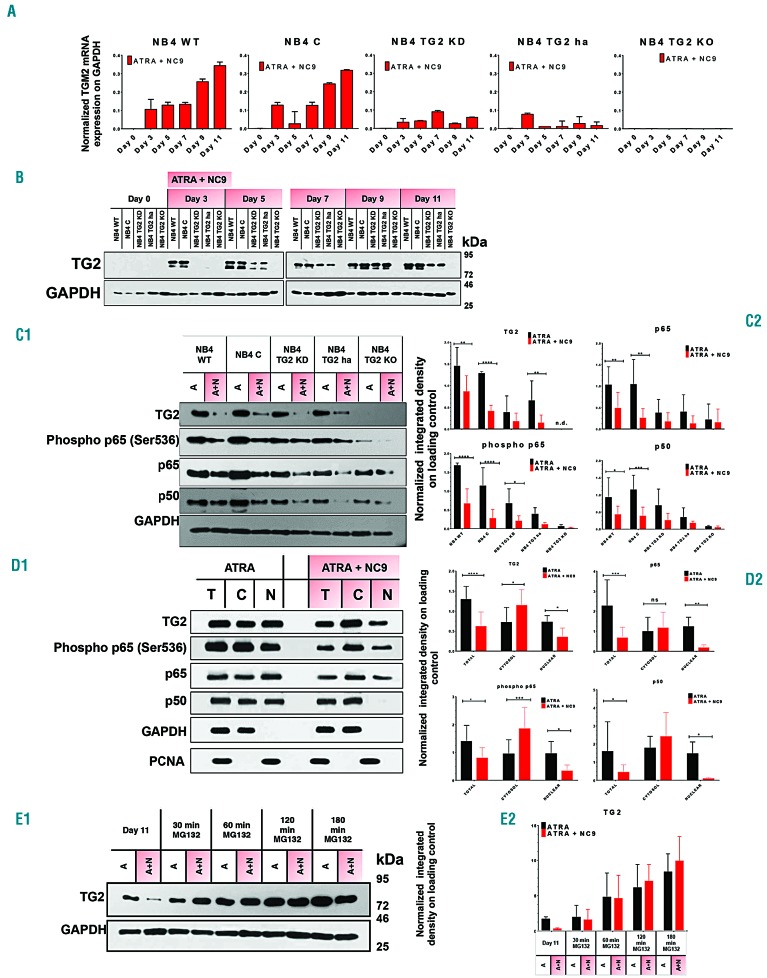
The NBT test is a simple method for examining phagocytic and oxygen-dependent antimicrobial ability of neutrophil granulocytes. We previously reported that NB4 TG2-KD (TG2 knockdown) cells reduced NBT but to a lesser extent than wild-type NB4 (NB4-WT) cells after three days of ATRA treatment.21 To determine the contribution and correlation of TG2 expression levels to differentiated neutrophil granulocyte status, NB4 human acute promyelocytic leukemia cells (NB4-WT) and sublines NB4 TG2-C (virus control), NB4 TG2-KD, NB4 TG2-ha (heterozygous allele), NB4 TG2-KO (knockout) (see Online Supplementary Figures S1-S3) were treated with ATRA. First, the level of TG2 mRNA transcription and protein expression was determined in cell lines; it was found that while the TG2-KO cell line did not express TG2, the NB4-WT and NB4 TG2-C cell lines expressed TG2 abundantly and comparably. However, the NB4 TG2-KD and NB4 TG2-ha cell lines expressed TG2 at intermediate levels between the total and TG2-deficient level (Figure 1A and B). TG2-deficient conditions (TG2-KO) led to a rightward shift in the NBT-positivity response curve compared to NB4-WT cells, indicating that the moderate or total expression of TG2 accelerated the differentiation process in NB4 TG2-KD or TG2-ha and NB4 TG2-C or NB4-WT cells, respectively, resulting in an early increase in NBT-positivity and a saturation curve over the 11-day time scale (Figure 1C and Online Supplementary Figure S4).
Figure 1.
Tissue transglutaminase (TG2) accelerates antimicrobial ability of differentiating NB4 cell lines. (A) Relative mRNA expression of TG2 in NB4-wild-type (WT) treated with 1 μM all-trans retinoic acid (ATRA), virus control TG2-C, shRNA-silenced TG2-KD, hetero-allelic TG2-ha, and TALEN-TG2 knocked-out (KO) cells measured at the indicated days by real-time Q-PCR and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression (n=3). (B) Representative Western blot showing TG2 protein expression levels upon ATRA treatment over 11 days (n=3). (C) NB4 cell lines undergoing differentiation characterized by ability to reduce nitro-blue-tetrazolium (NBT). The assays were performed at the indicated time points in triplicates. Percentage of the NBT-positivity expressed as mean %±Standard Deviation for 3 parallel experiments. Statistical analysis was performed via two-way analysis of variance (ANOVA; Bonferroni post-hoc test; *P<0.05, **P<0.01, ***P<0.005 ****P<0.001).
