Abstract
A tendon transfers force from the contracting muscle to the skeletal system to produce movement and is therefore a crucial component of the entire muscle‐tendon complex and its function. However, tendon research has for some time focused on mechanical properties without any major appreciation of potential cellular and molecular changes. At the same time, methodological developments have permitted determination of the mechanical properties of human tendons in vivo, which was previously not possible. Here we review the current understanding of how tendons respond to loading, unloading, ageing and injury from cellular, molecular and mechanical points of view. A mechanistic understanding of tendon tissue adaptation will be vital for development of adequate guidelines in physical training and rehabilitation, as well as for optimal injury treatment.
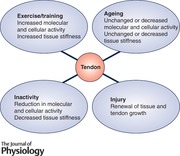
Keywords: collagen, extracellular matrix, tendon fibroblasts, viscoelastic properties, tendon structure
Introduction
Cellular and molecular aspects
The tendon is a connective tissue characterized by a relatively low abundance of cells (primarily fibroblasts) that are elongated in the direction of the collagen fibrils, and a richness of matrix proteins dominated by fibrillar collagen (especially collagen type I) embedded with proteoglycans (PGs), glycoproteins (GPs) and glycosaminoglycans (GAGs) (Kjaer, 2004). The larger PG molecules are dominated by versican and aggrecan, and the smaller PGs (small leucine‐rich proteoglycans, SLRPs) contain decorin, biglycan, lumican and fibromodulin. In addition, cartilage oligomorphometric protein (COMP), lubricant, tenascin‐C, fibronectin, elastin and cross‐link molecules of both an enzymatic and a non‐enzymatic nature each have a role in the tissue's mechanical properties and thus in tendon strength, stiffness and elasticity (Halper & Kjaer, 2014).
For many years the focus in tendon physiology has been on its biomechanical properties without any major appreciation of potential cellular biochemical changes in response to loading or unloading. Conversely, the focus in skeletal muscle research has been on the cellular biochemistry and protein synthesis of structural contractile components, but little attention has been given to the dynamics of intramuscular connective tissue and its biomechanical properties. Interestingly, early work by Vihko and colleagues (Savolainen et al. 1988; Karpakka et al. 1990) focusing on connective tissue in skeletal muscle demonstrated in a rat model that mechanical loading and unloading influenced the activity of enzymes involved in collagen synthesis in skeletal muscle and tendon (Savolainen et al. 1988; Karpakka et al. 1990). As summarized by Michael Rennie in the 1996 Handbook of Physiology (Rennie, 1996), it has been reported that whereas de‐tensioning of skeletal muscle in a shortening (rather than in a lengthening) position led to a marked decrease in the enzymatic activity of the collagen synthesizing enzymes propyl 4‐hydroxylase (PH) and galactosylhydroxylysyl glucosyltransferase (GGT) (Karpakka et al. 1990), mild mechanical loading after muscle‐tendon immobilization did not, in their model, alter PH or GGT activity (Savolainen et al. 1988). These early studies indicated that loading of tendon is important for maintenance of matrix protein synthesis, and indirectly suggested that a certain magnitude of loading is needed to obtain an increase in matrix protein synthesis in tendon.
The study of protein dynamics in tendon has developed markedly in recent years. Initially indirect determinations of protein synthesis were made using circulating or tissue concentrations of hydroxyprolin and collagen regulating enzymes (Weiss & Klein, 1969; Hart et al. 1976). Later, the ability to determine the cleavage products of collagen synthesis or degradation allowed estimates of tissue turnover (Shinmei et al. 1993), and the development of the microdialysis method allowed sampling of these substances in the proximity of the tendon, including in humans (Langberg et al. 1999; Miller et al. 2005; Hansen et al. 2009a; Miller et al. 2011). Some of these studies indicated a relatively fast turnover of tendon collagen tissue (Langberg et al. 1999). Subsequently the use of stable isotope infusion of labelled amino/imino acids or heavy water (deuterium) and tendon biopsy sampling allowed a more accurate evaluation of collagen dynamics both in relation to more acute exercise and immobilization interventions. Some studies in humans have found relatively high collagen synthesis rates in tendons of ∼1% per 24 h (Miller et al. 2005), while others have found more moderate values of around 0.2% per 24 h (de Boer et al. 2007b; Nielsen et al. 2014). Finally, tissue sampling of tendon autopsies, in both humans and animals, and analysis of 14C content (bomb pulse method) and L‐to‐D conversion of amino acids (racemization) have permitted ‘lifetime’ estimation of the matrix tissue turnover in tendon (Libby et al. 1964; Helfman & Bada, 1976; Lynnerup et al. 2008; Alkass et al. 2013). Using racemization, it has been demonstrated that the half‐life of horse tendon is around 200 years in high strain, injury‐prone superficial digital flexor tendon, and far less (∼34 years) in low strain and rarely injured common digital extensor tendon (Thorpe et al. 2010). The human Achilles tendon can be considered a high strain and injury‐prone tendon, and data obtained using the bomb pulse method indicates that the collagen structure of the core of the human Achilles tendon changes until about the age of 17 years and thereafter the tissue structure is stable (Heinemeier et al. 2013b). The latter findings are supported by data from rats showing that growth and turnover of tendon tissue ceased shortly after 60 days of life and remained relative stable throughout the entire lifespan of around 500 days (Bechshoft et al. 2017).
This leaves us with a challenge regarding our understanding of tendon tissue turnover in human tendons. It is suggested that the tendon collagen tissue consists of two pools of tissue, one that is relatively stable with relatively dormant cells, which is formed during childhood and adolescence, and one much smaller pool with a fast turnover of cells which provides a form of ‘daily maintenance’ of tissue homeostasis. Theoretically, such a model could work with all available methodologies, and fits with the view that a major part of the tendon is developed during childhood and adolescence, while at the same time allowing for a daily adjustment mechanism and thus the ability to adapt to changes in mechanical loading patterns. Presently, the anatomical locations of the proposed pools of tissue remain an enigma.
Effect of loading on tendon
Cellular and molecular aspects
Mechanical stimuli can lead to tendon cell responses that result in changes in the extracellular matrix. Cell culture studies on tendon show that fibroblasts respond to mechanical stretch by increasing production and secretion of certain growth factors that in turn act on the fibroblasts in an autocrine or paracrine fashion to induce expression and synthesis of collagen (Chiquet et al. 2009). Early growth response transcription factor 1(EGR‐1) is an important factor for embryonic and postnatal tendon development (Lejard et al. 2011). It is also known that EGR‐1 is regulated by mechanical loading (Olesen et al. 2006), and that a lack of EGR‐1 is associated with weaker tendon formation (Guerquin et al. 2013). With respect to other tendon growth factors, it has been shown, using a model that creates tendon‐like structures from isolated animal and human cells within 2–3 weeks (Bayer et al. 2010; Paxton et al. 2010), that phosphorylation of p38, S6K1 and ERK1/2 is associated with mechanical loading of tendon constructs (Paxton et al. 2010, 2012). Whether these factors are crucial for tendon growth can be debated, as blockade of, for example, p38 was found not to be important for tendon growth during development (Schwartz et al. 2015), but probably plays a role in mechanotransduction (Paxton et al. 2012). Interestingly, a study of the relation between loading and signalling for collagen synthesis based on the phosphorylation of ERK1/2 suggested that 10 min of 2.5%stretch every 6 h was most favourable for improving tendon function(Paxton et al. 2012). In addition, it should be noted that in a rat model it has been demonstrated that the stimulatory effect of loading on collagen and IGF‐I expression was similar whether the muscle contraction mode was concentric, isometric or eccentric, irrespective of the fact that the force‐time integral was far greater for the eccentric contraction (Heinemeier et al. 2007a).
The enzyme responsible for cross‐link formation, lysyl oxidase (LOX), has been demonstrated to be necessary for optimal tendon fibrillogenesis, since blocking of LOX does not influence the total amount of collagen synthesized, but markedly weakens the tendon structure and makes it more compliant (Herchenhan et al. 2015b). Several experiments on small animals with electrically induced muscle training or synergist ablation leads to substantial increases in mRNA expression of the collagen‐inducing growth factors IGF‐I and TGFb1, in parallel with increased mRNA expression of collagen type I and III in the tendon tissue (Olesen et al. 2006; Heinemeier et al. 2007a, b ). In addition, repeated bouts of treadmill running have been shown to elevate levels of IGF‐I protein in rat tendons (Hansson et al. 1988). Thus, it seems likely that the tendon cells respond to loading by increasing growth factor production, and that the action of these growth factors leads to induction of collagen expression, although this remains to be proven.
Growth factors involved in modulating the signalling cascade include transforming growth factor b1 (TGFb1), plasma‐derived growth factor (PDGF) and connective tissue growth factor (CTGF) (Schild & Trueb, 2002; Yang et al. 2004; Sugg et al. 2017). In addition, insulin like growth factor‐I (IGF‐I) can also act as a link between mechanical load and collagen synthesis in tendon tissue (Hansson et al. 1988; Abrahamsson & Lohmander, 1996; Herchenhan et al. 2015a). In humans the administration of growth hormone for 14 days, or local injection into tendon of IGF‐1 resulted in increased collagen synthesis (Doessing et al. 2010; Nielsen et al. 2014). Further, oestrogen also influences tendon protein metabolism, and can inhibit collagen synthesis (Hansen et al. 2009b) and lower the expression of lysyl oxidase (LOX), which is important for cross‐link formation (Lee et al. 2015). Very recently, the addition of vitamin C‐enriched gelatin (along with many amino acids) produced an increase in collagen synthesis in engineered ligament and amino terminal propeptide of collagen I in the blood of humans (Shaw et al. 2017). Regarding degradation, it has been demonstrated that matrix metalloproteinase (MMP) activity is increased with exercise (Koskinen et al. 2004), and that the activation of ERK1/2 seems important for this MMP response (Sugg et al. 2017). Finally, an investigation of the breakdown products of collagen revealed a higher release of these in response to exercise and prolonged physical training (Langberg et al. 1999, 2001).
