Abstract
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs), one of the most commonly used medications worldwide, are frequently associated with gastrointestinal adverse events. Primary care physicians often face the challenge of achieving adequate pain relief with NSAIDs, while keeping their adverse events to a minimum. This is especially true when long-term use of NSAIDs is required such as in patients with osteoarthritis and rheumatoid arthritis. To help primary care physicians deal with such challenges more effectively, a panel of expert gastroenterologists came together with the aim of developing practice recommendations.
Methods
A modified ‘Delphi’ process was used to reach consensus and develop practice recommendations. Twelve gastroenterologists from nine countries provided their expert inputs to formulate the recommendations. These recommendations were carefully developed taking into account existing literature, current practices, and expert opinion of the panelists.
Results
The expert panel developed a total of fifteen practice recommendations. Following are the key recommendations: NSAIDs should be prescribed only when necessary; before prescribing NSAIDs, associated modifiable and non-modifiable risk factors should be considered; H. pylori infection should be considered and treated before initiating NSAIDs; patients should be properly educated regarding NSAIDs use; patients who need to be on long-term NSAIDs should be prescribed a gastroprotective agent, preferably a proton pump inhibitor and these patients should be closely monitored for any untoward adverse events.
Conclusion/clinical significance
These practice recommendations will serve as an important tool for primary care physicians and will guide them in making appropriate therapeutic choices for their patients.
How to cite this article: Hunt R, Lazebnik LB, Marakhouski YC, Manuc M, Ramesh GN, Aye KS, Bordin DS, Bakulina NV, Iskakov BS, Khamraev AA, Stepanov YM, Ally R, Garg A. International Consensus on Guiding Recommendations for Management of Patients with Nonsteroidal Anti-inflammatory Drugs Induced Gastropathy-ICON-G. Euroasian J Hepatogastroenterol, 2018;8(2):148-160.
Keywords: Gastropathy, Gastroprotective agents, Non-prescription drugs, Nonsteroidal Anti-inflammatory Agents, Proton pump inhibitor.
BACKGROUND
Nonsteroidal anti-inflammatory drugs (NSAIDs) are used by over 30 million people daily across the globe.1 The use of NSAIDs has become widespread due to the availability of these agents both as prescription and as over-the-counter (OTC) medicines.2 Even though NSAIDs have proven efficacy in managing pain, fever, and inflammation, they are frequently associated with several untoward adverse events (AEs).3 While NSAIDs are also associated with renal (fluid retention, hyper-kalemia, secondary hypertension) and cardiovascular (vascular events, hypertension) AEs, the most common are gastrointestinal (GI) complications, which include-gastritis, ulcers, perforation, and enteropathy.3,4A study from Denmark reported an increase in the prevalence of NSAID-related peptic ulcers from 39% in 1993 to 53% in 2002.5 Further, the point prevalence of GI complications related to NSAID exposure in the Indian subcontinent in the year 2014 was found to be 30%.6 Overall, mortality in patients suffering from an upper gastrointestinal (UGI) bleed or perforation related to NSAIDs use is estimated to be about 1 in 5.7 Additionally, the prevalence of gas-troduodenal ulcers in patients taking low-dose aspirin (LDA) is about 10%.8
The NSAID-induced gastropathy (NIG) develops at doses that inhibit prostaglandin production, enhance gastric motility, and increase mucosal permeability. This results in neutrophil infiltration and free radical production, and eventually mucosal lesions.9 Risk factors include increasing age, the presence of comorbidities especially a history of peptic ulcer disease, liver cirrhosis, and cardiovascular disease. Long-term use of NSAIDs, Helicobacter pylori (H. pylori) infection, smoking, chronic alcohol abuse, and concomitant use of other medications increase the risk of developing NIG.3,10-13
The NSAIDs have remained the first-line for controlling pain and inflammation particularly in patients with osteoarthritis (OA). Complete withdrawal from NSAIDs is not always practical, particularly in patients with chronic musculoskeletal disorders. Therefore, it is important that clinicians prescribe NSAIDs wiselyto ensure maximum benefits and minimize AEs. All healthcare practitioners, particularly primary care physicians (PCP) can reduce the risk of NIG by careful patient assessment and identification of the risk factors before prescribing an NSAID, educating patients against the addition of OTC NSAIDs, using selective cyclooxygenase-2 (COX-2) inhibitors as first-line medications where appropriate,and co-therapy with a gastroprotective agent (GPA).1,3,9
Several international and regional guidelines have been developed to manage NSAID-induced GI complications.10-17 However, none specifically focus on management of NIG highlighting the need for a comprehensive clinical guidelineto guide PCPs in the management of NIG, particularly in resource-limited regions of the world. This article presents practice recommendations primarily targeted towards primary care providers, for prevention, early detection, and management of NIG formulated at a meeting held in Dubai, UAE on December 1st, 2016.
OBJECTIVE
The objective of this consensus meeting was to identify the advances in disease management and the opportu-nitiesfor prevention and management of NIG in nine nations. Further, we attempted to develop definitive clinical practice guidelines for the management of patients with NIG based on the existing literature, real-world evidence, and evidence-based practice.
Ameeting was held before the International Congress of GI Experts, Gastrosphere 2.0 (in Dubai, UAE) in association with the healthy stomach initiative (HSI). The committee of experts from nine nations was named the ICON-G group. Expert representatives proposed recommendations for use by PCPs and internists in the prevention, identification, and management of NIG.
METHODS
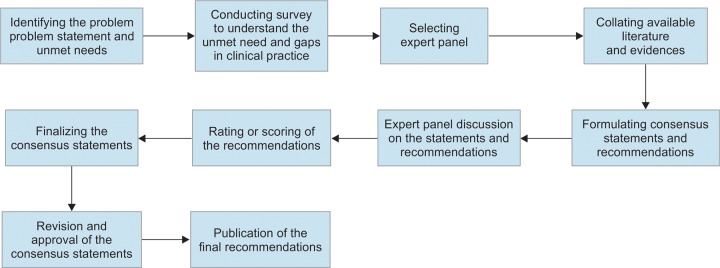
A modified Delphi consensus process (Fig. 1) was implemented to develop the recommendations.18,19 Literature was searched to provide evidence, and recommendations were developed by combining evidence-based and expert consensus-based approach. A comprehensive methodology and transparency in reporting were adopted to develop these clinical practice recommendations.
Fig. 1:
Modified Delphi protocol for consensus development
The process was conducted in two phases. Phase one included an online survey and literature search. The online survey(on online portal survey monkey. com) included twenty questions to establish the current clinical practice in the nine countries. The responses collected from the survey were then used to quantify theknowledgeand practice gap in each of the countries.
An electronic literature search was conducted in PubMed and MEDLINE. The search strategy was developed by combining Medical Subject Headings (MeSH) and free-text keywords using Boolean operators (’OR’ and ‘AND’). Keywords used were “nonsteroidal antiinflammatory drugs”, “NSAIDS”, “gastropathy”, “gastritis”, “ulcers”, “gastric bleeding”, “gastric complications”, and “gastroprotective agents”. Relevant literature from published clinical studies, narrative reviews, systematic reviews, and meta-analyses was collected through September 2016. No additional filters were used during the search. An extensive manual search of literature references was also done, and relevant articles retrieved. Several published international and regional clinical practice guidelines on the management of GI complications were also retrieved.10-17All the available literature and evidence were collated and developed into practice recommendations. These were discussed at the meeting and categorized according to the level of evidence.
In phase two, core-committee of ICON-G members comprising of a chairperson and twelve expert gastroen-terologists from Russia, India, Romania, Ukraine, South Africa, Kazakhstan, Belarus, Uzbekistan, and Myanmar, with knowledge and experience of NSAID-induced GI injury, was formed. A pre-meeting draft depicting the purpose of the guidelines, the target patient population, clinical outcomes of interest, key features of the systematic literature review, and a proposed timeline for guideline completion was shared with all panel members. An advisory board meeting chaired by Professor Richard Hunt was conducted with the core-committee of ICON-G on December 1st, 2016 in Dubai (UAE), to discuss and establish the process for the consensus guideline development.
