Abstract
Background/purpose
Langerhans cells (LCs) are antigen-presenting cells. This study assessed the LC counts in 80 dentigerous cysts (DCs).
Materials and methods
The S100-positive LC numbers in the lining epithelia and subepithelial connective tissues were counted at 80 DC sites without inflammation, 33 DC sites with mild/moderate inflammation, and 9 DC sites with severe inflammation from 80 DC specimens.
Results
The mean S100-positive LC counts in the lining epithelia and subepithelial connective tissues increased significantly from no inflammation (0.6 ± 0.6 and 0.7 ± 0.6 cell/high-power field or HPF, respectively) through mild/moderate inflammation (8.1 ± 2.0 and 4.5 ± 2.3 cells/HPF, respectively) to severe inflammation DC sites (21.0 ± 7.0 and 11.1 ± 6.5 cells/HPF, respectively; P-value < 0.001). DC sites with inflammation had thicker lining epithelia than those without inflammation. Moreover, the mean LC counts in the lining epithelia and subepithelial connective tissues of DCs were significantly higher in the thicker lining epithelium (>50 μm) group (8.6 ± 7.1 and 4.8 ± 4.5 cells/HPF, respectively) than in the thinner lining epithelium (≦50 μm) group (0.6 ± 0.6 and 0.6 ± 0.6 cells/HPF, respectively; both P-values < 0.001).
Conclusion
A significant association of high-grade inflammation and thick lining epithelium with the increased LC number in DCs is found. Very few LCs in the lining epithelia of DCs without inflammation indicate the reduced immunosurveillance ability against DC lining epithelial cells in DC patients. It needs further studies to confirm the role of reduced immunosurveillance in the enlargement of the DC.
Keywords: Langerhans cell, dentigerous cyst, inflammation, lining epithelium, immunosurveillance
Introduction
Dentigerous cyst (DC) is the most common type of developmental odontogenic cyst, consisting of approximately 20% of all epithelium-lined cysts of the jaws.1 Although the pathogenesis of the DC is still not clear, apparently it develops by accumulation of fluid between the reduced enamel epithelium and the tooth crown. Radiographically, three different types of the DC are found. The central-typed DC encloses the crown of an unerupted tooth and is attached to the tooth at the cementoenamel junction. The lateral-typed DC grows laterally along the root surface and partially surrounds the crown. The circumferential-typed DC extends along the mesial and distal root surfaces of an unerupted tooth so that a significant portion of the root appears to be within the cyst. Histopathologically, the uninflamed DC is usually lined by thin nonkeratinized stratified squamous epithelium. Mucous cells may be found in the focal areas of the lining epithelium. The inflamed DC may be lined by thick nonkeratinized stratified squamous epithelium with hyperplastic rete ridges.1
Langerhans cells (LC) are dendritic cells that reside within the stratified squamous epithelium of skin and the mucosa of the upper gastrointestinal and female genital tracts. LCs are usually located at the suprabasal and spinous cell layers of the epithelium and constitute 2–8% of the intra-epithelial cell content.2 LCs have Fc-IgG and C3 receptors, express immune response-associated (Ia) antigens, and function as antigen-presenting cells and allogeneic stimulatory cells to primed T lymphocytes.3 Previous studies have shown that after 3 weeks, the majority of LCs in parental skin which has been transplanted on to F1 hybrid are of recipient origin, indicating that the LCs are derived from a mobile pool of cells. Moreover, in skin from radiation-induced bone marrow chimaeric animals, up to 80% of the epidermal LCs are derived from the bone marrow of the donor animals; this further suggests the bone marrow origin of the epidermal LCs.3
There are few previous studies describing the presence of LCs in the lining epithelia and subepithelial connective tissues of DCs.4, 5, 6 In addition, it was still not clear whether the LC counts in the lining epithelia and in the subepithelial connective tissues of DCs were associated with the grade of inflammation in the subepithelial connective tissue, the thickness of the lining epithelium, and clinical parameters of the DCs. In this study, we used the anti-S100 protein immunostaining to study the LCs in a relatively large series of 80 DCs. The LC numbers in the lining epithelia and subepithelial connective tissues of DC samples were separately counted at many DC sites with or without inflammation. We tried to elucidate whether the LC counts in the lining epithelia and subepithelial connective tissues of DC samples were associated with the grade of inflammation in the focal fibrous cystic wall, the thickness of the lining epithelium, and the clinical parameters including the patients' age and gender as well as the location, associated tooth, and size of the DC.
