Abstract
Background:
There are limited data on downstream effects of hepatocellular carcinoma (HCC) surveillance, including frequency of false positive results. We aimed to quantify the incidence of indeterminate nodules and follow-up testing needed to resolve these findings among patients enrolled in a structured HCC surveillance program.
Methods:
We retrospectively analyzed adult cirrhosis patients enrolled in a structured HCC surveillance program in a large tertiary care center. Outcomes included surveillance benefits, defined as early HCC detection, and harms, defined as indeterminate nodules prompting additional diagnostic evaluation.
Results:
Among 999 patients followed for a median of 2.2 years, HCC surveillance imaging was consistently completed every 6, 9, and 12 months in 46%, 51% and 68% of patients, respectively. Of 256 (25.6%) patients with abnormal imaging 69 (26.9%) were diagnosed with HCC and 187 (73.1%) with indeterminate nodules. Most HCC (n=54, 78.3%) were found within Milan Criteria. Among those with an indeterminate nodule, 78.1% returned to ultrasound surveillance after a median of 2 (IQR 1–3) negative CT/MRI and 21.8% continued CT/MRI imaging (median 2; IQR 1–2). Eleven patients underwent diagnostic liver biopsy. Hypoalbuminemia, thrombocytopenia and larger nodule size were independently associated with HCC diagnosis.
Conclusion:
One in four patients enrolled in an HCC surveillance program had abnormal surveillance imaging, but three-fourths of the lesions were indeterminate nodules, resulting in downstream harms. Improved risk-stratification tools are needed to identify nodules that are benign to reduce follow-up diagnostic evaluation.
Keywords: HCC, indeterminate, nodule, screening
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related death worldwide.(1,2) In the United States, the annual incidence and mortality of HCC is increasing, largely due to a peak in hepatitis C virus (HCV)-related complications and emergence of nonalcoholic fatty liver disease (NAFLD) as a rapidly growing cause of chronic liver disease.(3) This high HCC-related mortality is due to a significant proportion of patients presenting with late stage tumors, which only have palliative treatment options.(4,5) Accordingly, several professional society guidelines recommend HCC surveillance using ultrasound +/− alpha fetoprotein (AFP) in patients with cirrhosis to improve early tumor detection and curative treatment receipt. (6–8)
The value of a cancer screening program must weigh any benefits against potential harms of the screening tests. The benefit of HCC surveillance is dependent on adherence to the surveillance program and its effectiveness.(9) Prior studies suggest adherence to HCC surveillance in real-world clinical practice is low at 15–20% for one-time screening and 5–10% for biannual surveillance, highlighting a need for interventions to increase surveillance utilization.(10–13) Similarly, studies have suggested variability in sensitivity to detect HCC at an early stage, with high rates of surveillance failure even in high-volume academic centers.(14) Conversely, finding of nodules during ultrasound surveillance that do not have the characteristic features of HCC on multi-phasic computed tomography (CT) or magnetic resonance imaging (MRI) nor typical features of benign lesions, i.e. indeterminate nodules (INs) can result in physical harms (e.g. radiation exposure, contrast nephropathy, and biopsy complications), financial harms (e.g. co-pays or lost wages), and/or psychological harms (e.g. worries about cancer), particularly if the nature of these nodules cannot be resolved after initial cross-sectional imaging.(15–17) Excess diagnostic testing are well established as physical harms in patients undergoing colon, breast and prostate cancer screening; however, this has been underexplored in patients undergoing HCC surveillance.(18–20) A randomized trial comparing 3-month and 6 month surveillance using US found that 70% of focal lesions that were detected on US we not HCC, however the downstream harms to those patients with indeterminate nodules were not characterized.(21) Similarly, a recent study from a safety-net hospital suggested up to 27.5% of patients may experience physical harms related to false positive findings; however, data in other practice settings are limited.(22)
The aims of this study were to determine (1) the incidence of abnormal imaging results in a large cohort of cirrhosis patients enrolled in a structured HCC surveillance program at a high-volume academic liver center, (2) the frequency in which the nodule(s) detected on ultrasound were indeterminate and the number of cross-sectional imaging needed to resolve the benign vs. malignant nature of these nodules, and (3) factors predictive of HCC among patients with abnormal imaging.
Methods
Patient Population and HCC Surveillance Program
All adult patients (age ≥18) with cirrhosis followed in outpatient hepatology clinics at the University of Michigan between January 2010 and December 2015 were prospectively enrolled in a chronic disease management program. Enrollment in this program was previously shown to increase one-time screening after implementation.(23) The diagnosis of cirrhosis for entry into the chronic disease management program was based clinically on histology, transient elastography, or imaging showing a nodular liver with or without associated signs of portal hypertension. The chronic disease management program included serial tracking of all laboratory and imaging results, including HCC surveillance, with a capacity to generate reminders at designated intervals. Clinic nurses contacted patients to complete any necessary surveillance testing at recommended intervals. Per the American Association for the Study of Liver Diseases guideline recommendations during the study period, abdominal imaging was required for completion of HCC surveillance, while alpha-fetoprotein (AFP) testing was optional and its measurement was provider dependent, although 94% of patients in the cohort had at least one AFP measurement.(8) The program captured outside imaging if results were scanned into the electronic medical record and patients were logged into the reminder system. Abdominal imaging done for non-surveillance purposes were also logged, given this satisfies the need for surveillance testing. For this study, included patients were required to have had at least one surveillance US without IN or HCC at baseline. We excluded patients with a history of liver transplantation, and those who exclusively received MRI/CT-based surveillance. Given our aim was to quantify incident HCC and INs, we also excluded patients who had any history of HCC or any IN at baseline. Abnormal imaging was defined as any imaging with a nodule that required follow-up multiphasic cross-sectional imaging. An IN was defined as any solid lesion greater than 1 cm in diameter that could not be categorized as definitely benign (i.e. cyst or hemangioma) and did not meet diagnostic criteria for HCC on cross sectional imaging. The recently adopted LIRADs imaging criteria were not available during the study period; however, most of the INs in this study would likely be classified as LR-3 or LR-4 lesions.