ATRA induces expression of leukocyte β2 integrin receptors MAC-1 and p150,95 and their high-affinity state on the cell surface of NB4 cell lines
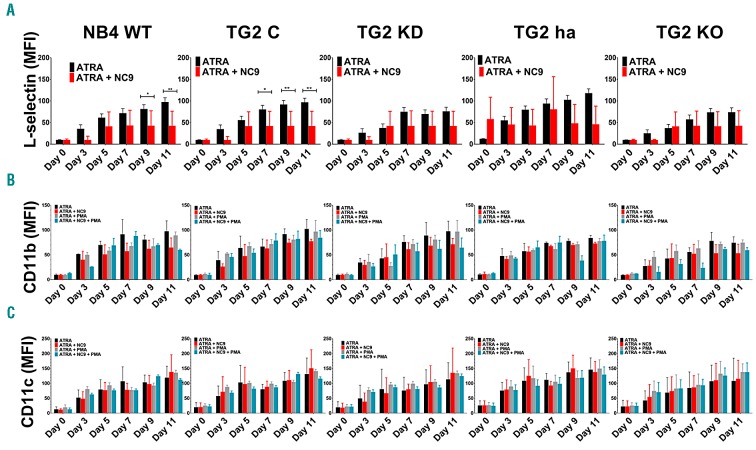
Analysis of the cell-surface expression of L-selectin, CD11b, and CD11c, which are indicators of the degree of differentiation on ATRA-treated NB4 cell lines, showed that while the expression of L-selectin, CD11b, and CD11c increased significantly from day 0 to day 3, subsequently remaining almost unaltered regarding cell surface positivity (Online Supplementary Figure S5), the mean fluorescence intensity (MFI) of the cells increased during the 11-day treatment. ATRA-induced differentiation is associated with increasing TG2 expression parallel to the progression of the differentiation of NB4 cell lines. Neither knockdown of TG2 expression nor use of the NB4 TG2-KO cell line changed the cell-surface expression of L-selectin, CD11b, and CD11c in ATRA-induced differentiation (Figure 2A-C). In addition, PMA did not stimulate cell-surface expression of CD11b over basal expression (Figure 2B). Notably, some non-activated adhesion receptors were observed on the cell surface, while others were restricted to cytoplasmic granules, like CD11b.26 To gain insight into the activation states of ATRA-treated NB4 cell lines, we used CBRM1/5 mAb, which is specific for high-affinity CD11b and does not recognize CD11b on resting myeloid cells, and examined the cell-surface expression and affinity status of CD11b. We found that ATRA not only enhanced the cell-surface expression of CD11b but also induced its high-affinity state (Figure 2B). Assuming that the measured surface expression levels of CD11b might be the “basal expression levels” on NB4 cell lines, we determined the PMA-stimulated CD11b cell-surface expression levels by CBRM1/5, revealing that PMA cannot further increase cell-surface expression of CD11b integrin (Figure 2B). Antibody anti-CD11c Clone 3.9 binds to CD11c in an activation-dependent manner and is specific to the I domain of CD11c.27 Anti-CD11c antibody revealed gradually increasing expression of activated CD11c integrin receptors on ATRA-treated NB4 cell lines, which could not be further enhanced by PMA (Figure 2C).
Figure 2.
All-trans retinoic acid (ATRA) induces both CD11b and CD11c β2 integrin expression and their high-affinity state on the cell surface of NB4 cell lines. FACS analysis of (A) cell-surface expression of L-selectin, differentiation marker (B) CD11b and (C) CD11c in 1 μM ATRA, 1 μM ATRA + 30 μM NC9, or after 20 minutes stimulation of 10 nM PMA-treated NB4-WT, TG2-C, NB4 TG2-KD, TG2-ha, and TG2-KO cells, respectively, at the indicated days. Measurements were conducted in triplicates, and values were validated via Flowing software. Graphs show the representation of the mean±Standard Deviation fluorescent intensity (MFI) values, in parallel. MFI values were calculated based on each treatment’s respective isotype control (n=9). Statistical analysis was performed via two-way analysis of variance (ANOVA; Bonferroni post-hoc test; *P<0.05, **P<0.01, ***P<0.005 ****P<0.001).
TG2 drives the respiratory burst of NB4 cell lines
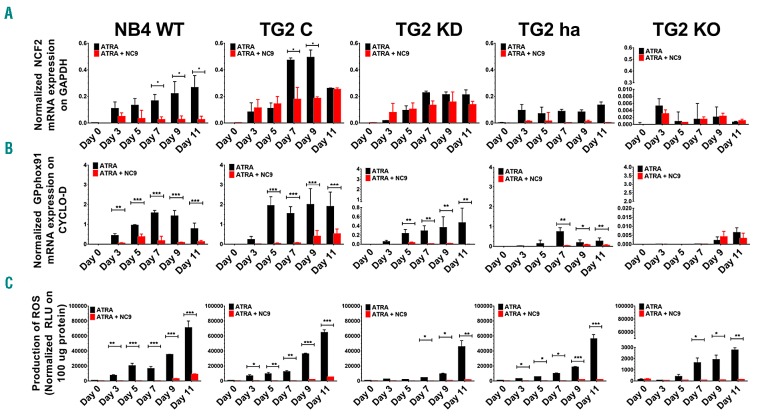
Some phagocytes and neutrophil granulocytes can generate large amounts of reactive oxidants in response to particulate or soluble inflammatory stimuli. We had previously found that neutrophils of TG2-KO mice showed lower expression of both GP91PHOX mRNA and protein with less ROS production compared to wild-type mice.20 The TG2-dependence of PMA-induced ROS capacity was further investigated by measuring the expression levels of GP91PHOX and NCF-2/P67PHOX mRNA, the two major components of the neutrophil NADPH-oxidase system. The TG2-dependent expression pattern of these genes (Figure 3A and B) was also consistent with the findings that ATRA-differentiated total TG2 expressing NB4-WT cells produced more than 20-fold higher levels of ROS (70996.33±9034.96: RLU/100 μg protein) than the ATRA-differentiated TG2 deficient NB4 TG2-KO cells (2767.33±205.90 RLU/100 μg protein) (Figure 3C).
Figure 3.
Tissue transglutaminase (TG2) expression drives both expression of NCF2 and GP91PHOX respiratory burst oxidase genes and the generation of reactive oxygen species (ROS). (A and B) Relative mRNA expressions of NCF2 and GP91PHOX upon ATRA and ATRA + NC9 treatment over 11 days were determined at the indicated time points by real-time Q-PCR and normalized to cyclophilin-D mRNA levels in NB4-WT, TG2-C, TG2-KD, TG2-ha, and TG2-KO cells. Results are the mean±Standard Deviation of 3 independent experiments. (C) Production of ROS was determined for each cell line by using a luminescence-based method in triplicates (n=5) and reported as relative light units (RLU). Values are normalized to 100 μg protein of cell lysate content, respectively. Statistical significance was determined via two-way analysis of variance (ANOVA; Bonferroni post-hoc test; *P<0.05, **P<0.01, ***P<0.005 ****P<0.001). GAPDH: glyceraldehyde 3-phosphate dehydrogenase; ATRA: all-trans retinoic acid.
