Abstract
Cleidocranial dysplasia (CCD) is an autosomal-dominant malformation syndrome affecting bones and teeth. The most common skeletal and dental abnormalities in affected individuals are hypoplastic/aplastic clavicles, open fontanelles, short stature, retention of primary teeth, delayed eruption of permanent teeth, supernumerary teeth, and multiple impacted teeth. Treatment of CCD requires a multidisciplinary approach that may include dental corrections, orthognathic surgery and cranioplasty along with management of any complications of CCD. Early diagnosis of this condition enables application of the treatment strategy that provides the best quality of life to such patients. Notably, Runx2 gene mutations have been identified in CCD patients. Therefore, further elucidation of the molecular mechanism of supernumerary teeth formation related to Runx2 mutations may improve understanding of dental development in CCD. The insights into CCD pathogenesis may assist in the development of new treatments for CCD.
Keywords: cleidocranial dysplasia, mutation, Runx2, supernumerary teeth
Introduction
The term cleidocranial dysplasia (CCD; OMIM 119600) is derived from the ancient Greek words cleido (collar bone), kranion (head), and dysplasia (abnormal formation). This rare hereditary skeletal disorder, which is also known as Scheuthauer-Marie-Sainton syndrome or cleidocranial dysostosis, is characterized by abnormal skeletal and dental development. The prevalence of CCD is an estimated one per million and does not differ by race or by gender.1 In most cases, the disorder is an inherited autosomal dominant trait. In 20–40% of reported cases, however, the disorder occurs sporadically.1 This syndrome is characterized by hypoplastic and/or aplastic clavicles, patent sutures and fontanelles, wormian bones, wide pubic symphysis, supernumerary teeth, short stature, and various other skeletal changes. Although clavicular defects have been reported in the literature as early as 1765,2 Scheuthauer3 in 1871 was apparently the first to describe the syndrome accurately. Marie and Sainton4 in 1898 coined the term “dysostose cléidocrânienne héréditaire” for this condition.
The term “cleidocranial dysostosis” was originally used because CCD was thought to involve only bones of intramembranous origin, i.e., bones of the skull, clavicles and flat bones. Subsequent studies showed that bones of endochondral ossification are also affected and that CCD is a generalized disorder of many skeletal structures. Therefore the term “cleidocranial dysostosis” was changed to “cleidocranial dysplasia” to reflect the more generalized nature of the condition.5, 6
Clinical features
The clinical appearance of CCD is so distinct that it is pathognomonic. The main clinical features of CCD are recognized during early childhood and include a short stature, delayed closure of fontanelles, prominent forehead, and abnormal dental development. The head of a CCD patient usually shows frontal and parietal bossing and a groove along the metopic suture. The neck appears to be abnormally long, and the shoulders are narrow with marked drooping. Clavicular abnormalities with associated muscle defects allow excessive mobility of the shoulder girdle. For example, many CCD patients can approximate their shoulders in front of the chest for variable levels. The clinical spectrum is extremely variable even within families and ranges from mild cases with only dental abnormalities to severe cases with pronounced skeletal deformities.7, 8
Radiographic features
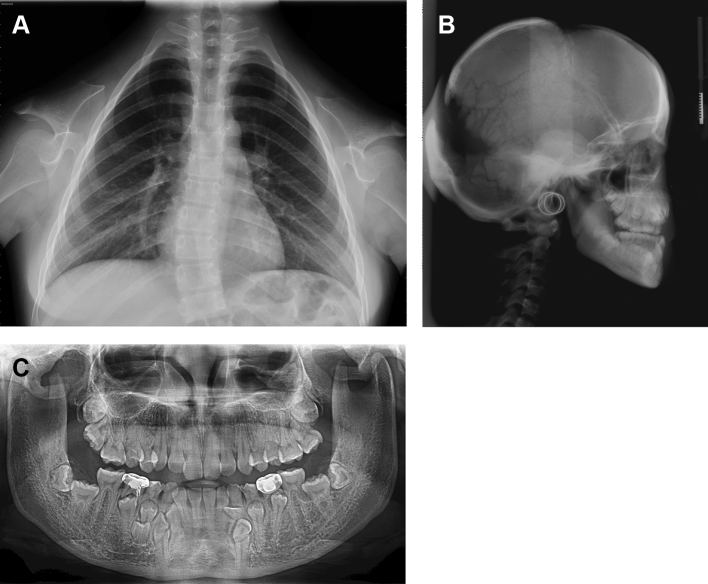
The distinctive radiological features of CCD are shortened or absent clavicles, delayed ossification of the skull bones, and delayed ossification of pelvic bones.1 The chest radiographs for CCD patients in Fig. 1A show that the clavicles may be completely absent (aplasia) or smaller than normal (hypoplasia). The clavicles are typically hypoplastic or discontinuous, either unilaterally of bilaterally; the clavicles are completely absent in 10% of cases. Hypoplastic clavicles include hypoplasia of the acromial end or absence of the sternal end with the acromial end present. The missing segment may cause fibrous pseudoarthrosis or may be replaced by a fibrous tether or cord.9
Figure 1.