Materials and methods
Patients and specimens
This study included 80 formalin-fixed, paraffin-embedded specimens collected from 80 DC patients (57 men and 23 women, mean age 34 ± 18 years, range 5–67 years). Diagnosis of the DC was based on histological examination of hematoxylin and eosin-stained tissue sections. The DC was characterized as having flattened nonkeratinized stratified squamous epithelium of 2–4 cells in thickness overlying a thin layer of fibrous connective tissue wall without inflammation or with a focal mild, moderate or severe chronic inflammatory cell infiltrate. All patients received total surgical enucleation of their DC lesions at the Department of Oral and Maxillofacial Surgery, National Taiwan University Hospital (NTUH), Taipei, Taiwan during the period from 2005 to 2009. Specimens were obtained from total surgical excision of the lesions. Of the 80 DC lesions, 30 (37.5%) were found in the maxilla (23 in the anterior and 7 in the posterior region) and 50 (62.5%) in the mandible (all in the posterior region). Moreover, the 80 DC-associated teeth contained 30 maxillary teeth (including 2 right upper central incisors, 1 right upper lateral incisor, 3 right upper canines, 1 right upper second premolar, 3 right upper third molars, 2 left upper central incisors, 1 left upper canine, 3 left upper third molars, and 14 mesiodentes) and 50 mandibular teeth (including 1 right lower first premolar, 1 right lower second premolar, 23 right lower third molars, 4 left lower second premolars, 1 left lower first molar, 1 left lower second molar, 18 left lower third molars, and 1 supernumerary left lower second premolar). The mean greatest dimension of the DC measured from the panoramic radiographs was 2.7 ± 1.0 (range 0.9–5.6) cm. All the 80 DCs were primary DCs and no recurrent DC was included. This study has been reviewed and approved by the Institutional Review Board of NTUH.
Immunohistochemical staining for Langerhans cells
The immunohistochemistry for identification of LCs in various lesional tissues has been described previously.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 In brief, all the specimens for immunostaining were fixed in 10% neutral formalin, embedded in paraffin, and cut in serial sections of 4 μm. Immunohistochemical staining was performed using a super-sensitive polymer- horseradish peroxidase (HRP) technique. Tissues sections were first deparaffinized and rehydrated. Then, sections were heated in a plastic slide holder (Dako, Copenhagen, Denmark) containing 0.01 M citrate buffer in a microwave oven for 15 min to retrieve antigenicity, and then treated with 3% H2O2 in methanol for 10 min to quench endogenous peroxidase activity. After washing in 10 mM Tris-buffered saline (TBS), pH 7.6, sections were incubated with 10% normal goat serum (BioGenex, San Ramon, CA, USA) to block nonspecific binding. Sections were then incubated overnight at 4 °C with mouse anti-S100 protein monoclonal antibody (Ab-1, Thermo Fisher Scientific, Runcorn, UK) at a dilution of 1: 100. The BioGenex Super Sensitive TM detection systems® were used for detection of bound antibodies. After washing in TBS, sections were treated with super enhancer reagent for 10 min, and subsequently reacted with the polymer-HRP reagent for another 10 min. The 0.02% diaminobenzidine hydrochloride (DAB, Zymed Laboratories, San Francisco, CA, USA) containing 0.03% H2O2 was used as chromogen to visualize the peroxidase activity. The preparations were lightly counterstained with hematoxylin, mounted with Permount, and examined by light microscopy. Human Langerhans cell histiocytosis gingival tissue sections that were previously shown to contain S100-positive LCs were used as positive controls. TBS instead of primary antibody was used as negative controls.
The LC in the lining epithelia and subepithelial fibrous connective tissues was counted positive when its cell body and at least one associated dendritic process showed S100-positive brown staining. The sections were initially scanned at the low power. For sections that demonstrated heterogeneous patterns of positive stain, the predominant pattern was taken into account for scoring. At least three high-power (200×) fields of the epithelium or of the subepithelial connective tissue were chosen randomly, and the LC number in each high-power field was counted. The LC count for each DC site with mild, moderate or severe inflammation was the mean of three or more LC counts per high-power field. The lining epithelial thickness was measured from the surface of the stratified squamous lining epithelium to the basement membrane. The lining epithelial thickness for each DC site with mild, moderate or severe inflammation was also the mean of three or more epithelial thickness measurements. The inflammatory cell infiltrate in the focal fibrous cystic wall was arbitrarily classified into three grades (mild, moderate and severe) according to the number of chronic inflammatory cells in the subepithelial connective tissue wall of the DC. In general, chronic inflammatory cell infiltrates extended to the superficial, middle, and deep layers of the subepithelial fibrous connective tissue for those sites classified into mild, moderate, and severe inflammation DC sites. Each of these assessments was independently carried out by two investigators. The LC counts with an interobserver variation of more than 10% and the disagreement in the grade of inflammation in the focal fibrous cystic wall of DC were reassessed using a double-headed light microscope to achieve consensus. In this study, the interobserver reproducibility was approximately 95%.
Statistical analyses
The mean S100-positive LC counts in the lining epithelia or in the subepithelial connective tissues of DC sites with mild, moderate or severe inflammation were compared first among 3 groups by analysis of variance (ANOVA) and then between any 2 groups by Student's t-test. The correlation between S100-positive LC counts in the lining epithelia or in the subepithelial connective tissues of DC sites with mild, moderate or severe inflammation and clinicopathological parameters of DC patients was analyzed by Student's t-test. A P-value of less than 0.05 was considered statistically significant.