On December 3rd, 2016, the members of Consensus Group (i.e., members of core-committee of ICON-G together with delegates attending the International Congress of GI Experts) discussed and rated their agreement for each recommendation on a four-point Likert scale. Open discussion was conducted for each recommendation, considering evidence and rationale. Voting then took place. About 60% of the voters were gastroenterologists, and 40% were general physicians , with extensive experience of delivering patient care across a wide range of specialties relevant to primary care. The core-committee determined the strength of agreement for each recommendation as follows:
Strong Consensus: If ≥90% of respondents rated the recommendation as “strongly agree” and/or “agree”
Consensus: If ≥80% and <90% of respondents ratedthe recommendation as “strongly agree” and/or “agree”
No Consensus: If <80% of the respondents rated the recommendation as “strongly agree” and/or “agree”
PRACTICE RECOMMENDATIONS
Practice Recommendation 1
The NSAID prescription is associated with a high-risk of NIG.
(Consensus: Vote; strongly agree 76%, agree 24%)
Rationale
Studies have shown that NSAIDs are associated with gastropathy irrespective of the duration of use.
The evidence study (N = 4144) was the largest prospective study of the real-life management of European patients treated with NSAIDs for rheumatic diseases, who wereat an increased GI risk due to advanced age (>60 years), history of peptic ulcer, or concomitant therapy (corticosteroids, anticoagulants).20 The study found that NSAIDs were associated with an incidence of 18.5 per 100 person-years for uncomplicated GI events and 0.7 per 100 person-years for complicated GI events. During a median follow-up of 6 months, UGI events (12%) were more common than the lower GI events (1%).20
A systematic review (N = 19841) reported that the risk of developing serious NSAID-related GI events is essentially constant over time.21 A large autopsy series on patients with a history of NSAID use found a slightly higher number of nonspecific ulcers in the small intestine in long-term NSAID users, and a slightly higher number ofgastric ulcersin short-term NSAID users when compared to long-term users.22
A nested case-control study found that, compared to non-users, current NSAID users are at a higher risk of developing serious UGI complications by a factor of 3.7 [95% confidence interval (CI ), 3.1-4.3], and selective COX-2 inhibitors by a factor of 2.6 (95% CI, 1.9-3.6).23 This study also foundthat short- and long-term NSAIDs use was associated with a similar risk of UGI complications. Similar findings were reported inother meta-analyses where the relative risk (RR) of serious GI complications was found to be 3 to 4 fold higher in NSAID users compared with non-users irrespective of the duration of use.24-26
A multicenter study (N = 187) reported a high point prevalence rate for ulcers (10.7%) and erosions (63.1%) in patients taking LDA. After a follow-up of 3 months (N = 113) the incidence of ulcers anderosions was 7.1% and 60.2%, respectively, suggesting ulcers developed in almost 1 in 10 LDA users.27A meta-analysis (N~66000) to assess the incidence of GI hemorrhage associated with aspirin therapy showed that long-term (≥12 months) aspirin treatment was associated with a significant increase in the incidence of GI bleeding. Bleeding was reported in 2.47% of patients taking aspirin compared with 1.42% taking a placebo [odds ratio (OR) 1.68; 95% CI,1.51-1.88].28
Practice Recommendation 2
All NSAIDs, irrespective of their route of administration, have the potential to NIG.
(Consensus: Vote; strongly agree 64%, agree 34%, disagree 2%)
Rationale
Although topical NSAIDs are generally safer than oral NSAIDs withfewer severe GI AEs, 17.5% of patients receiving topical NSAIDs in onestudy reported systemic AEs. Topical NSAIDs potentiated warfarin in five cases, leading to GI bleeding in one case.29
A meta-analysis comparing the safety profile of different types of NSAIDs confirmed the variability inRR among various NSAIDs.30 Irrespective of the type and formulation, all NSAIDs were associated with GI AEs. The lowest RR was observed with aceclofenac, celecoxib, and ibuprofen, and the highest with piroxicam, ketoro-lac, and azapropazone. Rofecoxib, sulindac, diclofenac, meloxicam, nimesulide, ketoprofen, tenoxicam, naproxen, indomethacin, and diflunisal were associated with moderate risks.30 In another meta-analysis, nabumetone was associated with a very low GI risk when compared to other NSAIDs (p = 0.007).31
Somenovel NSAID formulationsare safer compared to the conventional forms. A meta-analysis of randomized controlled trials (RCTs) concluded that AMG is an effective anti-inflammatory drug with an improved GI tolerability profile, and lower incidence of GI AEs compared to traditional NSAIDs. The endoscopy scores were higher for other NSAIDs than AMG (27.6% versus 21.4%; p < 0.05). Likewise, post-treatment severity of gastric and/ or duodenal ulcer was higher in the NSAID group than AMG group (14.2% vs. 4.3%; p<0.05).32
Practice Recommendation 3
The most common non-modifiable risk factors for NIG includeage (>60 years), a prior history of peptic ulcers, and complications such asa history of gastrointestinal bleeding.
(Consensus: Vote; 65% strongly agree, 33% agree, 2% disagree)
Rationale
Age is one of the strongest predictors of NSAID related GI complications. A large prospective multicenter study in rheumatoid arthritis (RA) patients (N = 2747) found that the principal risk factors (p < 0.05) were increasing age, a history of NSAID-related ulcer and its complications, and corticosteroid use.33 Similarly, another study reported that age >60 years (OR, 5.52; 95% CI, 4.63-6.60) and previous history of GI complications (OR, 4.76; 95% CI, 4.05-5.59)were associated with an increased risk for serious GI AEs.21
Another study reported that the risk for serious NSAID induced GI eventswas nearly doubled with each of the following factors: a history of previous GI events, peptic ulcer, concomitant glucocorticoid treatment, and severe arthritis induced disability. Using multiple drugs, changing the NSAID, or using high dosesincreasedthe risk by at least 6-fold. Age > 65 years and a history of cardiovascular disease increased the risk by 2 to 3 fold. The presence of multiple risk factors also increased the incidence of GI complications during NSAID therapy.34
Although increasing age is a major risk factor for NSAID-induced GI complications, these problems are also seen in the pediatric population. In a retrospective, multicenter study of Italian children (N = 51) attending the emergency unit with GI bleeding following NSAIDs use, 62% of patients had endoscopically confirmed gastric lesions, 33% had duodenal lesions, and 15% had esophageal lesions. Of particular concern, 6% required endoscopic hemostasis to control GI bleeding. These findings emphasize that children are also vulnerable to NSAID-induced gastric complications.35
Practice Recommendation 4
The modifiable risk factors for NIG include concomitant use of aspirin and/or other NSAIDs, systemic corticosteroids, anticoagulants/antiplatelet, and selective serotonin reup-take inhibitors (SSRIs).
(Consensus: Vote; strongly agree 59%, agree 37%, disagree 4%)
Rationale
Results from the Italian pharmaco vigilance reporting system show that the combined use of LDA and another NSAID or use of multiple NSAIDs is associated with an increased incidence of GI adverse events.36 Further, the Spanish safe prescription recommendations emphasize that two or more NSAIDs simultaneously do not increase effectiveness but do increase toxicity.37
A meta-analysis summarized the results from 16 studies reporting an almost twofold increase in the risk of serious GI complications when NSAIDs were used concomitantly with corticosteroids compared to NSAIDs alone (OR, 1.83; CI, 1.20-2.78).21
A case-control study from the National Health System in Spain reported that concomitant use of NSAIDs with clopidogrel/ticlopidine (RR, 15.2; 95% CI, 4.1-56.5)oranti-coagulants (RR, 19.3; 95% CI 8.2-45.3) increased the risk of UGI bleeding substantially.38 Similarly, in another case-control study from the United Kingdom General Practice Research Database, combining NSAIDs with clopidogrel (RR, 2.93; 95% CI, 1.74-4.93) or warfarin (RR, 4.60; 95% CI, 2.77-7.64) was associated with an increased risk of GI bleeding.39 Another study found that combined use of an NSAID with LDA (OR, 4.3; 95% CI, 1.7-11; p < 0.01) or other antiplatelet drugs (OR, 4.9; 95% CI, 1.4-17; p = 0.01) was associated with a greater risk of lower GI bleeding than when used alone (OR, 2.3; 95% CI, 1.6-3.2; p < 0.01). Furthermore, a combination of NSAIDs was associated with a higher risk than use of a single NSAID (OR, 4.9; 95% CI, 2-12; p < 0.01).40
A population-based retrospective cohort study reported that there could be up to a 50% increase in the risk of GI bleeding with dabigatran compared with warfarin and a more than the twofold higher risk of bleeding with rivaroxaban compared with warfarin.41 Combining these newer antiplatelet drugs with NSAIDs carries a definite increased GI risk of bleeding.