TG2 induces typical proinflammatory cytokines and chemokine expression through NF-κB and transcriptional activation
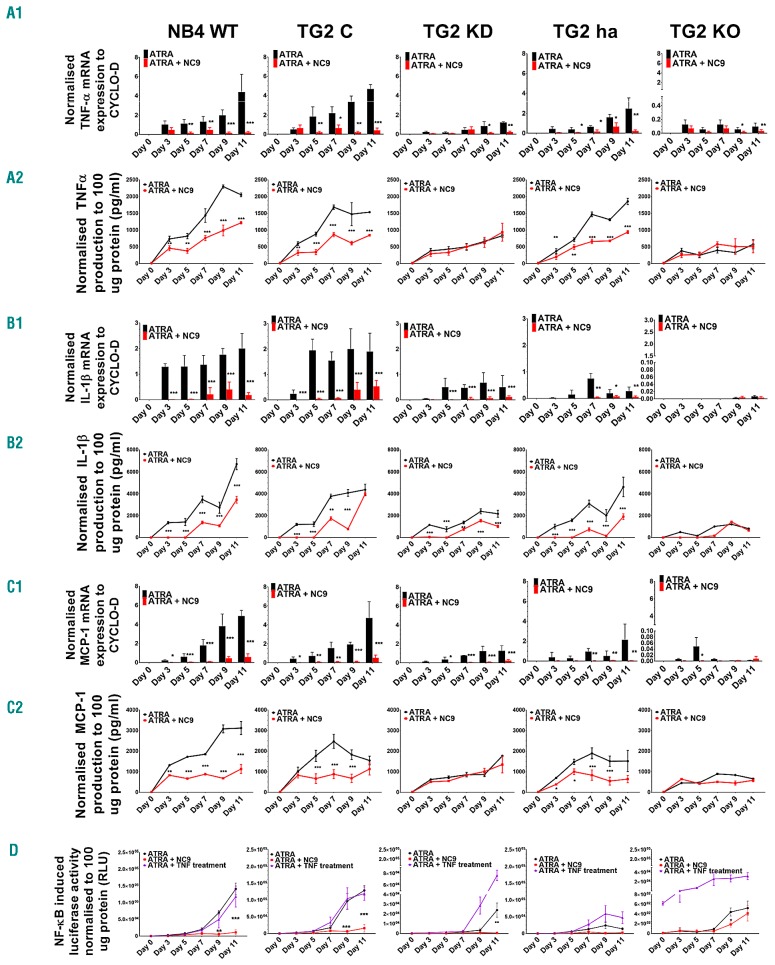
Proinflammatory cytokine TNFα, IL-1β, and chemokine monocyte chemoattractant protein-1 (MCP-1/CCL2) have many functions in the progression of inflammation and activation of other leukocytes. In the case of NB4 cell lines, the expression patterns of TNFα, IL-1β, and MCP-1 in both mRNA and protein levels showed very similar trends in the context of TG2 expression. While high TG2 expression was accompanied by elevated mRNA and protein expression of cytokines and MCP-1 chemokine (NB4-WT; TNFα 3120.74±321.39 pg/mL, IL-1β 6721.27±510.05 pg/mL, and MCP-1 2053.95±55.53 pg/mL) at low (NB4 TG2-KD and TG2-ha) or deficient expression of TG2-KO, both remained low in each case (TG2-KO; TNFα 658.28±36.33 pg/mL, IL-1β 818.00±10.02 pg/mL, and MCP-1, 576.16±133.86 pg/mL) (Figure 4A1-C2, black bars and lines).
Figure 4.
Tissue transglutaminase (TG2) up-regulates both expression of tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and monocyte chemoattractant protein 1 (MCP-1) through nuclear factor kappa (κ)-light-chain-enhancer of the activated B-cell (NF-κB) pathway transcriptional activation and their secretion. Relative mRNA expression of (A1) TNF-α, (B1) IL-1β and (C1) MCP-1 in 1 μM ATRA or 1 μM ATRA plus 30 μM NC9-treated NB4-WT, TG2-C, TG2-KD, TG2-ha, and TG2-KO cells measured at the indicated days by real-time Q-PCR and normalized to cyclophilin-D mRNA expression. In the cell culture, supernatant-secreted (A2) TNF-α chemokine, (B2) IL-1β and (C2) MCP-1 inflammatory cytokines were quantified by ELISA and normalized to 100 μg protein content of cell lysate. Figures show secreted protein levels from 3 independent experiments measured in triplicate and graphs show the representation of the mean±Standard Deviation. (D) All-trans retinoic acid (ATRA) induced NF-κB transcriptional activity on NF-κB responsive luciferase reporter gene. Treated and harvested cells were analyzed for luciferase reporter activity as relative light units (RLU). Luciferase activity measurements were performed in triplicates (n=9). Statistical significance was determined via the two-way analysis of variance (ANOVA; Bonferroni post-hoc test; *P<0.05, **P<0.01, ***P<0.005 ****P<0.001).
Because the expression levels of GP91PHOX (Figure 3B), MCP-1/CCL2, MDC/CCL22,21 TNF-α, and IL1β had changed proportionally with the amount of TG2 in differentiating NB4 cell lines, and as all these proteins were found to be NF-κB-dependent genes, we hypothesized that TG2 mediated the transcriptional activity of NF-κB proportional to TG2 abundance. To confirm that TG2 level supports NF-κB-mediated transcriptional activity, we carried out a luciferase reporter assay using an NF-κB promoter-driven luciferase construct that was stably integrated into the genomic DNA of the NB4 cell lines. We found that the various expression levels of TG2 in the cell lines during the 11-day time frame were associated with proportionate NF-κB promoter-driven luciferase reporter activity (WT: 1.41×105±3.14×104, TG2-C 1.29×105±1.46×104, TG2-KD 2.40×104±7.35×103, TG2-ha 1.39×104±3.63×103, TG2-KO 5.22×102±1.44×102/100 μg protein) and could not be further enhanced by exogenous TNF-α (Figure 4D).
