Abstract
Pests and pathogens restrict the production potential of many crop plants. The losses incurred due to pests and diseases are huge threatening food security. Management strategies include use of chemical pesticides which can be detrimental to human health and environment and other physical and biological methods which have serious limitations. An alternative would be to utilize the advanced technology such as RNA interference (RNAi) to engineer disease resistance in crop plants. The phenomenon of RNAi is very well studied in organisms across genera and found to be conserved. Taking advantage of this, dsRNAs have been delivered into pests and pathogens and showed significant growth inhibition. Banana is susceptible to various groups of pathogens which results in poor yield. The proof-of-principle studies using RNAi technology have already been demonstrated in banana to develop resistance to two important groups of pathogens. Transgenic banana plants expressing small interfering RNA targeting BBTV and Fusarium pathogen have shown high level of resistance. In this review, we summarize and discuss the studies utilizing RNAi as a strategy to develop resistance to major banana diseases and encourage further research in exploiting RNAi-based resistance in other crop plants.
Keywords: Banana, RNAi, Fusarium oxysporum f. sp. cubense, BBTV, PTGS, Resistance
Introduction
Pests and diseases cause significant losses to the crop plants thereby threatening food security (Strange and Scott 2005). Migration of plant pests and diseases to different regions of the world can cause serious epidemics thus menacing vulnerable farmers and food security on a global scale (Bebber et al. 2014). Banana is the staple food of many people in developing countries and ranks as the fourth most important crop (Pillay et al. 2012). Banana and plantains are sensitive to abiotic and biotic factors which reduces production drastically. Due to the shallow root system and round the year green canopy, banana plant is vulnerable to damage occurring due to water stress (Dash and Rai 2016). The plant cannot withstand temperatures lower than the ambient temperature of the tropics and sub-tropics (Turner and Lahav 1983; Sreedharan et al. 2013). Moreover, banana plants are constantly under the risk of being attacked by viruses, fungi, bacteria, nematodes and weevil which infects them and cause significant yield losses (Table 1). Banana and plantains were domesticated by humans, right from the time of the recorded history (Heslop-Harrison and Schwarzacher 2007). The popular cultivars are vegetatively propagated and cultivated as monocultures all over the world. As there is no sexual reproduction in the major edible cultivars of banana, the gene pool remained more or less dormant without any genomic variations. However, the pathogens infecting banana were constantly mutating to adapt to the host defense strategies. In the nineteenth century, when the superior clone Gros Michel was infected with the fungus Fusarium oxysporum f. sp. cubense (Foc) race 1, the banana industry had to fetch its substitute for its sustenance (Ploetz 2006; Ghag et al. 2015a). The present day Cavendish are resistant to race 1 strain, but the recently evolved Tropical race 4 (TR4) has started infecting them. Again the banana growers have come to the same juncture where they need to identify a substitute for Cavendish. Since the gene pool of banana and plantains is limited, there are very few known cultivars which show resistance to TR4, and those are diploid wild bananas unsuitable for consumption. Same is the case with other banana diseases such as banana bunchy top disease, Sigatoka disease and weevil infestation. It is essential to identify the genetic basis of resistance that can be transferred to the superior elite varieties. Banana biotechnologists all over the world are putting consorted efforts in developing varieties which can resist these pests and diseases. Elite cultivars of banana are triploid which do not set seeds and so breeding for disease resistance in banana is difficult (Bakry et al. 2009). Nonetheless, researchers in CIRAD (Guadeloupe), CARBAP (Cameroon), IITA (Nigeria), EMBRAPA (Brazil), NRCB (India) and TNAU (India) are able to breed bananas with valuable trait/s.
Table 1.
