Abstract
Endogenous hydrogen sulfide (H2S), synthesized by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), is a potent vasodilator that can be stimulated by estradiol-17β (E2β) in uterine artery (UA) smooth muscle (UASMC) in vivo; however, the underlying mechanisms are unknown. This study tested a hypothesis that E2β stimulates H2S biosynthesis by upregulating CBS expression via specific estrogen receptor (ER). Treatment with E2β stimulated time- and concentration- dependent CBS and CSE mRNA and protein expressions and H2S production in cultured primary UASMC isolated from late pregnant ewes, which were blocked by ICI 182, 780. Treatment with specific ERα or ERβ agonist mimicked these E2β-stimulated responses, which were blocked by specific ERα or ERβ antagonist. Moreover, E2β activated both CBS and CSE promoters and ICI 182,780 blocked the E2β-stimulated responses. Thus, E2β stimulates H2S production by upregulating CBS and CSE expression via specific ER-dependent transcription in UASMC in vitro.
Keywords: Estrogens, hydrogen sulfide, CBS, CSE, uterine artery smooth muscle cells, in vitro
INTRODUCTION
Estrogens potently dilate selected vasculatures throughout the body, with the greatest response in the uterus as reflected by dramatic rise in uterine blood flow (UBF) following local or systemic administration of 17β-estradiol (E2β) in various nonpregnant (NP) and pregnant (P) animal models (Ford et al., 1982; Magness and Rosenfeld, 1989; Rosenfeld et al., 1973). Estrogen-induced uterine vasodilation is important for pregnancy since circulating estrogens increase nearly 1000-fold, accompanied by as much as 50–80 fold rise in UBF in P vs. NP states (Magness and Rosenfeld, 1989; O’Leary et al., 1991). Estrogen-induced and pregnancy-associated rises in UBF facilitate the bidirectional maternal-fetal exchange of gases (e.g., O2 and CO2) and provide the sole nutrients to support fetal development and survival (Rosenfeld, 1977). Any constraints in UBF during pregnancy lead to preeclampsia and intrauterine growth restriction, threatening the health of both the mother and her fetus during pregnancy and after birth (Barker, 1995; Osol and Moore, 2014). Enhanced uterine artery (UA) endothelial production of nitric oxide (NO) has been recognized as a key player in estrogen-induced and pregnancy-associated uterine vasodilation because: 1) estrogens stimulate NO production via endothelial NO synthase (eNOS) in UA endothelial cells (EC) in vivo (Magness et al., 2001) and in vitro (Chen et al., 2004) and 2) local NO inhibition significantly attenuates estrogen-induced rise in UBF in NP ewes (Magness et al., 2005; Rosenfeld et al., 1996). However, local NO inhibition blocks only ~65% estrogen-induced uterine vasodilation (Magness et al., 2005; Rosenfeld et al., 1996), suggesting that mechanisms in addition to NO exist.
Hydrogen sulfide (H2S) is a gaseous signaling molecule of the gasotransmitter family after NO and carbon monoxide (Wang, 2012) that function as potent vasodilators (Yang et al., 2008). Endogenous H2S is primarily synthesized from L-cysteine by two enzymes: cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) (Bukovska et al., 1994; Erickson et al., 1990; Kredich et al., 1973); their expression can be tissue/cell specific as both are needed to generate H2S in some tissues while one enzyme is sufficient in others (Gadalla and Snyder, 2010; Mustafa et al., 2009). Our recent work first shows H2S to be a novel UA vasodilator since H2S production and the expression of UA smooth muscle (SM) and EC CBS, but not CSE, are significantly stimulated by exogenous estrogens in ovariectomized (OVX) NP ewes (Lechuga et al., 2015) and also are significantly augmented and positively linked to endogenous estrogens during pregnancy in human UA (Sheibani et al., 2017); moreover, a slow releasing H2S donor GYY4137 dose-dependently relaxes NP and P rat UAs, with greater potency in P state and vascular bed-specific effects (Sheibani et al., 2017). However, the mechanisms by which E2β stimulate UASM and EC H2S biosynthesis remains to be elusive.