Selective serotonin reuptake inhibitors may also lead to GI bleeding when given with NSAIDs, by impairing the metabolism of NSAIDs leading to an increase in their blood levels,and also by inhibiting hemostasis.42,43A systematic review showed that the RR for UGI bleeding from NSAID and SSRI combination compared to use of neither drug was 3.3-15.6 and that for GI AEs was 12.4.44 A case-control study showed a moderate increase in UGI risk with concurrent use of SSRIs and NSAIDs when com pared to NSAIDs use alone (OR, 1.57; 95% CI, 1.24-1.99) and higher increase, when compared to use of neither drug (OR, 4.19; 95% CI, 3.30-5.31).45 Similar results, were found in several other studies where the combined use of NSAIDs and SSRIs was associated with an OR of about 4 for GI AEs.46,47
Practice Recommendation 5
H. pylori infection increases the risk of developing NIG. It is suggested that physicians consider the possibility of H. pylori infection and treat it, if present, prior to prescribing NSAIDs.
(Consensus: Vote; strongly agree 58%, agree 38%, disagree 4%)
Rationale
H. pylori infection together with the use of NSAIDs is a well-known risk factor that induces gastroduodenal mucosal damage and ulcers.48A meta-analysis found that NSAIDs and H. pylori infection are not only independent risk factors, but they also have a synergistic effect on the development of peptic ulcer and ulcer bleeding.49 While the risk of ulcer bleeding increased 1.79-fold with H. pylori infection and 4.85-fold with NSAID use, the two together increased the risk 6.13-fold.49 In a systematic review of 21 studies (N = 10146), the peptic ulcer was more common in NSAID users who were H. pylori positive (OR, 1.81; 95% CI, 1.40-2.36).50 Furthermore, in a meta-analysis of RCTs, 7.4% of patients developed an ulcer in the H. pylori eradicated group compared with 13.3% in the control group (OR, 0.43; 95% CI, 0.20-0.93).51A recent retrospective study of 245 patients taking an NSAID or LDA continuously for at least 3 months reported that presence of H. pylori infection increases the risk of severe gastric mucosal injury (OR, 2.0; 95% CI, 1.2-3.5).52
All these studies highlight that NSAIDs and H. pylori infection synergistically cause gastric mucosal injury. However, H. pylori testing may not be practical for all patients especially in the primary care setting.53 Testing must be carried out when the patient is prescribed long-term NSAIDs in areas where H. pylori are common. In current practice, H. pylori eradication therapy comprises of PPIs and antimicrobials agents, including bismuth compounds, clarithromycin, amoxicillin, metronidazole, levofloxacin, furazolidone, doxycycline, nitazoxanide, andrifabutin.54,55
Practice Recommendation 6
It is suggested that physicians consider prescribing NSAIDs only when indicated.
The prescribing physician should consider patient-specific risk factors before prescribing NSAIDs.
(Consensus: Vote; strongly agree 69%, agree 29%, disagree 2%)
Rationale
Clinicians must identify NSAIDs use as a risk factor for GI complications and initiate preventive treatment. A population-based cohort study showed that despite the risk of UGI events, guidelines for GPA use were followed in less than half of the cases. GPAs were prescribed in 31.8% of high-risk patients receiving LDA and 48.0% of those receiving NSAIDs.56 Similarly, the Canadian Osteoarthritis Rx (CANOAR) study examined NSAID use in clinical practice in a cohort of PCPs and compared it with the osteoarthritis treatment guidelines. Overall, 58% of the prescriptions were found to be appropriate considering the GI risk of the patients.57 Thus, there is an opportunity for improvement in implementing strategies by adherence to guidelines.56,57
A RCT evaluated a physician education program, communicating OA management guidelines in elderly patients, which emphasized avoidance of NSAIDs. Physicians (N = 209) were visited with reminders for a re-evaluation of their patients’ NSAIDs. Brief physician educational visits resulted in a 7% (95% CI, 3-11%) reduction in NSAIDs use without undesirable substitution of other medications or detectable worsening of musculo-skeletal symptoms.58
A Korean observational study of NSAID prescription patterns in orthopedic patients highlighted the need for better understanding of patient-specific risk factors among prescribing physicians. The study showed that, despite being identified as high-risk or at very high GI risk (by a risk scoring scale), only 51% of patients were given a COX-2 selective inhibitor instead of a traditional NSAID. Physician’s preference for a particular NSAID and not considering a patient’s GI risk factors may expose patients to increased risk of NSAID-induced GI compli-cations.59
Practice Recommendation 7
It is recommended that the prescribing physician educates the patient against self-medication with NSAIDs and the importance of regular follow-up, particularly in those on long-term and/or high-dose NSAIDs.
(Consensus: Vote; strongly agree 54%, agree 41%, disagree 4%)
Rationale
When the prescribed NSAIDs do not adequately control pain, patients may often seek additional OTC NSAIDs.60 They may not be aware that OTC pain-relief medications belong to the same class as their prescribed NSAIDs, resulting in high dose NSAID treatment. Patients may thus increase their risk of developing GI complications. In one study, total 26% of the participants were users of two NSAIDs and had poorer health-related quality of life (QoL) compared to those not on high doses of NSAIDs.60 A cross-sectional study, conducted in the Netherlands, assessed the prevalence of OTC-NSAID use in a sample of the general population (N = 118) and in a sample of patients (N = 264) with a high risk of developing serious NSAID-related AEs.2 The results of this study showed high and unregulated use of NSAIDs. OTC NSAIDs were used by 30% of the general population and 13% of the high-risk sample. OTC NSAIDs dose exceeded the recommended daily maximum by 9% and 3% in the general population and high-risk sample respectively.2 Proper patient education, is, therefore, of the utmost importance.
Patients must also be informed about the importance of adherence to GPAs. One study evaluated the association between adherence to GPA and UGI events among NSAID users. Among those who were non-adherent, the OR was 2.39 (95% CI, 1.66-3.44) for all UGI events and 1.89 (95% CI, 1.09-3.28) for UGI bleeding alone, compared to those who adhered fully, emphasizing the importance of implementing strategies to follow and improve GPA adherence.61
Practice Recommendation 8
In patients on long-term NSAID treatment, regular and patient-specific monitoring (with due consideration to comorbidities, other medications, and the presence of all risk factors) is recommended for evidence of gastrointestinal bleeding and/or gastrointestinal side-effects.
(Consensus: Vote; strongly agree 67%, agree 27%, disagree 4%, strongly disagree 2%)
Rationale
Scarpignato and Hunt (2010) suggested that physicians should not prescribe NSAIDs before taking a careful history and undertaking a physical examination, to evaluate patient-specific risks and benefits for NSAID therapy.62 Furthermore, in the presence of GI and/or cardiovascular risk factors, appropriate preventive strategies (i.e., COX-2 selective inhibitors and/or PPI use as well as the need for LDA) should be implemented from the start of treatment with compliance assessed regularly, especially in high-risk patients.62
Practice Recommendation 9
Co-therapy with a PPI is the preferred approach for prevention of NIG. Alternatively, a histamine-2 receptor antagonist (H2RAs) or misoprostolcan be used.