TG2 expression level drives inflammatory cytokine expression quantitatively in resting ATRA-differentiated NB4 cells
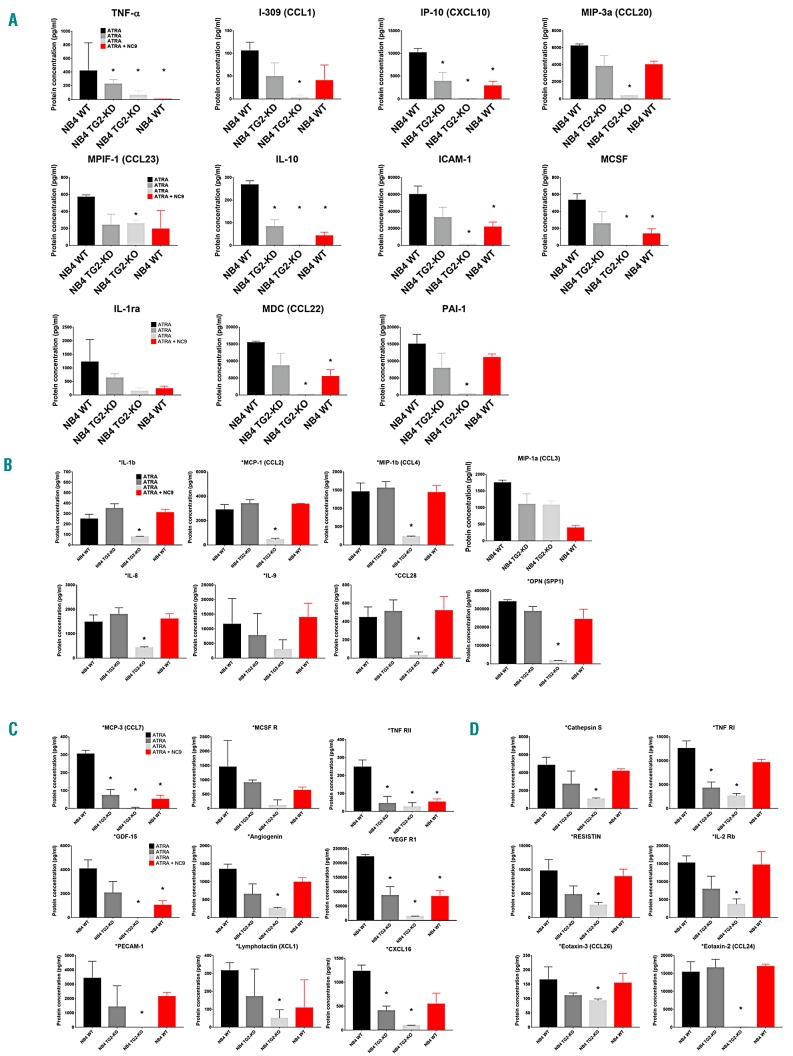
We determined the secreted proteins appearing in the supernatant of NB4 cells at normal, reduced, or abrogated TG2 expression. Inflammatory biomarkers were quantified from the supernatants of ATRA-differentiated NB4-WT, TG2-KD, TG2-KO, and NB4-WT NC9-treated cells using the ELISA-based RayBiotech 200 Human Biomarker Testing Service. Among the 200 proteins available, 50 were detectable in the supernatants. Among the 50 detectable proteins, 44 were expressed in a TG2-dependent manner, from which 18 were identified as NF-κB transcription-factor target genes in the Boston NF-κB target gene database.28 Of these 18 proteins, the expression level of the following 10 cytokines was observed to change in parallel with changes in the expression of TG2 : TNFα, I-309 (CCL-1), IP-10 (CXCL10), MIP-3α (CCL20), IL-10, ICAM-1, MCSF, IL-1ra, MDC (CCL22), and PAI-1 in NB4-WT, TG2-KD, and TG2-KO cells (Figure 5A). The levels of 8 NF-κB-controlled chemokines [MCP-1 (CCL2), MIP-1a (CCL3), MIP-1b (CCL4), cytokines IL-1b, IL-8, and IL-9, CCL-28, and OPN (SPP1)] were found to vary in parallel with TG2 expression (Figure 5B). Furthermore, expression of MCP-3 (CCL7), MCSF R, TNF RII, GDF-15, angiogenin, VEGF R1, PECAM-1, lymphotactin (XCL1), and CXCL16, which do not depend on NF-κB, was also TG2 expression-dependent (Figure 5C). Similarly, Cathepsin S, TNF RI, Resistin, and IL-2 Rb, but not Eotaxin-3 (CCL26) or Eotaxin-2 (CCL24), were expressed in a TG2-dependent manner (Figure 5D). The supernatant concentration of the remaining 16 biomarkers is shown in Online Supplementary Figure S6.
Figure 5.
Atypical expression levels of tissue transglutaminase (TG2) drives inflammatory cytokine expression. In the cell culture, supernatant-secreted chemokines and cytokines were quantified by ELISA-based fluorescent detection cytokine array (RayBiotech Human Cytokine Array). Figures represent the mean±Standard Deviation of secreted protein levels from 3 independent experiments. (A) TG2-quantity-dependent-regulated nuclear factor kappa (κ)-light-chain-enhancer of the activated B-cell (NF-κB) transcription factor target genes, and TG2 inhibitor, NC9-sensitive secretory proteins. (B) TG2-modulated NF-κB transcription factor target genes, and TG2 inhibitor, NC9-insensitive secretory proteins. (C) TG2-dependent and TG2 inhibitor, NC9-sensitive secretory proteins. (D) TG2-dependent and TG2 inhibitor, NC9-insensitive secretory proteins. Statistical significance was determined via the Student t-test; asterisks show the significant differences compared to the wild type (Student t-test; *P<0.05, **P<0.01, ***P<0.005 ****P<0.001).