The role of inflammation in acute physiological changes in tendon has been investigated in several ways. It has been demonstrated that inhibition of COX‐2 specific pathways abolishes the exercise‐induced rise in tendon blood flow in humans (Langberg et al. 2003). Further, stretching of tendon cells has resulted in prostaglandin E2 release in a dose‐dependent manner (Wang et al. 2003; Legerlotz et al. 2013), and in humans blockade of inflammation during acute exercise completely inhibited the rise in collagen synthesis normally seen in response to exercise (Christensen et al. 2011). In bovine models, tendon stretching has resulted in a rise in both IL‐6 and collagen type I mRNA (Legerlotz et al. 2013), while in humans a marked peritendinous rise in the interstitial tissue concentration of IL‐6 (and collagen synthesis) was found in association with exercise (Andersen et al. 2011), and an infusion of IL‐6 into the peritendinous tissue in the resting state caused a rise in collagen synthesis (Andersen et al. 2011). This suggests that a rise in inflammatory mediators is important for collagen turnover and for tendon adaptation to exercise. This mandatory effect of an increase in inflammatory parameters on activation of collagen synthesis may be independent of the potentially inhibiting effect on collagen synthesis of a chronic elevated inflammatory state (e.g. in ageing, disease or immobilization). In de‐tensioning of tendon, expression of collagen I is immediately reduced, together with a rise in expression of inflammatory signalling markers (Bayer et al 2014).
Comparable loading‐induced collagen synthesis in adult human tendon has been reported. Microdialysis studies showedincreased levels of markers for collagen synthesis in the peritendinous tissue surrounding the Achilles tendon in response to both acute exercise and long‐term training (Langberg et al. 1999, 2001). However, these microdialysis data probably reflect collagen synthesis at the very periphery of the tendon and might not reveal changes within tendon tissue. Using stable isotope infusion with tendon biopsy sampling, an increased rate of collagen synthesis was observed in patellar tendons of young men in response to acute kicking exercise (Miller et al. 2005). However, several other studies using the same technique, and the same exercise model, could not robustly confirm this loading‐induced collagen synthesis in adult human tendon (Hansen et al. 2009a, b ; Petersen et al. 2010; Dideriksen et al. 2013).
Several studies have investigated gene expression in human patellar tendon tissue in response to acute exercise. In two of these investigations a decrease or no change in growth factor and collagen mRNA expression in tendon biopsies from the mid‐portion of the tendon was found (Sullivan et al. 2009; Heinemeier et al. 2013a), while one study found modest increases in collagen and CTGF mRNA expression in tissue from the proximal part of the patellar tendon in response to exercise (Dideriksen et al. 2013). In other words, the response of adult human tendon tissue to acute loading does not seem to mimic that of rodent tendon tissue. This suggests that adult human tendon tissue is far less responsive than that of small animals, and such differences may relate to the fact that rats and mice are still in a growth phase when they are typically used in experiments (typically at 10–12 weeks of age for rats) (Olesen et al. 2006; Heinemeier et al. 2007a, b ). This hypothesis is supported by recent data on 6‐month‐old mice, which showed that overload‐induced plantaris tendon hypertrophy was based on growth and cell proliferation only in the most superficial layers of tendon tissue, while the ‘original’ core tendon remained relatively constant (Gumucio et al. 2014). A greater potential for growth at the tendon periphery is further suggested by an early study that showed higher levels of IGF‐I protein expression in cells located in the rat Achilles tendon periphery compared to the more central parts of the tendon(Hansson et al. 1988). More recent studies also suggest greater potential for growth and cell proliferation in the superficial parts of the tendon in rodents (Mendias et al. 2012; Tan et al. 2013). In other words, it may be speculated that a new layer of the collagen matrix is added at the periphery, analogous to growth of a tree, when the tendon grows in response to loading. An alternative explanation for contradictory results regarding adaptability of tendon tissue to loading and overall metabolic activity, could be that large differences exist between different types of tendons. Data from horses show that high strain‐injury prone tendons have slower turnover than low strain rarely injured tendons (Thorpe et al. 2010). Albeit counterintuitive, it may be that high‐strain tendons simply cannot ‘afford’ to have a constant ongoing remodelling as this may reduce the tendon strength. Therefore, the high‐strain Achilles tendon may well have slower turnover than tendons that are loaded less, such as the patellar tendon.
Structural and mechanical aspects
The chief function of tendon is force transmission, and yet the specific pathway of force transmission remains largely unresolved. The collagen fibrils of tendon, which have a diameter range of 30–200 nm (Williams et al. 1985; Magnusson et al. 2002; Lavagnino et al. 2005), are considered the principle load transmitting structure of tendons (Figure 1). The fibrils are surrounded by an extracellular matrix consisting of water, proteoglycans, glycosaminoglycans (GAGs), elastin and glycoproteins (Wang, 2006), and this structural composition is not unlike that of a composite material in which fibres transmit force laterally to adjacent fibres through the matrix. Fibre slippage at the microscopic level (Screen et al. 2004a, b ; Screen, 2008) and X‐ray diffraction studies showing that the constituent fibrils are stretched less than the whole structure (Fratzl et al. 1998; Puxkandl et al. 2002; Krauss et al. 2009) support such a composite concept. It has been suggested that force is transferred laterally between adjacent fibrils via proteoglycans and their associated GAG chains, including chondroitin‐ and dermatan‐sulfate (Scott & Thomlinson, 1998; Ryan et al. 2015). However, removing this complex in human tendon (Svensson et al. 2011) and ligament (Lujan et al. 2007, 2009) does not appreciably affect the mechanical properties of the tissue.
Figure 1. The principle load transmitting structure of tendons.
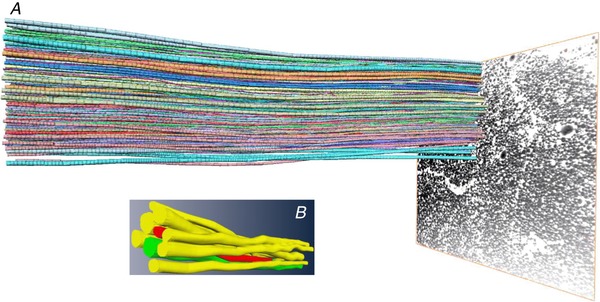
A, an illustration of tracing of collagen fibrils from a human patellar tendon obtained with FIB‐SEM on a nanoscale level, suggesting that the fibrils are continuous. B, close‐up of individual fibrils that typically range between 30 and 200 nm in diameter. For further detail, see Svensson et al. 2017.
The notion that there is a lateral pathway of force transmission largely rests on the assumption that fibrils in mature tissue are discontinuous, but determining the actual structural length of fibrils with diameters of 30–200 nm has proven rather challenging for several technical reasons. Studies based on direct structural observations indicate that fibrils may be structurally continuous whereas those based on more indirect methods tend to suggest that fibrils are discontinuous (Folkhard et al. 1987; Craig et al. 1989; Silver et al. 2000; Provenzano & Vanderby, 2006; Szczesny et al. 2015). However, in a recent study using serial block face‐scanning electron microscopy on tissue from adult human patellar and hamstring tendons, it was possible to track 2700 fibrils over a defined distance and only detect one single fibril tip (Svensson et al. 2017). Based on these data it was estimated that the fibril length was 67.5 mm, which strongly supports the notion that fibrils are continuous (Svensson et al. 2017).
The cross‐sectional area of the tendon will impact on the average stress (N/m2) that the tendon sees at a given force. It is therefore important to know if the tendon can hypertrophy in response to exercise. Animal studies have not provided conclusive data to answer this question (Sommer, 1987; Buchanan & Marsh, 2001; Heinemeier et al. 2012). In humans, cross‐sectional data suggests that endurance training is associated with a larger Achilles tendon cross‐sectional area that is more pronounced close to the insertion (Magnusson & Kjaer, 2003; Kongsgaard et al. 2005; Arampatzis et al. 2007; Couppe et al. 2014), and that resistance training yields fairly modest increases in tendon cross‐sectional area (Kongsgaard et al. 2007; Seynnes et al. 2009). However, a study performed on athletes who for years loaded one side more than the other has overcome some study limitations such as (i) training history, selection bias and inter‐subject variations, (ii) the relatively short duration of earlier training studies, and (iii) the lack of assessment of region specificity in existing training studies (Couppe et al. 2008). The data showed that subjects with a side‐to‐side difference (22%) in knee extensor strength as a result of habitual sport specific high loading also have a greater tendon cross‐sectional area (20%) on the stronger side (Couppe et al. 2008). These data are perhaps currently some of the most convincing evidence that tendons undergo tissue hypertrophy in response to loading. However, whether the hypertrophy represents added tensile bearing components, i.e. principally collagen fibrils, remains a largely unanswered question.
Relatively little is known about the effect of exercise on the collagen fibril itself. Animal data are essentially inconclusive (Michna, 1984; Patterson‐Kane et al. 1997, 1998). In recent years it has been possible to obtain human percutaneous tendon biopsies, which has opened up for new possibilities, although obtaining repeated biopsies remains a challenge (Heinemeier et al. 2016). It has been shown that the collagen of the Achilles tendon is not renewed after 17 years of age (Heinemeier et al. 2013b), and it has been proposed that exercise during skeletal maturation can influence the tendon fibril development (Smith et al. 2002). However, a recent study showed that the fibril morphology of long distance runners who were physically active did not differ from those who were physically inactive during their maturation (Lenskjold et al. 2015). Similarly, life‐long habitual running did not appear to appreciably influence fibril morphology compared to age‐matched non‐runners (Couppe et al. 2014). Albeit inconclusive, these relatively scant cross‐sectional human data suggest that the collagen fibrils are largely unaffected by exercise.