Results
LCs in DC samples
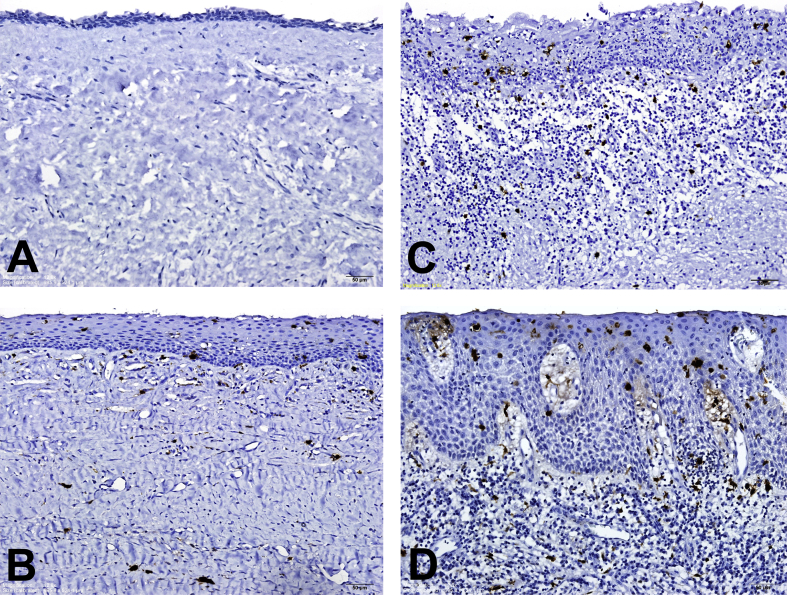
Representative anti-S100-immunostained microphotographs of LCs in the lining epithelia and in the subepithelial connective tissues of DC specimens are shown in Fig. 1. When there was no inflammation in the fibrous cystic wall of DC, dendritic LCs were not found or detected only occasionally in the lining epithelia or in the subepithelial connective tissues of DC specimens (Fig. 1A). When there was a mild, moderate or severe inflammatory cell infiltrate in the focal fibrous cystic wall of DC, dendritic LCs could be found mainly in the suprabasal and spinous cell layers of the lining epithelia and in the subepithelial connective tissues of DC specimens (Fig. 1B–D). Moreover, the number of S100-positive LCs in the lining epithelia or in the subepithelial connective tissues of DC specimens increased gradually from DC sites with mild inflammation, to those with moderate inflammation, and further to those with severe inflammation (Fig. 1B–D). Mucous cells could be discovered in the lining epithelia of 5% of 80 DC specimens.
Figure 1.
Anti-S100-immunostained microphotographs of Langerhans cells (LCs) in dentigerous cyst (DC) specimens. (A) A DC without inflammation showing none of LCs in both the thin lining epithelium and subepithelial connective tissue. (B) A DC with mild inflammation exhibiting few LCs in both the lining epithelium and the mildly-inflamed subepithelial connective tissue. (C) A DC with moderate inflammation demonstrating a few LCs in both the lining epithelium and the moderately-inflamed subepithelial connective tissue. (D) A DC with severe inflammation showing a relatively large number of LCs in both the thick lining epithelium and the severely-inflamed subepithelial connective tissue. (Original magnification; A, B, C and D: 12.6×).
LC counts in the lining epithelia and in the subepithelial connective tissues of DC specimens
Because the number of S100-positive LCs in the lining epithelia and in the subepithelial connective tissues of DC specimens varied according to the grade of inflammation in focal fibrous cystic wall sites, we observed the whole section of each DC specimen to identify all focal fibrous cystic wall sites with different grades of inflammation. In the 80 DC specimens, 80 sites without inflammation, 33 sites with mild/moderate inflammation, and 9 sites with severe inflammation could be identified. To avoid the bias, focal fibrous cystic wall sites with mild inflammation and those with moderate inflammation were integrated into one group of DCs with mild/moderate inflammation. We found that the mean LC count in either the lining epithelium or in the subepithelial connective tissue was significantly higher in the severe inflammation or mild/moderate inflammation group than in no inflammation group (both P-values < 0.001), and significantly higher in the severe inflammation group than in the mild/moderate inflammation group (P < 0.001) (Table 1). In addition, for the severe inflammation and mild/moderate inflammation groups, the mean LC counts in the lining epithelium were all greater than those in the subepithelial connective tissue (both P-values < 0.001) (Table 1).
Table 1.