TG2 is a new potential chemotherapeutic target in APL to prevent development of DS
NC9 is a novel penetrating, irreversible transamidase site-specific inhibitor of TG2, which can transform TG2 from its closed/folded (signaling) conformation to its open (non-signaling) form, modulating both its conformation and activity.29,30 We used NC9 to test its effect on NBT-positivity, cell-surface adhesion receptors, ROS, cytokine, and chemokine production in differentiating NB4 cell lines. We did not observe any significant difference in NBT-positivity (Figure 1C, right) between NC9-treated and -untreated NB4 TG2-KO cells. However, we did observe effective inhibition of ROS production, with almost 10 times lower magnitude at day 11 in all cell lines (WT 8884.00±759.29 RLU/100 μg protein and TG2-KO 133.66±17.67 RLU/100 μg protein) (Figure 3C). We also found that at low TG2 expression levels, the mRNA and protein expression levels of TNFα and IL-1β and the expression of MCP-1 remained low, similar to TG2-deficient states in the NB4 cells (Figure 4A-C, red bars and lines). In the ATRA-treated NB4-WT and NB4 TG2-C cells, where TG2 expression was maximal, the total expression levels of mRNA of TNFα, IL-1β , and MCP-1 were reduced by at least 50% or more in the presence of NC9. The lower mRNA levels were directly reflected in the amounts of secreted TNFα, IL-1β , and MCP-1 (WT; TNFα 1133.01±214.01pg/mL, IL-1β 3467.16±310.59 pg/mL, and MCP-1 1220.14±53.09pg/mL) (Figure 4A-C, red bars and lines). In parallel, the stably integrated NF-κB promoter-driven luciferase reporter activity was at least 15 times less inducible in the NB4-WT cell lines in the presence of the TG2 inhibitor (WT: 1.12×104±7.48×103, TG2-C 1.58×104±9.73×103, TG2-KD 6.15×102±3.40×102, TG2-ha 2.54×103±2.05×103, and TG2-KO 4.15×102±1.58×102/100 μg protein) (Figure 4D). The reduced endogenous NF-κB activities also became apparent in the amounts of secreted inflammatory proteins, which were expressed in a TG2-quantity-dependent manner (Figure 5A). Secretory proteins not targeting NF-κB genes but expressed in a TG2-dependent manner also included NC9 sensitive secretory proteins (Figure 5C).
TG2 contributes to expression and nuclear translocation of NF-κB, which is significantly reduced by the TG2 inhibitor NC9
NF-κB promoter-driven luciferase reporter activity and the extent of the expression of several NF-κB-controlled inflammatory biomarkers suggested that NF-κB transcriptional activity might depend on the magnitude of the expression of TG2 (Figures 4D and 5A). Our starting observation was that NB4 cells treated with ATRA plus NC9 expressed lower amounts of TG2 protein than cells treated only with ATRA (Figures 1B and 6B). First, we examined the impact of NC9 on the transcription level of TG2 mRNA and found that it did not change. The amount of TG2 protein was affected by NC9, while TG2 mRNA levels remained unaffected suggesting that the proteolytic degradation of TG2 might be increased by NC9. To test this hypothesis, 11-day differentiated NB4 cells were treated for 3 h with MG132 proteasome inhibitor. Western blot analysis of TG2 expression confirmed the enhanced proteolytic degradation of TG2 during the NC9 treatment. In the presence of NC9, the amount of TG2 decreased to approximately one-tenth that measured in cells treated with ATRA alone (Figure 6E1 and E2).
Figure 6.
Tissue transglutaminase (TG2) both induces and guides nuclear translocation of nuclear factor kappa (κ)-light-chain-enhancer of the activated B-cell (NFκB). (A) Relative mRNA expression of TG2 in 1 μM all-trans retinoic acid (ATRA) + 30 μM NC9 treated NB4-WT, TG2-C, TG2-KD, TG2-ha, and TG2-KO cells measured at the indicated days by real-time Q-PCR and normalized to GAPDH mRNA expression (n=3). (B) Representative Western blot showing TG2 protein expression levels upon ATRA + NC9 treatment through 11 days (n=3). (C1-2) Representative Western blots showing total cell lysate samples TG2, p65/RelA, phospho-p65/RelA, and p50 protein expression levels in NB4 cell lines and its densitometric analysis, where graphs show the representation of the mean±Standard Deviation values of integrated density (n=6). (D1-2) Western blots of total cell lysate, cytosolic and nuclear fractions of TG2, and p65/RelA, phospho-p65/RelA, and p50 protein expression and its densitometry, respectively (n=10). (E1-2) Western blot analysis of NB4-WT cells upon ATRA, ATRA + NC9, and 5 μM MG132 treatment (n=6). Statistical significance was determined via the two-way analysis of variance (ANOVA; Bonferroni post-hoc test; *P<0.05, **P<0.01, ***P<0.005 ****P<0.001).
Next, we investigated the effect of the lower TG2 protein levels on the expression of p65/RelA, p50, and phospho(Ser536)-p65/RelA components of NF-κB and found that the absence, or reduced gene expression, or suppression of TG2 by NC9 significantly restricted the expression of p65/RelA, phospho-p65/RelA and p50 proteins in NB4-WT, TG2-C, TG2-KD, TG2-ha, and TG2-KO cells (Figure 6C1 and C2, upper and lower, left and right panels). However, we have long been aware that in the ATRA-differentiated NB4-WT cells, significant amounts of TG2 protein translocate to the nucleus during differentiation.20 To demonstrate the role of nuclear TG2, after 11 days of differentiation, the cytosolic and nuclear fractions of NB4-WT were separated and analyzed for the expression of TG2, p65/RelA, phospho-p65/RelA, and p50 (Figure 6D1 and D2). In the differentiated cells, NC9 treatment was associated with significantly increased cytosolic TG2 levels and lower levels in the nucleus compared to cells treated only with ATRA (Figure 6D2, upper left). Concurrently, the amount of total and nuclear p65/RelA also decreased with NC9 treatment (Figure 6D2, upper right). Distribution patterns of phospho(Ser536)-p65/RelA, which is the transcriptionally active form of p65/RelA, were very similar to the TG2 protein detected in the total, cytosolic, and nuclear fractions (Figure 6D2, upper and lower right, black and red bars). Both total and nuclear phospho-p65/RelA protein contents were significantly reduced in the presence of NC9 inhibitor (Figure 6D2, lower left, black and red bars). The p50 subunit of NF-B showed patterns of cellular distribution very similar to p65/RelA (Figure 6D2, lower right and upper right, black bars), with a distinctive difference in the amount of nuclear p50 protein remaining low, around the detection limit, in NC9-treated NB4-WT cells (Figure 6D2, lower right, black and red bars). The cellular distribution of TG2 indicated that NC9 inhibits nuclear translocation of TG2 and considerably increases cytosolic levels (Figure 6D2, upper left, red bar). In addition, both phospho-p65/RelA and p50 showed similar distribution patterns upon NC9 treatment (Figure 6D2, lower left and right, black and red bars).