Data Collection and Definition of Outcomes
Baseline demographics (age, gender, race, ethnicity), etiology of cirrhosis, body mass index (BMI) and labs (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase, albumin, total bilirubin, AFP, creatinine, international normalized ratio [INR] and platelet count) at time of enrollment were abstracted from the electronic medical record. Complete records were available for all reviewed patients. Dates and results of all liver imaging (US, multiphasic contrast-enhanced CT or MRI) during the study period were recorded. For each imaging study, presence of any suspicious lesions was documented; and number and size of the lesions was recorded when available. For CT/MRI, diagnostic characteristics for HCC as defined by AASLD guidelines were noted including arterial enhancement and delayed washout.(24) A small proportion of patients (n=6) were treated for HCC based on liver tumor board review and recommendations, despite not meeting all imaging criteria for HCC. Data were collected from enrollment in the surveillance program until end of the study period (12/2015), last outpatient clinic visit, or development of HCC.
To evaluate the effectiveness of the surveillance program, we measured the proportion of patients who underwent imaging at 6-month, 9-month, and 12-month intervals.
Statistical Analysis
The primary outcome of interest was surveillance harms, defined as the proportion of patients with INs that underwent subsequent follow-up testing (e.g. 4-phase CT, contrast-enhanced MRI, or biopsy).(16,25) Diagnostic testing for INs was defined as physical harm, consistent with the definition adopted in screening literature for other malignancies (e.g. breast cancer(26) and prostate cancer(27)). We further stratified patients by mild harms (1–3 multiphasic cross-sectional imaging tests without a diagnosis of HCC) and severe harms (>= 4 multiphasic cross-sectional imaging tests without a diagnosis of HCC or performance of a liver biopsy).
We also measured surveillance benefits, defined as early stage HCC detection. Early stage HCC was defined as being within Milan criteria, the most common criteria for liver transplantation in the United States. Bivariate analyses were performed to assess frequencies of IN and HCC and patient-level factors associated with each outcome. Chi-square tests and Fisher exact tests were used for categorical variables and t-tests were used for continuous variables. Variables with distributions that deviated from normality were reported by median and interquartile range (IQR, Q1–Q3) rather than by conventional mean ± standard deviation (SD) and were compared using the Kruskal-Wallis test. Multivariate analysis was conducted to identify factors associated with HCC and INs in the entire cohort. We used pre-specified cutoffs for continuous variables including platelet count, albumin and nodule size. An additional multivariate analysis was conducted for correlates of HCC in patients with abnormal imaging. We included variables from the univariate analysis (i.e. baseline characteristics) with p-values <0.1 in our multivariate analyses, for which p-values <0.05 were considered statistically significant. All analyses were performed in STATA 14 (StataCorp, College Station, TX). This study was approved by the University of Michigan Institutional Review Board.
Results
Baseline Characteristics
A total of 999 patients met inclusion criteria (Table 1). The cohort consisted primarily of middle-aged (median age 58; IQR: 52.2–64.7), white (90.7%) individuals. Gender was evenly distributed (53.6% males) and the majority were overweight or obese (median baseline BMI 29; IQR:25–35). The etiology of cirrhosis was diverse (35% HCV, 17% alcohol, 16% NAFLD), and median baseline Model of End-Stage Liver Disease (MELD) score was 7 (IQR:6–10).
Table 1.
Baseline Characteristics of Patients with or without Abnormal Imaging.