Major diseases of banana and plantains
| Diseases | Causative agent | Symptoms | References |
|---|---|---|---|
| Viral | |||
| Banana bunchy top | Banana bunchy top virus (BBTV) | Stunted growth, leaf atrophy and chlorosis, young leaves appear choked or bunched appearance like rosette, leaves are upright with wavy margins, dark green dot or dash flecks along the veins | Dale and Harding (1998), Chen and Hu (2013) |
| Banana streak disease | Banana streak virus (BSV) | Yellow streaks on the banana leaves which later turns necrotic, splitting of pseudostem and rotting | Gayral et al. (2008), Manoranjitham et al. (2012) |
| Fungal | |||
| Fusarium wilt | Fusarium oxysporum f. sp. cubense (Foc) | Yellowing of the leaves, splitting of the pseudostem, falling off of the older leaves around the pseudostem, discoloration of the rhizome and pseudostem, wilting of the leaves | Ploetz (2006), Ghag et al. (2015a) |
| Black Sigatoka | Mycosphaerella fijiensis | Reddish-brown flecks on the lower leaf surface which runs parallel to leaf veins, lesions turns dark brown to black and appears sunken later, heavy infestation leads to necrotic lesions and falling off leaves | Carlier et al. (2000a); Etebu and Young-Harry (2011), Kumakech et al. (2015) |
| Yellow Sigatoka | Mycosphaerella musicola | Symptoms similar to black Sigatoka except the early streaks are seen more on the upper surface of the leaves which are yellow–green, narrower and shorter | Surridge et al. (2003), Perera and Kelaniyangoda (2013) |
| Anthracnose | Colletotrichum musae | Development of dark, sunken spots or lesions on affected leaves and fruit, fruit turns black and shrivels | Khan et al. (2001); Zakaria et al. (2009) |
| Septoria leaf spot | Mycosphaerella eumusae | Light brown streaks on the lower side of the leaf, later streaks developed into oval lesions with gray centers as small necrotic, and dark brown spots | Carlier et al. (2000b); Amani and Avagyan (2014) |
| Bacterial | |||
| Xanthomonas wilt | Xanthomonas campestris p. v. musacearum | Yellowing and wilting of leaves, diseased pseudostem shows yellowish bacterial ooze, immature fruits turn yellow, infected plants withers away | Biruma et al. (2007), Tripathi et al. (2009) |
| Moko | Ralstonia solanacearum (race 2) | Symptoms are similar to Fusarium wilt. Older leaves first turn chlorotic and wilt and then younger leaves are affected, internally fruit remains firm but becomes brown and later turns gray, vascular discoloration and presence of milky discharge after suspending in water | Ploetz (2003), Chaube and Pundhir (2005) |
| Rhizome rot |
Erwinia carotovora/ Erwinia chrysanthemi |
Wilting of the foliage, stunting and vascular discoloration of the pseudostem, water soaked lesions at the site of infection followed by necrotic lesions, loss of turgor in tissues, breaking of the pseudostem at the collar, rotting of rhizome and unpleasant odor | Gowen (2012), Arun et al. (2013), Nagrale et al. (2013) |
| Nematode | |||
| Root-knot | Meloidogyne incognita/ Meloidogyne javanica | Swollen, galled primary and secondary roots, root tip growth ceases, yellowing of the leaves, stunted plant growth and reduced fruit yield | Jaizme-Vega et al. (1997), De Waele and Davide (1998) |
| Nematode root rot | Radopholus similis | Root necrosis, stunted growth, delayed fruiting, toppling of the plants | Volcy (2011), Bartholomew et al. (2014) |
Several transgenic bananas have been developed in last few years which showed resistance to different groups of pests and pathogens. Genes such as defensins (Ghag et al. 2012, 2014a; Mohandas et al. 2013), chitinase (Kovács et al. 2013), pflp (Namukwaya et al. 2012), hrap (Tripathi et al. 2010), Xa21 (Tripathi et al. 2014a) cystatins (Atkinson et al. 2004), cysteine protease inhibitors (Roderick et al. 2012; Tripathi et al. 2015), and cell death-related genes namely, Ced9, Bcl-xL, BAG1, BI-1 and DAD1 (Paul et al. 2011; Ghag et al. 2014b) were used to generate resistant transgenic banana lines (Table 2). Expressing defensins, chitinases and cell death-related genes in transgenic banana plants have demonstrated fungal resistance, but ectopic and constitutive expression of these proteins in banana tissues have shown to affect growth and development of banana plants. Field trials were carried out for transgenic banana plants constitutively expressing hrap or pflp genes against Xanthomonas wilt disease. Fifty-nine out of 65 plants evaluated showed no growth anomaly (Tripathi et al. 2014b). Banana plants expressing the rice Xa21 gene were also developed and tested for Xanthomonas wilt disease resistance under glass house conditions (Tripathi et al. 2014a). Cysteine proteinase inhibitors or cystatins obstruct the activity of the major digestive enzymes, the cysteine proteinases, thereby suppressing nematode growth and reproduction. Expressing these inhibitors in transgenic banana plants offered resistance to nematodes under confined field conditions (Tripathi et al. 2015). These studies showed success under laboratory conditions barring a few, as they did not reach the farmers field due to worldwide GMO regulatory issues. Furthermore, expressing defense-related genes using strong promoters in transgenic banana plants have resulted in phenotypic abnormalities and can target beneficial organisms associated with banana plants (Paul et al. 2011; Stefani and Hamelin 2010). This state of affairs lead to the utilization of latest and robust technology like RNA interference (RNAi) for engineering disease resistance against important pests and pathogens in crop plants.
Table 2.
