Abstract
The maintenance of skeletal muscle mass and strength throughout life is a key determinant of human health and well‐being. There is a gradual loss of both skeletal muscle mass and strength with ageing (a process termed sarcopenia) that increases the risk of functional dependence, morbidity and mortality. Understanding the factors that regulate the size of human muscle mass, particularly during the later years of life, has therefore become an area of intense scientific inquiry. The amount of muscle mass is determined by coordinated changes in muscle protein synthesis (MPS) and muscle protein breakdown (MPB). In this review, we assess both classical and contemporary work that has examined how resistance exercise and nutrition impact on MPS and MPB. Special consideration is given to the role of different sources of dietary protein (food vs. supplements) and non‐protein nutrients such as omega‐3 fatty acids in regulating MPS. We also critically evaluate recent studies that have employed novel ‘omic’ technologies such as dynamic protein profiling to probe for changes in rates of MPS and MPB at the individual protein level following exercise. Finally, we provide suggestions for future research that we hope will yield important information for the development of exercise and nutritional strategies to counteract muscle loss in a variety of clinical settings.
Keywords: Food, Supplement, Omega‐3, Proteome, Exercise
Introduction
In the last century, the study of human health has shifted from one focused on the extension of lifespan to the protraction of healthspan. We loosely define healthspan as the amount of time spent in a state of functional independence and free of major disease. Critical to the sustenance of healthspan is the maintenance of skeletal muscle mass and strength throughout life. In particular, skeletal muscle provides the requisite contractile force for locomotive activities, which enables independent living and can enhance key aspects of health such as insulin sensitivity and joint mobility. Skeletal muscle also plays an important role in the recovery from major disease and it is now well established that possessing a greater proportion of skeletal muscle to total body mass is associated with increased survival and recovery rates from several disease states (van Venrooij et al. 2012; Cho et al. 2016; Shachar et al. 2016; Landi et al. 2017). However, as we age, there is a natural loss of skeletal muscle mass and strength, termed sarcopenia (Rosenberg, 1997), that is associated with a 2‐ to 3‐fold increased risk of falls, frailty, disability, loss of independence, and mortality (Cao & Morley, 2016). Sarcopenia affects ∼10–30% of independently living older adults without major illness, and is even more prevalent in those with chronic diseases and/or institutionalized older adults (Cao & Morley, 2016). Despite continued debate as to the cellular mechanisms and the exact definitions of what constitutes sarcopenia (Fielding et al. 2011), the negative health effects associated with sarcopenia have become such a world‐wide health concern (Drew, 2018) that it has recently been recognized as an independent condition having its own International Classification of Disease (Cao & Morley, 2016).
Regulation of skeletal muscle mass by exercise and feeding
The study of the biological mechanisms regulating the size of human muscle mass has, and continues to be, one of intense scientific inquiry. Over 30 years ago, Professor Mike Rennie and colleagues (Rennie et al. 1982) demonstrated for the first time that the incorporation rate of an intravenously infused stable isotope‐labelled amino acid tracer into skeletal muscle protein is stimulated by feeding. Compared to overnight fasting conditions, the muscle protein synthesis (MPS) rate doubled postprandially, and the postprandial increase in MPS accounted for the majority of the positive net protein balance at the whole‐body level (Rennie et al. 1982). Since then, a series of studies by Professor Rennie's group (e.g. Rennie et al. 2004; Atherton et al. 2010) and others (e.g. Biolo et al. 1995, 1997; Reidy et al. 2017) have explored the diurnal regulation of muscle protein turnover (e.g. the balance between synthesis and breakdown). From this work we have learned that during basal, postabsorptive conditions, the rate of muscle protein breakdown (MPB) exceeds the rate of MPS, causing net loss of protein (Rennie et al. 2004), and meal intake compensates for these losses because dietary protein‐derived amino acids stimulate MPS and insulin suppresses MPB (Rennie et al. 2004). The postprandial net protein gain is largely determined by the amount of protein ingested, because the resulting increase in essential amino acids in plasma stimulates MPS in a dose‐dependent manner up to ∼30 g of dietary protein (or an equivalent amount of amino acids) (Cuthbertson et al. 2005; Moore et al. 2015) whereas the concentration of insulin necessary to achieve maximal suppression of MPB (∼15–30 mU/L) already occurs after consuming a small amount of protein or carbohydrate (Bohe et al. 2001; Greenhaff et al. 2008; Moore et al. 2009). Accordingly, simply eating more protein results in increased rates of amino acid oxidation without a further increase in MPS (Moore et al. 2015).