Discussion
Treatment of APL principally comprises ATRA-based therapy, which leads to terminal differentiation of APL cells to neutrophil granulocytes. In adverse events, this treatment is frequently associated with severe hyper-inflammatory reactions, leading to organ infiltration of differentiating APL cells.
The pathogenic processes of the hyper-inflammatory cascade in DS are not completely understood. At least two different mechanisms appear to play important roles in DS development: differentiation of APL cells with cytokine release and adhesion, and migration of differentiated APL cells to different organs. ATRA-induced differentiation of APL cells is associated with elevated expression of inflammatory cytokines and adhesion molecules called integrins. Activation of neutrophils is elicited by mediators, including chemokines, selectins, and integrin-mediated outside-in signaling. Locally produced chemokines mediate a sequence of events leading to extravasation of leukocytes at the inflammatory site. Neutrophils express different chemokine receptors, including CXCR2, whose most potent ligand is IL8 (CXCL8).31 Shibakura et al. first demonstrated that ATRA could induce synthesis and secretion of IL8 in NB4 cells. Thereafter, it was shown that not only do ATRA-treated APL cells express IL8 mRNA in ex vivo cell cultures, but also IL8, as well as chemokines such as MCP-1 (CCL2), MIP-1a (CCL3), and MIP-1b (CCL4), are present in the serum of APL patients who developed DS during ATRA treatment.32 It has also become obvious that NB4 cells secrete IL8 constitutively, which is further enhanced during ATRA-induced differentiation, and cannot be inhibited by dexamethasone.33 In the presence of IL8, neutrophil granulocytes increase the amounts of CD11b integrin receptor along with its binding activity and the amounts of CD11c on their cell surfaces.34,35 Simultaneously, secreted TNF-α also stimulates neutrophils, triggering activation of CD11c.36 Since 1998, CD11b has been used as a surface marker of granulocytic cell differentiation of NB4 cells in published research, in spite of the fact that CD11b is mainly stored intracellularly in both specific and gelatinase granules and secretory vesicles under normal physiological conditions.37–39 Our observations confirm that during ATRA-induced differentiation of NB4 cell lines, CD11b and CD11c receptors were translocated in large quantities to the cell surface, and show that the amount of surface CD11b cannot be further increased by chemoattractant or phorbolesters (Figure 3A-C and Online Supplementary Figure S5). Nevertheless, Nupponen et al. found that while “neutrophil CD11b expression and circulating interleukin-8 represent a diagnostic marker for early-onset neonatal sepsis,” CD11c is also a potential diagnostic biomarker for sepsis and systemic inflammation.40 An antibody detecting activation-associated CD11c revealed gradually increasing cell-surface receptor expression in an activated state, with a high affinity for ligands on ATRA-treated NB4 cell lines (Figure 2C). In this manner, inflammatory cytokines could result in rolling, stable adhesion, microvascular sequestration, and infiltration of differentiated APL cells, with increasing potential for the APL-mediated organ damage observed in DS.38,41
TNFα is among the most effective physiological inducers of NF-κB activity. In NB4-WT cells, TNFα did not enhance further NF-κB activity already highly elevated by ATRA, but it increased NF-κB transcriptional activity from 5.2×102±1.4×102 to 3.2×104±7.2×103 RLU in NB4 TG2-KO cells (Figure 4D). This may indicate that NF-κB maintains a low basal transcriptional activity in the absence of TG2 in NB4 TG2-KO cells, and adding exogenous TNFα could trigger more NF-κB transcription activity from the IκB:p65:p50 complex. In NB4-WT cells, ATRA-induced atypical expression of TG2 can exacerbate low-level inflammation (IL8 expression, see above) as its expression is associated with both upregulation and activation of NF-κB, resulting in increased endogenous TNFα synthesis and secretion, which may become a sort of self-accelerating process (Figure 4A2-D and Figure 6C1 and 2).
Our findings strongly indicate that ATRA-induced TG2 expression is associated with translocation of NF-κB into the nucleus, upregulation of numerous inflammatory genes, and secretion of their products. These include TG2-quantity-dependent-regulated NF-κB transcription factor target genes: TNFα, I-309 (CCL-1), IP-10 (CXCL10), MIP-3α (CCL20), IL10, ICAM-1, MCSF, IL-1ra, MDC (CCL22), and PAI-1, whose amounts were significantly reduced in the absence of TG2 in NB4 TG2-KO cells, with the exception of IL-1ra (Figure 5A). Similarly, among TG2-modulated NF-κB transcription factor target genes and TG2 inhibitor NC9-insensitive secretory proteins [MCP-1 (CCL2), MIP-1a (CCL3), MIP-1b (CCL4), cytokines IL-1b, IL-8, IL-9, CCL-28, and OPN (SPP1)], only MIP-1a (CCL3) did not show a significant decrease in TG2 deficiency (Figure 5B). In addition, of the remaining 15 TG2 expression-dependent secretory proteins, only one was not significantly changed in the absence of TG2 expression. Together, these results suggest that expression of TG2 in ATRA-induced differentiating APL cells is a crucial factor in the developing inflammatory process.