Strategies employed to develop resistance to major diseases of banana
| Diseases | Resistance strategies employed | Level of progress | References |
|---|---|---|---|
| Banana bunchy top disease | RNAi | Laboratory level | Shekhawat et al. (2012) |
| Fusarium wilt | Expression of defensins, magainin (MSI-99) | Laboratory level | Chakrabarti et al. (2003), Ghag et al. (2012, 2014a) |
| Cell death genes | Laboratory level/field level | Paul et al. (2011), Ghag et al. (2014b) | |
| Resistance gene analogs | Field level | Dale et al. (2017) | |
| RNAi | Laboratory level | Ghag et al. (2014c) | |
| Sigatoka | Expression of chitinase | Laboratory level | Kovács et al. (2013) |
| Xanthomonas wilt | Expression of hrap and pflp genes | Field level | Tripathi et al. (2014b) |
| Root rot | Expression of cystatins | Field level | Tripathi et al. (2015) |
RNAi for disease resistance
RNAi is a sequence-specific gene silencing which involves the complex machinery called RNA-induced silencing complex (RISC) which identifies the homologous sequences and cleaves it (Hannon 2002). This mechanism is conserved across plant (called as cosuppression), animal (called as RNAi) and fungal (called as quelling) kingdom and was first elucidated in the nematode Caenorhabditis elegans (Fire et al. 1998). In fact, RNAi has been regarded as a natural component of antiviral defense mechanism in plants. Since the pest or pathogen populations are known to directly interact with host plants to derive nutrition, host plants can be engineered to express the RNAi construct targeting pest or pathogen transcripts. The small interfering RNA (siRNA) processed from the double-stranded RNA (dsRNA) generated in host plant finds entry into the pest or pathogen particularly pathogenic fungi and nematodes and cleave the transcripts important for its growth, development and/or pathogenicity (strategy known as host-induced gene silencing). Herein, no proteins are produced which is one of the important concerns to the GMO regulatory bodies. The dietary small RNAs are generally degraded in human gut due to its harsh environment and the ones enclosed in plant vesicles are poorly bioavailable, thereby reducing the chances of it causing any harm (Sherman et al. 2015; Chan and Snow 2017). Nonetheless, rigorous case by case investigations are warranted to establish potential effects of each of these siRNAs that finds entry into the human system. Further, since this strategy is highly sequence specific, selected genes can be targeted and, therefore, none of the other interacting or beneficial partners are affected. In addition to the detrimental effects of fungicide on environment and human health, some fungal pathogens are developing resistance to these chemicals (Hahn 2014; Hobbelen et al. 2014). Thus, RNAi technology seems to be the most sustainable solution to this problem with no significant environmental impact. This technology can be used to target single or multiple pathogens simultaneously. Moreover, this strategy can be applied using topical sprays or through transgene expression in host plant for durable resistance.
RNAi against banana diseases
The edible bananas (diploids, triploids, and tetraploids) have evolved from the natural hybridization of two originators, Musa acuminata (AA) and Musa balbisiana (BB) (Perrier et al. 2011). The triploid varieties have been cultivated throughout the world because they are more productive than the diploid counterparts. The edible bananas are propagated vegetatively and tissue culture has favored mass production of these varieties. The consumer demand for particular traits directed cultivation of certain varieties that led to monoculture cultivation over large acreage in the banana-growing regions. However, this monoculture practice increased the vulnerability to diseases causing huge economic losses. Banana plantations around the globe are devastated by the fungal diseases, Fusarium wilt and Sigatoka and viral disease, BBTD, while bacterial wilt is destroying plantations in East and central Africa (Dale 1987; Tripathi et al. 2009; Ghag et al. 2015a). Developing resistance in banana is difficult due to complex ploidy, limited gene pool and pathogen diversity. Rapid progress in developing strategies for resistance against different diseases of banana is warranted.
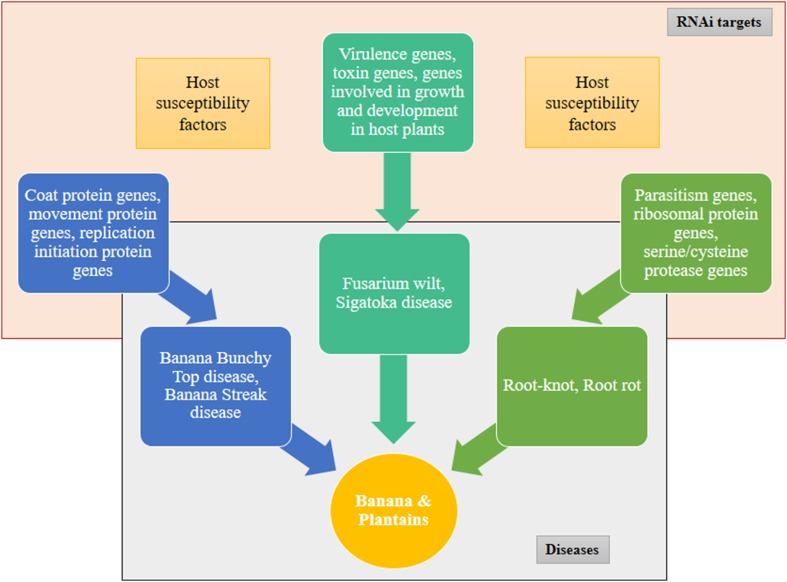
RNA silencing has been exploited as a dominant tool for engineering virus resistance in plants, because viral genome replicates within the host plant and is amenable for direct silencing (Wang et al. 2012). However, there are several suppressors of RNA silencing identified in plant viruses which act as counter defense (Burgyán and Havelda 2011; Pumplin and Voinnet 2013). RNAi strategy has proved effective in case of banana to develop resistance to important diseases such as Fusarium wilt and banana bunchy top disease. Banana bunchy top disease is a destructive viral disease which is transmitted by aphids causing severe stunting and distortion of leaves (Blomme et al. 2011). The infected plant do not produce bunch if infected before maturity or produces distorted bunch if infected later. RNAi-based resistance was introduced into susceptible cultivar of banana (cv. Rasthali), wherein the master replication protein gene ‘Rep’ full coding sequence or ‘Rep’ partial coding sequence together with its 5′ partial upstream regulatory region of the BBTV, was used to generate RNAi vector and successfully transformed into banana (Shekhawat et al. 2012). These plants were resistant to BBTV up to 6 months post-inoculation with viruliferous aphids. Moreover, RT-PCR failed to detect any BBTV-specific transcripts in these transgenic lines. A similar study was carried out using gene fragments from the four BBTV DNA segments namely DNA 1, DNA 3, DNA 4 and DNA 5 to generate RNAi vectors. The transgenic banana plants (cv. Grand Naine) generated showed delayed viral multiplication and development of symptoms (Krishna et al. 2011). It is very important to recognize a suitable target sequence and later to identify a transgenic line which expresses correct siRNA in proper amounts which is able to resist the virus (Fig. 1).