The large tube-shaped primary UA is composed of a thin layer of ECs covering the luminal surface that is surrounded by a thick layer of SM cells (SMCs). The role of ECs in uterine hemodynamics has been extensively studied; in comparison, the role of SMCs in estrogen-induced UA dilation is currently much less understood. Early studies show that estrogens stimulate UASMC proliferation during pregnancy-associated UA remodeling (Keyes et al., 1996). Our previous work shows that UASMCs are direct target cells of estrogens as they express ERα and ERβ (Byers et al., 2005; Liao et al., 2005). Indeed, estrogens stimulate endothelial-independent vasorelaxation (Jiang et al., 1991; Mugge et al., 1993) and various signaling pathways, including activation of extracellular signal-activated kinases (ERK½) and protein kinase C (Khan et al., 2010), cyclic GMP secretion, expression of large conductance potassium channels (BKCa) (Rosenfeld et al., 1996; Rosenfeld et al., 2002), and membrane hyperpolarization (White et al., 1995) in UASMCs. However, whether SMCs produces vasodilators is always neglected.
We hypothesize that E2β stimulates UASMC H2S biosynthesis via specific ER-dependent CBS transcription. The objective of this study were to first establish an in vitro UASMC culture model from late pregnant ewes by which we aimed to determine in UASMC in vitro if: 1) E2β stimulates H2S production via enhanced expression of CBS and/or CSE expression; 2) E2β-stimulated H2S biosynthesis is mediated by specific ERs; 3) ERα and ERβ play a role in E2β-stimulated H2S biosynthesis; and 4) E2β-stimulates ER-dependent CBS and/or CSE transcription.
MATERIALS & METHODS
Chemicals and antibodies
17β-estradiol, hydroxyethylpiperazine-N’−2-ethanesulfonic acid (HEPES), fatty acid free bovine serum albumin (BSA), O-(carboxymethyl)hydroxylamine hemihydrochloride (CHH), sodium dodecyl sulfate (SDS), and all other chemicals unless specified, were from Sigma (St. Louis, MO). ICI 182, 780 (ICI), 4,4,4-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT), diarylpropionitrile (DPN) 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylet hoxy)phenol]-1H-pyrazole dihydrochloride (MPP), 4-[2-Phenyl-5,7-bis (trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3yl]phenol (PHTPP), were from Tocris (Ellisville, MO). β-cyano-L-Alanine (BCA) was from Cayman Chemical (Ann Arbor, MI). Anti-β-actin monoclonal antibody was from Ambion (Austin, TX). Fetal bovine serum (FBS) was from Lonza (Walkersville, MD). Monoclonal antibodies against ERα and ERβ were from Fisher Scientific (Pittsburgh, PA). Antibodies against CBS, CSE, α-smooth muscle actin (α-SMA), eNOS, and caveolin-1 were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). DMEM and M199, Platinum Taq DNA polymerase, and Alexa488 and Alexa588-labeled immunoglobulin G (IgG) were from Invitrogen (Carlsbad, CA).
Primary UASMC isolation, culture, characterization, and treatment
Primary UASMCs were isolated from late pregnant ewes (120–130 days of gestation, normal term ≈145 days). The Institutional Animal Care and Use Committee of the University of California approved the animal use protocol. Immediately after sacrifice, the main UAs were dissected; the lumen was filled with 0.1% collagenase for a 45-min digestion at 37°C to remove the endothelium. Endothelium-denude UAs were cut into ~1-cm segments; after softening the tissue by incubation with 0.1% collagenase for 20 min, the SM layer was dissected mechanically under a Stereo Microscope (10x). The SM segments were then minced (<1 mm3) and digested with 0.1% collagenase in M-199–0.1% BSA at 37°C for 45 min. The cells were then collected and cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin. UASMCs were frozen in liquid N2. UASMCs at passage 2 were characterized by flow cytometry with SM and EC markers α-SMA and eNOS, respectively, as well as the expression of caveolin-1. Cells were used for later experiments within 7 passages.
Cells at ~80% confluence were cultured in serum/phenol red-free M-199 medium containing 0.1% fatty acid-free BSA, 0.5% charcoal stripped FBS, 1% penicillin/streptomycin, and 25 mM HEPES overnight. Following equilibration in fresh serum-free M-199, cells were treated with or without ER antagonists (1 μM) for 1 h, followed by treatment with or without E2β (10 nM) for 48 h. For time course experiments, cells were treated with E2β (10 nM) for up to 72 h. For dose response experiments, cells we treated with 0–1μM E2β for 48 h. For ER agonist experiments, cells were treated with E2β (10 nM) or specific ER agonists (10 nM) for 48 h. Ethanol was used to dissolve E2β and ER agonists and antagonists. Final ethanol concentrations were less than 0.5% and did not alter cellular responses surveyed in this study.
