Paroxysmal nocturnal hemoglobinuria (PNH), a rare hematological condition, presents with hemolytic or thrombotic symptoms. PNH stem cells arise due to somatic mutations in the phosphatidylinositol glycan A gene in bone marrow stem cells, resulting in loss of the glycosylphosphatidylinositol anchor protein (GPI anchor). Loss of this GPI anchor results in absence of CD55 and CD59, complement regulatory proteins on hematopoetic cells, rendering red blood cells susceptible to complement attack and intravascular hemolysis. However, GPI deficient white cells and platelets are likely to be behind the high thrombosis risk.1,2
Cumulative thrombosis incidence in PNH over an eight to ten-year period is 23-30% (in the pre-eculizumab era);3,4 20% of patients have multi-site thrombosis and 10% present with thrombosis.4 PNH thrombosis can also be subclinical affecting any system including the CNS, coronary arteries or pulmonary system.5 Patients with no known neurological symptoms have also been shown to have increased deep white matter chronic ischemic lesions on brain MRI, although clinical implications of this are less clear.6
Thrombosis in PNH is a clinical emergency due to risk of death, thrombosis extension and morbidity. Eculizumab (Soliris), a monoclonal antibody, is currently the only licenced treatment for PNH, significantly reducing thrombotic events from 7.37 to 1.07 per 100 patient-years, and a sustained, relative reduction of thrombotic events of 81.8%.7 Indications for consideration of anti-complement therapy in England, UK, include transfusions due to hemolytic PNH, hemolytic and symptomatic PNH without transfusions (LDH 1.5x upper limit of normal), PNH related thrombosis, pregnancy, or complications from PNH such as renal impairment and pulmonary hypertension.
Patients with PNH with low levels of hemolysis tend to present with thrombosis; however, the incidence of this is unclear and identifying patients at risk is difficult. Data of the Leeds PNH Service, UK, have been analysed to identify patients with PNH with low levels of hemolysis who present with thrombosis, to identify risk factors and consideration for patient management.
The Leeds (UK) PNH database was retrospectively interrogated. Patients with PNH granulocyte or monocyte cell proportions over 10% and an LDH level less than twice the upper limit of normal (ULN) for the testing laboratory were identified. Anonymised clinical data were then analysed to determine risk factors for thrombosis. Comparisons between those with and without thrombosis were assessed.
All patients who fulfilled the above requirements were then subdivided into groups as below:
Group 1: PNH white cells >30%, PNH red cells <10%, LDH <2xULN
Group 2: PNH white cells >30%, PNH red cells >10% with higher proportion of type II red cells than type III red cells, LDH <2xULN
Group 3: PNH white cells 10-30%, PNH red cells <10%, LDH <2xULN
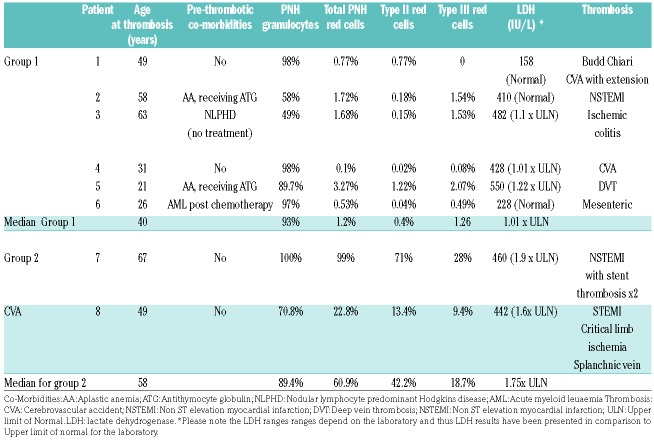
A total of 429 patients were analysed and 25 patients who fit the criteria were identified. Median age of the patient cohort was 60 years (range 23-80 years). Eleven patients fit the criteria for group 1, of whom 6 (54%) had thrombosis. Eleven patients fit the criteria for group 2, of whom 2 (18.1%) had thrombosis. Three patients fit the criteria for group 3, with no thrombosis. Median age at thrombosis was 49 years (range 21-67 years).