The suppression of TG2 parallels the considerably low levels of p50 and phospho (Ser536) p65/RelA (Figure 6C2), suggesting that ATRA-induced expression of TG2 reprograms APL cells to be inflammatory neutrophils and induces NF-κB nuclear translocation. Destruction of IκB is stimulated by several signals such as lipopolysaccharide, ROS, TNFα, and Il-β. One possibility for TG2-dependent NF-κB activation is that TG2 interacts with IκB to initiate its non-proteasomal degradation, causing both activation and nuclear translocation of NF-κB-s.42 Alternatively, TG2-mediated cross-linking of IκBα and activation of NF-κB has been previously described, yet we could not detect any forms of IκBα polymer in differentiated NB4 cells (K Jambrovics, 2018, unpublished observation). It was demonstrated that TG2 can form a complex in cytosol as well as in nuclei, with p65 binding to the promoter of the HIF-1α transcription factor, and in this sense, TG2 could become a transcriptional co-regulator in the nucleus.42
In the presence of NC9, an irreversible transamidase site-specific inhibitor of TG2, the conformational equilib rium of TG2 shifts from the closed GTP-binding form to the open conformation characterized by disorganized GTP-binding sites.25 According to our studies, NC9-induced conformational changes in TG2 significantly affect the NF-κB signaling pathway. In the presence of NC9, total TG2 is reduced, as is the nuclear translocation of the decreased amount of TG2, significantly increasing the amount of cytosolic TG2. In turn, the reduced amount of nuclear TG2 correlates with reduced total levels of nuclear p50, p65/RelA, and phospho-p65/RelA proteins in NB4-WT cells, while the level of the transcriptionally active form of p65 and phospho-p65 is increased significantly in the cytosol. This raises the possibility that TG2 in its GTP-bound closed conformation can readily translocate to the nucleus and assist the nuclear translocation of p65/RelA. When TG2 is no longer capable of adopting its GTP-bound closed conformation, due to modification by NC9, the accumulation of both TG2 and p65/RelA in the cytosol results in low NF-κB transcription activity, and, consequently, in the significantly reduced production of inflammatory cytokines and chemokines. Finally, the balance of TG2 synthesis and degradation is changed by degradation triggered by NC9.
Among the secreted cytokines and chemokines, TNFα and IL-1β are the most powerful inflammatory agents. At the highest concentrations of TNFα (0.8-1.2 ng/L in DS patients), they cause capillary leakage and reduced cardiac, lung, and renal function.38,43,44 Our data indicate that TG2 is a critical component of this process, and NC9-induced inhibition of TG2 may prevent development of this dangerous side effect of retinoid therapy.
Overall, our study revealed a novel, active role of TG2 in expression and activation of the components of NF-κB and thus in the development of atypical response to conventional ATRA treatment of APL. Targeted suppression of the TG2-dependent process may alleviate the common and potentially fatal toxicity of retinoid treatment in APL, representing an important potential therapeutic strategy.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. István Szatmári and Pál Botó for assistance with cell sorting, Orsolya Molnár for clone selection and Western blot analyses of the KO cell lines.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/3/505
Funding
This work was supported by Hungarian grants from the National Research Found OTKA NK105046, and in part by both OTKA K 129139 and TÁMOP-4.2.2.D-15/1/KONV-2015-0016 project implemented through the New Széchenyi Plan, co-financed by the European Social Fund. The work was in part also supported by the GINOP-2.3.2-15-2016-00020 |2.3.2-15-2016-00020 MolMedEx TUMORDNS grant and the EFOP-3.6.1-16-2016-00022|3.6.1-16-2016-00022 “Debrecen Venture Catapult Program”. The work is supported by the GINOP-2.3.2-15-2016-00006|2.3.2-15-2016-00006 project co-financed by the European Union and the European Regional Development Fund. KJ received a fellowship from the DOTE Apoptosis Research Foundation. The research was partly financed by the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the Biotechnology thematic programme of the University of Debrecen.
References
- 1.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the Classification of the Acute Leukaemias French-American-British (FAB) Co-operative Group. Br J Haematol. 1976;33(4):451–458. [DOI] [PubMed] [Google Scholar]
- 2.Bennett J, Catovsky D, Daniel M, et al. A variant form of hypergranular promyelocytic leukemia (M3). Ann Intern Med. 1980;92:261. [DOI] [PubMed] [Google Scholar]
- 3.Bennet J. Proposed revised criteria for the classification of acute leukemia: A report from the French-American-British Cooperative Group. Ann Intern Med. 1985; 3:620–625. [DOI] [PubMed] [Google Scholar]
- 4.Arber DA. Realistic pathologic classification of acute myeloid leukemias. Am J Clin Pathol. 2001;115(4):552–560. [DOI] [PubMed] [Google Scholar]
- 5.Kühnl A, Grimwade D. Molecular markers in acute myeloid leukaemia. Int J Hematol. 2012;96(2):153–163. [DOI] [PubMed] [Google Scholar]
- 6.Wiernik PH, Gallagher RE, Tallman MS. Acute promyelocytic leukemia. Neoplastic Diseases of the Blood: Springer, 2013:403–453. [Google Scholar]
- 7.Grignani F, Ferrucci PF, Testa U, et al. The acute promyelocytic leukemia-specific PML-RAR fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993;74(3):423–431. [DOI] [PubMed] [Google Scholar]
- 8.Kogan SC, Hong SH, Shultz DB, et al. Leukemia initiated by PMLRAR : the PML domain plays a critical role while retinoic acid–mediated transactivation is dispensable. Blood. 2000;95(5):1541–1550. [PubMed] [Google Scholar]
- 9.Ablain J. Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood. 2011;117(22):5795–5802. [DOI] [PubMed] [Google Scholar]
- 10.Breitman T, Collins SJ, Keene B. Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood. 1981; 57(6):1000–1004. [PubMed] [Google Scholar]
- 11.Raelson JV, Nervi C, Rosenauer A, et al. The PML/RAR alpha oncoprotein is a direct molecular target of retinoic acid in acute promyelocytic leukemia cells. Blood. 1996;88(8):2826–2832. [PubMed] [Google Scholar]