| Overall N=999 |
Normal Imaging (No IN or HCC) N=743 |
Abnormal Imaging (IN or HCC) N=256 |
P value | |
|---|---|---|---|---|
| Baseline Clinical Characteristics | ||||
| Age, median (IQR) | 58.0 (52.2–64.7) | 57.9 (51.8–64.5) | 58.7 (52.7–65.2) | 0.32 |
| Male Gender, N (%) | 535 (53.6%) | 383 (51.6%) | 152 (59.3%) | 0.03 |
| Race N (%) | 0.32 | |||
| White | 849 (90.7%) | 630 (91.0%) | 219 (89.7%) | |
| Black | 42 (4.4%) | 30 (4.3%) | 12 (4.9%) | |
| Asian | 29 (3.1%) | 19 (2.7%) | 10 (4.1%) | |
| Hispanic/Latino (N=974), N (%) | 24 (2.5%) | 16 (2.2%) | 8 (3.1%) | 0.41 |
| Etiology of Cirrhosis, N (%) | 0.01 | |||
| HCV | 356 (35.7%) | 248 (33.4%) | 108 (42.2%) | |
| Alcoholic cirrhosis | 175 (17.5%) | 143 (19.3%) | 32 (12.5%) | |
| NASH/NAFLD* | 165 (16.5%) | 124 (16.7%) | 41 (16%) | |
| PBC/PSC | 73 (7.3%) | 60 (8.1%) | 13 (5.0%) | |
| HBV | 47 (4.7%) | 30 (4.0%) | 17 (6.7%) | |
| Other | 182 (18.2%) | 137 (18.4%) | 45 (17.5%) | |
| BMI, median (IQR) | 29 (25–35) | 29 (25–35) | 29 (25–35) | 0.84 |
| Follow-up Duration (yr), median (IQR) |
2.17 (0.92–3.89) | 1.91 (0.77–3.73) | 2.9 (1.35–3.95) | <0.001 |
| Baseline Labs (median, IQR) | ||||
| MELD | 7 (6–10) | 6 (6–10) | 7 (7–10) | 0.24 |
| Platelet Count K/μL | 104 (74–147) | 108.5 (75–156) | 97 (67–125) | <0.001 |
| Alpha Fetoprotein ng/mL | 3.4 (2.1–6.4) | 3.1 (2–5.8) | 4.4 (2.7–8.4) | <0.001 |
| AST IU/L | 49 (34–75) | 47 (33–73) | 58 (34–75) | <0.001 |
| ALT IU/L | 37 (25–61) | 35 (24–57) | 45 (28–79) | 0.001 |
| Total Bilirubin mg/dL | 1.0 (0.7–1.7) | 1.0 (0.7–1.7) | 1.1 (0.8–1.8) | 0.03 |
| Alkaline Phosphatase IU/L | 116 (87–163) | 115 (87–167) | 117 (89–160) | 1.0 |
| Albumin g/dL | 3.8 (3.3–4–2) | 3.8 (3.3–4.2) | 3.7 (3.2–4.2) | 0.36 |
| INR | 1.1 (1.–1.3) | 1.1 (1–1.2) | 1.2 (1.1–1.3) | 0.01 |
| Imaging (median, IQR; range) | ||||
| Total US | 4 (2–7; 1–19) | 4 (2–6;1–19) | 5 (3–7; 1–14) | <0.001 |
| Total CT/MRI | 1 (0–2; 0–12) | 0 (0–1;0–12) | 2 (1–3; 0–12) | <0.001 |
| Outcomes, N (%) | ||||
| HCC or empiric treatment for HCC | 69 (6.91%) | 0 | 69 (26.9%) | <0.001 |
| Hepatic Decompensation | 532 (53.5%) | 394 (53.0%) | 138 (53.9%) | 0.87 |
| Variceal Bleeding | 112 (11.2%) | 87 (11.7%) | 25 (9.7%) | |
| Ascites | 319 (31.9%) | 227 (30.5%) | 92 (35.9%) | |
| Hepatic Encephalopathy | 99 (9.9%) | 79 (10.6%) | 20 (7.8%) |
Includes 22 cryptogenic cirrhosis
HCV – hepatitis C, HBV – hepatitis B, PBC – primary biliary cholangitis, PSC – primary sclerosing cholangitis, BMI – Body mass index, MELD – Model of end-stage liver disease
Receipt of HCC Surveillance
During the study period (median follow-up 2.17 years (IQR: 0.92–3.89), HCC surveillance imaging was consistently completed every 6, 9, and 12 months in 46%, 51% and 68% of patients, respectively. Of the patients who did not complete surveillance every 6 months, nearly half (44%) only missed one surveillance imaging appointment during follow-up; one-third (30%) missed two and 26% missed 3 or more imaging appointments.
The median number of US was 4 (IQR 2–7; range 1–19) and median number of CT/MRIs was 1 (IQR 0–2, range 0–12). Among all imaging studies performed for HCC surveillance or for clinical reasons other than follow-up of INs, 89.6% were conducted as an outpatient, 7.4% as an inpatient and 2.9% in the emergency department. The indication for most imaging studies was primarily for HCC surveillance in 81.5%, diagnostic purposes in 18%, and unspecified in 0.5%.