Fig. 1.
RNAi targets to manage important diseases and pests of banana
RNAi has also been successfully applied to protect plants against fungal pathogens (Ghag 2017). Fungal pathogens are known to accumulate small RNAs from host plants during colonization (Zhang et al. 2016). The ones colonizing the host vasculature such as Fusarium oxysporum are more likely to take up these silencing signals from the host plants as these are its sites of transport and storage (Chitwood and Timmermans 2010). Host plant-generated RNAi has proved efficient in managing Fusarium wilt disease (Ghag et al. 2014c). Partial sequences of two crucial genes namely, the velvet protein gene and Fusarium transcription factor 1 gene were assembled separately in the RNAi vectors and transformed into banana plants (cv. Rasthali). The transgenic banana plants did not show external or internal symptoms of Fusarium wilt disease. These plants resisted the infection till 8 months post-inoculation with Foc after which the experiment was terminated. Moreover, small RNAs of 21 nucleotides were formed all along the length of the transgene that targeted the vital Foc transcripts. This indicates that there is no directionality or specificity to form siRNAs when it comes to transgene expression. Therefore, several crucial gene segments from single or multiple pathogens can be stitched together to target all of them simultaneously. Nevertheless, when the ‘Rep’ RNAi vector and velvet RNAi vector were co-expressed in the transgenic banana plants it imparted resistance to both the pathogens (BBTV and Foc) (Ghag et al. 2015b).
There are no studies to demonstrate RNAi effectiveness against banana nematodes and weevil. However, in vitro RNAi-based studies were conducted on nematode Radopholus similis (causative agent of banana root lesion) and banana weevil Cosmopolites sordidus. R. similis Cathepsin B (Rs-cb-1) mRNA is expressed in the esophageal glands, intestines and gonads of the females, testes of males, juveniles and eggs which when targeted using dsRNA showed significant inhibition in development and hatching and also greatly reduced its pathogenicity (Li et al. 2015). Four pests of banana, Radopholus similis, Pratylenchus coffeae, Meloidogyne incognita and Helicotylenchus multicinctus were targeted by generating dsRNA against the conserved domains of Proteasomal alpha subunit 4 and Actin-4. In vitro Actin-4 dsRNA treatment on R. similis impaired motility and reduced nematode multiplication on carrot discs (Roderick et al. 2018). In vitro feeding of ubiquitin E2 gene dsRNA to banana weevil larva significantly arrested banana weevil larval growth and caused up to 100% mortality at 21 days (Ocimati et al. 2016). Transgenic banana plants expressing siRNA or hairpin RNA of the above validated genes could, therefore, potentially be used for controlling the banana nematode and weevil infestation (Fig. 1).