Immunofluorescence microscopy and image analysis
UASMCs were grown on glass coverslips to reach ~80% confluency. Following treatments, cells were washed in cold phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde for 20 min at room temperature. After permeablized with 0.2% Triton-100 in PBS for 15 min at room temperature, the cells were labeled with 1 μg/mL of anti-α-SMA, caveolin-1, CBS, or CSE overnight at 4°C, followed by Alex486 or Alex588-labeled corresponding IgGs (2μg/ml) for 1 h at room temperature. Coverslips were mounted with Prolong Gold antifade reagent containing 4’,6-Diamidine-2’-phenylindole dihydrochloride (DAPI) for labeling cell nuclei. Samples were examined under a fluorescence microscope (Leica Corporation, Deerfield, IL) and digital images were acquired using a CCD camera and SimplePCI image analysis software (Hamamatsu Corporation, Sewickley, PA). The images were used to determine relative CBS and CSE proteins by averaging mean fluorescence intensity (15 cells/image and 5 images/animal) by using SimplePCI as described (Lechuga et al., 2015). Normalized CBS and CSE proteins were presented as fold change of vehicle (veh) treated control cells.
Flow cytometry
UASMCs were fixed with 70% ethanol for 1 h at 4°C. The cells were washed with PBS and resuspended in PBS containing 1% BSA at a concentration of 1× 106/ml. After incubation with 10% goat serum for 30 min, the cells (2.5×105/ml) were incubated with PBS containing 1 μg/ml anti-α-SMA, anti-eNOS, or anti-caveolin-1 for 1 h at room temperature, followed by FITC-labeled anti-mouse (SC2010, 1:200) or anti-rabbit (SC2012, 1:200) IgG (Santa Cruz) overnight at 4°C in dark. Positive staining was verified by omitting first antibody and with only secondary antibodies. The stained cells were analyzed by FACSCalibur using CellQuest software (Becton Dickinson, Mountain View, CA).
SDS-PAGE and immunoblotting
SDS-PAGE and immunoblotting was performed as previously described (Lechuga et al., 2015; Sheibani et al., 2017).
Methylene Blue Assay
UASMCs (5×105/treatment in duplicate) were homogenized in 50 mM ice-cold potassium phosphate buffer pH 8.0. H2S production was determined by using the methylene blue assay as previously described (Lechuga et al., 2015; Sheibani et al., 2017). H2S concentration was calculated based on a calibration curve generated from NaHS solutions. For CBS and CSE inhibition experiments, their respective inhibitor CHH or BCA, were added separately or in combination (final conc. = 2 mM) to the reaction mixtures prior to initiating the assay.
Reverse transcription (RT-PCR) and quantitative real-time RT-PCR (qPCR)
Total RNAs were extracted from UASMCs using Trizol reagent (Invitrogen, Carlsbad, CA). RNA was quantified by OD260/280 and first-strand complementary DNA (cDNA) was synthesized by using random primers and AMV Reverse Transcriptase (Promega, Madison, WI) with 2 μg RNA template as previously described (Lechuga et al., 2015; Liao et al., 2005). RT-PCR using ERα and ERβ specific primers was conducted to assess ERα and ERβ mRNAs reference to ribosomal protein L19 as described previously (Liao et al., 2005). qPCR using CBS and CSE specific primers was conducted to assess CBS and CSE mRNAs reference to ribosomal protein L19 by using the comparative CT method (ΔΔCT method) as described previously (Lechuga et al., 2015; Sheibani et al., 2017).
Cell transfection and luciferase assay
RenSP luciferase-reporter constructs containing the promoters of human CBS (S711027), CTH (S712215), β-actin (S717678) and all transfection and luciferase reagents were purchased from Switchgear Genomics (Carlsbad, CA). The RenSP luciferase plasmid DNA and cypridina luciferase TK control construct were co-transfected into UASMCs by using FuGENE HD transfection reagents (1:3, μl/ng) for 24 h at 37°C. Cells transfected with an empty vector or β-actin promoter vector (S717678) were served as negative and positive transfection controls, respectively. After recovery in DMEM medium containing 10% FBS for 18–20 h, cells were serum-starved overnight and then treated with vehicle or 10 nM E2β for 24 h with or without ICI 182,780. The RenSP luciferase activity normalized by cypridina luciferase activity in both cells and supernatant were measured to assess CBS and CSE transcription as previously described (Mata-Greenwood et al., 2010).