Proportion of patients with INs and HCC
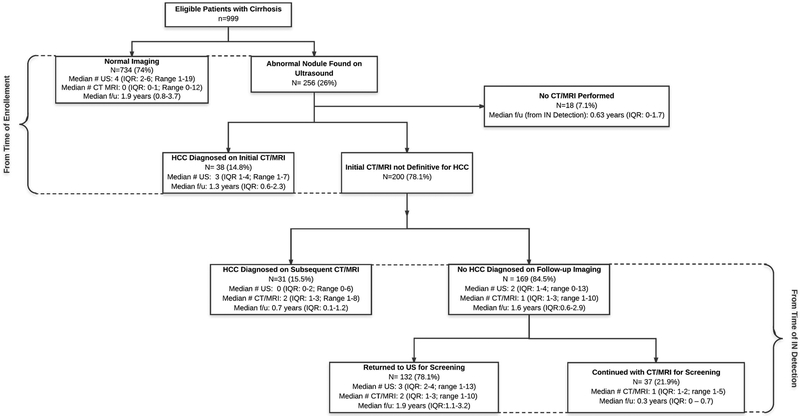
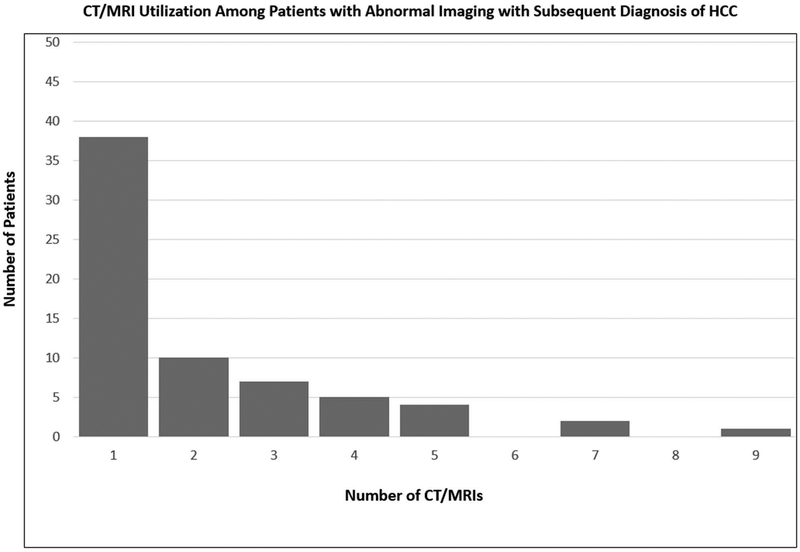
A summary of surveillance results and follow-up evaluation is depicted in Figure 1. A suspicious nodule was detected in 256 (26%) patients during ultrasound surveillance over a median period of 2.2 (IQR 0.9–3.9) years. The median number of nodules seen on US was 1 (IQR: 1–2) and the median size was 1.7 cm (IQR: 1.2–2.5). HCC was diagnosed in 69 (6.9%) patients, of whom 78% were within Milan Criteria or less. Specifically, 14 had T1 tumor burden; 36 had T2; and 3 with advanced HCC (mets). Of these patients, 38 were diagnosed on initial CT/MRI after abnormal US, and 31 patients required multiple (median 2, IQR 1 – 3; range 1 – 12) CT/MRIs after an abnormal US to make a diagnosis of HCC during a median follow-up time of 0.7 years (IQR: 0.1–1.2) after IN was detected (Figure 2A and 2B). A total of 10 patients had missed lesions with HCC diagnosed on initial CT/MRI in the setting of a normal US. There was an median time of 18 days (IQR; 3–163) from the normal US to HCC diagnosis in these 10 patients. In these patients, cross sectional imaging was prompted by elevated AFP in one patient, poor US quality in two patients, reasons other than surveillance in two patients, and unclear reasons in five patients. These patients had similar clinical and demographic characteristics when compared to the remainder of the cohort with similar age (60.0 vs 58.0), BMI (29 vs 29) and similar liver function (all NS).
Figure 1: Summary of Imaging Findings and Subsequent Evaluation.
US, ultrasound; CT, CAT scan; MRI, magnetic resonance imaging; HCC, hepatocellular carcinoma;
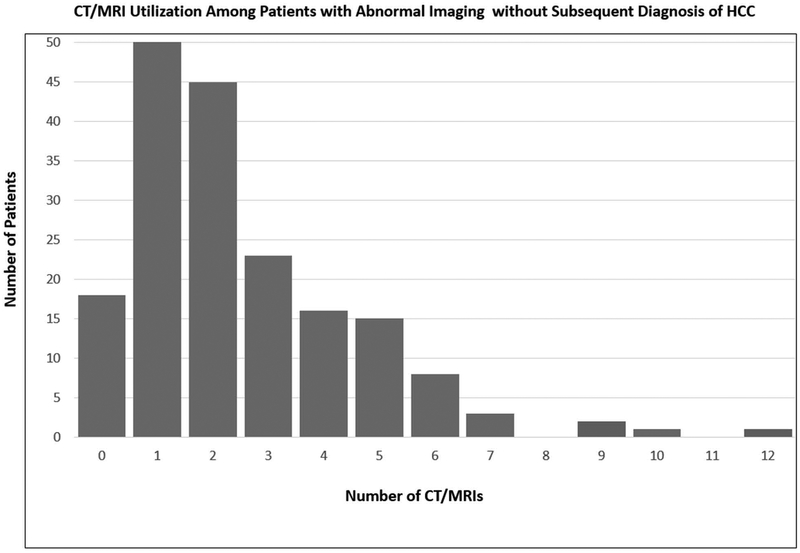
Figure 2. CT/MRI Utilization Among Patients with Abnormal Imaging A. Without and B. With Diagnosis of Hepatocellular Carcinoma.
CT, CAT scan; MRI, magnetic resonance imaging
Among the 187 patients with an IN on US but without HCC during the study period, 18 (9.6%) had not undergone CT/MRI for diagnostic evaluation after a median follow-up of 0.63 years (IQR: 0–1.7). Of the 169 patients who had further evaluation with CT/MRI, 132 (78.1%) were determined to be false positive results and returned to surveillance US after a median of 2 (IQR 1–3, range 1–10) CT/MRIs during a median follow-up of 1.9 years (IQR: 1.1–3.2) after the IN was detected. Among these patients, 49.5% had one CT/MRI, 18.9% had two, 13.6% had three, and 18.9% had ≥4 CT/MRI after IN detection. The remaining 37 (21.8%) patients were still categorized as indeterminate and undergoing CT/MRI imaging (median 1; IQR 1–2; range 1–5) at the end of the study period after a median follow-up of 0.32 years (IQ: 0.1–0.74) following IN detection on US. Among these patients, 62.2% had one CT/MRI, 18.9% had two, 10.8% had three, and 8.1% had ≥4 CT/MRI after IN detection.