Future prospects
RNAi is a potential strategy to control an array of pests and pathogens without having any significant side effects; which is quite common with transgenics expressing potent defense molecules and/or using harmful chemical pesticides. Although, there are off-target effects seen in RNAi-based approaches when there is more than 95% similarity in the sequences (Xing and Zachgo 2007). Identifying suitable target sequence which is specific for a given pest or pathogen population and with no corresponding homologous sequence in the host plant or any other non-target organisms resolves this problem. With the existing technology, it is becoming easier to identify such target sequences because of the availability of large-scale sequencing data derived from the genome, transcriptome or secretome of the host and pathogen species and accessibility of numerous bioinformatic tools. Particular gene sequences can be targeted and its homologous sequences can be identified in other organism just by a simple in silico homology search tool. Nevertheless, the non-exonic regions which are poorly conserved across species can be targeted to avoid off-target and non-target effects. Moreover, particular sequence stretches from different pathogens can be stacked together in a single RNAi vector to achieve resistance to different groups of pathogens. Further, to avoid ectopic expression and causing any undesirable effect, siRNAs can be expressed particularly in specified tissues using tissue-specific promoters. For example, in banana, siRNAs targeting root pathogens such as Fusarium or banana nematodes can be expressed specifically in banana roots using root-specific promoters and, therefore, it will not be expressed in fruits. This approach will not only help in mitigating biosafety concerns but might also relieve some hurdles in GMO regulatory approvals. Biosafety studies are essential to determine the safety of these siRNA in food and feed. Much more research is still warranted to understand the environmental fate of these siRNAs. Once all these studies are accomplished and we have enough knowledge and understanding supported with the experimental findings, undoubtedly RNAi technology will be highly efficient in managing pests and pathogens affecting susceptible crop plants.
Acknowledgements
SBG thank Department of Science and Technology, India and Indian National Science Academy for the INSPIRE Faculty award.
Compliance with ethical standards
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
References
- Amani M, Avagyan G. First report of banana septoria leaf spot disease caused by Septoria eumusae in Iran. Int J Farming Allied Sci. 2014;3:1140–1144. [Google Scholar]
- Arun WA, Bohra P, Umesha K, Chandrashekar SC, Sathyanarayana BN, Sreeramu BS. Successful rescue and field establishment of native banana varieties severely affected by rhizome rot. J Agric Rural Dev Trop Subtrop (JARTS) 2013;113:147–154. [Google Scholar]
- Atkinson HJ, Grimwood S, Johnston K, Green J. Prototype demonstration of transgenic resistance to the nematode Radopholus similis conferred on banana by a cystatin. Transgenic Res. 2004;13:135–142. doi: 10.1023/B:TRAG.0000026070.15253.88. [DOI] [PubMed] [Google Scholar]
- Bakry F, Carreel F, Jenny C, Horry JP. Genetic improvement of banana. In: Jain SM, Priyadarshan PM, editors. Breeding plantation tree crops: tropical species. New York: Springer; 2009. pp. 3–50. [Google Scholar]
- Bartholomew ES, Brathwaite RA, Isaac WAP. Control of root-burrowing nematode (Radopholus similis) in banana using extracts of Azadirachta indica and Allium sativum. J Org Syst. 2014;9:49–55. [Google Scholar]
- Bebber DP, Holmes T, Gurr SJ. The global spread of crop pests and pathogens. Glob Ecol Biogeogr. 2014;23:1398–1407. doi: 10.1111/geb.12214. [DOI] [Google Scholar]
- Biruma M, Pillay M, Tripathi L, Blomme G, Abele S, Mwangi M, Bandyopadhyay R, Muchunguzi P, Kassim S, Nyine M, Turyagyenda L. Banana Xanthomonas wilt: a review of the disease, management strategies and future research directions. Afr J Biotechnol. 2007;6:953–962. [Google Scholar]
- Blomme G, Eden-Green S, Mustaffa M, Nwauzoma B, Thangavelu R. Major diseases of banana. Banana breeding: progress and challenges. Boca Raton: CRC Press; 2011. pp. 85–119. [Google Scholar]
- Burgyán J, Havelda Z. Viral suppressors of RNA silencing. Trends Plant Sci. 2011;16:265–272. doi: 10.1016/j.tplants.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Carlier J, Fouré E, Gaul F, Jones DR, Lepoivre P, Mourichon X, Pasberg-Gauhl C, Romero RA. Fungal diseases of the foliage. In: Jones DR, editor. Diseases of banana, abaca and enset. Wallingford: CABI; 2000. pp. 37–79. [Google Scholar]