Statistical analysis
Each experiment was repeated at least three times with cells derived from different ewes. Data were presented as mean ± SEM and analyzed by one- or two-way analysis of variance (ANOVA), followed by Bonferroni test for multiple comparisons using SigmaPlot (Systat Software Inc.). Paired Student’s t-test was used for data comparison between two groups. Significance was defined as P<0.05, unless higher statistical power is indicated in the figure legends.
RESULTS
Characterization of a primary UASMC model
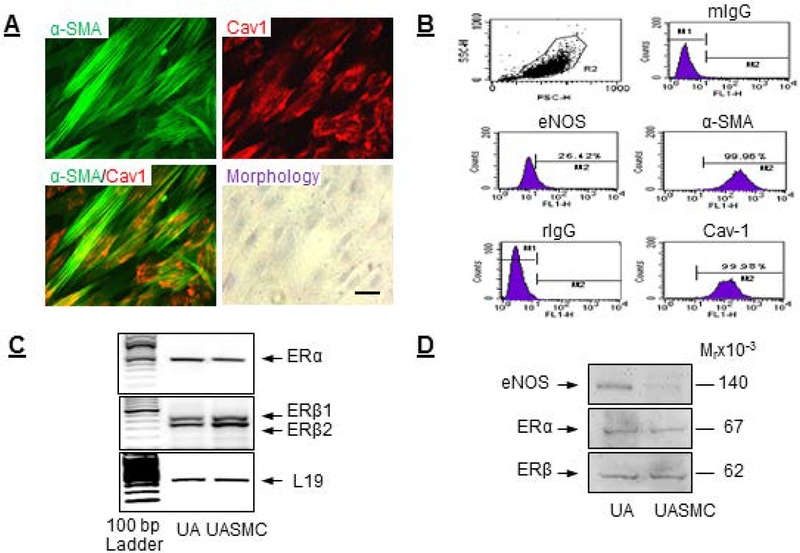
Primary UASMCs exhibited spindle-shaped classical smooth muscle cell morphology in culture and immunostained positively with the SM marker α-SMA and expressed high levels of the scaffolding protein caveolin-1 (Fig. 1A). Flow cytometry analysis showed that UASMCs expressed 99.9% α-SMA and caveolin-1, with low eNOS expression (Fig. 1B). UASMCs expressed ERα and ERβ mRNAs (Fig. 1C) and proteins (Fig. 1D) with levels quantitatively comparable to that of intact UAs. Immunoblot analysis revealed eNOS protein in intact UA but not in UASMCs (Fig. 1D).
Fig. 1: Establishment and characterization of an ovine uterine artery smooth muscle cells (UASMC) model.
Primary UASMCs from late pregnant ewes were isolated and characterized. (A) Cellular expression and co-localization of α-SMA and caveolin-1 by immunocytochemistry and assessment of cell morphology. (B) Flow cytometry for cellular expression of α-SMA, caveolin-1, and eNOS. ERα and ERβ (C) mRNA and (D) protein expressions in UASMCs and intact UAs. Scale bar is 50 μm.
E2β stimulates UASMC H2S production in vitro: role of CBS and CSE.
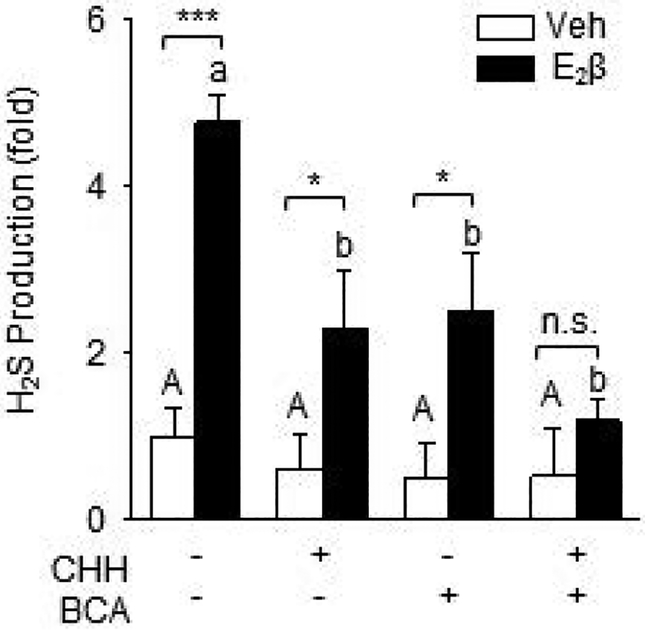
Treatment with 10 nM E2β resulted in a 4.77 ± 0.34 fold increase (P <0.01) in H2S production in UASMCs. Addition of CHH, BCA, or their combination did not alter baseline H2S production in Veh-treated cells. Either CHH or BCA alone significantly inhibit E2β-stimulated H2S production, although the inhibition was partial. Their combination did not fully enhance either CHH or BCA inhibition of E2β-stimulated H2S production; it did further reduce E2β-stimulated H2S production to baseline as seen in Veh-treated cells (Fig. 2).