Percutaneous liver biopsy was performed in 11 patients with IN, of whom 6 were diagnosed with HCC and 5 were benign. One patient, who had a benign lesion on biopsy, experienced a biopsy-related complication of abdominal pain, which required an emergency department visit without need for transfusion or hospitalization. Overall 17.1% of patients with INs on US experienced severe harms with either >= 4 cross sectional imaging tests or a liver biopsy with no diagnosis of HCC.
Characteristics Associated with IN and HCC
Characteristics of patients with normal imaging compared to those with abnormal imaging (IN or HCC) are displayed in Table 1. Patients with abnormal imaging were more commonly male, more likely to have HCV cirrhosis, had lower platelet count, and higher AFP, AST, ALT, total bilirubin, and INR; however, there was no difference in presence of any hepatic decompensation including ascites, hepatic encephalopathy or variceal bleeding. Compared to those with IN, patients with HCC had significantly higher MELD scores, alkaline phosphatase, and AFP, lower platelet count and albumin, larger nodule size and were more likely to have hepatic decompensation (Table 2).
Table 2.
Characteristics of Patients with Abnormal Imaging with or without Subsequent HCC Diagnosis.
| Abnormal Imaging no HCC N=187 |
Abnormal Imaging with HCC N=69 |
P value | |
|---|---|---|---|
| Baseline Clinical Characteristics | |||
| Age, median (IQR) | 58.2 (52.2–65.9) | 61.1 (55.4–64.6) | 0.12 |
| Male Gender, N (%) | 105 (56.1%) | 47 (68.1%) | 0.08 |
| Race, N (%) | 0.74 | ||
| White | 162 (90.5%) | 57 (87.7%) | |
| Black | 8 (4.4%) | 4 (6.1%) | |
| Asian | 7 (3.9%) | 3 (4.6%) | |
| Hispanic/Latino, N (%) | 6 (3.2%) | 2 (2.9%) | 0.90 |
| Etiology of Liver Disease, N (%) | 0.76 | ||
| HCV | 75 (40.1%) | 33 (47.8%) | |
| Alcoholic cirrhosis | 23 (12.3%) | 9 (13.0%) | |
| NASH/NAFLD | 33 (17.6%) | 8 (11.6%) | |
| PBC/PSC | 10 (5.4%) | 3 (4.3%) | |
| HBV | 14 (7.5%) | 4 (4.3%) | |
| Other | 32 (17.1%) | 13 (18.8%) | |
| BMI, median (IQR) | 29 (25–35) | 30 (25–32) | 0.74 |
| Follow-up Duration (yr), median (IQR) | 3.28 (1.85–4.18) | 1.76 (1.1–2.9) | <0.001 |
| Baseline Labs (median, IQR) | |||
| MELD | 7 (6–10) | 8 (6–11) | 0.02 |
| Platelet Count K/μL | 103 (75–132) | 77 (52–106) | <0.001 |
| Alpha Fetoprotein ng/mL | 4 (2.3–7.3) | 6.0 (3.5–12.1) | 0.003 |
| AST IU/L | 55 (39–88) | 61 (45–103) | 0.07 |
| ALT IU/L | 45 (27.5–80) | 46 (32–77) | 0.88 |
| Total Bilirubin mg/dL | 1 (0.7–1.65) | 1.3 (0.9–2) | 0.02 |
| Alkaline Phosphatase IU/L | 111.5 (88–151.5) | 140 (95–174) | 0.01 |
| Albumin g/dL | 3.8 (3.4–4.25) | 3.3 (3.1–3.9) | <0.001 |
| INR | 1.1 (1.1–1.2) | 1.2 (1.1–1.3) | <0.001 |
| Imaging/Diagnostics | |||
| Total US, median (IQR; range) | 5 (4–8;1–14) | 3 (2–5;1–10) | <0.001 |
| Total CT/MRI, median (IQR; range) | 2 (1–3; 0–12) | 1 (1–3; 1–9) | 0.19 |
| Number of nodules on US (IQR) | 1 (1–2) | 1 (1–3) | 0.36 |
| Size of largest nodule on US, cm (IQR) | 1.4 (1.1–2.0) | 2.3 (1.8–2.8) | <0.001 |
HCV – hepatitis C, HBV – hepatitis B, PBC – primary biliary cholangitis, PSC – primary sclerosing cholangitis, BMI – Body mass index, MELD – Model of end-stage liver disease; US - ultrasound
In a multivariate analysis of the overall cohort, no factors were independently associated with IN, however baseline platelet count approached significance (Supplemental Table 1). Thrombocytopenia and hypoalbuminemia were independently associated with HCC diagnosis (OR 2.75 95% CI 1.37–5.53 and OR 2.77 95% CI 1.43–5.35 respectively) (Table 3). In a subgroup multivariate analysis among those with abnormal imaging, thrombocytopenia and hypoalbuminemia continued to be associated with HCC diagnosis (OR 3.67 95% CI 1.46–9.23 and OR 4.07 95% CI 1.56–10.63 respectively). Larger nodule size on US (≥2cm) was also associated with diagnosis of HCC (OR: 8.63; 95% CI: 3.55–20.92–1.07) (Table 3).