(Consensus: Vote; strongly agree 84%, agree 14%, disagree 2%)
Rationale
A systematic reviewand meta-analysis emphasized the superiority of PPI therapy over H2RA or placebo in reducing mortality among patients with an endoscopi-cally confirmed high-risk of peptic ulcer bleeding. PPI therapy also reduced rates of re-bleeding and the need for surgical intervention.63
In a meta-analysis including 10 RCTs (N = 8780), PPIs reduced the risk of LDA-associated UGI ulcers (OR = 0.16; 95% CI, 0.12-0.23) and bleeding (OR = 0.27; 95%CI, 0.16-0.43) compared with control (placebo, a cytoprotec-tive agent, or an H2RA).64
A double-blind RCT (OMNIUM study) compared omeprazole and misoprostol for NSAID-induced ulcers. Although the two drugs were overall equally effective, remission rates were better with omeprazole(61% vs 48%; p = 0.001). Moreover, omeprazole was better tolerated than misoprostol.65
A RCT comparing misoprostol with two doses of lansoprazole reported that both drugs were equally effective and superior to placebo. After considering the withdrawals due to AEs, treatment was deemed successful in 69% of patients in each treatment group vs. 35% for placebo.66
A Cochrane review found that misoprostol significantly reduced the RR of gastric ulcer by 74% (RR, 0.26; 95% CI, 0.17-0.39) corresponding to a 12% absolute risk reduction.67 The review also reported that, while a standard dose of H2RA was effective at reducing the risk of endoscopic duodenal ulcer (RR = 0.36; 95% CI, 0.18-0.74), a double dose was required toreduce the risk of endoscopic gastric ulcer (RR = 0.44; 95% CI, 0.26-0.74).67
Practice Recommendation 10
There is no difference in effectiveness between different PPIs (esomeprazole, lansoprazole, omeprazole, pantopra-zole, and rabeprazole) available in the market. All available PPIs at recommended doseshave similar efficacy in reducing the risk of NIG.
(Consensus: Vote; strongly agree 76%, agree 24%)
Rationale
Few head-to-head studies are comparing the different PPIs in the managementof NIG. However, the available data suggest that all PPIs are essentially equivalentin the management of NIG. A randomized, double-blind study (N = 595) to compare pantoprazole (20 mg OD and 40 mg OD) withomeprazole (20 mg OD) for the prevention of GI lesions associated with NSAIDs found that both PPIs were similarly effective (remission rates for lack of “therapeutic failure” were 90%, 93%, and 89%, and for lack of “endoscopic failure” were 91%, 95%, and 93% (for pantoprazole 20 mg OD, pantoprazole 40 mg OD, and omeprazole 20 mg OD, respectively).68
A systematic review comparing the efficacy of different available PPIs found that for symptom relief in gastroesophageal reflux disease lansoprazole was faster than omeprazole, and esomeprazole was faster than both lansoprazole and omeprazole. However, none of these-drugs was found to be superior over the other.69
A few novels or immediate-release formulations of PPIs have also been studied.70 The immediate-release omeprazole (Zegerid®, Omez-Insta®) are associated with faster absorption of omeprazole, the more rapid onset of anti-secretory activity, and a slightly longer duration of acid suppression. This formulation is not food dependent and provides dosing flexibility which is considered apt for suppressing nocturnal gastric acid secretion.71 Immediate-release omeprazole has also shown superiority over once daily dosing of delayed-release PPIs in control of nocturnal gastric acidity.72 Dexlansoprazole is a PPI with adual delayed-release formulation, which produces a dual-peak pharmacokinetic profile unlike the single peak profile of the first generation or delayed release PPIs. Thus, dexlansoprazole maintains therapeutic plasma drug concentrations longer than lansoprazole and other PPIs.73
Practice Recommendation 11
It is suggested that physicians consider continued prophylaxis with a PPI when NSAIDs are prescribed.
(Consensus: Vote; strongly agree 69%, agree 31%)
Rationale
In a large international multicenter study (N = 610), both omeprazole and misoprostol improved QoL in chronic NSAID users with NSAID-associated gastroduodenal lesions. However, omeprazole relieved gastrointestinal symptoms better than misoprostol. Hence, it is appropriate to co-prescribe a PPI in this patient group for prophylaxis as long as NSAIDs are prescribed and taken.74
Long-term PPI treatment is associated with better-outcomes in patients continuing NSAID therapy. A large RCT in OA and RA patients compared omeprazole with ranitidine in the prevention of gastric and duodenal ulcers. Omeprazole healed and prevented ulcers more effectively than ranitidine over 8 weeks of the study. During the subsequent 6-month maintenance treatment, 72% and 59% of patients were in remission in the omepra-zole and ranitidine groups respectively. Relapses were more common with ranitidine than with omeprazole.75
In long-term NSAID users, a PPI can substantially reduce the occurrence of GI complications. A 3-month studyto evaluate omeprazole 20 mg every morning as primary prophylaxis against NSAID-induced ulcer and dyspepsia, found that the estimated probability of remaining symptom-free for 6 months for patients taking omeprazole was 0.78 compare d to 0.53 for placebo (p = 0.004).76 Thus, continuing a PPI is important in the management of NIG. Although long-term PPIs could be associated with some AEs, in patients with a clear indication for the PPI, the risk-benefit ratio favors PPI use.43
Practice Recommendation 12
The following clinical presentations in a patient taking an NSAID to raise the suspicion of NIG: abdominal cramps/ pain, dyspepsia, nausea, and vomiting.
In patients with pre-existing risk factors for gas-tropathy, it is suggested that the physician maintain a high index of suspicion of onset of NSAID-induced gastropathy in patients on NSAID therapy developing such symptoms.
(Consensus: Vote; strongly agree 43%, agree 40%, disagree 9%, and strongly disagree 9%)
Rationale
Upper GI symptoms including dyspepsia, heartburn, bloating or cramping, nausea, and vomiting are reported in up to 40% of patients taking NSAIDs.77 However, in 50 to 60% NSAIDs users, GI complications may be clinically asymptomatic.78 Hence, close monitoring is advisable forall patients on long-term NSAIDs, particularly those with the presence of one or more risk factors.
Practice Recommendation 13
It is suggested that to determine the risk, physicians take a careful history andperform necessary laboratory tests when starting NSAID treatment.
If a patient presents with a history, or clinical symptoms and signs suggesting NSAID-related GI problems, the PCP should consider referring the patient to a specialist for further management.
(Consensus: Vote; strongly agree 38%, agree 54%, disagree 16%, and strongly disagree 2%)
Rationale
Standard approaches for diagnosing NIG include identification of high-risk populations; history, clinical presentation, and examination of the patient; tests for H. pylori infection in suspected cases; and hemoglobin and hematocrit tests. Upper GI endoscopy is required to confirm the diagnosis.79
Practice Recommendation 14
While PPIs are the preferred drugs, some choice of GPA (in treatment doses) is recommended for patients diagnosed with NIG.
(Consensus: Vote; strongly agree 40%, agree 40%, disagree 17%, and strongly disagree 2%)
Rationale
Several studies have highlighted the efficacy of PPIs in the treatment of LDA- and NSAID-induced GI injury.63,64,80-82 Evidence also indicates that PPIs are superior to other GPAs.63-65,74,75 Therefore, PPIs remain the preferred treatment. Nevertheless, other GPAs, including H2RAs and misoprostol, have also demonstrated effectiveness in the treatment of NIG,66,67,83,84 and may be considered when PPIs cannot be prescribed.
Practice Recommendation 15
A fixed-dose combination (FDC) of an NSAID and a GPA is not recommended in patients with NIG.
(Consensus: Vote; strongly agree 59%, agree 28%, disagree 13%)
Rationale
The ketoprofen-omeprazole FDC was the first NSAID GPA combination to be approved.85 Other commonly used FDCs include ibuprofen-famotidine and naproxen-esomeprazole.86,87 Although FDCs are believed to be associated with better compliance; sufficient clinical evidence is not available to support their superiority regarding efficacy and safety.86,87 Furthermore, the high-cost of FDC makes prescription difficult in long-term users, such as patients with OA.