Fig. 2: E2β stimulates UASMC H2S production in vitro.
Cells were treated with vehicle or 10 nM E2β for 48 h. Cell lysates were subjected to the methylene blue assay to determine H2S production with CHH and/or BCA. Data (mean ± SEM) were collected from cells prepared from 3–5 different ewes. Bars with different letters differ significantly (p < 0.05), capital letters pertain to Veh and lowercase to E2β, * P<0.05, *** P<0.001.
E2β stimulates CBS and CSE in time- and concentration-dependent manners in UASMCs
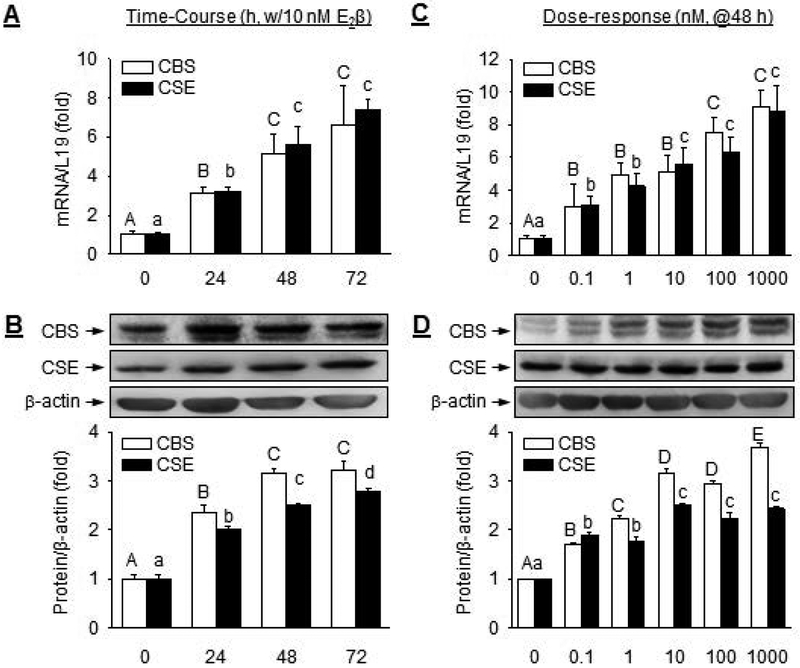
E2β significantly stimulated CBS and CSE mRNAs in a time-dependent manner in UASMC in vitro. Following treatment with 10 nM E2β, levels of both CBS and CSE mRNAs began to increase significantly at 24 h, maximized at 48 h (CBS: 5.11 ± 1.03 fold vs. control; P<0.01; CSE: 5.61 ± 0.93 fold vs. control, P<0.01), and plateaued at 72 h (Fig. 3A). E2β also significantly stimulated CBS and CSE proteins in a time-dependent manner. Following treatment with 10 nM E2β, levels of both CBS and CSE proteins began to significantly increase at 24 h and maximized by 48 h (Fig. 3B).
Fig. 3: E2β stimulates CBS and CSE mRNA and protein expression in a time- and concentration-dependent manner.
UASMCs were treated with 10 nM E2β for up to 3 days (time-course) to asses CBS and CSE (A) mRNA and protein (B), or with increasing concentrations of E2β (0–1 μM) for 48 h (dose-response) to asses CBS and CSE mRNAs (C) and proteins (D). Data (mean ± SEM) were collected from cells prepared from 3–5 different ewes. Bars with different letters differ significantly (p < 0.05), capital letters pertain to CBS and lowercase to CSE.