Mean anti-S100-stained Langerhans cell (LC) number in the lining epithelium and subepithelial connective tissue of 80 dentigerous cysts.
| Inflammation in focal fibrous cystic wall site | Mean LC count ± SD (cells/high-power field) |
||
|---|---|---|---|
| Lining epithelium | Subepithelial connective tissue | P-value | |
| Severe inflammation (n = 9) | 21.0 ± 7.0 | 11.1 ± 6.5 | 0.007 |
| aP-value | <0.001 | <0.001 | |
| bP-value | <0.001 | <0.001 | |
| Mild/moderate inflammation (n = 33) | 8.1 ± 2.0 | 4.5 ± 2.3 | <0.001 |
| aP-value | <0.001 | <0.001 | |
| No inflammation (n = 80) | 0.6 ± 0.6 | 0.7 ± 0.6 | 0.879 |
Comparison of mean LC number between severe or mild/moderate inflammation and no inflammation groups by Student's t-test.
Comparison of mean LC number between severe inflammation and mild/moderate groups by Student's t-test.
To find out whether there were differences in the LC counts in the lining epithelia and subepithelial connective tissues of DCs stained with either anti-S100 or anti-CD1a antibody, we compared the mean S100-positive LC counts with the previously-published mean CD1a-positive LC counts in each of three different groups of DC with severe, mild/moderate, and no inflammation in the focal fibrous cystic walls.22 In the mild/moderate inflammation group, the mean S100-positive LC counts in the lining epithelium (8.1 ± 2.0 cells/HPF) and in the subepithelial connective tissue (4.5 ± 2.3 cells/HPF) were significantly higher than the mean CD1a-positive LC counts in the lining epithelium (6.8 ± 1.8 cells/HPF, P = 0.010) and in the subepithelial connective tissue (2.4 ± 2.0 cells/HPF, P < 0.001), respectively (Table 2). In addition, in the no inflammation group, the mean S100-positive LC count in the subepithelial connective tissue (0.7 ± 0.6 cell/HPF) was significantly higher than the mean CD1a-positive LC count in the subepithelial connective tissue (0.2 ± 0.3 cell/HPF, P < 0.001) (Table 2).
Table 2.
Comparisons of mean anti-S100-stained Langerhans cell (LC) count with mean anti-CD1a-stained LC count in the lining epithelium or in the subepithelial connective tissue of 80 dentigerous cysts.
| Inflammation in focal fibrous cystic wall site | Mean LC count ± SD (cells/high-power field) |
|
|---|---|---|
| Lining epithelium | Subepithelial connective tissue | |
| Severe inflammation (n = 9) | ||
| Anti-CD1a stain | 18.9 ± 7.0 | 6.7 ± 5.8 |
| Anti-S100 stain | 21.0 ± 7.0 | 11.1 ± 6.5 |
| aP-value | 0.540 | 0.149 |
| Mild/moderate inflammation (n = 33) | ||
| Anti-CD1a stain | 6.8 ± 1.8 | 2.4 ± 2.0 |
| Anti-S100 stain | 8.1 ± 2.0 | 4.5 ± 2.3 |
| aP-value | 0.010 | <0.001 |
| No inflammation (n = 80) | ||
| Anti-CD1a stain | 0.5 ± 0.5 | 0.2 ± 0.3 |
| Anti-S100 stain | 0.6 ± 0.6 | 0.7 ± 0.6 |
| aP-value | 0.130 | <0.001 |
Comparison of mean LC counts in sections stained with either anti-CD1a antibody or anti-S100 antibody between two corresponding groups by Student's t-test.
The mean thickness of epithelium overlying the focal fibrous cystic wall with severe, mild/moderate or no inflammation was 426 ± 281 μm, 137 ± 78 μm, or 31 ± 19 μm, respectively. There was a significant difference in the mean lining epithelial thickness among severe, mild/moderate, and no inflammation groups (P < 0.001). In addition, the mean lining epithelial thickness was significantly greater in the severe inflammation group than in the mild/moderate inflammation or in no inflammation group (both P-values < 0.001), and was significantly greater in the mild/moderate inflammation group than in no inflammation group (P < 0.001).
When the 122 data of LC counts were divided into two groups according to the thickness of lining epithelium, the mean S100-positive LC count in the lining epithelia or in the subepithelial connective tissues of DCs was significantly higher in the thicker lining epithelium (>50 μm) group than in the thinner lining epithelium (≦50 μm) group (both P-values < 0.001) (Table 3). Moreover, in the thinner lining epithelium (≦50 μm) group, the mean LC count in the lining epithelium was significantly higher than that in the subepithelial connective tissue (P < 0.001) (Table 3).
Table 3.
Correlations between the anti-S100-stained Langerhans cell (LC) counts in the lining epithelia or in the subepithelial connective tissues of 80 dentigerous cysts and lining epithelial thickness.
| Thickness of lining epithelium | Mean LC count ± SD (cells/high-power field) |
||
|---|---|---|---|
| Lining epithelium | Subepithelial connective tissue | aP-value | |
| ≤50 μm (n = 68) | 0.6 ± 0.6 | 0.6 ± 0.6 | 0.886 |
| >50 μm (n = 54) | 8.6 ± 7.1 | 4.8 ± 4.5 | 0.001 |
| aP-value | <0.001 | <0.001 | |
Comparison of mean LC counts between two different groups by Student's t-test.