Boxes 1 and 2 list the recommendations for doctors in the treatment of NIG for highrisk and moderate risk patients respectively.
Box 1: Recommendations for doctors-high risk patients
| 1. | Document patient’s history and carry out necessary laboratory investigations before starting any treatment | ||
| 2. | For high-risk patients, avoid NSAIDs and use alternate management strategies like physiotherapy and/or exercise to ease pain and inflammation in diseases like OA | ||
| 3. | Consider prescribing low doses and shorter durations for diseases that require instant relief (headache, dysmenorrhea, post-operative pain) | ||
| 4. | Consider pulse therapy - prescribing large doses NSAIDs in an intermittent manner to enhance the therapeutic effect and reduce the GI complications/other adverse events in chronic diseases | ||
| 5. | Avoid prescribing NSAIDs with other drugs (SSRIs, antiplatelet drugs, corticosteroids) | ||
| 6. | Inform the patient that NSAIDs can be taken “as required” and generally do not have a fixed schedule | ||
| 7. | Prefer selective COX-2 inhibitors like coxibs or safer drugs like amtolmentinguacyl over conventional NSAIDs | ||
| 8. | Prescribe double dose of gastroprotective agents like PPIs in patients undergoing long-term (>30 days) NSAID treatment | ||
| 9. | Prescribe a single dose of PPI (up to 30 days) in patients undergoing NSAID treatment | ||
| 10. | Perform periodic assessment of patients on long-term NSAID therapy for early identification of signs/symptoms of gastropathy |
Abbreviations: COX-2, cyclooxygenase-2; GI, gastrointestinal; NSAID, non-steroidal anti-inflammatory drug; OA, osteoarthritis; SSRI, selective serotonin reuptake inhibitor
Box 2: Recommendations for doctors-moderate risk patients
| 1 | Document patient’s history and carry out necessary laboratory investigations before starting any treatment | ||
| 2. | Consider prescribing topical NSAIDs over oral NSAIDs in patients experiencing mild pain like muscle pain, or low back pain | ||
| 3 | Initiate prophylactic therapy with PPI (e.g. Omeprazole 20 mg once daily) to reduce the risk of gastropathy | ||
| 4. | Consider H. pylori eradication in case it was diagnosed, and eradication therapy not given | ||
| 5. | Include an antacid in the drug regimen as antacids neutralize existing stomach acid and can provide rapid pain relief | ||
| 6. | Consider probiotic supplement (containing Lactobacillus acidophilus). Probiotics or “friendly” bacteria may help maintain a balance in the digestive system between good and harmful bacteria | ||
| 7. | Suggest a multivitamin daily to improve digestive health (e.g. multivitamins containing the antioxidant vitamins A, C, E, the B vitamins, and trace minerals, such as magnesium, calcium, zinc, and selenium) | ||
| 8. | Guide patients to avoid self-medication, high dose unregulated use of NSAIDs, and to never combine OTC medications without consultation | ||
| 9. | Counsel the patients well and educate them on the signs and symptoms of gastropathy so that they can reach for help at the earliest | ||
| 10. | Suggest the patients to visit regularly so that the need for further treatment with NSAIDs can be reviewed |
Abbreviations: COX-2, cyclooxygenase-2; GI, gastrointestinal; NSAID, non-steroidal anti-inflammatory drug; OA, osteoarthritis; OTC, over-the-counter; SSRI, selective serotonin reuptake inhibitor
CONCLUSION
NSAIDs are the most widely used drugs for controlling pain and inflammation. Easy availability (as OTC drugs) and good efficacy of these drugs increase their use. However, the gastrointestinal complications, particularly gastropathy, remain a matter of concern for the prescribing physician. The risks of developing gastropathy further increase with high-dose, long-term, or inappropriate use of NSAIDs especially in the elderly. Hence, recommendations are necessary to guide a PCP in making safe and sensible use of NSAIDs and to minimize the risk of gastropathy. The Consensus Group recommends the use of NSAIDs only as and when indicated. Associated risk factors and a risk profile of the patient is important and should be determined before prescribing NSAIDs. Additionally, physicians are encouraged to take time to educate their patients about the safe use of NSAIDs properly, to avoid dose creep, and not combine their prescribed drugs with OTC medications without prior discussion with their physician. Furthermore, risk monitoring of patients undergoing long-term NSAID treatment and co-therapy with a GPA usually a PPI is also recommended. The consensus group emphasizes the importance of the patient’s age, history, clinical presentation, and presence of H. pylori infection for timely management of NIG; and the need to develop clinical strategies to ensure GI safety for patients undergoing NSAID treatment.
Voting Participants
| Participant Name | Country | ||
| Julia Gorgun | Belarus | ||
| Jan Tack | Belgium | ||
| Richard Hunt | Canada | ||
| G N Ramesh | India | ||
| Almagul Kuzgibekova | Kazakhstan | ||
| Yerlan Bazargaliyev | Kazakhstan | ||
| Gyuzel Jakupova | Kazakhstan | ||
| Baurzhan Iskakov Samikovich | Kazakhstan | ||
| Thein Saw | Myanmar | ||
| Win Phyu Phyu Myint | Myanmar | ||
| Khin San Aye | Myanmar | ||
| Paul Jurgen Porr | Romania | ||
| Elena-Tatiana Ivan | Romania | ||
| Radu-Bogdan Mateescu | Romania | ||
| Cristina Daniela Bura | Romania | ||
| Ligia Ariana Bancu | Romania | ||
| Victor-CatalinSfarti | Romania | ||
| Mircea Manuc | Romania | ||
| Lucian Negreanu | Romania | ||
| Elena Onuchina | Russia | ||
| Mikhail Sheviakov | Russia | ||
| Tatiana Sviridova | Russia | ||
| Oksana Pozdniakova | Russia | ||
| Elina Petrova | Russia | ||
| Svetlana Turkina | Russia | ||
| Elena Vyuchnova | Russia | ||
| AimanSarsenbaeva | Russia | ||
| Irina Kozlova | Russia | ||
| Aleksandr Stepchenko | Russia | ||
| Oleg Mironchev | Russia | ||
| Alexey Okhlobystin | Russia | ||
| Tatiana Iankovaia | Russia | ||
| Elena Li | Russia | ||
| Inna Putintseva | Russia | ||
| Zarina Galeeva | Russia | ||
| Sergei Alekseenko | Russia | ||
| Elena Miguskina | Russia | ||
| EmiliyaYakovenko | Russia | ||
| Elena Kashkina | Russia | ||
| Leonid Lazebnik | Russia | ||
| Natalia Bakulina | Russia | ||
| Victor Pasechnikov | Russia | ||
| Dmitry Bordin | Russia | ||
| Anell Meyer | South Africa | ||
| Reidwaan Ally | South Africa | ||
| Bilal Bobat | South Africa | ||
| Vasudevan Naidoo | South Africa | ||
| Fritz Potgieter | South Africa | ||
| Monique Marais | South Africa | ||
| Hitendrakumar Bhaga | South Africa | ||
| Sandie Thomson | South Africa | ||
| Yurii Stepanov | Ukraine | ||
| Olha Bondarenko | Ukraine | ||
| Andriy Dorofyeyev | Ukraine | ||
| Abror Khamraev | Uzbekistan | ||
| Feruza Khamrabaeva | Uzbekistan |
ACKNOWLEDGMENTS
The consensus conference was supported by an unrestricted educational grant from Dr Reddy’s Laboratories Ltd. We would like to thank all the clinicians who actively participated in the survey, discussions, and voting process. The names of all the voters are included in the appendix. The authors acknowledge Turacoz Healthcare Solutions (www.Turacoz.com), Gurugram, India, for helping in the conduct of survey, coverage of advisory board meeting, and writing support.