Besides protein/amino acid feeding, exercise serves as the other main anabolic stimulus to skeletal muscle. Classic work by Rennie et al. (1981) revealed that an acute bout of exercise resulted in an improved post‐exercise whole body net protein balance through a rise of whole body protein synthesis in excess of protein breakdown. Over a decade later, similar findings were made at the level of the skeletal muscle showing that a single bout of resistance exercise increased both MPS and MPB for up to 48 h, but while the relative stimulation of MPS was greater than MPB, MPB still exceeded MPS in the fasted state, resulting in no net muscle protein accretion (Phillips et al. 1997). Importantly, the effects of exercise and protein ingestion are additive (Witard et al. 2009; Pennings et al. 2011). Exercise training therefore increases muscle mass largely because of an increase in MPS rather than suppression of MPB.
Ageing results in characteristic changes in muscle protein turnover, referred to as age‐associated ‘anabolic resistance’ (Moore et al. 2015), which is characterized by a blunted response of both MPS and MPB to the anabolic effects of amino acids and exercise and the antiproteolytic effect of insulin, which leads to a gradual loss of muscle mass. The first evidence for this phenomenon was provided by Professor Rennie and colleagues (Cuthbertson et al. 2005; Wilkes et al. 2009; Kumar et al. 2009a), who demonstrated that, although the basal rates of MPS and MPB are not different in young and older adults, the increase in MPS to amino acid ingestion is diminished in older adults. Later, they demonstrated that the insulin‐mediated suppression of MPB (Wilkes et al. 2009) and the exercise‐induced increase in MPS (Kumar et al. 2009b) are also reduced in older compared to young adults. The presence of anabolic resistance in older adults has since been confirmed by several other investigators (Moore et al. 2015). In addition, this anabolic resistance affects older women more than older men (Smith et al. 2012). Strategies to prevent and treat age‐associated sarcopenia therefore focus on overcoming this anabolic resistance (Bauer et al. 2013). These efforts have led to novel discoveries related to the potential importance of non‐protein food components for the regulation of muscle protein turnover.
Muscle protein remodelling after ingestion of protein‐rich foods
Although we have learned a lot about the regulation of muscle protein turnover from studies that administered isolated protein or amino acids, food is what people eat and it has become evident that although protein/amino acids are the primary anabolic stimulus for muscle, the source of dietary protein and interactions with the food matrix could have important effects on the biological activity of protein/amino acids. While ingestion of protein‐rich lean meat was found to stimulate MPS in a dose‐dependent curvilinear manner in both young and older adults (Symons et al. 2009; Robinson et al. 2013), and the MPS response to lean beef ingestion was maximal with a serving size of ∼113–170 g (4–6 oz), which provides ∼30–35 g of protein. Studies of whole food proteins are rare, but have been made. For example, ingestion of isonitrogenous skimmed milk vs. lean (97% fat‐free) beef resulted in greater availability of circulating dietary amino acids after ingestion of beef than milk in the early postprandial phase (0–2 h), yet paradoxically the MPS response was greater after milk than beef during this time (Burd et al. 2015). When assessed over a 5 h period, no differences were observed in MPS rates between lean beef and skimmed milk, despite a trend towards higher dietary amino acid availability after beef ingestion. A recent comparison of egg white vs. whole egg (both providing 18 g of dietary protein) consumption during post‐exercise recovery in healthy young men revealed a superior 5 h MPS response after whole egg ingestion, despite similar postprandial amino acid profiles after ingestion of these two protein sources (van Vliet et al. 2017). These findings confirmed the results of a previous study in which whole milk consumption resulted in a greater net uptake of amino acids by skeletal muscle (presumably for use in protein synthesis) compared to ingestion of skimmed milk matched for protein content (Elliot et al. 2006).