- Carlier J, Zapater MF, Lapeyre F, Jones DR, Mourichon X. Septoria leaf spot of banana: a newly discovered disease caused by Mycosphaerella eumusae (anamorph Septoria eumusae) Phytopathology. 2000;90:884–890. doi: 10.1094/PHYTO.2000.90.8.884. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Ganapathi TR, Mukherjee PK, Bapat VA. MSI-99, a magainin analogue, imparts enhanced disease resistance in transgenic tobacco and banana. Planta. 2003;216:587–596. doi: 10.1007/s00425-002-0918-y. [DOI] [PubMed] [Google Scholar]
- Chan SY, Snow JW. Formidable challenges to the notion of biologically important roles for dietary small RNAs in ingesting mammals. Genes Nutr. 2017;12:13. doi: 10.1186/s12263-017-0561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaube HS, Pundhir VS. Crop diseases and their management. Prentice Hall: PHI Learning Pvt. Ltd; 2005. [Google Scholar]
- Chen Y, Hu X. High-throughput detection of banana bunchy top virus in banana plants and aphids using real-time TaqMan® PCR. J Virol Methods. 2013;193:177–183. doi: 10.1016/j.jviromet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Chitwood DH, Timmermans MC. Small RNAs are on the move. Nature. 2010;467:415. doi: 10.1038/nature09351. [DOI] [PubMed] [Google Scholar]
- Dale JL. Banana bunchy top: an economically important tropical plant virus disease. Adv Virus Res. 1987;33:301–326. doi: 10.1016/S0065-3527(08)60321-8. [DOI] [PubMed] [Google Scholar]
- Dale JL, Harding RM. Banana bunchy top disease: current and future strategies for control. In: Hadidi A, Khertarpal RK, Koganezawa H, editors. Plant virus disease control. St. Paul: APS Press; 1998. pp. 659–669. [Google Scholar]
- Dale J, James A, Paul JY, Khanna H, Smith M, Peraza-Echeverria S, Garcia-Bastidas F, Kema G, Waterhouse P, Mengersen K, Harding R. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat Commun. 2017;8:1496. doi: 10.1038/s41467-017-01670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Rai R. Translating the “Banana Genome” to delineate stress resistance, dwarfing, parthenocarpy and mechanisms of fruit ripening. Front Plant Sci. 2016;7:1543. doi: 10.3389/fpls.2016.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waele D, Davide RG (1998) The root- knot nematodes of Banana. In: Musa Pest Fact, International Network for the improvement of Banana and Plantain (INIBAP), Parc Scientifique Agropolis II, 34 397 Montpellier Cedex 5, France. Sheet No. 3
- Etebu E, Young-Harry W. Control of black Sigatoka disease: challenges and prospects. Afr J Agric Res. 2011;6:508–514. [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Gayral P, Noa-Carrazana JC, Lescot M, Lheureux F, Lockhart BE, Matsumoto T, Piffanelli P, Iskra-Caruana ML. A single banana streak virus integration event in the banana genome as the origin of infectious endogenous pararetrovirus. J Virol. 2008;82:6697–6710. doi: 10.1128/JVI.00212-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghag SB. Host induced gene silencing, an emerging science to engineer crop resistance against harmful plant pathogens. Physiol Mol Plant Pathol. 2017;100:242–254. doi: 10.1016/j.pmpp.2017.10.003. [DOI] [Google Scholar]
- Ghag SB, Shekhawat UKS, Ganapathi TR. Petunia floral defensins with unique prodomains as novel candidates for development of Fusarium wilt resistance in transgenic banana plants. PLoS One. 2012;7:e39557. doi: 10.1371/journal.pone.0039557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghag SB, Shekhawat UKS, Ganapathi TR. Transgenic banana plants expressing a Stellaria media defensin gene (Sm-AMP-D1) demonstrate improved resistance to Fusarium oxysporum. Plant Cell Tiss Org Cult. 2014;119:247–255. doi: 10.1007/s11240-014-0529-x. [DOI] [Google Scholar]
- Ghag SB, Shekhawat UKS, Ganapathi TR. Native cell-death genes as candidates for developing wilt resistance in transgenic banana plants. AoB Plants. 2014;6:plu037. doi: 10.1093/aobpla/plu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghag SB, Shekhawat UK, Ganapathi TR. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol J. 2014;12:541–553. doi: 10.1111/pbi.12158. [DOI] [PubMed] [Google Scholar]
- Ghag SB, Shekhawat UKS, Ganapathi TR. Fusarium wilt of banana: biology, epidemiology and management. Int J Pest Manag. 2015;61:250–263. doi: 10.1080/09670874.2015.1043972. [DOI] [Google Scholar]
- Ghag SB, Shekhawat UKS, Hadapad AB, Ganapathi TR. Stacking of host-induced gene silencing mediated resistance to banana bunchy top virus and Fusarium wilt disease in transgenic banana plants. Curr Trends Biotechnol Pharm. 2015;9:212–221. [Google Scholar]
- Gowen S, editor. Bananas and plantains. New York: Springer Science & Business Media; 2012. [Google Scholar]