Table 3.
Multivariable Analysis of Predictors of HCC Diagnosis.
| Multivariable Analysis of Diagnosis of HCC Within Overall Cohort | |||
| Odds Ratio | 95% CI | P value | |
| Age (year) | 1.01 | (0.97–1.04) | 0.51 |
| Sex (male) | 1.56 | (0.82–2.99) | 0.17 |
| Baseline MELD | 0.98 | (0.91–1.06) | 0.74 |
| Baseline Low Platelets (<100 K/μL) | 2.75 | (1.37–5.53) | 0.004 |
| Baseline Alpha Fetoprotein | 1.00 | (0.99–1.02) | 0.37 |
| Baseline Albumin (<3.4 g/dL) | 2.77 | (1.43–5.35) | 0.002 |
| Multivariable Analysis of Diagnosis of HCC Among Patients with Abnormal Imaging | |||
| Odds Ratio | 95% CI | P value | |
| Age (year) | 1.00 | (0.96–1.04) | 0.83 |
| Sex | 1.02 | (0.43–2.41) | 0.06 |
| Baseline MELD | 0.92 | (0.81–1.05) | 0.23 |
| Baseline Thrombocytopenia (<100 K/μL) | 3.67 | (1.46–9.23) | 0.006 |
| Baseline Alpha Fetoprotein | 1.02 | (0.99–1.05) | 0.15 |
| Baseline Hypoalbuminemia (<3.4 g/dL) | 4.07 | (1.56–10.63) | 0.004 |
| Maximum Nodule Size ≥ 2.0 cm | 8.63 | (3.55–20.92) | <0.001 |
MELD – model of endstage liver disease
Discussion
Our study is one of the first to quantify the benefits and harms of a structured ultrasound-based HCC surveillance program in a large cohort of cirrhosis patients followed in an academic tertiary care center. The structured surveillance program was able to achieve consistent surveillance completion in nearly half of all patients over a 2.2-year median follow-up and detected over 75% of HCC patients at an early stage. However, these benefits were accompanied by physical harms including nearly 20% of patients having an indeterminate nodule requiring additional diagnostic evaluation.
Our study builds upon our prior results demonstrating the benefits of a structured surveillance program.(23) Our program using electronic medical record reminders achieved consistent HCC surveillance every 6 months in 46% of enrolled cirrhosis patients over a median of 2.2 years. Further, most patients without consistent surveillance only missed 1 or 2 surveillance exams. These results are notable, as most prior studies in the U.S. report consistent surveillance rates of only 5–10% when assessed over extended study periods.(11) For example, a study investigating the effectiveness of a mailed outreach program doubled one-time HCC screening (47%) compared to usual care, but longitudinal surveillance was only 5% over an 18-month period.(28) A review of hepatology provider orders who were noted that nearly all (>95%) patients enrolled in the program had orders for HCC surveillance at 6-month intervals. Therefore, this structured program addresses provider oversight as a source of surveillance failure, which has been reported as the most common failure in the HCC screening process.(29) A potential contributing factor to suboptimal surveillance completion rates is that outside imaging reports may not have been accurately captured. To circumvent this issue, we now try to schedule imaging the same day as patients’ clinic visit. Further research to optimize this multi-step process and achieve higher rates of HCC surveillance completion is needed to improve early HCC detection. Increased HCC surveillance completion was associated with early HCC detection, as over 75% of HCC patients in our surveillance program were detected at an early stage. Ten patients were diagnosed in the setting of a normal US, however the reasons for subsequent cross-sectional imaging varied, and we could not find any age or BMI differences to explain the false negative US results.
The benefits of an HCC surveillance program must be weighed against observed HCC surveillance harms.(30) Over 15% of patients in our cohort had an indeterminate nodule that was determined to be benign or remains indeterminate and continues to undergo diagnostic evaluation. Our results are similar to a study from a safety-net health system, in which 22.7% of cirrhosis patients underwent “unnecessary” imaging due to indeterminate or false positive surveillance tests.(25) Most surveillance-related “harm” in the study by Atiq and colleagues was limited to a single CT or MRI diagnostic exam, although some patients experienced moderate to severe harm, defined as repeated cross-sectional imaging or invasive evaluation with biopsy or angiogram. In our study, over half of patients with INs also underwent repeated cross-sectional imaging or invasive evaluation with biopsy. We were able to quantify the number of cross sectional exams in all patients with INs, adding further granularity to the data on harms related to HCC surveillance. However, notably there were 31 patients who required multiple cross-sectional imaging tests and 6 patients who required biopsy to diagnose HCC, highlighting the fact that it can be difficult to define what is “excessive” or “unnecessary” diagnostic evaluation at the time.