A. Chest radiograph of a 15-year-old female showing aplasia of clavicles on both sides, scoliosis of the thoracic spines, a tapered thorax with oblique ribs, and incomplete closure of neural arches of the cervical vertebrae. Figure 1B Lateral cephalograph showing frontal, parietal and occipital bossing; patency of the anterior fontanelle; and persistently open skull sutures and multiple wormian bones in the coronal and lambdoid suture regions. Dense alveolar crestal bone is visible in the anterior mandible. Figure 1C Panoramic radiograph of a 17-year-old female showing multiple impacted supernumerary and permanent teeth, retained deciduous teeth, hypoplastic maxillary sinus, severe downward tilt and discontinuity of the right zygomatic arch, discontinuity of the left arch, narrow ascending ramus with nearly parallel anterior and posterior borders, and an abnormally slender and pointed coronoid process with an abnormally distal curvature. The trabecular patterns of the maxilla and mandible were very coarse. Dense alveolar crestal bone is visible in the anterior mandible.
Craniofacial morphological features
Fig. 1B shows that the skull in CCD is characterized by brachycephaly, delayed or failed closure of the fontanels, open skull sutures, and multiple wormian bones in the coronal and lambdoid suture regions. Defective fusion of frontal and parietal bones leading to open coronal and sagittal sutures are also visible. The nasal bones are missing or hypoplastic. Mandibular prognathism may be secondary to nasomaxillary deficiency. Dense alveolar crestal bone can be seen in the anterior mandible.
Other craniofacial morphological features of CCD include abnormally small or nonexistent maxillary sinuses, hypoplastic zygomatic bones, and patency of the mandibular symphysis.1, 10 The zygomatic arch may be thin or even discontinuous at the zygomaticotemporal suture. The zygomatic arch has a characteristic downward bend.1 The mandible is characterized by a narrow ascending ramus with nearly parallel anterior and posterior borders and by an abnormally slender and pointed coronoid process with an abnormally distal curvature.10 The trabecular pattern of the mandible is very coarse. Fig. 1C is a panoramic radiograph of these features.
Radiographic features associated with the teeth
Fig. 1C shows that CCD is characterized by prolonged retention and delayed shedding of the primary teeth and multiple unerupted permanent and supernumerary teeth.10 Dentigerous cysts occasionally arise in association with these unerupted teeth. Although development of the primary teeth is rarely affected, root resorption and exfoliation of the primary teeth may be delayed.
Cone-beam computed tomography (CBCT) imaging
Imaging by CBCT is now routinely used for three-dimensional dentition, which reduces guesswork and enables better anatomical localization of supernumerary and impacted teeth. Other pertinent information provided by CBCT include the precise location of a supernumerary tooth in relation to important structures such as the cortex of the nasal floor, labial cortex of the nasal ridge, nasopalatine duct, and the mandibular canal and adjacent root apices.11 Because CBCT clearly depicts the position and anatomy of impacted teeth, CBCT is useful for both diagnosis and treatment planning in CCD.