- Hahn M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Biol. 2014;7:133–141. doi: 10.1007/s12154-014-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon GJ. RNA interference. Nature. 2002;418:244. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison JS, Schwarzacher T. Domestication, genomics and the future for banana. Ann Bot. 2007;100:1073–1084. doi: 10.1093/aob/mcm191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbelen PH, Paveley ND, van den Bosch F. The emergence of resistance to fungicides. PLoS One. 2014;9:e91910. doi: 10.1371/journal.pone.0091910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaizme-Vega MC, Tenoury P, Pinochet J, Jaumot M. Interactions between the root-knot nematode Meloidogyne incognita and Glomus mosseae in banana. Plant Soil. 1997;196:27–35. doi: 10.1023/A:1004236310644. [DOI] [Google Scholar]
- Khan SH, Aked J, Magan N. Control of the anthracnose pathogen of banana (Colletotrichum musae) using antioxidants alone and in combination with thiabendazole or imazalil. Plant Pathol. 2001;50:601–608. doi: 10.1046/j.1365-3059.2001.00599.x. [DOI] [Google Scholar]
- Kovács G, Sági L, Jacon G, Arinaitwe G, Busogoro JP, Thiry E, Strosse H, Swennen R, Remy S. Expression of a rice chitinase gene in transgenic banana (‘Gros Michel’, AAA genome group) confers resistance to black leaf streak disease. Transgenic Res. 2013;22:117–130. doi: 10.1007/s11248-012-9631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna B, Kadu, AA, Vyavhare SN, Chaudhary RS, Joshi SS, Patil AB, Subramaniam VR, Sane PV (2011) RNAi-mediated resistance against banana bunchy top virus (BBTV) in ‘Grand Nain’ banana. In: II Genetically modified organisms in horticulture symposium, vol 974, pp 157–164
- Kumakech A, Jørgensen HL, Edema R, Okori P. Efficient screening procedure for black sigatoka disease of banana. Afr Crop Sci J. 2015;23:387–397. doi: 10.4314/acsj.v23i4.8. [DOI] [Google Scholar]
- Li Y, Wang K, Xie H, Wang DW, Xu CL, Huang X, Wu WJ, Li DL. Cathepsin B cysteine proteinase is essential for the development and pathogenesis of the plant parasitic nematode Radopholus similis. Int J Biol Sci. 2015;11:1073. doi: 10.7150/ijbs.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoranjitham SK, Kavino M, Thiribhuvanamala G, Ganapathy T, Rabindran R, Kumar N. Detection of banana streak virus (BSV) Tamil Nadu isolate (India) and its serological relationship with other badna viruses. Afr J Biotechnol. 2012;11:14632–14637. [Google Scholar]
- Mohandas S, Sowmya HD, Saxena AK, Meenakshi S, Rani RT, Mahmood R. Transgenic banana cv. Rasthali (AAB, Silk gp) harboring Ace-AMP1 gene imparts enhanced resistance to Fusarium oxysporum f. sp. cubense race 1. Sci Hortic. 2013;164:392–399. doi: 10.1016/j.scienta.2013.09.018. [DOI] [Google Scholar]
- Nagrale DT, Borkar SG, Gawande SP, Mandal AK, Raut SA. Characterization of a bacterial collar and rhizome rot of banana (Musa paradisiaca) caused by strains of Erwinia chrysanthemi pv. paradisiaca. J Appl Nat Sci. 2013;1:435–441. doi: 10.31018/jans.v5i2.349. [DOI] [Google Scholar]
- Namukwaya B, Tripathi L, Tripathi JN, Arinaitwe G, Mukasa SB, Tushemereirwe WK. Transgenic banana expressing Pflp gene confers enhanced resistance to Xanthomonas wilt disease. Transgenic Res. 2012;21:855–865. doi: 10.1007/s11248-011-9574-y. [DOI] [PubMed] [Google Scholar]
- Ocimati W, Kiggundu A, Bailey A, Niblett CL, Pedun H, Tazuba AF, Tushemereirwe WK, Karamura EB (2016) Suppression of the ubiquitin E2 gene through RNA interference causes mortality in the banana weevil, Cosmopolites sordidus (Germar). In: XXIX international horticultural congress on horticulture: sustaining lives, livelihoods and landscapes (IHC2014), vol IX 1114, pp 181–190.
- Paul JY, Becker DK, Dickman MB, Harding RM, Khanna HK, Dale JL. Apoptosis-related genes confer resistance to Fusarium wilt in transgenic ‘Lady Finger’ bananas. Plant Biotechnol J. 2011;9:1141–1148. doi: 10.1111/j.1467-7652.2011.00639.x. [DOI] [PubMed] [Google Scholar]
- Perera N, Kelaniyangoda D (2013) Fungal leaf spot diseases in banana (Musa spp.); symptom verification and their control (in vitro). In: Proceedings of 12th agricultural research symposium, vol 36, p 140
- Perrier X, De Langhe E, Donohue M, Lentfer C, Vrydaghs L, Bakry F, Carreel F, Hippolyte I, Horry JP, Jenny C, Lebot V. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc Natl Acad Sci USA. 2011;108:11311–11318. doi: 10.1073/pnas.1102001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay M, Ude G, Kole C, editors. Genetics, genomics, and breeding of bananas. Enfield: Science Publishers; 2012. [Google Scholar]
- Ploetz RC, editor. Diseases of tropical fruit crops. Wallingford: CABI; 2003. [Google Scholar]