There is a clear need for better risk stratification tools to differentiate HCC from benign lesions to reduce unnecessary imaging. Unfortunately, we did not identify any demographic or clinical factors correlated with IN; thus, we failed to identify subgroups who may benefit from alternate modalities of surveillance. However, other studies suggest ultrasound false positive and indeterminate results may be more likely in obese patients, those with alcohol or NASH-related cirrhosis, and those with more advanced liver dysfunction.(25,31,32) Controversy still surrounds the use of AFP in surveillance(33) and while AFP levels were statistically significantly higher in those patients with HCC when compared to those patients with IN in our cohort, this difference was not clinically significant. Accurate risk stratification for HCC development remains a challenging task with most studies only able to achieve modest predictive accuracy.(34,35) A study of 494 hepatitis B-infected patients with INs noted that age, nodule size, arterial enhancement, albumin and AFP levels were independently predictive of HCC progression. The associated predictive model had an area under the curve of 0.88 and 0.92 for 3- and 5-year risk prediction.(36) This model still requires external validation, as it is unclear how the model would perform in heterogeneous patient populations with different etiologies of liver disease. In our study, we found thrombocytopenia and hypoalbuminemia were independently predictive of HCC development among the overall cohort. In addition to these factors, nodule size was also associated with HCC in the subset of patients with abnormal imaging.
There are several notable limitations with our study. The study was performed at a single center so it is unknown if our results can be generalized to other centers but our data are consistent with what has been reported in the literature.(25) As demonstrated in Table 1, our patient population is relatively homogenous in terms of race and ethnicity which has been associated with variable incidence rates of HCC. However, we did have diverse etiologies of chronic liver disease, which represents a strength over much of the existing literature that focuses on HCC risk within disease specific groups (i.e. hepatitis B or hepatitis C). Additionally, our center does not routinely perform contrast-enhanced ultrasound, and could be included as part of a diagnostic algorithm for IN where available, which could be effective reducing the number of CT/MRIs performed. Secondly, some patients with abnormal imaging were still in the process of evaluation and we may have overestimated the IN rate as some of these patients could have been diagnosed with HCC after the data collection had been completed. We mitigated this by excluding patients who had not yet received multiphasic cross-sectional imaging after IN detection in calculating the IN rate. Patients with nodules on cross-sectional imaging are at increased risk of eventually developing HCC, however continued surveillance of nodules until they meet diagnostic criteria for HCC, can be prolonged incurring costs and causing patient anxiety. The optimal timing of when to return to US surveillance remains an open question. Third, given the study design, we did not capture the psychological harms of surveillance for HCC and the psychological burden of having an IN. Fourth, the study spanned the introduction of LIRADS radiographic criteria for HCC and nodule diagnosis on cross-sectional imaging, so the LIRADS classification for the INs were not readily available for this analysis. While the LIRADS classification is an important tool in classifying nodules seen on dynamic imaging, it lacks thorough validation. Importantly, there is little guidance on the management and follow-up for indeterminate (LR3 and LR4) lesions, especially ones that are identified on serial imaging.(37) Thus we believe these data in indeterminate nodules are relevant even without LIRADS classification of the INs. Lastly, as a retrospective analysis, there are inherent limitations in determining indication for imaging studies and the possibility of provider bias influencing which patients received cross-sectional imaging, whether liver tumor board review was conducted, and their follow-up.
In conclusion, a structured HCC surveillance program effectively promoted HCC surveillance completion and detected over 75% of HCC at an early stage. However, over 15% of patients in the surveillance program had suspicious nodules prompting CT/MRI evaluation that did not lead to HCC diagnosis. This information is critically important when counseling patients on risks and benefits upon entering an HCC surveillance program. Improved risk-stratification tools are needed to better predict HCC risk as well as better differentiate benign from malignant nodules to maximize the value of HCC surveillance in patients with cirrhosis.
Supplementary Material
Acknowledgments
Financial support: This study was funded in part by the National Institutes of Health T32DK062708 training grant (MAK), National Cancer Institute RO1CA212008 and RO1CA222900 (AGS), and the AASLD Advanced/Transplant Hepatology Fellowship (MAK).
Footnotes
Disclosure: All authors report no relevant conflicts of interest related to the content in this manuscript
Disclosures outside the current scope of this manuscript:
Monica Konerman: None reported
Aashesh Verma: None reported
Betty Zhao: None reported
Amit Singal: Advisory board: Exact Sciences and Bayer. Consultant: Glycotest, Roche, and Bayer. Research Support: Exact Sciences, Glycotest, and Wako Diagnostics.
Anna Lok: Research funding Target Pharmasolutions
Neehar Parikh: Consulting: Bristol Myers Squibb, Exelixis; Advisory Boards: Bayer, Eisai; Research funding: Bayer, Exact Sciences, Target Pharmasolutions
References
- 1.Organization WH. World Health Organization Fact Sheets-Cancer. 2017.
- 2.Cancer WHO-IAfRo. GLOBOCAN-Estimated number of incident cases, both sexes, worldwide in 2012 2017.