- 12.Fenaux P, Wang Z, Degos L. Treatment of acute promyelocytic leukemia by retinoids. Acute Promyelocytic Leukemia: Springer, 2007:101–128. [DOI] [PubMed] [Google Scholar]
- 13.Montesinos P, Sanz MA. The differentiation syndrome in patients with acute promyelocytic leukemia: experience of the pethema group and review of the literature. Mediterr J Hematol Infect Dis. 2011; 3(1):e2011059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patatanian E, Thompson D. Retinoic acid syndrome: a review. J Clin Pharm Ther. 2008;33(4):331–338. [DOI] [PubMed] [Google Scholar]
- 15.Luesink M, Pennings JL, Wissink WM, et al. Chemokine induction by all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia: triggering the differentiation syndrome. Blood. 2009;114(27):5512–5521. [DOI] [PubMed] [Google Scholar]
- 16.Montesinos P, Bergua JM, Vellenga E, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009; 113(4):775–783. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida H, Kitamura K, Tanaka K, et al. Accelerated degradation of PML-retinoic acid receptor (PML-RARA) oncoprotein by all-trans-retinoic acid in acute promyelocytic leukemia: possible role of the proteasome pathway. Cancer Res. 1996;56(13):2945–2948. [PubMed] [Google Scholar]
- 18.Lanotte M, Martin-Thouvenin V, Najman S, et al. NB4, a maturation inducible cell line with t (15; 17) marker isolated from a human acute promyelocytic leukemia (M3). Blood. 1991;77(5):1080–1086. [PubMed] [Google Scholar]
- 19.Tallman MS, Andersen JW, Schiffer CA, et al. Clinical description of 44 patients with acute promyelocytic leukemia who developed the retinoic acid syndrome. Blood. 2000;95(1):90–95. [PubMed] [Google Scholar]
- 20.Balajthy Z, Csomós K, Vámosi G, et al. Tissue-transglutaminase contributes to neutrophil granulocyte differentiation and functions. Blood. 2006;108(6):2045–2054. [DOI] [PubMed] [Google Scholar]
- 21.Csomós K, Német I, Fésüs L, et al. Tissue transglutaminase contributes to the all-trans-retinoic acid–induced differentiation syndrome phenotype in the NB4 model of acute promyelocytic leukemia. Blood. 2010;116(19):3933–3943. [DOI] [PubMed] [Google Scholar]
- 22.Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002; 27(10):534–539. [DOI] [PubMed] [Google Scholar]
- 23.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003; 4(2):140–156. [DOI] [PubMed] [Google Scholar]
- 24.Eckert RL, Fisher ML, Grun D, et al. Transglutaminase is a tumor cell and cancer stem cell survival factor. Mol Carcinog. 2015;54(10):947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerr C, Szmacinski H, Fisher ML, et al. Transamidase site-targeted agents alter the conformation of the transglutaminase cancer stem cell survival protein to reduce GTP binding activity and cancer stem cell survival. Oncogene. 2017;36(21):2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 26.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89(10):3503–3521. [PubMed] [Google Scholar]
- 27.Sadhu C, Hendrickson L, Dick KO, et al. Novel Tools for Functional Analysis of CD11c: Activation-Specific, Activation-Independent, and Activating Antibodies. J Immunoassay Immunochem. 2007; 29(1):42–57. [DOI] [PubMed] [Google Scholar]
- 28.Boston University Biology. 2018. Target Genes of NF-kB. [ONLINE] Available at: https://www.bu.edu/nf-kb/gene-resources/target-genes/ [Last accessed 15 May 2018]
- 29.Caron NS, Munsie LN, Keillor JW, et al. Using FLIM-FRET to measure conformational changes of transglutaminase type 2 in live cells. PloS One. 2012;7(8):e44159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keillor JW, Chica RA, Chabot N, et al. The bioorganic chemistry of transglutaminase: from mechanism to inhibition and engineering. Can J Chem. 2008;86(4):271–276. [Google Scholar]
- 31.Harada A, Sekido N, Akahoshi T, et al. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56(5):559–564. [PubMed] [Google Scholar]
- 32.Shibakura M, Niiya K, Niiya M, et al. Induction of CXC and CC chemokines by all-trans retinoic acid in acute promyelocytic leukemia cells. Leuk Res. 2005;29(7):755–759. [DOI] [PubMed] [Google Scholar]
- 33.Tsai W-H, Hsu H-C, Lin C-C, et al. Role of interleukin-8 and growth-regulated oncogene- in the chemotactic migration of all-trans retinoic acid-treated promyelocytic leukemic cells toward alveolar epithelial cells. Crit Care Med. 2007;35(3):879–885. [DOI] [PubMed] [Google Scholar]
- 34.Detmers PA, Lo SK, Olsen-Egbert E, et al. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990;171(4):1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takami M1, Terry V, Petruzzelli L. Signaling pathways involved in IL-8-dependent activation of adhesion through Mac-1. J Immunol. 2002;168(9):4559–4566. [DOI] [PubMed] [Google Scholar]
- 36.Loike JD, Sodeik B, Cao L, et al. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proc Natl Acad Sci. 1991;88(3):1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89(10):3503–3521. [PubMed] [Google Scholar]
- 38.Frankel SR, Eardley A, Lauwers G, et al. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med. 1992;117(4):292–296. [DOI] [PubMed] [Google Scholar]
- 39.Bainton DF, Miller LJ, Kishimoto T, et al. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987;166(6):1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nupponen I, Andersson S, Järvenpää A-L, et al. Neutrophil CD11b expression and circulating interleukin-8 as diagnostic markers for early-onset neonatal sepsis. Pediatrics. 2001;108(1):E12. [DOI] [PubMed] [Google Scholar]
- 41.Tang L, Chai W, Ye F, et al. HMGB1 promotes differentiation syndrome by inducing hyperinflammation via MEK/ERK signaling in acute promyelocytic leukemia cells. Oncotarget. 2017;8(16):27314–27327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar S, Mehta K. Tissue transglutaminase constitutively activates HIF-1 promoter and nuclear factor- B via a non-canonical pathway. PloS One. 2012;7(11):e49321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flombaum CD, Isaacs M, Reich L, et al. Acute renal failure associated with the retinoic acid syndrome in acute promyelocytic leukemia. Am J Kidney Dis. 1996; 27(1):134–137. [DOI] [PubMed] [Google Scholar]
- 44.Mitrovic M, Suvajdzic N, Elezovic I, et al. Thrombotic events in acute promyelocytic leukemia. Thromb Res. 2015;135(4):588–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.