Besides amino acids, protein‐rich foods often also provide a wide spectrum of lipids, vitamins, minerals and other bioactive compounds (growth factors, peptides, miRNAs, etc.; Moller et al. 2008; Zanovec et al. 2010; Phillips et al. 2015) that appear capable of modulating the MPS response upon ingestion. For instance, in animal models it has been demonstrated that the provision of oleate (Tardif et al. 2011), the vitamins A, D and E, and the minerals selenium and zinc can also directly influence the muscle anabolic response (Zhao et al. 2016; Chanet et al. 2017). Moreover, Smith et al. demonstrated that 8 weeks of fish oil‐derived omega‐3 fatty acid supplementation increased the EPA and DHA content in skeletal muscle phospholipids and enhanced MPS under hyperaminoacidaemic‐hyperinsulinaemic conditions in both older (Smith et al. 2011a) and younger adults (Smith et al. 2011b). The same authors extended this work by demonstrating that supplementation with fish oil‐derived omega‐3 fatty acids increased skeletal muscle mass and function in healthy older adults (Smith et al. 2015). This report corroborated findings by other investigators who have shown that dietary supplementation with omega‐3 fatty acids promoted gains in skeletal muscle strength during exercise training in older women (Rodacki et al. 2012; Da Boit et al. 2017). The biological mechanisms by which these fatty acids might regulate skeletal muscle anabolism remain unknown. Incorporation of the highly unsaturated fatty acids EPA and DHA into the diet has been associated with enhanced mechanistic target of rapamycin complex‐1 signalling (Smith et al. 2011a; Kamolrat et al. 2013; McGlory et al. 2014), which provides one potential mechanism through which anabolism might be augmented. It is also possible that omega‐3 fatty acid incorporation into skeletal muscle alters lipid raft formation (Hou et al. 2016) and the transmission of mechanical and nutritional cues to the translational machinery. Thus, omega‐3 fatty acids represent a promising area of research for the development of nutritional strategies to counteract anabolic resistance in older adults. The next logical step would be to investigate whether ingestion of fatty acids such as omega‐3s can offset skeletal muscle atrophy during periods of bed rest/muscle disuse in ageing persons. Such data would inevitably have an important clinical impact, given the pervasive metabolic consequences of periodic muscle disuse in older adults (English & Paddon‐Jones, 2010; McGlory et al. 2017).
In summary, we have only recently begun to understand, and perhaps appreciate, the complexity of various food sources and their role in stimulating remodelling of the muscle proteome. Clearly much more work is needed to understand how ingestion of food, as opposed to protein/amino acids alone, may affect daily protein requirements, particularly in older individuals. However, current evidence would suggest that the presence of vitamins, minerals, lipids and other bioactive nutrients in food work in concert with amino acids/protein to support the postprandial rise in MPS. Elucidating the active biological non‐protein components of food, such as specific lipid species, may yield important data relevant to the development of novel nutraceutical therapies to promote musculoskeletal health in older adults.
Proteomic/phosphoproteomic analysis of muscle protein turnover
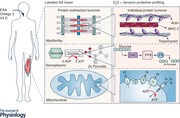
Traditional methods of assessing protein turnover in humans rely on the infusion of a stable isotope‐labelled amino acids coupled with serial muscle biopsies. The muscle biopsy samples are analysed for tracer incorporation into mixed or specific muscle protein fractions (i.e. myofibrillar, sarcoplasmic, mitochondrial) or a few select individual proteins (e.g. actin or myosin heavy chain). How resistance exercise and nutrition affect turnover rates of the numerous individual proteins that constitute skeletal muscle has remained unknown until lately. In an attempt to address this knowledge gap, Murphy and colleagues (2018) administered deuterated water (D2O) to their study participants and used tandem‐mass spectrometric proteomic analysis of muscle biopsy samples (Shankaran et al. 2016b) to identify changes in the synthetic rates of > 150 individual muscle proteins contained in the myofibrillar, mitochondrial and sarcoplasmic protein fractions. The primary finding from this study (Murphy et al. 2018) was that older adults performing resistance exercise during an energy restricted state enhanced the synthesis of 175 out of 195 measured individual skeletal muscle proteins in each of the specific protein fractions. Besides upregulation of individual skeletal muscle proteins in the myofibrillar (i.e. contractile) category, the synthesis of proteins involved in the regulation of numerous metabolic processes such as glycolysis (i.e. 6‐phosphofrucktokinase) and the electron transport chain (i.e. ATP synthase subunits) were also increased after resistance exercise.