- 3.Parikh ND, Marrero WJ, Wang J, Steuer J, Tapper EB, Konerman M, et al. Projected increase in obesity and non-alcoholic steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology 2017. doi 10.1002/hep.29473. [DOI] [PubMed] [Google Scholar]
- 4.Ulahannan SV, Duffy AG, McNeel TS, Kish JK, Dickie LA, Rahma OE, et al. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology 2014;60(5):1637–44 doi 10.1002/hep.27288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh ND, Marshall VD, Singal AG, Nathan H, Lok AS, Balkrishnan R, et al. Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: An analysis of the SEER-Medicare database. Hepatology 2017;65(1):122–33 doi 10.1002/hep.28881. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Hepatocellular carcinoma. The New England journal of medicine 2011;365(12):1118–27 doi 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 7.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology 2012;56(4):908–43 doi 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53(3):1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mourad A, Deuffic-Burban S, Ganne-Carrié N, Renaut-Vantroys T, Rosa I, Bouvier AM, et al. Hepatocellular carcinoma screening in patients with compensated hepatitis C virus (HCV)-related cirrhosis aware of their HCV status improves survival: A modeling approach. Hepatology (Baltimore, Md) 2014;59(4):1471–81. [DOI] [PubMed] [Google Scholar]
- 10.Singal AG, Tiro J, Li X, Adams-Huet B, Chubak J. Hepatocellular Carcinoma Surveillance Among Patients With Cirrhosis in a Population-based Integrated Health Care Delivery System. Journal of clinical gastroenterology 2017;51(7):650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 2012;27(7):861–7 doi 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus–infected veterans in the United States. Ann Intern Med 2011;154(2):85–93. [DOI] [PubMed] [Google Scholar]
- 13.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology 2010;52(1):132–41 doi 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singal A, Volk M, Waljee A, Salgia R, Higgins P, Rogers M, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor EJ, Jones RL, Guthrie JA, Rowe IA. Modelling the benefits and harms of surveillance for hepatocellular carcinoma: information to support informed choices. Hepatology 2017. doi 10.1002/hep.29315. [DOI] [PubMed] [Google Scholar]
- 16.Harris RP, Sheridan SL, Lewis CL, Barclay C, Vu MB, Kistler CE, et al. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA internal medicine 2014;174(2):281–6. [DOI] [PubMed] [Google Scholar]
- 17.DeFrank JT, Barclay C, Sheridan S, Brewer NT, Gilliam M, Moon AM, et al. The psychological harms of screening: the evidence we have versus the evidence we need. Journal of general internal medicine 2015;30(2):242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin K, Lipsitz R, Miller T, Janakiraman S. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the US Preventive Services Task Force. Ann Intern Med 2008;149(3):192–9. [DOI] [PubMed] [Google Scholar]
- 19.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2009;59(1):27–41. [DOI] [PubMed] [Google Scholar]
- 20.Screening IUPoBC. The benefits and harms of breast cancer screening: an independent review. The Lancet 2012;380(9855):1778–86. [DOI] [PubMed] [Google Scholar]
- 21.Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3-and 6-month periodicities. Hepatology 2011;54(6):1987–97. [DOI] [PubMed] [Google Scholar]
- 22.Wilt TJ, Harris RP, Qaseem A. Screening for Cancer: Advice for High-Value Care From the American College of PhysiciansScreening for Cancer: Advice for High-Value Care From the ACP. Annals of internal medicine 2015;162(10):718–25. [DOI] [PubMed] [Google Scholar]
- 23.Aberra FB, Essenmacher M, Fisher N, Volk ML. Quality improvement measures lead to higher surveillance rates for hepatocellular carcinoma in patients with cirrhosis. Dig Dis Sci 2013;58(4):1157–60 doi 10.1007/s10620-012-2461-4. [DOI] [PubMed] [Google Scholar]
- 24.Heimbach J, Kulik LM, Finn R, Sirlin CB, Abecassis M, Roberts LR, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017. doi 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 25.Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65(4):1196–205 doi 10.1002/hep.28895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers ER, Moorman P, Gierisch JM, Havrilesky LJ, Grimm LJ, Ghate S, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015;314(15):1615–34. [DOI] [PubMed] [Google Scholar]
- 27.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. Early detection of prostate cancer: AUA Guideline. The Journal of urology 2013;190(2):419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singal AG, Tiro JA, Marrero JA, McCallister K, Mejias C, Adamson B, et al. Mailed Outreach Program Increases Ultrasound Screening of Patients With Cirrhosis for Hepatocellular Carcinoma. Gastroenterology 2017;152(3):608–15.e4 doi 10.1053/j.gastro.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singal AG, Yopp AC, Gupta S, Skinner CS, Halm EA, Okolo E, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prevention Research 2012;5(9):1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? The American journal of gastroenterology 2013;108(3):425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Poggio P, Olmi S, Ciccarese F, Di Marco M, Rapaccini GL, Benvegnu L, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12(11):1927–33 e2 doi 10.1016/j.cgh.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Simmons O, Fetzer D, Yokoo T, Marrero J, Yopp A, Kono Y, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45(1):169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018. doi 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma X, Yang Y, Tu H, Gao J, Tan YT, Zheng JL, et al. Risk prediction models for hepatocellular carcinoma in different populations. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu 2016;28(2):150–60 doi 10.21147/j.issn.1000-9604.2016.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flemming JA, Yang JD, Vittinghoff E, Kim WR, Terrault NA. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer 2014;120(22):3485–93 doi 10.1002/cncr.28832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho HJ, Kim B, Lee JD, Kang DR, Kim JK, Lee JH, et al. Development of Risk Prediction Model for Hepatocellular Carcinoma Progression of Indeterminate Nodules in Hepatitis B Virus-Related Cirrhotic Liver. The American journal of gastroenterology 2017;112(3):460–70 doi 10.1038/ajg.2016.480. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61(3):1056–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.