All 8 patients with thrombosis are detailed in the table, including co-morbidities, PNH clone size and site of thrombosis. Clinical data provided is from time of the thrombosis or at the time of diagnosis for those without thrombosis. Patients in the cohort also had immature red cell clones assessed (CD71+ cells), which were all less than 2%, reflecting comparably with the red cell clone data provided in the table. No patient was on eculizumab at the time of their initial thrombosis.
Table 1.
Patient characteristics for those experiencing thrombosis without hemolysis.
Seven patients were not on anticoagulation at the time of the thrombosis, with one patient extending their thrombosis whilst on warfarin (patient 1). One patient had a further multi-territory acute infarct six days after eculizumab dose, with complete complement blockade on CH50 lysis analysis (patient 4).
In total, 3 of the 25 patients (12%) have died; one in the thrombosis cohort, and two in the non-thrombosis group. Causes of death include dementia, metastatic cancer, and presumed complications of allograft for aplastic anemia.
We report the first case series of patients with PNH who experience thrombosis with low levels of hemolysis. Patients at higher risk of presenting with thrombosis were those with high PNH white cell proportions and low PNH red cells (group I). Patients with a greater proportion of type II red cells (partial deficiency of GPI linked proteins) compared to type III red cells (complete deficiency of GPI linked proteins) and LDH <2x ULN were also at risk of thrombosis. This is an under-recognised risk for patients with PNH cells.
It should be noted that only one patient was anticoagulated at the time of their CVA extension. Others were not anticoagulated due to patients not being diagnosed with PNH at the time of thrombosis or thought to be unsuitable for anticoagulation due to thrombocytopenia.
Recurrent thrombosis is a recognised risk factor in patients with PNH;8 patients in this series had recurrent thrombosis or extension of thrombosis resulting in significant morbidity, emphasising the importance of recognition of PNH as a contributing factor for thrombosis and education of physicians to request PNH testing.
Mechanisms for thrombosis in PNH are not completely understood with both hemolytic and non-hemolytic mediated processes described. The complement and coagulation systems are intimately linked.9 This study lends further support to non-hemolytic mechanisms playing a major role in the pathophysiology of thrombosis in PNH. PNH platelets may play a key role through platelet derived microparticles production and activation of the coagulation system, in particular due to CD59 deficiency.2,10 Endothelial activation may also be a significant contributor due to endothelial microparticle release and inflammation.11 The formation of neutrophil extracellular traps (NETs) stimulated by infection or inflammation can also directly promote thrombin activation, even in platelet-poor plasma.12 Differences in nucleosome levels in PNH patients with a history of thrombosis and those without thrombosis support the formation of low grade NETs in PNH, although further studies are required.13
Whilst hemolytic PNH rarely presents as an acute emergency, thrombosis can occur without preceding symptoms and can be catastrophic. Managing this risk is difficult. Granulocyte cell proportion correlates with thrombosis risk;3,14 however, decisions to commence complement inhibition is not solely based on this. In those whom complement inhibition is not indicated, a granulocyte cell proportion over 50% is usually utilised as an ‘arbitrary’ level for commencement of prophylactic anticoagulation. However, it must be recognised that patients with smaller PNH cell proportions remain at an increased risk of thrombosis thus clinical vigilance and patient education is essential.15 Patients should have an informed discussion with their hematologist regarding the risks and benefits of anticoagulation.