Correlation between LC counts in DC samples and clinical parameters of DC patients
Correlations between the anti-S100-stained LC counts in the lining epithelia or in the subepithelial connective tissues of 80 DC samples and clinical parameters of 80 DC patients according to the grade of inflammation (severe, mild/moderate, and none) in the focal fibrous cystic wall are shown in Table 4. The 122 data of LC counts in the lining epithelia or in the subepithelial connective tissues of DC samples were divided into three groups: severe, mild/moderate, and no inflammation groups. At the no inflammation DC sites, the mean LC count in the subepithelial connective tissue was greater in patients with the age >30 years than in patients with the age ≤30 years (P = 0.009). Moveover, at the mild/moderate inflammation DC sites, the mean LC count in the lining epithelium was higher in the DCs located in the posterior regions of the jaw bones than in those located in the anterior regions of the jaw bones (P = 0.044). However, the LC counts in the lining epithelia or in the subepithelial connective tissues of DC samples had no association with the gender of DC patients as well as the associated tooth and size of DCs.
Table 4.
Correlations between the anti-S100-stained Langerhans cell (LC) counts in the lining epithelia or in the subepithelial connective tissues of 80 dentigerous cysts (DC) samples and clinical parameters of 80 DC patients according to the grade of inflammation (severe, mild/moderate, and none) in the focal fibrous cystic wall.
| Parameter | Mean LC count ± SD (cells/high-power field) |
|||||
|---|---|---|---|---|---|---|
| Lining epithelium |
Subepithelial connective tissue |
|||||
| Severe | Mild/moderate | None | Severe | Mild/moderate | None | |
| Gender | ||||||
| Male (n = 57) | 23.0 ± 7.6 (n = 6) | 8.2 ± 2.0 (n = 26) | 0.6 ± 0.5 (n = 57) | 12.8 ± 7.2 (n = 6) | 4.6 ± 2.4 (n = 26) | 0.7 ± 0.6 (n = 57) |
| Female (n = 23) | 16.9 ± 3.9 (n = 3) | 7.7 ± 2.3 (n = 7) | 0.7 ± 0.8 (n = 23) | 7.7 ± 3.5 (n = 3) | 4.0 ± 2.3 (n = 7) | 0.5 ± 0.7 (n = 23) |
| aP-value | 0.238 | 0.608 | 0.611 | 0.301 | 0.595 | 0.265 |
| Age (yr) | ||||||
| ≤30 (n = 37) | 23.9 ± 7.8 (n = 4) | 8.7 ± 2.6 (n = 13) | 0.6 ± 0.6 (n = 37) | 10.7 ± 7.1 (n = 4) | 3.6 ± 2.4 (n = 13) | 0.5 ± 0.5 (n = 37) |
| >30 (n = 43) | 18.7 ± 6.2 (n = 5) | 7.7 ± 1.6 (n = 20) | 0.7 ± 0.7 (n = 43) | 11.4 ± 6.8 (n = 5) | 5.0 ± 2.2 (n = 20) | 0.8 ± 0.7 (n = 43) |
| aP-value | 0.303 | 0.163 | 0.751 | 0.869 | 0.079 | 0.009 |
| Location | ||||||
| Maxilla (n = 30) | 20.6 ± 5.4 (n = 3) | 7.0 ± 1.2 (n = 8) | 0.7 ± 0.8 (n = 30) | 11.2 ± 8.6 (n = 3) | 4.2 ± 2.5 (n = 8) | 0.6 ± 0.6 (n = 30) |
| Mandible (n = 50) | 21.2 ± 8.2 (n = 6) | 8.4 ± 2.1 (n = 25) | 0.6 ± 0.5 (n = 50) | 11.0 ± 6.1 (n = 6) | 4.6 ± 2.3 (n = 25) | 0.7 ± 0.7 (n = 50) |
| aP-value | 0.918 | 0.080 | 0.371 | 0.974 | 0.679 | 0.704 |