At about same time as Murphy and colleagues (2018) reported their results, Camera and colleagues (2017a) employed the dynamic proteome profiling approach to evaluate the effect of resistance exercise during a high‐fat, low‐carbohydrate diet on the synthesis and breakdown of individual muscle proteins in young adults. They found the abundance of 28 out of 90 measured myofibrillar and sarcoplasmic proteins increased and that this was due to both increased synthesis and decreased breakdown rates of these proteins. Interestingly, the initial increase in protein abundance after resistance exercise occurred with little to no change in protein synthesis, thus highlighting the potential key role played by MPB in the remodelling of the muscle proteome with resistance exercise. Such findings would seem at odds with extant knowledge that changes in the proteome with resistance exercise are predominantly driven by changes in rates of MPS. However, it is important to interpret these findings with a degree of caution as the rate of breakdown was estimated from the difference between the change in protein abundance and synthesis over time. Thus, protein breakdown was not directly assessed and 11 proteins even demonstrated a physiologically impossible negative value for the estimated breakdown rate, highlighting the need for further improvement in ‘omics’ approaches used to study the role of MPB in the skeletal muscle adaptive response to physical activity and nutritional interventions. As the authors acknowledge, their findings related to MPB could be attributable to technical errors but it is also possible that amino acid recycling may have increased protein abundance to a greater extent than predicted by synthesis. Nevertheless, this study (Camera et al. 2017a) demonstrates the power of dynamic proteome profiling applied to human skeletal muscle research, which could be used to discover novel and clinically important interventions to improve musculoskeletal health. For instance, one finding of the study (Camera et al. 2017b) was that in response to resistance exercise there was a significant increase in the synthesis of myosin heavy chain protein, but only that of fast‐twitch 2a (MYHC2), which is a fascinating discovery given that sarcopenia is characterized by a reduction in fast twitch fibre expression (Lexell et al. 1988).
Although advances in molecular/cell biology techniques over the past 20 years have provided important insights into the regulation of muscle protein turnover, our understanding of the mechanisms responsible for muscle loss with advancing age remain elusive. There is evidence that failure to adequately activate mTORC‐1 and subsequently translate genes that encode muscle proteins plays some role. However, such signalling networks are multifaceted and interrelated, and unravelling the complexity of these interactions is unlikely to be achieved by employing contemporary approaches such as immunoblotting or immunohistochemistry alone. One interesting avenue of research that may provide some answers is phosphoproteomics. Phosphoproteomics provides a global and unbiased approach to studying changes in phosphorylation networks in response to anabolic stimulation. As an example, two recent studies have identified novel phosphorylation sites on numerous protein kinases following endurance exercise in humans (Hoffman et al. 2015) and maximal eccentric contractions in rodents (Potts et al. 2017). One of these two studies (Potts et al. 2017) discovered a striated muscle‐specific serine/threonine protein kinase and obscurin as contraction‐sensitive z‐disk kinases that may sense mechanical cues for transmission to the translational machinery. Whether failure to fully activate these and/or other related kinases plays some role in the anabolic resistance of older adults to exercise and even nutrition remains unknown. Future research that combines proteomic (Shankaran et al. 2016b), phosphoproteomic (Potts et al. 2017) and, potentially, lipidomic (Jeromson et al. 2017) technologies to study skeletal muscle plasticity following exercise and feeding in both older and young adults would no doubt address this important gap in our understanding (Figure 1).
Figure 1.