Histopathological features
Tooth formation and eruption occur in a series of complex and highly regulated process. The reasons for failure of permanent tooth eruption and retention of the primary teeth in CCD patients are poorly understood. Absence of cellular cementum at the root apex is presumably one factor in failed or delayed eruption of permanent teeth and retention of the primary teeth in CCD.12, 13 The lack of cellular cementum is presumed to increase the number of unerupted teeth in patients with CCD. However, recent reports of a lack of cellular cementum in normal teeth do not support this presumption.14, 15, 16
Studies of bone from the alveolus overlying unerupted teeth in CCD patients have a higher than normal density as well as reversal lines, which suggest an abnormal resorption pattern.17, 18 Possible explanations for delayed eruption of teeth included increased density and coarse trabecular pattern of the jaw bone, decreased resorption, and multiple reversal lines. A delayed eruption may also be attributable to various other factors such as mechanical obstruction of multiple supernumerary teeth. Therefore, the most likely causes of extreme delay or arrested eruption of permanent teeth in CCD are diminished bone resorption, delayed resorption of the roots of primary teeth, and, less commonly, multiple supernumerary teeth.19
One proposed explanation is that supernumerary tooth formation results from hyperactive dental lamina, i.e., over-proliferation or prolonged survival of dental lamina epithelial cells.20 Another hypothesis is that formation of supernumerary permanent teeth in CCD patients results from markedly delayed resorption or from dental lamina of permanent dentition that is normal but does not resolve completely at the expected time.19
Molecular genetics
The Runx2 gene is a master transcription factor of bone and plays a role in all stages of bone formation. Core binding factor (Cbf) plays crucial roles during skeletal development. Cbf consists of two subunits: Cbf alpha (Cbfα) and Cbf beta (Cbfβ). Runt-related transcription factor 2 (Runx2) has been shown to be critical for the differentiation of osteoblasts and skeletal development.21, 22 CCD results from a Runx2 gene mutation in the small arm of chromosome 6 at 6p21.1.23, 24 A heterozygous mutation in the Runx2 gene encodes runt-related transcription factor 2, also termed core-binding factor alpha1 (CBFA1). Researchers generally agree that the underlying mechanism of CCD pathogenesis is haploinsufficiency or loss of Runx2 function.8, 25 The Runx2 contains a DNA-binding domain (runt domain) which is necessary for transcriptional activation of target genes, a region of glutamine and alanine repeats in the N-terminal region (Q/A domain), and a region rich in proline-serine-threonine (PST). The Runx2 is a key transcription factor involved in osteoblastic differentiation and skeletal morphogenesis.26 Studies also suggest that Runx2 plays an important role in odontogenesis via participation in odontoblast differentiation, enamel organ formation, and dental lamina proliferation.27 Disruption of these functions might explain the distinct dental anomalies associated with this disorder. To date, over 90 Runx2 gene mutations in 500 independent cases of CCD have been reported in the literature, including deletions, insertions, translocations, missense, frameshift, and splice mutations.28 In most cases mutations occur in the runt domain.23, 29 Mutations in Runx2 have a high penetrance and extreme variability. The Runx2 mutation is currently the only known molecular etiology of CCD. Notably, individuals who have CCD and identical Runx2 gene mutations show a wide variation in the number of asymmetrical supernumerary teeth in the maxilla and the mandible, which implies that the number and position of supernumerary teeth are not governed solely by Runx2 mutations.
Runx2 mutations, which functions as a heterodimer with core binding factor β (Cbfβ), are found in most individuals with CCD.21, 22 Cbfβ forms a heterodimer with Runx family proteins and enhances their DNA-binding capacity. Multiple functions of Cbfβ are required for skeletal development and homeostasis in postnatal skeletogenesis. Cbfβ deficiency reduced the expression of several key factors that mediate osteoblast formation and/or function. Cbfβ is crucial for the later stages of chondrocyte differentiation as its deletion affects chondrocyte maturation and the formation of the growth plate. Although no Cbfβ mutation has yet been identified in classical CCD patients, genetic alterations in the Cbfβ gene may be responsible for CCD in those patients with no Runx2 mutation. Because Runx2 functions as a heterodimer with CBFβ, it has been suspected that Cbfβ may be responsible for some cases of CCD. In terms of the pathogenesis of CCD, Cbfβ deficiency may be equivalent to Runx2 haploinsufficiency as it relates to the function of the Runx2/Cbfβ complex in skeletogenesis.21
Fibroblast growth factor (FGF) signaling is one molecular mechanism of supernumerary teeth formation in CCD patients.30 Runx2 might indirectly inhibit FGF signaling by antagonizing Twist1 function. Twist1 is a basic helix-loop-helix-containing transcription factor that is expressed in the dental mesenchyme in early stages of tooth development. A relative abundance of unbound Twist1 caused by Runx2 haploinsufficiency may elevate FGF signaling, which then causes formation of supernumerary teeth in human CCD.30
Treatment
Managing the dental and orofacial manifestations of CCD is a challenging long-term process that requires careful planning and execution by an interdisciplinary team. The treatment strategies may differ according to the age of the patient. Surgical exposure of unerupted permanent teeth with orthodontic guided eruption is the preferred treatment for adolescent CCD patients. Generally, deciduous and supernumerary teeth should be removed to improve the possibility of orthodontic guided eruption.31, 32 Bone overlying permanent teeth should also be removed since histology studies show that alveolar bone in CCD has abnormal dense trabeculation with multiple reversal lines.17 Orthodontic treatment with mini-implant screws for traction of impacted teeth can reduce the treatment time for CCD patients.33 Leaving numerous deeply unerupted teeth in place is not an acceptable practice. The dentition associated with CCD is usually responsive to skillful orthodontic therapy and obviates the need for partial dentures. In adults with fully developed jaws, dental implants and fixed prostheses are the preferred therapeutic measures in adult CCD cases requiring multiple extractions of teeth.