Although it is well known that the tendon cells (fibroblasts) respond to strain, the dose response to strain magnitude is not well established. Most (Webb et al. 2006; Joshi & Webb, 2008; Gauvin et al. 2011), but not all (Feng et al. 2006) studies have found that the adaptive response of fibroblasts to dynamic load is superior to that of static load. In healthy human Achilles tendons, it has been shown that exercising at 90% of maximum voluntary contraction (MVC; ∼5% tendon strain) yields increased stiffness and cross‐sectional area compared to working at 55% MVC (∼3% tendon strain), and that the tendon was more responsive to long duration loading(6 s cycle) than high faster loads (2 s cycle) (Arampatzis et al. 2007, 2010). Studies specifically addressing the effects of recovery and how it affects tendon adaptation are lacking.
Unlike muscle, the tendon is a mechanically passive structure that lengthens or shortens in response to the load placed on it, and it is therefore unlikely that direction of the movement of the muscle‐tendon unit will have any distinct effect on the tissue of the tendon. In fact, the magnitude of human Achilles tendon stretch is identical during the concentric and eccentric component of a heel rise/drop against body weight (Rees et al. 2008; Chaudhry et al. 2015). The cellular response to concentric or eccentric contractions to the same force level is similar with respect to the expression of collagen (Garma et al. 2007). In healthy subjects 12 weeks of resistance training comprising either concentric or eccentric knee extensions produced similar magnitudes of tendon hypertrophy (Farup et al. 2014). These findings reinforce the notion that the cellular and tissue response in healthy tendon is independent of contraction mode. Finally, some of the confounding results with regard to changes in tendon mechanical properties in response to mechanical loading may have to do with the different tendons examined (e.g. patellar vs. Achilles vs. supraspinatus etc.), in addition to the methods, different animal/human models, and inherent biology.
Unloading
Cellular and molecular aspects
De‐tensioning of tissue removes the constant signal for mechanotransduction in tendon. In tendon constructs made from human tenocytes it has been demonstrated that even a few days after removing tensile loading there is a downregulation of mRNA for both tenomodulin and collagen, and this is accompanied by a disorganization of the aligned fibrils in the tendon structure (Bayer et al. 2014). This downregulation could not be counteracted by addition of TGF‐beta to the medium. The findings indicate that tension influences the phenotypical characteristics of tendon. Clearly, protein signalling and tissue turnover in this in vitro model of tendon may occur much more rapidly and pronouncedly than in vivo, but even 2–3 weeks of immobilization of a lower limb in both young and old individuals will lead to an 80% reduction in synthesis rate of collagen (de Boer et al. 2007b; Dideriksen et al. 2017). In line with these changes, 2 weeks of immobilization resulted in a downregulation of LOX and scleraxis and an upregulation of MMP2 in human patella tendon (Boesen et al. 2013; Dideriksen, 2014). It is interesting that administration of both growth hormone and NSAID counteracted some of the signalling, and, taken together, it appears that inactivity in humans favours reduced collagen synthesis, reduced collagen synthesis stimulating signalling and accelerated proteolytic activity. Both growth hormone and, to some extent, NSAID are able to act as matrix stabilizers during immobilization during rehabilitation (Boesen et al. 2013, 2014; Dideriksen et al. 2017).
Structural and mechanical aspects
The effects of immobilization on the tendon have been studied in animal models, and they overwhelming show a decline in mechanical properties (Woo et al. 1982; Yamamoto et al. 1993; Hannafin et al. 1995; Almeida‐Silveira et al. 2000; Matsumoto et al. 2003; Rumian et al. 2009). It seems that the human data on the effects of immobilization on tendon properties largely mirror those of the animal models (Reeves et al. 2005; de Boer et al. 2007a; Seynnes et al. 2008; Shin et al. 2008; Kinugasa et al. 2010; Couppe et al. 2012). Interestingly, rather short‐term (1–2 weeks) limb unloading seems to significantly reduce the stiffness of the tendon. Moreover, the changes in mechanical properties take place in the absence of any change in cross‐section of the tendon (Reeves et al. 2005; de Boer et al. 2007a; Christensen et al. 2008; Shin et al. 2008; Couppe et al. 2012). In fact, it appears that the cross‐section of tendon will only be reduced during extremely protracted periods of inactivity (Maganaris et al. 2006). The mechanism for this relatively rapid change in mechanical properties in the absence of an overall change in the (MRI determined) size of the structure is elusive, but may relate to LOX‐derived cross‐links.
Ageing
Cellular and molecular aspects
Tendon cell numbers and density have so far not been studied systematically in humans. Instead animal models have mostly been used, making it difficult to separate changes in the number of tendon cells during development and growth from those related to ageing (Svensson et al. 2016). Within the confines of these limitations, it has been shown that cell density is reduced with age in rabbits and rats (Nagy et al. 1969; Nakagawa et al. 1994), whereas in horses there is no decline in cell density (or DNA content) from 2 to 30 years of age (Stanley et al. 2007; Thorpe et al. 2015). Interestingly, there is very little or no difference in metabolic activity of cultured cells from rats and in collagen synthesis capacity in horse tendons (Tsai et al. 2011; Thorpe et al. 2015) in regard to ageing. This suggests that there is only a limited decline in synthesis capacity of tendon cells with ageing, and observations in animal models may reflect an effect of maturation than ageing per se. When cell function is evaluated by migration and proliferation capacity, an age‐related reduced cell proliferation has been shown in injured human supraspinatus tendon (Klatte‐Schulz et al. 2012), and in rat and mouse tendon a reduction in both migration and proliferation was shown (Arnesen & Lawson, 2006; Torricelli et al. 2013). Further, data using progenitor cells from aged tendons in humans and animals suggest both lower proliferation and migration with ageing (Zhou et al. 2010; Kohler et al. 2013; Zhang & Wang, 2015). This indicates that ageing does impair cell capacity in a way that probably cannot be counteracted by changes in the circulating environment. In the study on human tendon progenitor cells, it was shown that the potential for differentiation into adipogenic, chondrogenic and osteogenic cells was unaltered with ageing (Kohler et al. 2013). Given the very limited human data, it is difficult to firmly conclude anything, and the findings in animal models are still impaired by the limited ability to separate ageing and tissue maturation. In regard to expression and synthesis of proteins from tendon cells, it has been shown that older rats demonstrated a reduced mRNA expression of collagen (I, III and V) whereas the protein content of collagen types estimated by immunohistochemistry was unchanged with ageing (Goh et al. 2008; Dyment & Galloway, 2015; Zhang et al. 2016). Further, ageing in rats led to decreased expression of elastin but no change in expression of the factors involved in tissue growth (CTGF and TGF‐beta; Kostrominova & Brooks, 2013). Finally, high levels of extracellular proteoglycan, calcium deposits and lipid have been found in elderly mice (Zhang & Wang, 2015). In a recent study with young and old rats it was demonstrated that ageing was associated with a downregulation of genes that regulate matrix remodelling (Marqueti et al. 2018). Further, aged rats show a reduced density of blood vessels and a few examples of calcification in older tendons were shown (Marqueti et al. 2018). Interestingly, the intervention of regular strength training in older rats upregulated mRNA expression of CTGF, decorin and VEGF, and no calcifications were found in tendons of old trained rats (Marqueti et al. 2018). Although the study found differences between young and old rats, it also demonstrated that physical training can counteract several age‐related changes in tendon connective tissue, which is similar to findings in relation to ageing and oxidative and metabolic capacity in skeletal muscle (Larsen et al. 2012).
Structural and mechanical aspects
The effect of ageing is challenging to study because it is hard to separate the effects of age per se from the related inactivity associated with ageing (Lazarus & Harridge, 2017). Animal data show that tendon cross‐sectional area (CSA) may increase (Nakagawa et al. 1996; Birch et al. 1999) or remain unchanged (Wood et al. 2011) with age, and, similarly, cross‐sectional studies in humans indicate that tendon CSA may increase (Magnusson et al. 2003; Stenroth et al. 2012; Couppe et al. 2014) or remain unchanged (Carroll et al. 2008; Couppe et al. 2009a, 2014) with ageing. However, not accounting for reduced physical activity with ageing (Lazarus & Harridge, 2017) and its potential effects on tendon CSA may be a key limitation. It was recently shown that young and old men of similar height, weight and activity level also displayed similar tendon CSA (Couppe et al. 2014), which suggests that, unlike muscle, there is no loss of tendon tissue with increasing age. In support of this, the total content of collagen fibrils (volume fraction) remains largely unaltered with ageing in animal and human models (Parry & Craig, 1978; Wood et al. 2011; Couppe et al. 2014).