Footnotes
Source of support: Nil
Conflict of interest: Richard Hunt has served as a consultant for INSYS, Dr Reddy’s, Takeda, and Novar-tis. He has received an honorarium from Novartis, Danone, Dr Reddy’s, and Takeda. He has been on the speaker’s bureau for Takeda and Dr Reddy’s and on scientific advisory board for INSYS. Dmitry S Bordin has served as a lecturer for Astellas, AstraZeneca, KRKA and Abbott. For the remaining authors, there are no conflicts of interest.
REFERENCES
- 1.Singh G. Gastrointestinal complications of prescription and over-the-counter nonsteroidal anti-inflammatory drugs: a view from the ARAMIS database. Arthritis, Rheumatism, and Aging Medical Information System. Am J Ther. 2000 Mar;7(2):115–121. doi: 10.1097/00045391-200007020-00008. [DOI] [PubMed] [Google Scholar]
- 2.Koffeman AR, Valkhoff VE, ×elik S, W’t Jong G, Sturkenboom MC, Bindels PJ et al. High-risk use of over-the-counter non- steroidal anti-inflammatory drugs: a population-based cross-sectional study. Br J Gen Pract. 2014 Apr;64(621):e191–198. doi: 10.3399/bjgp14X677815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peura DA. Gastrointestinal safety and tolerability of nonse-lective nonsteroidal anti-inflammatory agents and cyclooxy-genase-2-selective inhibitors. Cleve Clin J Med. 2002;69(Suppl 1):SI31–139. doi: 10.3949/ccjm.69.suppl_1.si31. [DOI] [PubMed] [Google Scholar]
- 4.Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16(5):821–847. doi: 10.18433/j3vw2f. [DOI] [PubMed] [Google Scholar]
- 5.Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993-2002: a population-based cohort study. Am J Gastroen- terol. 2006 May;101(5):945–953. doi: 10.1111/j.1572-0241.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, Dureja GP, Kadhe G, Mane A, Phansalkar AA, Sawant S et al. Cross-Sectional Study for Prevalence of Non-Steroidal Anti-Inflammatory Drug-Induced Gastrointestinal, Cardiac and Renal Complications in India: Interim Report. Gastroenterology Res. 2015 Aug;8(3-4):216–221. doi: 10.14740/gr658w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straube S, Tramèr MR, Moore RA, Derry S, McQuay HJ. Mortality with upper gastrointestinal bleeding and perforation: effects of time and NSAID use. BMC Gastroenterol. 2009 Jun;9(1):41. doi: 10.1186/1471-230X-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeomans ND, Lanas AI, Talley NJ, Thomson AB, Daneshjoo R, Eriksson et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther. 2005 Nov;22(9):795–801. doi: 10.1111/j.1365-2036.2005.02649.x. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hyper- motility. World J Gastroenterol. 2012 May;18(18):2147–2160. doi: 10.3748/wjg.v18.i18.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unified Clinical Protocol for Primary and Secondary Medical Care Peptic Ulcer of Stomach and Duodenum in Adults, Ukraine, 2014 Sep. Available at: http://mtd.dec.gov.ua/images/dodatki/2014_613_PeptVyrazka_12paloi/2014_613y kpmd_PeptVyr.pdf.
- 11.Russian gastroenterological association clinical guidelines on diagnosis and treatment of NSAIDs-associated erosive and ulcerative lesions of the stomach and duodenum; 2014 Available at: http://www.gastro.ru/userfiles/R_npvp_2014_6.pdf.
- 12.Abraham NS, Hlatky MA, Antman EM, Bhatt DL, Bjorkman DJ, Clark CB, Furberg CD et al. ACCF/ACG/AHA. ACCF/ ACG/AHA 2010 Expert Consensus Document on the Concomitant Use of Proton Pump Inhibitors and Thienopyridines: A Focused Update of the ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010 Dec;122(24):2619–2633. doi: 10.1161/CIR.0b013e318202f701. [DOI] [PubMed] [Google Scholar]
- 13.Lanza FL, Chan FK, Quigley EM. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009 Mar;104(3):728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 14.NICE Guidelines: Acute upper gastrointestinal bleeding in over 16s: management. Available at: https://www.nice.org.uk/guidance/cg141. [PubMed]
- 15.Unified Clinical Protocol for Primary Medical Care Dyspepsia, Ukraine [August 2012]. Available at: http://mtd.dec.gov.ua/images/dodatki/2012_600/19_07_2012_ykpmd.pdf.
- 16.Rostom A, Moayyedi P, Hunt R. Canadian Association of Gastroenterology Consensus Group. Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks. Aliment PharmacolTher. 2009 Mar;29(5):481–496. doi: 10.1111/j.1365-2036.2008.03905.x. [DOI] [PubMed] [Google Scholar]
- 17.Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH. International NSAID Consensus Group. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis--an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015 Mar;13:55. doi: 10.1186/s12916-015-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalkey N, Helmer O. An Experimental Application of the DELPHI Method to the Use of Experts. Management Science. 1962 Jul;9(3):458–467. [Google Scholar]
- 19.Hsu Chia-Chien, Sandford BA. The Delphi Technique: Making Sense of Consensus. Practical Assessment, Research & Evaluation. 2007 Aug;12(10):1–8. [Google Scholar]
- 20.Lanas A, Boers M, Nuevo J. Gastrointestinal events in at-risk patients starting non-steroidal anti-inflammatory drugs (NSAIDs) for rheumatic diseases: the EVIDENCE study of European routine practice. Ann Rheum Dis. 2015 Apr;74(4):675–681. doi: 10.1136/annrheumdis-2013-204155. [DOI] [PubMed] [Google Scholar]
- 21.Schaffer D, Florin T, Eagle C, Marschner I, Singh G, Grobler M et al. Risk of serious NSAID-related gastrointestinal events during long-term exposure: a systematic review. Med J Aus. 2006 Nov;185(9):501–506. doi: 10.5694/j.1326-5377.2006.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 22.Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. Gastrointestinal damage associated with the use of non-steroidal anti-inflammatory drugs. N Engl J Med. 1992 Sep; 327(11):749–754. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
- 23.GarcíaRodríguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007 Feb;132(2):498–506. doi: 10.1053/j.gastro.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Hernández Díaz S, García Rodríguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation. An overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000 Jul;160(14):2093–2099. doi: 10.1001/archinte.160.14.2093. [DOI] [PubMed] [Google Scholar]
- 25.García Rodriguez LA, Hernández-Díaz S. Relative risk of upper gastrointestinal complications among users of acetaminophen and nonsteroidal anti-inflammatory drugs. Epidemiology. 2001 Sep;12(5):570–576. doi: 10.1097/00001648-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Tarone RE, Blot WJ, McLaughlin JK. Nonselective nonaspirin nonsteroidal anti-inflammatory drugs and gastrointestinal bleeding: relative and absolute risk estimates from recent epidemiologic studies. Am J Ther. 2004 Jan-Feb;11(1):17–25. doi: 10.1097/00045391-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Yeomans ND, Lanas AI, Talley NJ, Thomson AB, Daneshjoo R, Eriksson B et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther. 2005 Nov;22(9):795–801. doi: 10.1111/j.1365-2036.2005.02649.x. [DOI] [PubMed] [Google Scholar]
- 28.Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ. 2000 Nov;321(7072):1183–1187. doi: 10.1136/bmj.321.7270.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makris UE, Kohler MJ, Fraenkel L. Adverse Effects (AEs) of Topical NSAIDs in Older Adults with Osteoarthritis (OA): a Systematic Review of the Literature. J Rheumatol. 2010 Jun;37(6):1236–1243. doi: 10.3899/jrheum.090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Reglat A, Nicotra F et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project) Drug Saf. 2012 Dec;35(12):1127–1146. doi: 10.1007/BF03261999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang JQ, Sridhar S, Hunt RH. Gastrointestinal safety profile of nabumetone: a meta-analysis. Am J Med. 1999 Dec;107(6A):55S–61S; discussion 61S-64S. doi: 10.1016/s0002-9343(99)00368-x. [DOI] [PubMed] [Google Scholar]