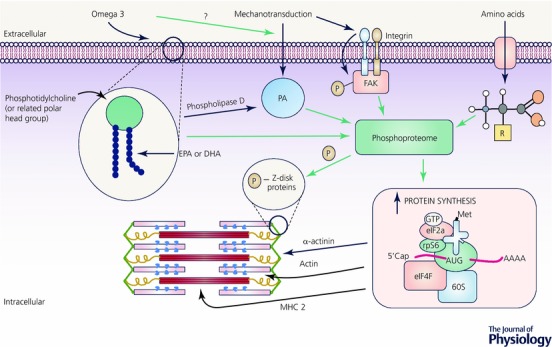
Illustration of lipidomic, proteomic, and phosphoproteomic interactions in skeletal muscle
Future directions
Since the first studies of protein turnover, which relied on the arterio‐venous balance approach and measuring labelled amino acid incorporation into muscle proteins, significant strides have been made in our understanding of the factors that regulate muscle protein turnover following exercise and nutrition. The next era of scientific advancement in this field will no doubt be underpinned by the use of ‘omic’ technologies (e.g. lipidomic, proteomic, metaboliomic, transcriptomic). A primary advantage of applying ‘omic’ technologies to study muscle protein turnover and associated intracellular signalling networks is that the impact of nutritional and exercise strategies can be examined in greater scope and detail. Unbiased global analysis of changes in muscle protein kinetics and the molecular factors that regulate these changes will help pinpoint age‐ and disease‐related alterations in entire biological networks. There is now also evidence that proteins assembled in muscle, such as creatine kinase M‐type and carbonic anhydrase, escape into the circulation and that the fractional turnover rates of these proteins in plasma can be used as a surrogate marker of muscle protein turnover (Shankaran et al. 2016a; Murphy et al. 2018). Such data open up the exciting possibility of studying the impact of exercise and nutrition on muscle protein turnover with minimally invasive (blood draw) techniques. This is particularly important when dealing with compromised individuals, such as those in intensive care units or other similarly precarious clinical scenarios, for whom skeletal muscle biopsy sampling is not practical or possible. Another area worthy of further interrogation is the study of how changes in muscle‐specific lipid species impact on muscle protein turnover. The discovery that omega‐3 fatty acids render skeletal muscle more ‘anabolically sensitive’ was a first step in this direction and requires interrogation of the mechanisms responsible for their beneficial effect on muscle protein turnover, which could include improved membrane fluidity and/or alterations in lipid raft formation. Future work that manipulates phospholipid species content and composition in both pre‐clinical and human models will no doubt help provide the answers.
To conclude, the ageing muscle proteome is an area of intense scientific investigation. By understanding the impact of physical activity and various nutrients sources on human muscle protein turnover at the individual protein level it will be possible to develop better strategies to counteract the normal age‐associated loss of muscle loss and the loss of muscle caused by the numerous age‐associated comorbidities. Achieving this aim will probably be expedited by employing a wide range of new and existing methodologies pari passu.
Additional information
Competing interests
None.
Author contributions
C.M. and S.v.V. wrote the initial draft of the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
C.M. is supported by Diabetes Canada Fellowship award. T.S. acknowledges support from the Labarge Mobility Scholarship support. S.M.P. receives funding from the National Science and Engineering Research Council of Canada, the Canadian Institutes for Health Research, and the Canada Research Chairs program. B.M. received salary support from NIH grants DK115400, DK56341 (Washington University School of Medicine Nutrition and Obesity Research Center), and UL1 TR000448 (Washington University School of Medicine Clinical Translational Science Award), a grant from the American Diabetes Association (ICTS 1‐18‐ICTS‐119), and the Atkins Obesity Award while working on this manuscript. S.v.V. received salary support from NIH grants DK115400 and a grant from the American Diabetes Association (ICTS 1‐18‐ICTS‐119) while working on this manuscript.
Biography
Chris McGlory is a Diabetes Canada‐funded Postdoctoral Research Fellow working with Professor Stuart Phillips at McMaster University, Canada where he is studying the influence of omega‐3 fatty acids on muscle mass, protein synthesis, and mitochondrial function in both humans and rodents. His work employs a range of specialist methodologies such as deuterium labelling of proteins and magnetic resonance imaging, as well as measurements of kinase activity, protein expression and post‐translational modification.