Factors such as the rate of force development, electromechanical delay and the elastic energy return of tendon influence the overall function of the muscle‐tendon complex, and can be affected by the mechanical properties of tendon (Bojsen‐Moller et al. 2005; Magnusson et al. 2008; Nordez et al. 2009; Quinlan et al. 2017). Again, data from animal models do not provide a coherent picture, as some show increased modulus and strength (Viidik et al. 1996; Nielsen et al. 1998; Wood et al. 2011), some a weaker and more compliant tendon (Vogel, 1978; Simonsen et al. 1995; Dressler et al. 2002; LaCroix et al. 2013), and yet others demonstrate no change with age (Haut, 1983; Haut et al. 1992; Nakagawa et al. 1996). In vitro data on human tendons suggest that there are no changes or a reduction in the mechanical properties of the tendon with ageing (Hubbard & Soutas‐Little, 1984; Johnson et al. 1994; Flahiff et al. 1995), while in vivo human studies have reported unchanged (Carroll et al. 2008; Couppe et al. 2009a) or reduced (Kubo et al. 2003; Onambele et al. 2006; Stenroth et al. 2012; Couppe et al. 2014; Quinlan et al. 2017) modulus values with ageing. Some of the discrepancies in the human in vivo data may relate to data analysis: strength tends to decline with age, and therefore a reduction in tendon strain may be a consequence of a lower force placed on the tendon (and as a result less strain). Therefore, it is necessary to evaluate strain to a common force when comparing mechanical properties between age groups. As previously mentioned it is probably of paramount importance to account for age‐related activity levels. It was recently shown that old subjects (66 years) had a lower modulus compared to young subjects (26 years), but when old and young were matched for activity level, there was no difference in mechanical properties (Couppe et al. 2014). Collectively, the data suggest that with age there is no change or a decrease in the modulus of the tendon. What is somewhat puzzling about these findings is that there is an increase in advance glycation endproducts (AGE) with age (Couppe et al. 2009b, 2014; Hansen et al. 2010), which can be mitigated by physical activity (Couppe et al. 2014), and an elevation of AGEs would typically increase tendon stiffness (Bai et al. 1992; Li et al. 2013; Svensson et al. 2018). It is unclear, but it is possible that the increase in AGEs is partly countered by a reduction in collagen content with age (Couppe et al. 2009b).
Injured tendon
Cellular and molecular aspect
The exact pathogenesis behind development of tendon overuse – tendinopathy – is still not completely solved. It is known that tendinopathic (and painful) tendon areas are associated with more rounded fibroblasts and cell accumulation (Glazebrook et al. 2008; de Mos et al. 2009). Further, there is an upregulation of mRNA for collagen I and II plus TGF‐beta (Pingel et al. 2013a, 2014), and increased amounts of proteoglycans, and of the proteins versican, aggrecan and fibromodulin, whereas decorin, which normally is associated with aligned tendon fibrils, was unchanged in content in tendinpathic tendons (Parkinson et al. 2010). To what extend tendinopathy is associated with inflammation is still debated, but in general long‐term chronic tendinopathy is not dominated by inflammation, whereas different indicators of inflammation have been demonstrated early in tendinopathy (Dakin et al. 2015).
After tendon rupture, a broad anabolic and inflammatory signalling occurs. A marked inflammatory response has been demonstrated both in the early, the proliferative and the remodelling phase of tendon healing in animal models (Eliasson et al. 2009; Schulze‐Tanzil et al. 2011). Interestingly, the addition of mechanical loading and activity to the tendon during healing stimulated the NO response (Eliasson et al. 2012) and lowered the inflammatory response, as indicated by TNF‐alpha and IL‐1. At the same it time caused an improvement in the synthesis of tendon matrix tissue (Eliasson et al. 2009).
Structural and mechanical aspects
In addition to rounding of fibroblasts, increased cell number, an increase in the content of proteoglycans glycosaminoglycans (GAGs), water and hypervascularization (with nerve ingrowth), the collagen fibrils appear disorganized (Glazebrook et al. 2008; Kongsgaard et al. 2009; Pingel et al. 2013b). The increase in water content, along with the hypervascularization, leaves the tendon with an overall increase in CSA. Tendon stiffness has been reported to decrease with tendinopathy (Arya & Kulig, 2010; Child et al. 2010; Helland et al. 2013), or remain unchanged (Kongsgaard et al. 2010). It remains unknown if such a change relates to the pathology itself or if it is an injury‐related response to inactivity (unloading).
Loading‐based interventions have become a principal theme in the treatment of tendinopathies (Malliaras et al. 2013), with positive clinical (Alfredson et al. 1996; Alfredson & Lorentzon, 2000; Mafi et al. 2001; Silbernagel et al. 2007), structural, and biochemical outcomes (Couppe et al. 2009b; Kongsgaard et al. 2010). An isolated eccentric loading protocol has mostly been regarded as the treatment of choice, although there is a lack of support for its superior effective (Malliaras et al. 2013). Other loading‐based exercise regimes, including isolated concentric training (Mafi et al. 2001), heavy slow resistance training (Kongsgaard et al. 2009; Beyer et al. 2015) and concentric‐eccentric progressing to eccentric training (Silbernagel et al. 2001, 2007) also appear to be clinically effective. How a loading regime influences tendon composition in patients with tendinopathy has been very little investigated. It has been shown that fibril morphology is abnormal in tendinopathy, but that heavy slow resistance training can change fibril morphology towards normal fibril density and mean fibril area (Kongsgaard et al. 2010). It has also been shown that the cross‐link composition can be changed with heavy slow resistance training in this patient group (Kongsgaard et al. 2009).
Despite the tendon's remarkable ability to transfer large magnitudes of muscular force to the skeletal system for locomotion purposes, there are instances when the tendon ruptures completely. The aetiology remains largely unknown, and much research has focused on tendon repair, and rehabilitation and clinical outcome in order to rapidly return patients to normal function. However, it was recently shown that cellular activity, as measured by the glucose uptake associated with ambulation, is higher in repaired than in intact Achilles tendons at 3 months (6x), 6 months (3x) and even 12 months (1.6x) after a surgical repair (Eliasson et al. 2016). Furthermore, it was recently shown that the repaired Achilles tendon increases its stiffness up to at least 6 months (P. Eliasson and others, unpublished data). Collectively, these data indicate that the tendon response to loading is rather slow and is not normalized until sometime after 6–12 months after injury (Eliasson et al. 2016).
In conclusion, methodological improvements in the last 20 years or so have permitted determination of cellular, molecular and mechanical changes in response to loading or unloading of tendon tissue (summarized in Table 1), which was previously difficult to study. This new knowledge of tendon physiology will undoubtedly contribute to the unravelling of the optimal adaptation of tendon to loading throughout life, as well as the aetiology of tendon injuries. With that knowledge, it will be possible to develop more effective treatment and prevention strategies than we have today. To ensure optimal guidelines for both normal physiological adaptation to physical training and for rehabilitation after tendon injury, we need a tight interplay between the description of clinical presentation and associated tissue changes and a basic biomedical understanding of the mechanisms behind adaptive changes in tendon to mechanical challenges throughout life.
Table 1.
Tendon responses to loading, unloading, ageing and injury: arrows indicate the overall direction
| Cellular/molecular | Structural/mechanical | ||||
|---|---|---|---|---|---|
| Anabolic/catabolic | Cell number | Tissue synthesis | CSA | Stiffness | |
| Loading | ↑ | → | ↑ | ↑ | ↑ |
| Unloading | ↓ | → | ↓ | → | ↓ |
| Ageing | →↘ | ↓* | → | → | →↘ |
| Injury | ↑ | ↓ | ↑ | ↑ | →↘** |
*Only animal data. **Only tendinopathy.
Additional information
Competing interests
None declared.
Author contributions
Both authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The work was supported by the Danish Council for Independent Research, Lundbeck Foundation and the Center for Healthy Aging.
Biographies
Peter Magnusson obtained his doctorate of medical science from University of Copenhagen. Since 2008 he has been Professor of Musculoskeletal Rehabilitation at the University of Copenhagen. His research focuses on increasing understanding of tendon structure and function with regards to injury, rehabilitation, loading/unloading and ageing.

Michael Kjaer has a medical degree from University of Copenhagen. He did his thesis work on hormonal regulation during exercise, and specialized in rheumatology. He is Professor of Sports Medicine at the University of Copenhagen and heads the Institute of Sports Medicine at Bispebjerg Hospital. His research focuses on adaptive responses of tendon and skeletal muscle to physical exercise.