E2β also significantly stimulated CBS and CSE mRNAs in a concentration-dependent manner in UASMC in vitro. At 48 h post-treatment, 0.1 nM E2β effectively stimulated both CBS and CSE mRNA expressions. CBS and CSE mRNA expression was increased with increasing doses of E2β and was maximized with 100 nM E2β and plateaued with 1 μM E2β (Fig. 3C). At 48 h post-treatment, 0.1 nM E2β effectively stimulated both CBS and CSE proteins. The responses increased with increasing concentrations of E2β, and maximized with 1μM E2β for CBS (3.69 ± 0.09 fold vs. control, P<0.01) and 10nM E2β for CSE (2.51 ± 0.03 fold vs. control, P<0.01) (Fig. 3D).
E2β stimulates ER-dependent CBS and CSE transcription in UASMCs
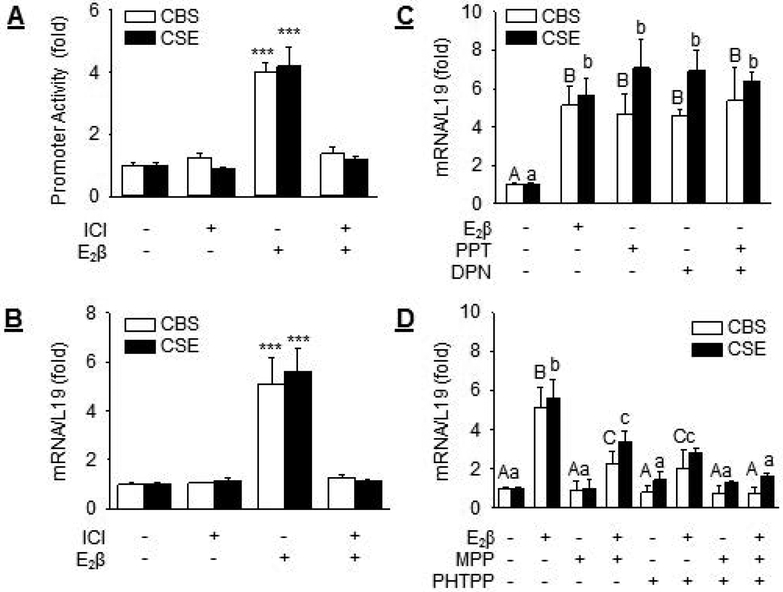
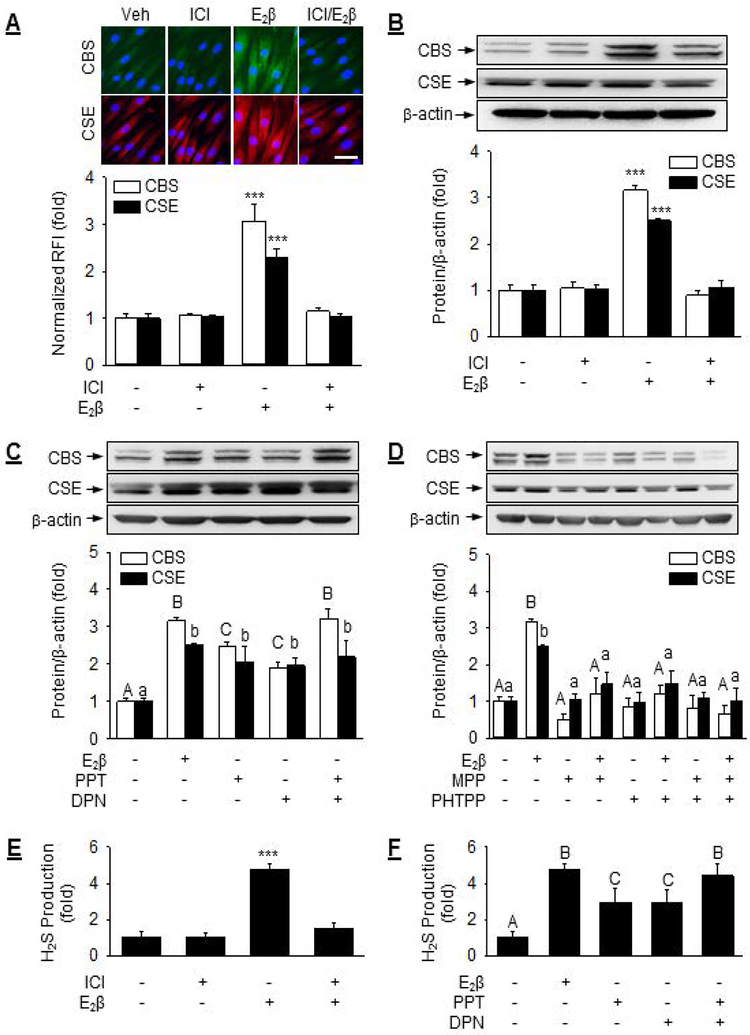
Treatment with E2β provoked 4.00 ± 0.30 (P<0.001) and 4.21 ± 0.61 (P<0.001) fold increases in CBS and CSE promoter activities in UASMCs. Treatment with ICI was able to completely attenuated E2β-stimulated CBS and CSE promoter activation (Fig. 4A). Consistent with these findings, treatment with 10 nM E2β resulted in 5.11 ± 1.03 (P< 0.001) and 5.61 ± 0.93 (P< 0.001) fold increases in CBS and CSE mRNAs in UASMCs, respectively. Treatment with 1 μM ICI did not alter baseline but completely attenuated E2β-stimulated CBS and CSE mRNA expressions (Fig. 4B).