| Anterior (n = 23) | 21.1 ± 7.5 (n = 2) | 6.6 ± 1.1 (n = 6) | 0.8 ± 0.8 (n = 23) | 6.5 ± 3.8 (n = 2) | 3.6 ± 2.5 (n = 6) | 0.6 ± 0.6 (n = 23) |
| Posterior (n = 57) | 20.9 ± 7.5 (n = 7) | 8.4 ± 2.1 (n = 27) | 0.6 ± 0.5 (n = 57) | 12.4 ± 6.7 (n = 7) | 4.6 ± 2.3 (n = 27) | 0.7 ± 0.7 (n = 57) |
| aP-value | 0.980 | 0.044 | 0.086 | 0.284 | 0.330 | 0.338 |
| Associated tooth | ||||||
| Supernumerary teeth/mesiodentes (n = 15) | No cases | 6.4 ± 1.1 (n = 4) | 0.7 ± 0.8 (n = 15) | No cases | 4.0 ± 2.7 (n = 4) | 0.5 ± 0.5 (n = 15) |
| Maxillary anterior teeth (n = 9) | 21.1 ± 7.5 (n = 2) | 7.0 ± 1.0 (n = 3) | 1.1 ± 0.8 (n = 9) | 6.5 ± 3.8 (n = 2) | 2.3 ± 1.9 (n = 3) | 0.6 ± 0.7 (n = 9) |
| Maxillary posterior teeth (n = 7) | 19.6 ± 0.0 (n = 1) | 8.2 ± 0.8 (n = 2) | 0.4 ± 0.4 (n = 7) | 20.6 ± 0.0 (n = 1) | 5.8 ± 2.0 (n = 2) | 0.9 ± 0.7 (n = 7) |
| Mandibular posterior teeth (n = 49) | 21.2 ± 8.2 (n = 6) | 8.5 ± 2.2 (n = 24) | 0.6 ± 0.5 (n = 49) | 11.0 ± 6.1 (n = 6) | 4.7 ± 2.3 (n = 24) | 0.7 ± 0.7 (n = 49) |
| aP-value | 0.937 | 0.213 | 0.239 | 0.429 | 0.342 | 0.531 |
| Size (cm) | ||||||
| ≤2.5 cm (n = 35) | 17.1 ± 4.2 (n = 3) | 7.8 ± 2.4 (n = 9) | 0.5 ± 0.7 (n = 35) | 12.3 ± 9.4 (n = 3) | 4.3 ± 2.6 (n = 9) | 0.5 ± 0.6 (n = 35) |
| >2.5 cm (n = 45) | 22.9 ± 7.6 (n = 6) | 8.2 ± 1.9 (n = 24) | 0.7 ± 0.6 (n = 45) | 10.5 ± 5.5 (n = 6) | 4.5 ± 2.3 (n = 24) | 0.8 ± 0.6 (n = 45) |
| aP-value | 0.264 | 0.593 | 0.144 | 0.726 | 0.807 | 0.107 |
Comparison of mean LC counts between two different groups by Student's t-test.
Discussion
In this study, the LC was scarcely found in both the lining epithelium (mean, 0.6 ± 0.6 cell/high-power field) and subepithelial connective tissue (mean, 0.7 ± 0.6 cell/high-power field) of the DC site without inflammation. The paucity of LCs in the lining epithelia of DCs without inflammation indicates the reduced immunosurveillance ability against the lining epithelial cells in DC patients. The DC is a developmental odontogenic cyst that usually attaches to or surrounds the associated impacted tooth. When the DC and the associated impacted tooth are both intrabony and are not exposed to the oral cavity, there is usually no inflammatory cell infiltrate in the fibrous cystic wall of DC.1 In this study, the 80 DCs ranged from 0.9 cm to 5.6 cm with a mean of 2.7 ± 1.0 cm in greatest dimension measured from the panoramic radiographs. This finding indicates that some DCs may progress to a relatively large size. The pathogenesis of the DC has been reported to be due to accumulation of fluid between the reduced enamel epithelium and the tooth crown and the relatively high osmotic pressure of the cystic fluid contributes to the enlargement of the DC.23 We further suggest that the decreased immunosurveillance ability against the lining epithelial cells in DC patients may favor the growth of the lining epithelium and in turn leads to the progression of the DC to a greater size.