Edited by: Ole Petersen & Dario Farina
References
- Abrahamsson SO & Lohmander S (1996). Differential effects of insulin‐like growth factor‐I on matrix and DNA synthesis in various regions and types of rabbit tendons. J Orthop Res 14, 370–376. [DOI] [PubMed] [Google Scholar]
- Alfredson H & Lorentzon R (2000). Chronic Achilles tendinosis recommendations for treatment and prevention. Sports Med 29, 135–146. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Pietila T & Lorentzon R (1996). Chronic Achilles tendinitis and calf muscle strength. Am J Sports Med 24, 829–833. [DOI] [PubMed] [Google Scholar]
- Alkass K, Saitoh H, Buchholz BA, Bernard S, Holmlund G, Senn DR, Spalding KL & Druid H (2013). Analysis of radiocarbon, stable isotopes and DNA in teeth to facilitate identification of unknown decedents. PLoS One 8, e69597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida‐Silveira MI, Lambertz D, Perot C & Goubel F (2000). Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. Eur J Appl Physiol 81, 252–257. [DOI] [PubMed] [Google Scholar]
- Andersen MB, Pingel J, Kjaer M & Langberg H (2011). Interleukin‐6: a growth factor stimulating collagen synthesis in human tendon. J Appl Physiol 110, 1549–1554. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K & Albracht K (2007). Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J Exp Biol 210, 2743–2753. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Peper A, Bierbaum S & Albracht K (2010). Plasticity of human Achilles tendon mechanical and morphological properties in response to cyclic strain. J Biomech 43, 3073–3079. [DOI] [PubMed] [Google Scholar]
- Arnesen SM & Lawson MA (2006). Age‐related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mech Ageing Dev 127, 726–732. [DOI] [PubMed] [Google Scholar]
- Arya S & Kulig K (2010). Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol (1985) 108, 670–675. [DOI] [PubMed] [Google Scholar]
- Bai P, Phua K, Hardt T, Cernadas M & Brodsky B (1992). Glycation alters collagen fibril organization. Connect Tissue Res 28, 1–12. [DOI] [PubMed] [Google Scholar]
- Bayer ML, Schjerling P, Herchenhan A, Zeltz C, Heinemeier KM, Christensen L, Krogsgaard M, Gullberg D & Kjaer M (2014). Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype. PLoS One 9, e86078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer ML, Yeung CY, Kadler KE, Qvortrup K, Baar K, Svensson RB, Magnusson SP, Krogsgaard M, Koch M & Kjaer M (2010). The initiation of embryonic‐like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials 31, 4889–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechshoft CL, Schjerling P, Borno A & Holm L (2017). Existence of life‐time stable proteins in mature rats – dating of proteins' age by repeated short‐term exposure to labeled amino acids throughout age. PLoS One 12, e0185605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer R, Kongsgaard M, Hougs Kjaer B, Ohlenschlaeger T, Kjaer M & Magnusson SP (2015). Heavy slow resistance versus eccentric training as treatment for achilles tendinopathy: a randomized controlled trial. Am J Sports Med 43, 1704–1711. [DOI] [PubMed] [Google Scholar]
- Birch HL, McLaughlin L, Smith RK & Goodship AE (1999). Treadmill exercise‐induced tendon hypertrophy: assessment of tendons with different mechanical functions. Equine Vet J Suppl 30, 222–226. [DOI] [PubMed] [Google Scholar]
- Boesen AP, Dideriksen K, Couppe C, Magnusson SP, Schjerling P, Boesen M, Aagaard P, Kjaer M & Langberg H (2014). Effect of growth hormone on aging connective tissue in muscle and tendon: gene expression, morphology, and function following immobilization and rehabilitation. J Appl Physiol 116, 192–203. [DOI] [PubMed] [Google Scholar]
- Boesen AP, Dideriksen K, Couppe C, Magnusson SP, Schjerling P, Boesen M, Kjaer M & Langberg H (2013). Tendon and skeletal muscle matrix gene expression and functional responses to immobilisation and rehabilitation in young males: effect of growth hormone administration. J Physiol 591, 6039–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojsen‐Moller J, Magnusson SP, Rasmussen LR, Kjaer M & Aagaard P (2005). Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol 99, 986–994. [DOI] [PubMed] [Google Scholar]
- Buchanan CI & Marsh RL (2001). Effects of long‐term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J Appl Physiol 90, 164–171. [DOI] [PubMed] [Google Scholar]
- Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP & Trappe TA (2008). Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105, 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry S, Morrissey D, Woledge RC, Bader DL & Screen HRC (2015). Eccentric and concentric loading of the triceps surae: an in vivo study of dynamic muscle and tendon biomechanical parameters. J Appl Biomech 31, 69–78. [DOI] [PubMed] [Google Scholar]
- Child S, Bryant AL, Clark RA & Crossley KM (2010). Mechanical properties of the achilles tendon aponeurosis are altered in athletes with Achilles tendinopathy. Am J Sports Med 38, 1885–1893. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Gelman L, Lutz R & Maier S (2009). From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta 1793, 911–920. [DOI] [PubMed] [Google Scholar]
- Christensen B, Dandanell S, Kjaer M & Langberg H (2011). Effect of anti‐inflammatory medication on the running‐induced rise in patella tendon collagen synthesis in humans. J Appl Physiol 110, 137–141. [DOI] [PubMed] [Google Scholar]
- Christensen B, Dyrberg E, Aagaard P, Kjaer M & Langberg H (2008). Short‐term immobilization and recovery affect skeletal muscle but not collagen tissue turnover in humans. J Appl Physiol 105, 1845–1851. [DOI] [PubMed] [Google Scholar]
- Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M & Magnusson SP (2009a). Mechanical properties and collagen cross‐linking of the patellar tendon in old and young men. J Appl Physiol 107, 880–886. [DOI] [PubMed] [Google Scholar]
- Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M & Magnusson SP (2009b). Mechanical properties and collagen cross‐linking of the patellar tendon in old and young men. J Appl Physiol 107, 880–886. [DOI] [PubMed] [Google Scholar]
- Couppe C, Kongsgaard M, Aagaard P, Hansen P, Bojsen‐Moller J, Kjaer M & Magnusson SP (2008). Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol 105, 805–810. [DOI] [PubMed] [Google Scholar]
- Couppe C, Suetta C, Kongsgaard M, Justesen L, Hvid LG, Aagaard P, Kjaer M & Magnusson SP (2012). The effects of immobilization on the mechanical properties of the patellar tendon in younger and older men. Clin Biomech (Bristol, Avon) 27, 949–954. [DOI] [PubMed] [Google Scholar]
- Couppe C, Svensson RB, Grosset JF, Kovanen V, Nielsen RH, Olsen MR, Larsen JO, Praet SF, Skovgaard D, Hansen M, Aagaard P, Kjaer M & Magnusson SP (2014). Life‐long endurance running is associated with reduced glycation and mechanical stress in connective tissue. Age (Dordr) 36, 9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AS, Birtles MJ, Conway JF & Parry DAD (1989). An estimate of the mean length of collagen fibrils in rat tail‐tendon as a function of age. Connect Tissue Res 19, 51–62. [DOI] [PubMed] [Google Scholar]
- Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJ, Smith RD, Wheway K, Watkins B, Roche L & Carr AJ (2015). Inflammation activation and resolution in human tendon disease. Sci Transl Med 7, 311ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MD, Maganaris CN , Seynnes OR, Rennie MJ & Narici MV (2007a). Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower‐limb suspension in young men. J Physiol 583, 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MD, Selby A , Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV & Rennie MJ (2007b). The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mos M, Koevoet W, van Schie HT, Kops N, Jahr H, Verhaar JA & van Osch GJ (2009). In vitro model to study chondrogenic differentiation in tendinopathy. Am J Sports Med 37, 1214–1222. [DOI] [PubMed] [Google Scholar]
- Dideriksen K ( 2014). Muscle and tendon connective tissue adaptation to unloading, exercise and NSAID. Connect Tissue Res 55, 61–70. [DOI] [PubMed] [Google Scholar]
- Dideriksen K, Boesen AP, Reitelseder S, Couppe C, Svensson R, Schjerling P, Magnusson SP, Holm L & Kjaer M (2017). Tendon collagen synthesis declines with immobilization in elderly humans: no effect of anti‐inflammatory medication. J Appl Physiol 122, 273–282. [DOI] [PubMed] [Google Scholar]
- Dideriksen K, Sindby AK, Krogsgaard M, Schjerling P, Holm L & Langberg H (2013). Effect of acute exercise on patella tendon protein synthesis and gene expression. SpringerPlus 2, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doessing S, Heinemeier KM, Holm L, Mackey AL, Schjerling P, Rennie M, Smith K, Reitelseder S, Kappelgaard AM, Rasmussen MH, Flyvbjerg A & Kjaer M (2010). Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol 588, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F & Boivin GP (2002). A potential mechanism for age‐related declines in patellar tendon biomechanics. J Orthop Res 20, 1315–1322. [DOI] [PubMed] [Google Scholar]
- Dyment NA & Galloway JL (2015). Regenerative biology of tendon: mechanisms for renewal and repair. Curr Mol Biol Rep 1, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson P, Andersson T & Aspenberg P (2009). Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol 107, 399–407. [DOI] [PubMed] [Google Scholar]
- Eliasson P, Andersson T & Aspenberg P (2012). Influence of a single loading episode on gene expression in healing rat Achilles tendons. J Appl Physiol 112, 279–288. [DOI] [PubMed] [Google Scholar]
- Eliasson P, Couppe C, Lonsdale M, Svensson RB, Neergaard C, Kjaer M, Friberg L & Magnusson SP (2016). Ruptured human Achilles tendon has elevated metabolic activity up to 1 year after repair. Eur J Nuc Med Mo Imaging 43, 1868–1877. [DOI] [PubMed] [Google Scholar]
- Farup J, Rahbek SK, Vendelbo MH, Matzon A, Hindhede J, Bejder A, Ringgard S & Vissing K (2014). Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand J Med Sci Sports 24, 788–798. [DOI] [PubMed] [Google Scholar]
- Feng Z, Tateishi Y, Nomura Y, Kitajima T & Nakamura T (2006). Construction of fibroblast‐collagen gels with orientated fibrils induced by static or dynamic stress: toward the fabrication of small tendon grafts. J Artificial Organs 9, 220–225. [DOI] [PubMed] [Google Scholar]