Calvarial defects in the open anterior fontanelle, sagittal suture, and metopic suture have been successfully corrected by cranioplasty using bone cement.34 Midface deficiency can be corrected by orthognathic surgery after growth is complete.35, 36 In patients who meet the defined criteria, the above treatments can obtain substantial esthetic and functional benefits.
Discussion
CCD is a generalized skeletal dysplasia affecting bones of intramembranous and endochondral ossification. The phenotypic spectrum of the condition varies from mild cases presenting with only supernumerary teeth to cases with the phenotypic features that characterize CCD. Timely recognition of CCD and counseling for patients with hereditary risk factors are mandatory. Although CCD is associated with various skeletal abnormalities, CCD patients typically visit dental clinics only when they require treatment for dental and orofacial problems. Therefore, dentists have essential roles in identifying CCD and then planning and implementing a multidisciplinary therapeutic treatment aimed at improving quality of life in patients with this condition.
Different approaches to the treatment of the dentition in CCD have been proposed in the past. The method suggested by Becker et al.31, 32 may be viewed as the most promising. The proposed method is founded on several premises: (1) the need for early removal of all obstacles to the eruption of the unerupted permanent teeth and application of traction forces at the biologically appropriate time, (2) extraneous force needs to be provided to bring about an eruption of the teeth, along with an accompanying vertical alveolar development, and (3) concentrating initial efforts towards bringing anterior teeth into mouth early, for the patient's psychological well-being.
Extract the anterior deciduous teeth and all supernumerary teeth, and expose unerupted permanent incisor teeth. The timing of surgical exposure of unerupted teeth is governed by appropriate root development. Root development should be two-thirds their expected length and is suitable for its active eruption. The approximately 3-year discrepancy in development of the dentition in these cases of dental age 7–8 years generally dictates that the chronological age of the patient is usually around 10–12 years.31 Further development of the roots of the posterior successional teeth will have increased their length to around two-thirds of the expected final length and are suitable for their active eruption at dental age of 10–11 years and chronological age 13+ years.
Surgical and orthodontic difficulties and complications abound during the treatment of CCD and there is a risk for the failure of one or other of the many aspects of the treatment or the prognosis of the result. An inordinately long period involved in the completion of last orthodontic treatment stage.32 The displacement of the roots of several of the teeth is often extreme and many months of root torqueing and uprighting are needed to bring them into their proper positions. Long-term retention of the treatment result is advised.
FGF signaling is reportedly a molecular mechanism of supernumerary teeth formation in CCD patients.30 However, wide variation in the dental phenotype of CCD patients suggests that genetic modifiers and interacting partners await discovery.37 Twist1 is the functional antagonist of the Runx2. Excess of unbound Twist1 caused by Runx2 haploinsufficiency enhances FGF signaling, which then promotes formation of supernumerary teeth.30 Runx2 haploinsufficiency in humans affects permanent dentition but not primary dentition.19 It is difficult to establish direct genotype–phenotype correlation for Runx2 because of very variable phenotypic penetrance of the mutations.38 There is also a weak genotype–phenotype correlation in case of dental aspect of CCD phenotypes, especially with respect to teeth development.39 Further elucidation of molecular mechanisms of supernumerary teeth formation related to Runx2 mutations will improve insight into dental development. The insights into CCD pathogenesis may assist in development of novel therapies for CCD.
Conflict of interest
All authors have no conflicts of interest relevant to this article.
Acknowledgment
The authors thank Dr. Shih-Chieh Chen for his assistance in gathering the radiographs.