Edited by: Ole Petersen & Bruno Grassi
References
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K & Rennie MJ (2010). Muscle full effect after oral protein: time‐dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92, 1080–1088. [DOI] [PubMed] [Google Scholar]
- Bauer J, Biolo G, Cederholm T, Cesari M, Cruz‐Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D, Visvanathan R, Volpi E & Boirie Y (2013). Evidence‐based recommendations for optimal dietary protein intake in older people: a position paper from the PROT‐AGE Study Group. J Am Med Dir Assoc 14, 542–559. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD & Wolfe RR (1995). Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268, E514–E520. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S & Wolfe RR (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab 273, E122–E129. [DOI] [PubMed] [Google Scholar]
- Bohe J, Low JF, Wolfe RR & Rennie MJ (2001). Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532, 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, Gorissen SH, van Vliet S, Snijders T & van Loon LJ (2015). Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am J Clin Nutr 102, 828–836. [DOI] [PubMed] [Google Scholar]
- Camera DM, Burniston JG, Pogson MA, Smiles WJ & Hawley JA (2017a). Dynamic proteome profiling of individual proteins in human skeletal muscle after a high‐fat diet and resistance exercise. FASEB J 31, 5478–5494. [DOI] [PubMed] [Google Scholar]
- Camera L, Liccardo I, Romano F, Liuzzi R, Rispo A, Imbriaco M, Testa A, Luglio G, De Fronzo S, Castiglione F, Bucci L & Brunetti A (2017b). Diagnostic efficacy of single‐pass abdominal multidetector‐row CT: prospective evaluation of a low dose protocol. Br J Radiol 90, 20160612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L & Morley JE (2016). Sarcopenia is recognized as an independent condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD‐10‐CM) Code. J Am Med Dir Assoc 17, 675–677. [DOI] [PubMed] [Google Scholar]
- Chanet A, Salles J, Guillet C, Giraudet C, Berry A, Patrac V, Domingues‐Faria C, Tagliaferri C, Bouton K, Bertrand‐Michel J, Van Dijk M, Jourdan M , Luiking Y, Verlaan S, Pouyet C, Denis P, Boirie Y & Walrand S (2017). Vitamin D supplementation restores the blunted muscle protein synthesis response in deficient old rats through an impact on ectopic fat deposition. J Nutr Biochem 46, 30–38. [DOI] [PubMed] [Google Scholar]
- Cho HM, Lee K, Min W, Choi YS, Lee HS, Mun HJ, Shim HY, Lee da G & Yoo MJ (2016). Survival and functional outcomes after hip fracture among nursing home residents. J Korean Med Sci 31, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM & Rennie MJ (2005). Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19, 422–424. [DOI] [PubMed] [Google Scholar]
- Da Boit M, Sibson R, Sivasubramaniam S, Meakin JR, Greig CA, Aspden RM, Thies F, Jeromson S, Hamilton DL, Speakman JR, Hambly C, Mangoni AA, Preston T & Gray SR (2017). Sex differences in the effect of fish‐oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am J Clin Nutr 105, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew L (2018). Fighting the inevitability of ageing. Nature 555, S15–S17. [DOI] [PubMed] [Google Scholar]
- Elliot TA, Cree MG, Sanford AP, Wolfe RR & Tipton KD (2006). Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc 38, 667–674. [DOI] [PubMed] [Google Scholar]
- English KL & Paddon‐Jones D (2010). Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S & Zamboni M (2011). Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A & Rennie MJ (2008). Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295, E595–E604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman NJ, Parker BL, Chaudhuri R, Fisher‐Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, Stockli J, Burchfield JG, Jensen TE, Jothi R, Kiens B, Wojtaszewski JF, Richter EA & James DE (2015). Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise‐regulated kinases and AMPK substrates. Cell Metab 22, 922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou TY, McMurray DN & Chapkin RS (2016). Omega‐3 fatty acids, lipid rafts, and T cell signaling. Eur J Pharmacol 785, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeromson S, Mackenzie I, Doherty MK, Whitfield PD, Bell G, Dick J, Shaw A, Rao F, Ashcroft S, Philp A, Galloway S, Gallagher I & Hamilton DL (2017). Lipid remodeling and an altered membrane proteome may drive the effects of EPA and DHA treatment on skeletal muscle glucose uptake and protein accretion. Am J Physiol Endocrinol Metab 314, E605–E619. [DOI] [PubMed] [Google Scholar]