- Flahiff CM, Brooks AT, Hollis JM, Vander Schilden JL & Nicholas RW (1995). Biomechanical analysis of patellar tendon allografts as a function of donor age. Am J Sports Med 23, 354–358. [DOI] [PubMed] [Google Scholar]
- Folkhard W, Geercken W, Knorzer E, Mosler E, Nemetschekgansler H, Nemetschek T & Koch MHJ (1987). Structural dynamic of native tendon collagen. J Mol Biol 193, 405–407. [DOI] [PubMed] [Google Scholar]
- Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H & Bernstorff S (1998). Fibrillar structure and mechanical properties of collagen. J Struct Biol 122, 119–122. [DOI] [PubMed] [Google Scholar]
- Garma T, Kobayashi C, Haddad F, Adams GR, Bodell PW & Baldwin KM (2007). Similar acute molecular responses to equivalent volumes of isometric, lengthening, or shortening mode resistance exercise. J Appl Physiol 102, 135–143. [DOI] [PubMed] [Google Scholar]
- Gauvin R, Parenteau‐Bareil R, Larouche D, Marcoux H, Bisson F, Bonnet A, Auger FA, Bolduc S & Germain L (2011). Dynamic mechanical stimulations induce anisotropy and improve the tensile properties of engineered tissues produced without exogenous scaffolding. Acta Biomater 7, 3294–3301. [DOI] [PubMed] [Google Scholar]
- Glazebrook MA, Wright JR Jr, Langman M, Stanish WD & Lee JM (2008). Histological analysis of Achilles tendons in an overuse rat model. J Orthop Res 26, 840–846. [DOI] [PubMed] [Google Scholar]
- Goh KL, Holmes DF, Lu HY, Richardson S, Kadler KE, Purslow PP & Wess TJ (2008). Ageing changes in the tensile properties of tendons: influence of collagen fibril volume fraction. J Biomech Eng 130, 021011. [DOI] [PubMed] [Google Scholar]
- Guerquin MJ, Charvet B, Nourissat G, Havis E, Ronsin O, Bonnin MA, Ruggiu M, Olivera‐Martinez I, Robert N, Lu Y, Kadler KE, Baumberger T, Doursounian L, Berenbaum F & Duprez D (2013). Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest 123, 3564–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio JP, Phan AC, Ruehlmann DG, Noah AC & Mendias CL (2014). Synergist ablation induces rapid tendon growth through the synthesis of a neotendon matrix. J Appl Physiol 117, 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halper J & Kjaer M (2014). Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol 802, 31–47. [DOI] [PubMed] [Google Scholar]
- Hannafin JA, Arnoczky SP, Hoonjan A & Torzilli PA (1995). Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res 13, 907–914. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kongsgaard M, Holm L, Skovgaard D, Magnusson SP, Qvortrup K, Larsen JO, Aagaard P, Dahl M, Serup A, Frystyk J, Flyvbjerg A, Langberg H & Kjaer M (2009a). Effect of estrogen on tendon collagen synthesis, tendon structural characteristics, and biomechanical properties in postmenopausal women. J Appl Physiol 106, 1385–1393. [DOI] [PubMed] [Google Scholar]
- Hansen M, Miller BF, Holm L, Doessing S, Petersen SG, Skovgaard D, Frystyk J, Flyvbjerg A, Koskinen S, Pingel J, Kjaer M & Langberg H (2009b). Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J Appl Physiol 106, 1435–1443. [DOI] [PubMed] [Google Scholar]
- Hansen P, Haraldsson BT, Aagaard P, Kovanen V, Avery NC, Qvortrup K, Larsen JO, Krogsgaard M, Kjaer M & Peter Magnusson S (2010). Lower strength of the human posterior patellar tendon seems unrelated to mature collagen cross‐linking and fibril morphology. J Appl Physiol 108, 47–52. [DOI] [PubMed] [Google Scholar]
- Hansson HA, Engstrom AM, Holm S & Rosenqvist AL (1988). Somatomedin C immunoreactivity in the Achilles tendon varies in a dynamic manner with the mechanical load. Acta Physiol Scand 134, 199–208. [DOI] [PubMed] [Google Scholar]
- Hart W, van den Hamer CJ & van der Sluys Veer J (1976). The use of hydroxy‐DL‐proline‐2‐(14)C in the investigation of hydroxyproline metabolism in normal subjects and in patients with renal insufficiency. Clin Nephrol 6, 379–387. [PubMed] [Google Scholar]
- Haut RC ( 1983). Age‐dependent influence of strain rate on the tensile failure of rat‐tail tendon. J Biomech Eng 105, 296–299. [DOI] [PubMed] [Google Scholar]
- Haut RC, Lancaster RL & DeCamp CE (1992). Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J Biomech 25, 163–173. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Bjerrum SS, Schjerling P & Kjaer M (2013a). Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sports 23, e150‐161. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Lorentzen MP, Jensen JK, Schjerling P, Seynnes O, Narici MV & Kjaer M (2016). Local trauma in human patellar tendon leads to widespread changes in the tendon gene expression. J Appl Physiol 120, 1000–1010. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM & Schjerling P (2007a). Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol 582, 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM & Kjaer M (2007b). Short‐term strength training and the expression of myostatin and IGF‐I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol 102, 573–581. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP & Kjaer M (2013b). Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J 27, 2074–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeier KM, Skovgaard D, Bayer ML, Qvortrup K, Kjaer A, Kjaer M, Magnusson SP & Kongsgaard M (2012). Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J Appl Physiol 113, 827–836. [DOI] [PubMed] [Google Scholar]
- Helfman PM & Bada JL (1976). Aspartic acid racemisation in dentine as a measure of ageing. Nature 262, 279–281. [DOI] [PubMed] [Google Scholar]
- Helland C, Bojsen‐Moller J, Raastad T, Seynnes OR, Moltubakk MM, Jakobsen V, Visnes H & Bahr R (2013). Mechanical properties of the patellar tendon in elite volleyball players with and without patellar tendinopathy. Br J Sports Med 47, 862–868. [DOI] [PubMed] [Google Scholar]
- Herchenhan A, Bayer ML, Eliasson P, Magnusson SP & Kjaer M (2015a). Insulin‐like growth factor I enhances collagen synthesis in engineered human tendon tissue. Growth Horm IGF Res 25, 13–19. [DOI] [PubMed] [Google Scholar]
- Herchenhan A, Uhlenbrock F, Eliasson P, Weis M, Eyre D, Kadler KE, Magnusson SP & Kjaer M (2015b). Lysyl oxidase activity is required for ordered collagen fibrillogenesis by tendon cells. J Biol Chem 290, 16440–16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RP & Soutas‐Little RW (1984). Mechanical properties of human tendon and their age dependence. J Biomech Eng 106, 144–150. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY & Woo SLY (1994). Tensile and viscoelastic properties of human patellar tendon. J Orthop Res 12, 796–803. [DOI] [PubMed] [Google Scholar]
- Joshi SD & Webb K (2008). Variation of cyclic strain parameters regulates development of elastic modulus in fibroblast/substrate constructs. J Orthop Res 26, 1105–1113. [DOI] [PubMed] [Google Scholar]
- Karpakka J, Vaananen K, Virtanen P, Savolainen J, Orava S & Takala TE (1990). The effects of remobilization and exercise on collagen biosynthesis in rat tendon. Acta Physiol Scand 139, 139–145. [DOI] [PubMed] [Google Scholar]
- Kinugasa R, Hodgson JA, Edgerton VR, Shin DD & Sinha S (2010). Reduction in tendon elasticity from unloading is unrelated to its hypertrophy. J Appl Physiol 109, 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M ( 2004). Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84, 649–698. [DOI] [PubMed] [Google Scholar]
- Klatte‐Schulz F, Pauly S, Scheibel M, Greiner S, Gerhardt C, Schmidmaier G & Wildemann B (2012). Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte‐like cells. Eur Cell Mater 24, 74–89. [DOI] [PubMed] [Google Scholar]
- Kohler J, Popov C, Klotz B, Alberton P, Prall WC, Haasters F, Muller‐Deubert S, Ebert R, Klein‐Hitpass L, Jakob F, Schieker M & Docheva D (2013). Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging cell 12, 988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsgaard M, Aagaard P, Kjaer M & Magnusson SP (2005). Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J Appl Physiol 99, 1965–1971. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Kovanen V, Aagaard P, Doessing S, Hansen P, Laursen AH, Kaldau NC, Kjaer M & Magnusson SP (2009). Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scand J Med Sci Sports 19, 790–802. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Qvortrup K, Larsen J, Aagaard P, Doessing S, Hansen P, Kjaer M & Magnusson SP (2010). Fibril morphology and tendon mechanical properties in patellar tendinopathy: effects of heavy slow resistance training. Am J Sports Med 38, 749–756. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M & Magnusson SP (2007). Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol 191, 111–121. [DOI] [PubMed] [Google Scholar]
- Koskinen SO, Heinemeier KM, Olesen JL, Langberg H & Kjaer M (2004). Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon‐related connective tissue. J Appl Physiol 96, 861–864. [DOI] [PubMed] [Google Scholar]
- Kostrominova TY & Brooks SV (2013). Age‐related changes in structure and extracellular matrix protein expression levels in rat tendons. Age (Dordr) 35, 2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Fratzl P, Seto J, Currey JD, Estevez JA, Funari SS & Gupta HS (2009). Inhomogeneous fibril stretching in antler starts after macroscopic yielding: indication for a nanoscale toughening mechanism. Bone 44, 1105–1110. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Miyatani M, Tachi M & Fukunaga T (2003). Effect of low‐load resistance training on the tendon properties in middle‐aged and elderly women. Acta Physiol Scand 178, 25–32. [DOI] [PubMed] [Google Scholar]
- LaCroix AS, Duenwald‐Kuehl SE, Brickson S, Akins TL, Diffee G, Aiken J, Vanderby R, Jr. & Lakes RS (2013). Effect of age and exercise on the viscoelastic properties of rat tail tendon. Ann Biomed Eng 41, 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Boushel R, Skovgaard D, Risum N & Kjaer M (2003). Cyclo‐oxygenase‐2 mediated prostaglandin release regulates blood flow in connective tissue during mechanical loading in humans. J Physiol 551, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Rosendal L & Kjaer M (2001). Training‐induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol 534, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J & Kjaer M (1999). Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol 521, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Hey‐Mogensen M, Rabol R, Stride N, Helge JW & Dela F (2012). The influence of age and aerobic fitness: effects on mitochondrial respiration in skeletal muscle. Acta Physiol (Oxf) 205, 423–432. [DOI] [PubMed] [Google Scholar]