References
- 1.McNamara C.M., O'Riordan B.C., Blake M., Sandy J.R. Cleidocranial dysplasia: radiological appearances on dental panoramic radiography. Dentomaxillofac Radiol. 1999;28:89–97. doi: 10.1038/sj/dmfr/4600417. [DOI] [PubMed] [Google Scholar]
- 2.Martin S. Sur un déplacement natural de la clavicule. J Med Chir Pharmacol. 1765;23:456–460. [Google Scholar]
- 3.Scheuthauer G. Kombination rudimentärer Schüsselbeine mit Anomalien des, Schädels biem erwachsenen Menschen. Allg Wien Med Ztg. 1871;16:293–295. [Google Scholar]
- 4.Marie P., Sainton P. Sur la dysostose cléido-cranienne héréditaire. Rev Neurol. 1898;6:835–838. [PubMed] [Google Scholar]
- 5.McKusick V.A., Scott C.I. A nomenclature for constitutional disorders of bone. J Bone Jt Surg Am. 1971;53A:978–986. [PubMed] [Google Scholar]
- 6.Rimoin D.L. International nomenclature of constitutional diseases of bone. J Pediatr. 1978;93:614–616. doi: 10.1016/s0022-3476(78)80897-x. [DOI] [PubMed] [Google Scholar]
- 7.Chitayat D., Hodgkinson K.A., Azouz E.M. Intrafamilial variability in cleidocranial dysplasia: a three generation family. Am J Med Genet. 1992;42:298–303. doi: 10.1002/ajmg.1320420307. [DOI] [PubMed] [Google Scholar]
- 8.Quack I., Vonderstrass B., Stock M. Mutation analysis of core binding factor A1 in patients with cleidocranial dysplasia. Am J Hum Genet. 1999;65:1268–1278. doi: 10.1086/302622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mundlos S. Cleidocranial dysplasia: clinical and molecular genetics. J Med Genet. 1999;36:177–182. [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen B.L., Kreiborg S. Craniofacial abnormalities in 52 school-age and adult patients with cleidocranial dysplasia. J Craniofac Genet Dev Biol. 1993;13:98–108. [PubMed] [Google Scholar]
- 11.Dalessandri D., Laffranchi L., Tonni I. Advantages of cone beam computed tomography (CBCT) in the orthodontic treatment planning of cleidocranial dysplasia patients: a case report. Head Face Med. 2011;7:6. doi: 10.1186/1746-160X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rushton M.A. An anomaly of cementum in cleido-cranial dysostosis. Br Dent J. 1956;100:81–83. [Google Scholar]
- 13.Smith N.H., Sydney N.S. A histologic study of cementum in a case of cleidocranial dysostosis. Oral Surg. 1968;25:470–478. doi: 10.1016/0030-4220(68)90023-6. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto H., Sakae T., Davies J.E. Cleidocranial dysplasia: a light microscope, electron microscope, and crystallographic study. Oral Surg Oral Med Oral Pathol. 1989;68:195–200. doi: 10.1016/0030-4220(89)90192-8. [DOI] [PubMed] [Google Scholar]
- 15.Counts A.L., Rohrer M.D., Prasad H., Bolen P. An assessment of root cementum in cleidocranial dysplasia. Angle Orthod. 2001;71:293–298. doi: 10.1043/0003-3219(2001)071<0293:AAORCI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Yang X., Zhang C.Y., Zheng S.G. Analysis of root resorption and dental structure in patients with cleidocranial dysplasia. J Peking Univ Heal Sci. 2011;43:98–101. [PubMed] [Google Scholar]
- 17.Hitchin A.D., Faily J.M. Dental management in cleido-cranial dysostosis. Brit J Oral Surg. 1974;12:46–55. doi: 10.1016/0007-117x(74)90060-2. [DOI] [PubMed] [Google Scholar]
- 18.Kreiborg S., Jensen B.L., Björk A., Skieller V. Abnormalities of the cranial base in cleidocranial dysostosis. Am J Orthod. 1981;79:549–557. doi: 10.1016/s0002-9416(81)90465-6. [DOI] [PubMed] [Google Scholar]
- 19.Jensen B.L., Kreiborg S. Development of the dentition in cleidocranial dysplasia. J Oral Pathol Med. 1990;19:89–93. doi: 10.1111/j.1600-0714.1990.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 20.Järvinen E., Tummers M., Thesleff I. The role of the dental lamina in mammalian tooth replacement. J Exp Zool B Mol Dev Evol. 2009;312B:281–291. doi: 10.1002/jez.b.21275. [DOI] [PubMed] [Google Scholar]