- Kamolrat T, Gray SR & Thivierge MC (2013). Fish oil positively regulates anabolic signalling alongside an increase in whole‐body gluconeogenesis in ageing skeletal muscle. Eur J Nutr 52, 647–657. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N & Rennie MJ (2009a). Age‐related differences in the dose‐response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N & Rennie MJ (2009b). Age‐related differences in the dose‐response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F, Calvani R, Ortolani E, Salini S, Martone AM, Santoro L, Santoliquido A, Sisto A, Picca A & Marzetti E (2017). The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in‐hospital rehabilitation. Osteoporos Int 28, 1569–1576. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC & Sjostrom M (1988). What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15‐ to 83‐year‐old men. J Neurol Sci 84, 275–294. [DOI] [PubMed] [Google Scholar]
- McGlory C, Galloway SD, Hamilton DL, McClintock C, Breen L, Dick JR, Bell JG & Tipton KD (2014). Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot Essent Fatty Acids 90, 199–206. [DOI] [PubMed] [Google Scholar]
- McGlory C, von Allmen MT, Stokes T, Morton RG, Hector AJ, Lago BA, Raphenya AR, Smith BK, McArthur AG, Steinberg GR, Baker SK & Phillips SM (2017). Failed recovery of glycemic control and myofibrillar protein synthesis with 2 wk of physical inactivity in overweight, pre‐diabetic older adults. J Gerontol A Biol Sci Med Sci 73, 1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller NP, Scholz‐Ahrens KE, Roos N & Schrezenmeir J (2008). Bioactive peptides and proteins from foods: indication for health effects. Eur J Nutr 47, 171–182. [DOI] [PubMed] [Google Scholar]
- Moore DR, Churchward‐Venne TA, Witard O, Breen L, Burd NA, Tipton KD & Phillips SM (2015). Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70, 57–62. [DOI] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA & Phillips SM (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89, 161–168. [DOI] [PubMed] [Google Scholar]
- Murphy CH, Shankaran M, Churchward‐Venne TA, Mitchell CJ, Kolar NM, Burke LM, Hawley JA, Kassis A, Karagounis LG, Li K, King C, Hellerstein M & Phillips SM (2018). Effect of resistance training and protein intake pattern on myofibrillar protein synthesis and proteome kinetics in older men in energy restriction. J Physiol 596, 2091–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings B, Koopman R, Beelen M, Senden JM, Saris WH & van Loon LJ (2011). Exercising before protein intake allows for greater use of dietary protein‐derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 93, 322–331. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Fulgoni VL 3rd, Heaney RP, Nicklas TA, Slavin JL & Weaver CM (2015). Commonly consumed protein foods contribute to nutrient intake, diet quality, and nutrient adequacy. Am J Clin Nutr 101, 1346S–1352S. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE & Wolfe RR (1997). Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273, E99–E107. [DOI] [PubMed] [Google Scholar]
- Potts GK, McNally RM, Blanco R, You JS, Hebert AS, Westphall MS, Coon JJ & Hornberger TA (2017). A map of the phosphoproteomic alterations that occur after a bout of maximal‐intensity contractions. J Physiol 595, 5209–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy PT, Borack MS, Markofski MM, Dickinson JM, Fry CS, Deer RR, Volpi E & Rasmussen BB (2017). Post‐absorptive muscle protein turnover affects resistance training hypertrophy. Eur J Appl Physiol 117, 853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL & Millward DJ (1982). Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 63, 519–523. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Krywawych S, Davies CT, Halliday D, Waterlow JC & Millward DJ (1981). Effect of exercise on protein turnover in man. Clin Sci (Lond) 61, 627–639. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H, Spangenburg EE & Booth FW (2004). Control of the size of the human muscle mass. Annu Rev Physiol 66, 799–828. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, Baker SK & Phillips SM (2013). Dose‐dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle‐aged men. Appl Physiol Nutr Metab 38, 120–125. [DOI] [PubMed] [Google Scholar]