- Lavagnino M, Arnoczky SP, Frank K & Tian T (2005). Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress‐deprived tendons. J Biomech 38, 69–75. [DOI] [PubMed] [Google Scholar]
- Lazarus NR & Harridge SDR (2017). Declining performance of master athletes: silhouettes of the trajectory of healthy human ageing? J Physiol 595, 2941–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Lee‐Barthel A, Marquino L, Sandoval N, Marcotte GR & Baar K (2015). Estrogen inhibits lysyl oxidase and decreases mechanical function in engineered ligaments. J Appl Physiol 118, 1250–1257. [DOI] [PubMed] [Google Scholar]
- Legerlotz K, Jones GC, Screen HR & Riley GP (2013). Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin‐6 expression by tenocytes. Scand J Med Sci Sports 23, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejard V, Blais F, Guerquin MJ, Bonnet A, Bonnin MA, Havis E, Malbouyres M, Bidaud CB, Maro G, Gilardi‐Hebenstreit P, Rossert J, Ruggiero F & Duprez D (2011). EGR1 and EGR2 involvement in vertebrate tendon differentiation. J Biol Chem 286, 5855–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenskjold A, Kongsgaard M, Larsen JO, Nielsen RH, Kovanen V, Aagaard P, Kjaer M & Magnusson SP (2015). The influence of physical activity during youth on structural and functional properties of the Achilles tendon. Scand J Med Sci Sports 25, 25–31. [DOI] [PubMed] [Google Scholar]
- Li Y, Fessel G, Georgiadis M & Snedeker JG (2013). Advanced glycation end‐products diminish tendon collagen fiber sliding. Matrix Biol 32, 169–177. [DOI] [PubMed] [Google Scholar]
- Libby WF, Berger R, Mead JF, Alexander GV & Ross JF (1964). Replacement Rates for Human Tissue from Atmospheric Radiocarbon. Science 146, 1170–1172. [DOI] [PubMed] [Google Scholar]
- Lujan TJ, Underwood CJ, Henninger HB, Thompson BM & Weiss JA (2007). Effect of dermatan sulfate glycosaminoglycans on the quasi‐static material properties of the human medial collateral ligament. J Orthop Res 25, 894–903. [DOI] [PubMed] [Google Scholar]
- Lujan TJ, Underwood CJ, Jacobs NT & Weiss JA (2009). Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J Appl Physiol 106, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C & Heinemeier J (2008). Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS One 3, e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafi N, Lorentzon R & Alfredson H (2001). Superior short‐term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc 9, 42–47. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K & De Haan A (2006). Adaptive response of human tendon to paralysis. Muscle Nerve 33, 85–92. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K & Kjaer M (2003). Increased cross‐sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci 58, 123–127. [DOI] [PubMed] [Google Scholar]
- Magnusson SP & Kjaer M (2003). Region‐specific differences in Achilles tendon cross‐sectional area in runners and non‐runners. Eur J Appl Physiol 90, 549–553. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Narici MV, Maganaris CN & Kjaer M (2008). Human tendon behaviour and adaptation, in vivo. J Physiol 586, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Qvortrup K, Larsen JO, Rosager S, Hanson P, Aagaard P, Krogsgaard M & Kjaer M (2002). Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol 21, 369–377. [DOI] [PubMed] [Google Scholar]
- Malliaras P, Barton CJ, Reeves ND & Langberg H (2013). Achilles and patellar tendinopathy loading programmes: a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med 43, 267–286. [DOI] [PubMed] [Google Scholar]
- Marqueti RC, Durigan JLQ, Oliveira AJS, Mekaro MS, Guzzoni V, Aro AA, Pimentel ER & Selistre‐de‐Araujo HS (2018). Effects of aging and resistance training in rat tendon remodeling. FASEB J 32, 353–368. [DOI] [PubMed] [Google Scholar]
- Matsumoto F, Trudel G, Uhthoff HK & Backman DS (2003). Mechanical effects of immobilization on the Achilles' tendon. Arch Phys Med Rehabil 84, 662–667. [DOI] [PubMed] [Google Scholar]
- Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB & Brooks SV (2012). Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res 30, 606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna H ( 1984). Morphometric analysis of loading‐induced changes in collagen‐fibril populations in young tendons. Cell Tissue Res 236, 465–470. [DOI] [PubMed] [Google Scholar]
- Miller BF, Ellis D, Robinson MM, Rivera JD, Kjaer M & Langberg H (2011). Measurement of skeletal muscle collagen breakdown by microdialysis. Scand J Med Sci Sports 21, e1–8. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K & Rennie MJ (2005). Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567, 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy IZ, Von Hahn HP & Verzar F (1969). Age‐related alterations in the cell nuclei and the DNA content of rat tail tendon. Gerontologia 15, 258–264. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Hayashi K, Yamamoto N & Nagashima K (1996). Age‐related changes in biomechanical properties of the Achilles tendon in rabbits. Eur J Appl Physiol Occup Physiol 73, 7–10. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Majima T & Nagashima K (1994). Effect of ageing on ultrastructure of slow and fast skeletal muscle tendon in rabbit Achilles tendons. Acta Physiol Scand 152, 307–313. [DOI] [PubMed] [Google Scholar]
- Nielsen HM, Skalicky M & Viidik A (1998). Influence of physical exercise on aging rats. III. Life‐long exercise modifies the aging changes of the mechanical properties of limb muscle tendons. Mech Ageing Dev 100, 243–260. [DOI] [PubMed] [Google Scholar]
- Nielsen RH, Holm L, Malmgaard‐Clausen NM, Reitelseder S, Heinemeier KM & Kjaer M (2014). Increase in tendon protein synthesis in response to insulin‐like growth factor‐I is preserved in elderly men. J Appl Physiol 116, 42–46. [DOI] [PubMed] [Google Scholar]
- Nordez A, Gallot T, Catheline S, Guevel A, Cornu C & Hug F (2009). Electromechanical delay revisited using very high frame rate ultrasound. J Appl Physiol 106, 1970–1975. [DOI] [PubMed] [Google Scholar]
- Olesen JL, Heinemeier KM, Haddad F, Langberg H, Flyvbjerg A, Kjaer M & Baldwin KM (2006). Expression of insulin‐like growth factor I, insulin‐like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J Appl Physiol 101, 183–188. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Narici MV & Maganaris CN (2006). Calf muscle‐tendon properties and postural balance in old age. J Appl Physiol 100, 2048–2056. [DOI] [PubMed] [Google Scholar]
- Parkinson J, Samiric T, Ilic MZ, Cook J, Feller JA & Handley CJ (2010). Change in proteoglycan metabolism is a characteristic of human patellar tendinopathy. Arthritis Rheum 62, 3028–3035. [DOI] [PubMed] [Google Scholar]
- Parry DAD & Craig AS (1978). Collagen fibrils and elastic fibers in rat‐tail tendon: an electron microscopic investigation. Biopolymers 17, 843–845. [DOI] [PubMed] [Google Scholar]
- Patterson‐Kane JC, Firth EC, Parry DA, Wilson AM & Goodship AE (1998). Effects of training on collagen fibril populations in the suspensory ligament and deep digital flexor tendon of young thoroughbreds. Am J Vet Res 59, 64–68. [PubMed] [Google Scholar]
- Patterson‐Kane JC, Parry DA, Birch HL, Goodship AE & Firth EC (1997). An age‐related study of morphology and cross‐link composition of collagen fibrils in the digital flexor tendons of young thoroughbred horses. Connect Tissue Res 36, 253–260. [DOI] [PubMed] [Google Scholar]
- Paxton JZ, Grover LM & Baar K (2010). Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng Part A 16, 3515–3525. [DOI] [PubMed] [Google Scholar]
- Paxton JZ, Hagerty P, Andrick JJ & Baar K (2012). Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A 18, 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SG, Saxne T, Heinegard D, Hansen M, Holm L, Koskinen S, Stordal C, Christensen H, Aagaard P & Kjaer M (2010). Glucosamine but not ibuprofen alters cartilage turnover in osteoarthritis patients in response to physical training. Osteoarthritis Cartilage 18, 34–40. [DOI] [PubMed] [Google Scholar]
- Pingel J, Fredberg U, Mikkelsen LR, Schjerling P, Heinemeier KM, Kjaer M, Harisson A & Langberg H (2013a). No inflammatory gene‐expression response to acute exercise in human Achilles tendinopathy. Eur J Appl Physiol 113, 2101–2019. [DOI] [PubMed] [Google Scholar]
- Pingel J, Lu Y, Starborg T, Fredberg U, Langberg H, Nedergaard A, Weis M, Eyre D, Kjaer M & Kadler KE (2014). 3‐D ultrastructure and collagen composition of healthy and overloaded human tendon: evidence of tenocyte and matrix buckling. J Anat 224, 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingel J, Wienecke J, Kongsgaard M, Behzad H, Abraham T, Langberg H & Scott A (2013b). Increased mast cell numbers in a calcaneal tendon overuse model. Scand J Med Sci Sports 23, e353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP & Vanderby R (2006). Collagen fibril morphology and organization: Implications for force transmission in ligament and tendon. Matrix Biol 25, 71–84. [DOI] [PubMed] [Google Scholar]
- Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, Purslow P & Fratzl P (2002). Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci 357, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan JI, Maganaris CN, Franchi MV, Smith K, Atherton PJ, Szewczyk NJ, Greenhaff PL, Phillips BE, Blackwell JI, Boereboom C, Williams JP, Lund J & Narici MV (2017). Muscle and tendon contributions to reduced rate of torque development in healthy older males. J Gerontol A Biol Sci Med Sci 73, 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JD, Lichtwark GA, Wolman RL & Wilson AM (2008). The mechanism for efficacy of eccentric loading in Achilles tendon injury; an in vivo study in humans. Rheumatology 47, 1493–1497. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Ferretti G & Narici MV (2005). Influence of 90‐day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol 98, 2278–2286. [DOI] [PubMed] [Google Scholar]