Anticoagulation alone is not wholly effective in reducing the thrombosis risk in patients with PNH compared with the general population. Patients are at risk of thrombosis extension and recurrence despite prophylactic anticoagulation, in some reports as high as 57%.15 This risk, particularly in countries where anti-complement therapy is not available, causes increased morbidity and mortality. Reasons for ongoing thrombosis in patients with PNH on prophylactic anticoagulation are multifactorial with several non-hemolytic mechanisms. Complement activation results in increased levels of interleukin 6, interleukin 8 and tumour necrosis factor alpha, which activate endothelium-releasing microparticles and promote further thrombosis. Interleukin 6 can also activate thrombin directly, bypassing the coagulation cascade. Thrombin activates the complement system independently, cleaving C3 and C5 perpetuating the thrombotic state.2
Arterial thrombosis risk is around 15-30%.7,15 Interestingly and surprisingly, arterial thrombosis in this cohort was disproportionately high, with 62.5% experiencing either arterial or mixed venous and arterial thrombosis. Utilisation of non-invasive methods including echocardiogram, MRI and cardiac markers can be useful to identify patients who may benefit from complement inhibition.
This study supports the theory that while hemolysis may be associated with a thrombotic event, white cell and platelet factors appear to have a more pivotal role than previously thought in the mechanisms of thrombosis in PNH. Ongoing laboratory research into this area will provide further valuable data. As per the national recommendations in England, PNH-related thrombosis, regardless of LDH level, is an indication to commence treatment.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hillmen P, Lewis S, Bessler M, Luzatto L, Dacie J. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:(19)1253–1258. [DOI] [PubMed] [Google Scholar]
- 2.Hill A, Kelly R, Hillmen P. Thrombosis in paroxymal nocturnal hemoglobinuria. Blood. 2013;121(25):4985–4996. [DOI] [PubMed] [Google Scholar]
- 3.Hall C, Richards S, Hillmen P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood. 2003;102(10):3587–3591. [DOI] [PubMed] [Google Scholar]
- 4.de Latour R, Mary J, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112(8):3099–3106. [DOI] [PubMed] [Google Scholar]
- 5.Hill A, Sapsford RJ, Scally A, et al. Under-recognized complications in patients with paroxysmal nocturnal haemoglobinuria: raised pulmonary pressure and reduced right ventricular function. Br J Haematol. 2012;158(3):409–414. [DOI] [PubMed] [Google Scholar]
- 6.Barcellini W, Scola E, Lanfranconi S, et al. Paroxysmal nocturnal hemoglobinuria (Pnh): brain MRI ischemic lesions In neurologically asymptomatic patients. Sci Rep. 2018;8(1):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillmen P, Muus P, Duhrsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–4128. [DOI] [PubMed] [Google Scholar]
- 8.Meppiel E, Crassard I, de Latour R, et al. Cerebral venous thrombosis in paroxysmal nocturnal hemoglobinuria A series of 15 cases and review of the Literature. Medicine (Baltimore). 2015;94(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young B, Macrae F, Newton D, Hill A, Ariëns R. The prothrombotic state in paroxysmal nocturnal hemoglobinuria: a multifaceted source. Haematologica. 2018;103(1):9–17. [DOI] [PubMed] [Google Scholar]
- 10.Hugel B, Socié G, Vu T et al. Elevated levels of circulating procoagulant microparticles in patients with paroxysmal nocturnal hemoglobinuria and aplastic anemia. Blood. 1999;93(10):3451–3456. [PubMed] [Google Scholar]
- 11.Simak J, Holanda K, Risitano A, Zivny J, Young N, Vostal J. Elevated circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2004;125(6):804–813. [DOI] [PubMed] [Google Scholar]
- 12.Gould T, Vu T, Swystun L, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34(9):1977–1984. [DOI] [PubMed] [Google Scholar]
- 13.Zeerleder S, van Bijnen S, Wouters D, van Mierlo G, Muus P. Neutrophil extracellular trap formation in PNH patients with and without a history of thrombosis - effects of eculizumab. Blood. 2013;122(21):1235. [Google Scholar]
- 14.Moyo V, Mukhina G, Garrett E, Brodsky R. Natural history of paroxysmal nocturnal haemoglobinuria using modern diagnostic assays. Br J Haematol. 2004;126(1):133–138. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Jang J, Kim J. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int J Hematol. 2013;97(6):749–757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.