- Rodacki CL, Rodacki AL, Pereira G, Naliwaiko K, Coelho I, Pequito D & Fernandes LC (2012). Fish‐oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr 95, 428–436. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH (1997). Sarcopenia: origins and clinical relevance. J Nutr 127, 990s–991s. [DOI] [PubMed] [Google Scholar]
- Shachar SS, Williams GR, Muss HB & Nishijima TF (2016). Prognostic value of sarcopenia in adults with solid tumours: A meta‐analysis and systematic review. Eur J Cancer 57, 58–67. [DOI] [PubMed] [Google Scholar]
- Shankaran M, King CL, Angel TE, Holmes WE, Li KW, Colangelo M, Price JC, Turner SM, Bell C, Hamilton KL, Miller BF & Hellerstein MK (2016a). Circulating protein synthesis rates reveal skeletal muscle proteome dynamics. J Clin Invest 126, 288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran M, Shearer TW, Stimpson SA, Turner SM, King C, Wong PY, Shen Y, Turnbull PS, Kramer F, Clifton L, Russell A, Hellerstein MK & Evans WJ (2016b). Proteome‐wide muscle protein fractional synthesis rates predict muscle mass gain in response to a selective androgen receptor modulator in rats. Am J Physiol Endocrinol Metab 310, E405–E417. [DOI] [PubMed] [Google Scholar]
- Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ & Mittendorfer B (2011a). Dietary omega‐3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 93, 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ & Mittendorfer B (2011b). Omega‐3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia‐hyperaminoacidaemia in healthy young and middle‐aged men and women. Clin Sci (Lond) 121, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S & Mittendorfer B (2015). Fish oil‐derived n‐3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr 102, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GI, Reeds DN, Hall AN, Chambers KT, Finck BN & Mittendorfer B (2012). Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biol Sex Differ 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons TB, Sheffield‐Moore M, Wolfe RR & Paddon‐Jones D (2009). A moderate serving of high‐quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 109, 1582–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif N, Salles J, Landrier JF, Mothe‐Satney I, Guillet C, Boue‐Vaysse C, Combaret L, Giraudet C, Patrac V, Bertrand‐Michel J, Migne C, Chardigny JM, Boirie Y & Walrand S (2011). Oleate‐enriched diet improves insulin sensitivity and restores muscle protein synthesis in old rats. Clin Nutr 30, 799–806. [DOI] [PubMed] [Google Scholar]
- van Venrooij LM, Verberne HJ, de Vos R, Borgmeijer‐Hoelen MM, van Leeuwen PA & de Mol BA (2012). Postoperative loss of skeletal muscle mass, complications and quality of life in patients undergoing cardiac surgery. Nutrition 28, 40–45. [DOI] [PubMed] [Google Scholar]
- van Vliet S, Shy EL, Abou Sawan S, Beals JW, West DW, Skinner SK, Ulanov AV, Li Z, Paluska SA, Parsons CM, Moore DR & Burd NA (2017). Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am J Clin Nutr 106, 1401–1412. [DOI] [PubMed] [Google Scholar]
- Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K & Rennie MJ (2009). Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age‐related sarcopenia. Am J Clin Nutr 90, 1343–1350. [DOI] [PubMed] [Google Scholar]
- Witard OC, Tieland M, Beelen M, Tipton KD, van Loon LJ & Koopman R (2009). Resistance exercise increases postprandial muscle protein synthesis in humans. Med Sci Sports Exerc 41, 144–154. [DOI] [PubMed] [Google Scholar]
- Zanovec M, O'Neil CE, Keast DR, Fulgoni VL 3rd & Nicklas TA (2010). Lean beef contributes significant amounts of key nutrients to the diets of US adults: National Health and Nutrition Examination Survey 1999–2004. Nutr Res 30, 375–381. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Barcus M, Kim J, Lum KL, Mills C & Lei XG (2016). High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J Nutr 146, 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]