This study also demonstrated that the mean LC counts in the lining epithelia or in the subepithelial connective tissues increased significantly from no inflammation through mild/moderate inflammation to severe inflammation DC sites. Furthermore, DC sites with severe inflammation had the greatest mean thickness of lining epithelium, followed by the DC sites with mild/moderate inflammation and the DC sites without inflammation. In addition, the mean LC count in the lining epithelia or in the subepithelial connective tissues of DCs was significantly higher in the thicker lining epithelium (>50 μm) group than in the thinner lining epithelium (≦50 μm) group. When the part of the DC or its associated impacted tooth is exposed to the oral cavity or when the DC is involved by a periapical or a periodontal lesion of an adjacent tooth, it may be infected by oral bacteria resulting in an acute and chronic inflammatory cell infiltrate in the focal fibrous cystic wall of the DC. Thus, the bone marrow-derived LCs may migrate out from the blood vessels along with the acute and chronic inflammatory cells into the inflamed fibrous cystic wall. This can firstly explain why we can see LCs in the subepithelial connective tissues with inflammation and why more LCs can be discovered in the DC sites with severer inflammation. Both the acute and chronic inflammatory cells can secrete growth factors that stimulate the proliferation of lining epithelial cells, and this in turn results in the thickening of the lining epithelium at the DC site with inflammation. During the rapid epithelial cell proliferation process, some of the lining epithelial cells may undergo apoptosis and release epithelial antigens into the lining epithelial layer or degraded proteins into the cystic fluid. Because LCs have epithelial tropism, they may be attracted into the lining epithelia to phagocytose the released apoptotic antigens. These epithelial antigen-bearing LCs may migrate out from the lining epithelia into the subepithelial connective tissues and finally reach to the regional lymph nodes where they process the antigenic proteins into antigenic peptides and subsequently present the antigenic peptides to T cells in the paracortical area of the lymph node.2 The epithelial tropism and the migration process of LCs can further explain why we can see LCs among lining epithelial cells and in the subepithelial connective tissues of the DCs with inflammation. Furthermore, in DC sites of mild/moderate or severe inflammation, a significantly greater number of LCs were found in the lining epithelia than in the subepithelial connective tissues of the DCs. This finding can further provide the evidence that the LCs have an epithelial tropism. In addition, when the degraded epithelial protein is dissolved into the cystic fluid, the osmotic pressure may elevate, and this in turn absorbs more tissue fluid into the cystic cavity, finally leading to the further enlargement of the DC.23
Previous studies have demonstrated the presence of LCs in DC samples. Piattelli et al.4 showed the CD1a-positive LCs in 4 (22%) of 18 DCs. Murase et al.5 found the S100-positive LCs in 3 (11%) of 28 DCs. The LC-positive DCs are usually accompanied with a high degree of inflammatory cell infiltration in their lesions. On the contrary, the LC-negative DCs generally lack inflammatory responses. Wu et al.6 studied the LCs in a DC by anti-S100 immunostaining. Few LCs are found in the lining epithelium above the uninflamed fibrous connective tissue of the DC, but an elevated number of LCs are demonstrated in both the lining epithelium and inflamed subepithelial fibrous connective tissue of the DC. The results of the aforementioned last two studies showed the intimate association of inflammation with the high number of LCs in DCs; these findings were consistent with the results of the present study.
The LCs can be detected by either the anti-CD1a or anti-S100 immunostaining. This study found that the anti-S100 immunostaining can identify significantly higher mean LC counts in both the lining epithelia and subepithelial connective tissues at the DC sites with mild/moderate inflammation as well as in the subepithelial connective tissues at the DC sites without inflammation than the anti-CD1a immunostaining (Table 2).22 The CD1a is a relatively specific marker for the LC but the sensitivity of anti-CD1a antibody is slightly lower than that of anti-S100 antibody. This can initially explain why the anti-S100 immunostaining can detect a little bit more LCs in DCs than the anti-CD1a immunostaining. Moreover, S100 protein is not only a marker of LC but also markers of melanocytes, Schwann cells, nerve fibers, and myoepithelial cells.24 Because the melanocytes in the lining epithelia and Schwann cells in the subepithelial connective tissues of DCs can also be immunostained by anti-S100 antibodies, this can further explain why the anti-S100 immunostaining can identify a little bit more LCs in DCs than the anti-CD1a immunostaining. However, using the anti-S100 immunostaining, the LCs are dendritic cells, the nerve fibers are long fibers, and the Schwann cells are spindle cells without dendritic processes. Therefore, the nerve fibers and Schwann cells are easy to differentiate from the LCs by the morphology. Furthermore, the melanocytes are located in the basal cell layer only and the LCs are found mainly in the suprabasal and spinous cell layers of the lining epithelium. Thus, although both LCs and melanocytes are dendritic cells, they can be identified by the location of the cells in the lining epithelium of the DC.