- 21.Chen W., Ma J., Zhu G. Cbfβ deletion in mice recapitulates cleidocranial dysplasia and reveals multiple functions of Cbfβ required for skeletal development. Proc Natl Acad Sci U. S. A. 2014;111:8482–8487. doi: 10.1073/pnas.1310617111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaruga A., Hordyjewska E., Kandzierski G., Tylzanowski P. Cleidocranial dysplasia and Runx2-clinical phenotype-genotype correlation. Clin Genet. 2016;90:393–402. doi: 10.1111/cge.12812. [DOI] [PubMed] [Google Scholar]
- 23.Mundlos S., Mulliken J.B., Abramson D.L., Warman M.L., Knoll J.H., Olsen B.R. Genetic mapping of cleidocranial dysplasia and evidence of a microdeletion in one family. Hum Mol Genet. 1995;4:71–75. doi: 10.1093/hmg/4.1.71. [DOI] [PubMed] [Google Scholar]
- 24.Lee B., Thirunavukkarasu K., Zhou L. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 25.Kim H.J., Nam S.H., Kim H.J. Four novel RUNX2 mutations including a splice donor site result in the cleidocranial dysplasia phenotype. J Cell Physiol. 2006;207:114–122. doi: 10.1002/jcp.20552. [DOI] [PubMed] [Google Scholar]
- 26.Ducy P., Zhang R., Geoffroy V., Ridall A.L., Karsenty G. Osf2/Cbfa1 a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri S., McDonald F. Runx2 and dental development. Eur J Oral Sci. 2006;114:361–373. doi: 10.1111/j.1600-0722.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 28.Shen Z., Zou C.C., Yang R.W., Zhao Z.Y. Cleidocranial dysplasia: report of 3 cases and literature review. Clin Pediatr. 2009;48:194–198. doi: 10.1177/0009922808323107. [DOI] [PubMed] [Google Scholar]
- 29.Ducy P. Cbfa1: a molecular switch in osteoblast biology. Dev Dyn. 2000;219:461–471. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1074>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y., Li Y., Cavender A.C., Wang S., Mansukhani A., D'Souza R.N. Molecular studies on the roles of Runx2 and Twist1 in regulating FGF signaling. Dev Dyn. 2012;241:1708–1715. doi: 10.1002/dvdy.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker A., Lustmann J., Shteyer A. Cleidocranial dysplasia: Part 1–General principles of the orthodontic and surgical treatment modality. Am J Orthod Dentofac Orthop. 1997;111:28–33. doi: 10.1016/s0889-5406(97)70298-1. [DOI] [PubMed] [Google Scholar]
- 32.Becker A., Shteyer A., Bimstein E., Lustmann J. Cleidocranial dysplasia: Part 2 General principles of the orthodontic and surgical treatment modality. Am J Orthod Dentofac Orthop. 1997;111:173–183. doi: 10.1016/s0889-5406(97)70213-0. [DOI] [PubMed] [Google Scholar]
- 33.Kuroda S., Yanagita T., Kyung H.M., Takano-Yamamoto T. Titanium screw anchorage for traction of many impacted teeth in a patient with cleidocranial dysplasia. Am J Orthod Dentofac Orthop. 2007;131:666–669. doi: 10.1016/j.ajodo.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Kang N., Kim S.Z., Jung S.N. Correction of depressed forehead with bone source in cleidocranial dysplasia. J Craniofac Surg. 2009;20:564–566. doi: 10.1097/SCS.0b013e31819ba361. [DOI] [PubMed] [Google Scholar]
- 35.Hwang S.M., Park B., Hwang M.K., Kim M.W., Lee J.S. Aesthetic facial correction of cleidocranial dysplasia. Arch Craniofac Surg. 2016;17:82–85. doi: 10.7181/acfs.2016.17.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madeira M.F., Caetano I.M., Dias-Ribeiro E. Orthognathic surgery in patients with cleidocranial dysplasia. J Craniofac Surg. 2015;26:792–795. doi: 10.1097/SCS.0000000000001414. [DOI] [PubMed] [Google Scholar]
- 37.Lee K.E., Seymen F., Ko J. RUNX2 mutations in cleidocranial dysplasia. Genet Mol Res. 2013;12:4567–4574. doi: 10.4238/2013.October.15.5. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida T., Kanegane H., Osato M. Functional analysis of RUNX2 mutations in Japanese patients with cleidocranial dysplasia demonstrates novel genotype-phenotype correlations. Am J Hum Genet. 2002;71:724–738. doi: 10.1086/342717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryoo H.M., Kang H.Y., Lee S.K. RUNX2 mutations in cleidocranial dysplasia patients. Oral Dis. 2010;16:55–60. doi: 10.1111/j.1601-0825.2009.01623.x. [DOI] [PubMed] [Google Scholar]