- 32.Garg A, Shoeb A, Moodahadu LS, Sharma A, Gandhi A, Akku S. Amtolmeti: A Reappraisal of NSAID with Gastroprotection. Arthritis. 2016;2016:7103705. doi: 10.1155/2016/7103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal anti-inflammatory drug- associated gastropathy: incidence and risk factor models. Am J Med. 1991 Sep;91(3):213–222. doi: 10.1016/0002-9343(91)90118-h. [DOI] [PubMed] [Google Scholar]
- 34.Koch M, Dezi A, Tarquini M, Capurso L. Prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal mucosal injury: risk factors for serious complications. Dig Liver Dis. 2000 Nov;32(20):138–151. doi: 10.1016/s1590-8658(00)80402-8. [DOI] [PubMed] [Google Scholar]
- 35.Cardile S, Martinelli M, Barabino A, Gandullia P, Oliva S, Nardo GD et al. Italian survey on non-steroidal anti-inflammatory drugs and gastrointestinal bleeding in children. World J Gastroenterol. 2016 Feb;22(5):1877–1883. doi: 10.3748/wjg.v22.i5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rafaniello C, Ferrajolo C, Sullo MG, Sessa M, Sportiello L, Balzano A et al. Risk of gastrointestinal complications associated to NSAIDs, low-dose aspirin and their combinations: Results of a pharmacovigilance reporting system. Pharmacol Res. 2016 Feb;104:108–114. doi: 10.1016/j.phrs.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 37.Lanas A, Benito P, Alonso J, Hernández-Cruz B, Barón-Esquiv-ias G, Perez-Aísa Á et al. Safe prescription recommendations for non-steroidal anti-inflammatory drugs: consensus document ellaborated by nominated experts of three scientific associations (SER-SEC-AEG) ReumatolCli. 2014 Mar-Apr;10(2):68–84. doi: 10.1016/j.reuma.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Lanas A, García-Rodríguez LA, Arroyo MT, Gomollón F, Feu F, González-Pérez A et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006 Dec;55(12):1731–1738. doi: 10.1136/gut.2005.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007 Aug;177(4):347–351. doi: 10.1503/cmaj.070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagata N, Niikura R, Aoki T, Shimbo T, Kishida Y, Sekine K et al. Lower GI bleeding risk of nonsteroidal anti-inflammatory drugs and antiplatelet drug use alone and the effect of combined therapy. GastrointestEndosc. 2014 Dec;80(6):1124–1131. doi: 10.1016/j.gie.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 41.Chang HY, Zhou M, Tang W, Alexander GC, Singh S. Risk of gastrointestinal bleeding associated with oral anticoagulants: population based retrospective cohort study. BMJ. 2015 Apr;350:h1585. doi: 10.1136/bmj.h1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halperin D, Reber G. Influence of antidepressants on hemo-stasis. Dialogues Clin Neurosci. 2007;9(1):47–59. doi: 10.31887/DCNS.2007.9.1/dhalperin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scarpignato C, Gatta L, Zullo A, Blandizzi C. SIF-AIGO- FIMMG Group; Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016 Nov;14(1):179. doi: 10.1186/s12916-016-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mort JR, Aparasu RR, Baer RK. Interaction between selective serotonin reuptake inhibitors and nonsteroidal antiinflamma-tory drugs: review of the literature. Pharmacotherapy. 2006 Sep;26(9):1307–1313. doi: 10.1592/phco.26.9.1307. [DOI] [PubMed] [Google Scholar]
- 45.Helin-Salmivaara A, Huttunen T, Gronroos JM, Klaukka T, Huupponen R. Risk of serious upper gastrointestinal events with concurrent use of NSAIDs and SSRIs: a case-control study in the general population. Eur J Clin Pharmacol. 2007 Apr;63(4):403–408. doi: 10.1007/s00228-007-0263-y. [DOI] [PubMed] [Google Scholar]
- 46.Anglin R, Yuan Y, Moayyedi P, Tse F, Armstrong D. Leontia-dis GI. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent it nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014 Jun;109(6):811–819. doi: 10.1038/ajg.2014.82. [DOI] [PubMed] [Google Scholar]
- 47.Oka Y, Okamoto K, Kawashita N, Shirakuni Y, Takagi T. Meta-analysis of the risk of upper gastrointestinal hemorrhage with combination therapy of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs. Biol Pharm Bull. 2014;37(6):947–953. doi: 10.1248/bpb.b13-00885. [DOI] [PubMed] [Google Scholar]
- 48.Sostres C, Gargallo CJ, Lanas A. Interaction between Helico-bacter pylori infection, nonsteroidal anti-inflammatory drugs and/or low-dose aspirin use: old question new insights. World J Gastroenterol. 2014 Jul;20(28):9439–9450. doi: 10.3748/wjg.v20.i28.9439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002 Jan;359(9300):14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 50.Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006 Feb;4(2):130–142. doi: 10.1016/j.cgh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Vergara M, Catalán M, Gisbert JP, Calvet X. Meta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment PharmacolTher. 2005 Jun;21(!2):1411–1418. doi: 10.1111/j.1365-2036.2005.02444.x. [DOI] [PubMed] [Google Scholar]
- 52.Kono Y, Okada H, Takenaka R, Miura K, Kanzaki H, Hori K et al. Does Helicobacter pylori Exacerbate Gastric Mucosal Injury in Users of Nonsteroidal Anti-Inflammatory Drugs? A Multicenter, Retrospective, Case-Control Study. Gut Liver. 2016 Jan;10(1):69–75. doi: 10.5009/gnl14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pounder RE. Helicobacter pylori and NSAIDs--the end of the debate? . Lancet. 2002 Jan;359(9300):3–4. doi: 10.1016/S0140-6736(02)07267-7. [DOI] [PubMed] [Google Scholar]
- 54.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helico-bacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017 Jan;66(1):6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 55.Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016 Jul;151(1):51–69.e14. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Warlé-van Herwaarden MF, Koffeman AR, Valkhoff VE, ’t Jong GW, Kramers C, Sturkenboom MC et al. Time-trends in the prescribing of gastroprotective agents to primary care patients initiating low-dose aspirin or non-steroidal anti-inflammatory drugs: a population-based cohort study. Br J Clin Pharmacol. 2015 Sep;80(3):589–598. doi: 10.1111/bcp.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sebaldt RJ, Petrie A, Goldsmith CH, Marentette MA. Appropriateness of NSAID and Coxib prescribing for patients with osteoarthritis by primary care physicians in Ontario: results from the CANOAR study. Am J Manag Care. 2004;10(11):742–750. [PubMed] [Google Scholar]
- 58.Ray WA, Stein CM, Byrd V, Shorr R, Pichert JW, Gideon P et al. Educational program for physicians to reduce use of non-steroidal anti-inflammatory drugs among community-dwelling elderly persons: a randomized controlled trial. Med Care. 2001;39(5):425–435. doi: 10.1097/00005650-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Lee SH, Han CD, Yang IH, Ha CW. Prescription Pattern of NSAIDs and the Prevalence of NSAID-induced Gastrointestinal Risk Factors of Orthopaedic Patients in Clinical Practice in Korea. J Korean Med Sci. 2011 Apr;26(4):561–567. doi: 10.3346/jkms.2011.26.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovac SH, Saag KG, Curtis JR, Allison J. Association of health-related quality of life with dual use of prescription and over- the-counter nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008 Feb;59(2):227–233. doi: 10.1002/art.23336. [DOI] [PubMed] [Google Scholar]