- Rennie MJ ( 1996). Influence of exercise on protein and amino acid metabolism In Handbook of Physiology, eds Rowell LB. & Sheppard JT. Oxford University Press. [Google Scholar]
- Rumian AP, Draper ER, Wallace AL & Goodship AE (2009). The influence of the mechanical environment on remodelling of the patellar tendon. J Bone Joint Surg Br 91, 557–564. [DOI] [PubMed] [Google Scholar]
- Ryan CN, Sorushanova A, Lomas AJ, Mullen AM, Pandit A & Zeugolis DI (2015). Glycosaminoglycans in tendon physiology, pathophysiology, and therapy. Bioconjug Chem 26, 1237–1251. [DOI] [PubMed] [Google Scholar]
- Savolainen J, Vaananen K, Puranen J, Takala TE, Komulainen J & Vihko V (1988). Collagen synthesis and proteolytic activities in rat skeletal muscles: effect of cast‐immobilization in the lengthened and shortened positions. Arch Phys Med Rehabil 69, 964–969. [PubMed] [Google Scholar]
- Schild C & Trueb B (2002). Mechanical stress is required for high‐level expression of connective tissue growth factor. Exp Cell Res 274, 83–91. [DOI] [PubMed] [Google Scholar]
- Schulze‐Tanzil G, Al‐Sadi O, Wiegand E, Ertel W, Busch C, Kohl B & Pufe T (2011). The role of pro‐inflammatory and immunoregulatory cytokines in tendon healing and rupture: new insights. Scand J Med Sci Sports 21, 337–351. [DOI] [PubMed] [Google Scholar]
- Schwartz AJ, Sarver DC, Sugg KB, Dzierzawski JT, Gumucio JP & Mendias CL (2015). p38 MAPK signaling in postnatal tendon growth and remodeling. PLoS One 10, e0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE & Thomlinson AM (1998). The structure of interfibrillar proteoglycan bridges (shape modules') in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J Anat 192, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screen HRC ( 2008). Investigating load relaxation mechanics in tendon. J Mech Behav Biomed Mater 1, 51–58. [DOI] [PubMed] [Google Scholar]
- Screen HRC, Bader DL, Lee DA & Shelton JC (2004a). Local strain measurement within tendon. Strain 40, 157–163. [Google Scholar]
- Screen HRC, Lee DA, Bader DL & Shelton JC (2004b). An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng H 218, 109–119. [DOI] [PubMed] [Google Scholar]
- Seynnes OR, Erskine RM, Maganaris CN, Longo S, Simoneau EM, Grosset JF & Narici MV (2009). Training‐induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J Appl Physiol 107, 523–530. [DOI] [PubMed] [Google Scholar]
- Seynnes OR, Maganaris CN, de Boer MD, di Prampero PE & Narici MV (2008). Early structural adaptations to unloading in the human calf muscles. Acta Physiol (Oxf) 193, 265–274. [DOI] [PubMed] [Google Scholar]
- Shaw G, Lee‐Barthel A, Ross ML, Wang B & Baar K (2017). Vitamin C‐enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr 105, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Finni T, Ahn S, Hodgson JA, Lee HD, Edgerton VR & Sinha S (2008). Effect of chronic unloading and rehabilitation on human Achilles tendon properties: a velocity‐encoded phase‐contrast MRI study. J Appl Physiol 105, 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmei M, Ito K, Matsuyama S, Yoshihara Y & Matsuzawa K (1993). Joint fluid carboxy‐terminal type II procollagen peptide as a marker of cartilage collagen biosynthesis. Osteoarthritis Cartilage 1, 121–128. [DOI] [PubMed] [Google Scholar]
- Silbernagel KG, Thomee R, Eriksson BI & Karlsson J (2007). Continued sports activity, using a pain‐monitoring model, during rehabilitation in patients with Achilles tendinopathy: a randomized controlled study. Am J Sports Med 35, 897–906. [DOI] [PubMed] [Google Scholar]
- Silbernagel KG, Thomee R, Thomee P & Karlsson J (2001). Eccentric overload training for patients with chronic Achilles tendon pain – a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports 11, 197–206. [DOI] [PubMed] [Google Scholar]
- Silver FH, Christiansen DL, Snowhill PB & Chen Y (2000). Role of storage on changes in the mechanical properties of tendon and self‐assembled collagen fibers. Connect Tissue Res 41, 155–164. [DOI] [PubMed] [Google Scholar]
- Simonsen EB, Klitgaard H & Bojsen‐Moller F (1995). The influence of strength training, swim training and ageing on the Achilles tendon and m. soleus of the rat. J Sports Sci 13, 291–295. [DOI] [PubMed] [Google Scholar]
- Smith RK, Birch HL, Goodman S, Heinegard D & Goodship AE (2002). The influence of ageing and exercise on tendon growth and degeneration – hypotheses for the initiation and prevention of strain‐induced tendinopathies. Comp Biochem Physiol A Mol Integr Physiol 133, 1039–1050. [DOI] [PubMed] [Google Scholar]
- Sommer HM ( 1987). The biomechanical and metabolic effects of a running regime on the Achilles tendon in the rat. Int Orthop 11, 71–75. [DOI] [PubMed] [Google Scholar]
- Stanley RL, Fleck RA, Becker DL, Goodship AE, Ralphs JR & Patterson‐Kane JC (2007). Gap junction protein expression and cellularity: comparison of immature and adult equine digital tendons. J Anat 211, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenroth L, Peltonen J, Cronin NJ, Sipila S & Finni T (2012). Age‐related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol 113, 1537–1544. [DOI] [PubMed] [Google Scholar]
- Sugg KB, Markworth JF, Disser NP, Rizzi AM, Talarek JR, Sarver DC, Brooks SV & Mendias CL (2017). Postnatal tendon growth and remodeling requires platelet‐derived growth factor receptor signaling. Am J Physiol Cell Physiol 314, C389–C403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BE, Carroll CC, Jemiolo B, Trappe SW, Magnusson SP, Dossing S, Kjaer M & Trappe TA (2009). Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory mRNA expression. J Appl Physiol 106, 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson RB, Hassenkam T, Hansen P, Kjaer M & Magnusson SP (2011). Tensile force transmission in human patellar tendon fascicles is not mediated by glycosaminoglycans. Connect Tissue Res 52, 415–421. [DOI] [PubMed] [Google Scholar]
- Svensson RB, Heinemeier KM, Couppe C, Kjaer M & Magnusson SP (2016). The effect of aging and exercise on the tendon. J Appl Physiol (1985) 121, 1237–1246. [DOI] [PubMed] [Google Scholar]
- Svensson RB, Herchenhan A, Starborg T, Larsen M, Kadler KE, Qvortrup K & Magnusson SP (2017). Evidence of structurally continuous collagen fibrils in tendons. Acta Biomater 50, 293–301. [DOI] [PubMed] [Google Scholar]
- Svensson RB, Smith ST, Moyer PJ & Magnusson SP (2018). Effects of maturation and advanced glycation on tensile mechanics of collagen fibrils from rat tail and Achilles tendons. Acta Biomater 70, 270–280. [DOI] [PubMed] [Google Scholar]
- Szczesny SE, Caplan JL, Pedersen P & Elliott DM (2015). Quantification of interfibrillar shear stress in aligned soft collagenous tissues via notch tension testing. Sci Rep 5, 14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q, Lui PP & Lee YW (2013). In vivo identity of tendon stem cells and the roles of stem cells in tendon healing. Stem Cells Dev 22, 3128–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Chaudhry S, Lei, II , Varone A, Riley GP, Birch HL, Clegg PD & Screen HR (2015). Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand J Med Sci Sports 25, e381‐391. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD & Birch HL (2010). Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J Biol Chem 285, 15674–15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli P, Veronesi F, Pagani S, Maffulli N, Masiero S, Frizziero A & Fini M (2013). In vitro tenocyte metabolism in aging and oestrogen deficiency. Age (Dordr) 35, 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WC, Hsu CC, Chen CP, Chang HN, Wong AM, Lin MS & Pang JH (2011). Ciprofloxacin up‐regulates tendon cells to express matrix metalloproteinase‐2 with degradation of type I collagen. J Orthop Res 29, 67–73. [DOI] [PubMed] [Google Scholar]
- Viidik A, Nielsen HM & Skalicky M (1996). Influence of physical exercise on aging rats: II. Life‐long exercise delays aging of tail tendon collagen. Mech Ageing Dev 88, 139–148. [DOI] [PubMed] [Google Scholar]
- Vogel HG ( 1978). Influence of maturation and age on mechanical and biochemical parameters of connective tissue of various organs in the rat. Connect Tissue Res 6, 161–166. [DOI] [PubMed] [Google Scholar]
- Wang JH, Jia F, Yang G, Yang S, Campbell BH, Stone D & Woo SL (2003). Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res 44, 128–133. [DOI] [PubMed] [Google Scholar]
- Wang JHC ( 2006). Mechanobiology of tendon. J Biomech 39, 1563–1582. [DOI] [PubMed] [Google Scholar]
- Webb K, Hitchcock R, Smeal R, Li W, Gray S & Tresco P (2006). Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast‐seeded polyurethane constructs. J Biomech 39, 1136–1144. [DOI] [PubMed] [Google Scholar]
- Weiss PH & Klein L (1969). The quantitative relationship of urinary peptide hydroxyproline excretion to collagen degradation. J Clin Invest 48, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams IF, Craig AS, Parry DAD, Goodship AE, Shah J & Silver IA (1985). Development of collagen fibril organization and collagen crimp patterns during tendon healing. Int J Biol Macromol 7, 275–282. [Google Scholar]
- Woo SL, Gomez MA, Woo YK & Akeson WH (1982). Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology 19, 397–408. [DOI] [PubMed] [Google Scholar]
- Wood LK, Arruda EM & Brooks SV (2011). Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol 111, 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Ohno K, Hayashi K, Kuriyama H, Yasuda K & Kaneda K (1993). Effects of stress shielding on the mechanical properties of rabbit patellar tendon. J Biomech Eng 115, 23–28. [DOI] [PubMed] [Google Scholar]
- Yang G, Crawford RC & Wang JH (2004). Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum‐free conditions. J Biomech 37, 1543–1550. [DOI] [PubMed] [Google Scholar]
- Zhang J & Wang JH (2015). Moderate Exercise Mitigates the Detrimental Effects of Aging on Tendon Stem Cells. PLoS One 10, e0130454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yuan T & Wang JH (2016). Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats. Oncotarget 7, 8498–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Akinbiyi T, Xu L, Ramcharan M, Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL & Sun HB (2010). Tendon‐derived stem/progenitor cell aging: defective self‐renewal and altered fate. Aging cell 9, 911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]