This study showed a significant and gradual increase in the LC count from DC sites without inflammation to those with mild/moderate inflammation and further to those with severe inflammation. A significantly higher mean LC count was found in the thicker lining epithelium group than in the thinner lining epithelium group of the DC. These findings suggest the significant association of the high-grade inflammation and thick lining epithelium with the elevated number of LCs in DCs. The presence of very few LCs in the lining epithelia of DCs without inflammation indicates the reduced immunosurveillance ability against the DC lining epithelial cells in DC patients. However, further studies are needed to confirm the role of the reduced immunosurveillance ability against the lining epithelial cells in the enlargement and growth of the DC.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
References
- 1.Neville B.W., Damm D.D., Allen C.M., Chi A.C. Odontogenic cysts and tumors. In: Neville B.W., Damm D.D., Allen C.M., Chi A.C., editors. Oral and Maxillofacial Pathology. 4th ed. Elsevier; St. Louis: 2016. pp. 632–635. [Google Scholar]
- 2.Barrett A.W., Cruchley A.T., Williams D.M. Oral mucosal Langerhans' cells. Crit Rev Oral Biol Med. 1996;7:36–58. doi: 10.1177/10454411960070010301. [DOI] [PubMed] [Google Scholar]
- 3.Katz S.I., Tamaki K., Sachs D.H. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979;282:324–326. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- 4.Piattelli A., Rubini C., Iezzi G., Fioroni M. CD1a-positive cells in odontogenic cysts. J Endod. 2002;28:267–268. doi: 10.1097/00004770-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Murase N., Tatemoto Y., Iwai Y., Okada Y., Mori M. Langerhans cells in odontogenic tumours and cysts as detected by S-100 protein immunohistochemistry. Basic Appl Histochem. 1990;34:135–141. [PubMed] [Google Scholar]
- 6.Wu Y.C., Wang Y.P., Chang J.Y.F., Chiang C.P. Langerhans cells in lining epithelia of odontogenic cysts. J Formos Med Assoc. 2013;112:725–727. doi: 10.1016/j.jfma.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Cheng S.J., Wang Y.P., Chen H.M., Chiang C.P. Central granular cell odontogenic tumor of the mandible. J Formos Med Assoc. 2013;112:583–585. doi: 10.1016/j.jfma.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y.C., Wang Y.P., Chang J.Y.F., Chiang C.P. Langerhans cells in lining epithelia of epidermoid cysts. J Dent Sci. 2013;8:448–450. [Google Scholar]
- 9.Wu Y.C., Wang Y.P., Chang J.Y.F., Chen H.M., Sun A., Chiang C.P. Langerhans cells in odontogenic epithelia of odontogenic fibromas. J Formos Med Assoc. 2013;112:756–760. doi: 10.1016/j.jfma.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Chiang C.T., Hu K.Y., Tsai C.C. Central granular cell odontogenic tumor: the first reported case in oriental people and literature review. J Formos Med Assoc. 2014;113:321–325. doi: 10.1016/j.jfma.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.J., Wei L.Y., Wu Y.C., Chiang C.P. An early central granular cell odontogenic tumor arising from the dental follicle of an impacted mandibular third molar. J Formos Med Assoc. 2014;113:766–768. doi: 10.1016/j.jfma.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y.C., Chang J.Y.F., Wang Y.P., Chiang C.P. Langerhans cells in keratoacanthoma. J Formos Med Assoc. 2015;114:475–476. doi: 10.1016/j.jfma.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Tseng C.H., Wang Y.P., Lee J.J., Chang J.Y.F. Noncalcifying variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Formos Med Assoc. 2015;114:781–782. doi: 10.1016/j.jfma.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y.C., Wang Y.P., Liu Y.C., Chen H.M. Langerhans cells in lining epithelium of unicystic ameloblastoma. J Dent Sci. 2015;10:464–466. [Google Scholar]
- 15.Wu Y.H., Chang J.Y.F., Chang H.H., Chiang C.P. Langerhans cells in dermoid cyst lining epithelium. J Formos Med Assoc. 2016;115:57–58. doi: 10.1016/j.jfma.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Lin H.P., Kuo Y.S., Wu Y.C., Wang Y.P., Chang J.Y.F., Chiang C.P. Non-calcifying and Langerhans cell-rich variant of calcifying epithelial odontogenic tumor. J Dent Sci. 2016;11:117–122. doi: 10.1016/j.jds.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y.P., Chen I.C., Wu Y.H., Wu Y.C., Chen H.M., Chang J.Y.F. Langerhans cell counts in oral epithelial dysplasia and their correlations to clinicopathological parameters. J Formos Med Assoc. 2017;116:457–463. doi: 10.1016/j.jfma.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y.H., Chang J.Y.F., Wang Y.P., Chiang C.P. Langerhans cells in granular cell ameloblastoma. J Formos Med Assoc. 2017;116:642–644. doi: 10.1016/j.jfma.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y.H., Chang J.Y.F., Wang Y.P., Chiang C.P. Langerhans cells in plexiform ameloblastoma. J Dent Sci. 2017;12:195–197. doi: 10.1016/j.jds.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CH, Wu YC, Wu YH, Sun A, Cheng SJ, Chen HM. Significant association of inflammation grade with the number of Langerhans cells in odontogenic keratocysts. J Formos Med Assoc [in press]. [DOI] [PubMed]
- 21.Chang C.H., Wu Y.C., Wu Y.H., Sun A., Chen H.M., Lin H.P. Langerhans cells in 60 odontogenic keratocysts. J Dent Sci. 2017;12:283–290. doi: 10.1016/j.jds.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CH, Wu YC, Wu YH, Sun A, Cheng SJ, Chen HM. Significant association of high-grade inflammation and thick lining epithelium with the increased number of Langerhans cells in dentigerous cysts. J Formos Med Assoc [in press]. [DOI] [PubMed]
- 23.Browne R.M. The pathogenesis of odontogenic cysts: a review. J Oral Pathol Med. 1975;4:31–46. doi: 10.1111/j.1600-0714.1975.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 24.Kahn H.J., Marks A., Thom H., Baumal R. Role of antibody to S100 protein in diagnostic pathology. Am J Clin Pathol. 1983;79:341–347. doi: 10.1093/ajcp/79.3.341. [DOI] [PubMed] [Google Scholar]