- Ploetz RC. Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology. 2006;96:653–656. doi: 10.1094/PHYTO-96-0653. [DOI] [PubMed] [Google Scholar]
- Pumplin N, Voinnet O. RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nat Rev Microbiol. 2013;11:745. doi: 10.1038/nrmicro3120. [DOI] [PubMed] [Google Scholar]
- Roderick H, Tripathi L, Babirye A, Wang D, Tripathi J, Urwin PE, Atkinson HJ. Generation of transgenic plantain (Musa spp.) with resistance to plant pathogenic nematodes. Mol Plant Pathol. 2012;13:842–851. doi: 10.1111/j.1364-3703.2012.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick H, Urwin PE, Atkinson HJ. Rational design of biosafe crop resistance to a range of nematodes using RNA interference. Plant Biotechnol J. 2018;16:520–529. doi: 10.1111/pbi.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhawat UKS, Ganapathi TR, Hadapad AB. Transgenic banana plants expressing small interfering RNAs targeted against viral replication initiation gene display high-level resistance to banana bunchy top virus infection. J Gen Virol. 2012;93:1804–1813. doi: 10.1099/vir.0.041871-0. [DOI] [PubMed] [Google Scholar]
- Sherman JH, Munyikwa T, Chan SY, Petrick JS, Witwer KW, Choudhuri S. RNAi technologies in agricultural biotechnology: the toxicology forum 40th annual summer meeting. Regul Toxicol Pharmacol. 2015;73:671–680. doi: 10.1016/j.yrtph.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Sreedharan S, Shekhawat UKS, Ganapathi TR. Transgenic banana plants overexpressing a native plasma membrane aquaporin MusaPIP1; 2 display high tolerance levels to different abiotic stresses. Plant Biotechnol J. 2013;11:942–952. doi: 10.1111/pbi.12086. [DOI] [PubMed] [Google Scholar]
- Stefani FOP, Hamelin RC. Current state of genetically modified plant impact on target and non-target fungi. Environ Rev. 2010;18:441–475. doi: 10.1139/A10-022. [DOI] [Google Scholar]
- Strange RN, Scott PR. Plant disease: a threat to global food security. Ann Rev Phytopathol. 2005;43:83–116. doi: 10.1146/annurev.phyto.43.113004.133839. [DOI] [PubMed] [Google Scholar]
- Surridge AK, Viljoen A, Crous RW, Wehner FC. Identification of the pathogen associated with Sigatoka disease of banana in South Africa. Australas Plant Pathol. 2003;32:27–31. doi: 10.1071/AP02058. [DOI] [Google Scholar]
- Tripathi L, Mwangi M, Abele S, Aritua V, Tushemereirwe WK, Bandyopadhyay R. Xanthomonas wilt: a threat to banana production in East and Central Africa. Plant Dis. 2009;93:440–451. doi: 10.1094/PDIS-93-5-0440. [DOI] [PubMed] [Google Scholar]
- Tripathi L, Mwaka H, Tripathi JN, Tushemereirwe WK. Expression of sweet pepper Hrap gene in banana enhances resistance to Xanthomonas campestris pv. musacearum. Mol Plant Pathol. 2010;11:721–731. doi: 10.1111/j.1364-3703.2010.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi JN, Lorenzen J, Bahar O, Ronald P, Tripathi L. Transgenic expression of the rice Xa21 pattern-recognition receptor in banana (Musa sp.) confers resistance to Xanthomonas campestris pv. musacearum. Plant Biotechnol J. 2014;12:663–673. doi: 10.1111/pbi.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi L, Tripathi JN, Kiggundu A, Korie S, Shotkoski F, Tushemereirwe WK. Field trial of Xanthomonas wilt disease-resistant bananas in East Africa. Nat Biotechnol. 2014;32:868–870. doi: 10.1038/nbt.3007. [DOI] [PubMed] [Google Scholar]
- Tripathi L, Babirye A, Roderick H, Tripathi JN, Changa C, Urwin PE, Tushemereirwe WK, Coyne D, Atkinson HJ. Field resistance of transgenic plantain to nematodes has potential for future African food security. Sci Rep. 2015;5:8127. doi: 10.1038/srep08127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DW, Lahav E. The growth of banana plants in relation to temperature. Funct Plant Biol. 1983;10:43–53. doi: 10.1071/PP9830043. [DOI] [Google Scholar]
- Volcy C. Past and present of the nematode Radopholus similis (Cobb) Thorne with emphasis on Musa: a review. Agron Colomb. 2011;29:433–440. [Google Scholar]
- Wang MB, Masuta C, Smith NA, Shimura H. RNA silencing and plant viral diseases. Mol Plant Microbe Interact. 2012;25(10):1275–1285. doi: 10.1094/MPMI-04-12-0093-CR. [DOI] [PubMed] [Google Scholar]
- Xing S, Zachgo S. Pollen lethality: a phenomenon in Arabidopsis RNA interference plants. Plant Physiol. 2007;145:330–333. doi: 10.1104/pp.107.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria L, Sahak S, Zakaria M, Salleh B. Characterizations of Colletotrichum species associated with Anthracnose of banana. Trop Life Sci Res. 2009;20:119. [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhao YL, Zhao JH, Wang S, Jin Y, Chen ZQ, Fang YY, Hua CL, Ding SW, Guo HS. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat Plants. 2016;2:16153. doi: 10.1038/nplants.2016.153. [DOI] [PubMed] [Google Scholar]