- 61.van Soest EM, Valkhoff VE, Mazzaglia G, Schade R, Molokhia M, Goldstein JL et al. Suboptimal gastroprotective coverage of NSAID use and the risk of upper gastrointestinal bleeding and ulcers: an observational study using three European databases. Gut. 2011;60:1650–1659. doi: 10.1136/gut.2011.239848. [DOI] [PubMed] [Google Scholar]
- 62.Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010 Sep;39(3):433–464. doi: 10.1016/j.gtc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW et al. Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol Assess. 2007 Dec;11(51):iii, iv, 1-164. doi: 10.3310/hta11510. [DOI] [PubMed] [Google Scholar]
- 64.Mo C, Sun G, Lu ML, Zhang L, Wang YZ, Sun X et al. Proton pump inhibitors in prevention of low-dose aspirin-associated upper gastrointestinal injuries. World J Gastroenterol. 2015 May;21(17):5382–5392. doi: 10.3748/wjg.v21.i17.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hawkey CJ, Karrasch JA, Szczepanski L, Walker DG, Barkun A, Swannell AJ, Yeomans ND. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal anti-inflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. N Engl J Med. 1998 Mar;338(11):727–734. doi: 10.1056/NEJM199803123381105. [DOI] [PubMed] [Google Scholar]
- 66.Graham DY, Agrawal NM, Campbell DR, Haber MM, Collis C, Lukasik NL et al. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs. lansoprazole. Arch Intern Med. 2002 Jan;162(2):169–175. doi: 10.1001/archinte.162.2.169. [DOI] [PubMed] [Google Scholar]
- 67.Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, McGowan J. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev. 2002;4:CD002296. doi: 10.1002/14651858.CD002296. [DOI] [PubMed] [Google Scholar]
- 68.Regula J, Butruk E, Dekkers CP, de Boer SY, Raps D, Simon L et al. Prevention of NSAID-associated gastrointestinal lesions: a comparison study pantoprazole versus omeprazole. Am J Gastroenterol. 2006;101(8):1747–1755. doi: 10.1111/j.1572-0241.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 69.Vakil N, Fennerty MB. Direct comparative trials of the efficacy of proton pump inhibitors in the management of gastro-oesophageal reflux disease and peptic ulcer disease. Aliment Pharmacol Ther. 2003 Sep;18(6):559–568. doi: 10.1046/j.1365-2036.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 70.Howden CW. Review article: immediate-release proton-pump inhibitor therapy--potential advantages Aliment Pharmacol- Ther. 2005 Dec;22(Suppl 3):25–30. doi: 10.1111/j.1365-2036.2005.02709.x. [DOI] [PubMed] [Google Scholar]
- 71.Castell D. Review of immediate-release omeprazole for the treatment of gastric acid-related disorders. Expert OpinPhar- macother. 2005 Nov;6(14):2501–2510. doi: 10.1517/14656566.6.14.2501. [DOI] [PubMed] [Google Scholar]
- 72.Castell D, Bagin R, Goldlust B, Major J, Hepburn B. Comparison of the effects of immediate-release omeprazole powder for oral suspension and pantoprazole delayed-release tablets on nocturnal acid breakthrough in patients with symptomatic gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2005 Jun;21(12):1467–1474. doi: 10.1111/j.1365-2036.2005.02513.x. [DOI] [PubMed] [Google Scholar]
- 73.Metz DC, Vakily M, Dixit T, Mulford D. Review article: dual delayed release formulation of dexlansoprazole MR, a novel approach to overcome the limitations of conventional single release proton pump inhibitor therapy. Aliment Pharmacol- Ther. 2009 May;29(9):928–937. doi: 10.1111/j.1365-2036.2009.03984.x. [DOI] [PubMed] [Google Scholar]
- 74.Yeomans N, Wilson I, Langstrom G, Hawkey C, Naesdal J, Walan A et al. Quality of life in chronic NSAID users: a com- parison of the effect of omeprazole and misoprostol. Scand J Rheumatol. 2001;30(6):328–334. doi: 10.1080/030097401317148516. [DOI] [PubMed] [Google Scholar]
- 75.Yeomans ND, Tulassay Z, Juhasz L, Rácz I, Howard JM, van Rensburg CJ et al. A comparison of omeprazole with raniti-dine for ulcers associated with nonsteroidal anti-inflammatory drugs. Acid Suppression Trial: Ranitidine vs. Omeprazole for NSAID-associated Ulcer Treatment (ASTRONAUT) Study Group. N Engl J Med. 1998 Mar;338(11):719–726. doi: 10.1056/NEJM199803123381104. [DOI] [PubMed] [Google Scholar]
- 76.Cullen D, Bardhan KD, Eisner M, Kogut DG, Peacock RA, Thomson JM et al. Primary gastroduodenal prophylaxis with omeprazole for non-steroidal anti-inflammatory drug users. Aliment PharmacolTher. 1998 Feb;12(2):135–140. doi: 10.1046/j.1365-2036.1998.00288.x. [DOI] [PubMed] [Google Scholar]
- 77.Brun J, Jones R. Non-steroidal anti-inflammatory drug-associated dyspepsia: the scale of the problem. Am J Med. 2001 Jan;110(1A):12S–13S. doi: 10.1016/s0002-9343(00)00629-x. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong CP, Blower AL. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987 May;28(5):527–532. doi: 10.1136/gut.28.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schellack N. An overview of gastropathy induced by non-steroidal anti-inflammatory drugs. S Afr Pharm J. 2012;79(4):12–18. [Google Scholar]
- 80.Sugano K, Choi MG, Lin JT, Goto S, Okada Y, Kinoshita Y et al. LAVENDER Study Group. Multinational, double- blind, randomised, placebo-controlled, prospective study of esomeprazole in the prevention of recurrent peptic ulcer in low-dose acetylsalicylic acid users: the LAVENDER study. Gut. 2014;63(7):1061–1068. doi: 10.1136/gutjnl-2013-304722. [DOI] [PubMed] [Google Scholar]
- 81.Scheiman JM, Lanas A, Veldhuyzen van Zanten S, Baldycheva I, Svedberg LE, Nagy P. Effect of esomeprazole on gastro-duodenal erosions in patients at increased gastrointestinal risk treated with low-dose acetylsalicylic acid: a post-hoc analysis of the OBERON trial. Int J Cardiol. 2015 Mar;182:500502. doi: 10.1016/j.ijcard.2014.12.140. [DOI] [PubMed] [Google Scholar]
- 82.Hooper L, Brown TJ, Elliott R, Payne K, Roberts C, Symmons D. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. BMJ. 2004 Oct;329(7472):948. doi: 10.1136/bmj.38232.680567.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taha AS, Hudson N, Hawkey CJ, Swannell AJ, Trye PN, Cottrell J et al. Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal antiinflammatory drugs. N Engl J Med. 1996 May;334(22):1435–1439. doi: 10.1056/NEJM199605303342204. [DOI] [PubMed] [Google Scholar]
- 84.Van Groenendael JH, Markusse HM, Dijkmans BA, Breed-veld FC. The effect of ranitidine on NSAID related dyspeptic symptoms with and without peptic ulcer disease of patients with rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 1996 Sep;15(5):450–456. doi: 10.1007/BF02229641. [DOI] [PubMed] [Google Scholar]
- 85.Gigante A, Tagarro I. Non-steroidal anti-inflammatory drugs and gastroprotection with proton pump inhibitors: a focus on ketoprofen/omeprazole. Clin Drug Investig. 2012 Apr;32(4):221–233. doi: 10.2165/11596670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 86.Weinblatt , Michael E, Genovese , Mark C, Kivitz , Alan J et al. Efficacy, Safety And Tolerability Of HZT-501, Including Users Of Low-Dose Aspirin, A Single-Tablet Combination Of Ibuprofen-Famotidine: Results Of Two Phase 3 Trials. [abstract] Arthritis Rheum. 2010 Oct;62(10 (Suppl 10)):945. [Google Scholar]
- 87.Sostek MB, Fort JG, Estborn L, Vikman K. Long-term safety of naproxen and esomeprazole magnesium fixed-dose combination: phase III study in patients at risk for NSAID-associated gastric ulcers. Curr Med Res Opin. 2011 Apr;27(4):847–854. doi: 10.1185/03007995.2011.555756. [DOI] [PubMed] [Google Scholar]