Fig. 4: Role of ER in E2β stimulation of CBS and CSE transcription.
(A) Human CBS and CSE promoter luciferase-reporter constructs were transfected into UASMCs. Twenty-four hours later cells were allowed to recover before treatment with vehicle or 10 nM E2β with or without 1 μM ICI for 24 h. Promoter activity was determined. (B) UASMCs were treated with vehicle or 10 nM E2β in the presence or absence of 1 μM ICI for 48 h. CBS and CSE mRNAs were determined by qPCR. (C) Cells were treated with vehicle, 10 nM E2β, 10 nM agonist of ERα (PPT) or ERβ (DPN), or PPT+DPN to assess the role of ERα and ERβ agonists on CBS and CSE mRNA expressions. (D) Cells were treated with vehicle or 10nM E2β in the presence or absence of 1 μM antagonist of ERα (MPP) or ERβ (PHTPP), or MPP+PHTPP to assess the role of ERα and ERβ antagonists on CBS and CSE mRNA expressions. Data (mean ± SEM) were collected from cells prepared from 3–5 different ewes. Bars with different letters differ significantly (P < 0.05), capital letters pertain to CBS and lowercase to CSE. *** P<0.001.
We next assessed the role of ERα and ERβ in E2β-stimulated CBS and CSE mRNA expression. Treatment with specific agonist of ERα (PPT, 10 nM) or ERβ (DPN, 10 nM) alone, or their combination, was able to fully mimic E2β-stimulated CBS and CSE mRNA expression (Fig. 4C). Treatment with the specific antagonist for ERα (MPP, 1 μM) or ERβ (PHTPP, 1 μM) alone partially blocked E2β-stimulated CBS and CSE mRNA expressions; their combination was able to fully attenuate E2β-stimulated CBS and CSE mRNA expressions (Fig. 4D). Either MPP or PHTPP alone or their combination did not alter baseline CBS and CSE mRNA levels in UASMCs.
E2β stimulates specific ER-dependent CBS and CSE protein expressions and activities in UASMCs
Immunocytochemistry analysis revealed that UASMCs exhibited low basal CBS and CSE proteins in culture. Both CBS and CSE proteins were mainly localized in the cytoplasm of Veh, E2β, and ICI-treated cells (Fig. 5A). Quantitative immunocytochemistry analysis showed that treatment with 10 nM E2β stimulated 3.08 ± 0.35 (P<0.001) and 2.29 ± 0.18 (P<0.001) fold increases in CBS and CSE proteins, respectively; the stimulations were completely blocked by ICI. Immunoblot analysis revealed that treatment with 10 nM E2β stimulated 3.17 ± 0.09 (P< 0.001) and 2.51 ± 0.04 (P< 0.001)-fold increases in CBS and CSE proteins, respectively; 1 μM ICI did not alter baseline expression but completely blocked E2β-stimulated CBS and CSE protein expressions (Fig. 5B).
Fig. 5: Role of ER in E2β stimulation of CBS and CSE protein expression and activity.
(A) UASMCs were treated with vehicle, 1 μM ICI 182,780, 10 nM E2β, or ICI + E2β for 48 h. Cells were then subjected to immunocytochemistry for microscopic analysis of CBS (labeled green) and CSE (labeled red) protein expressions and their cellular localization. Nuclei were labeled with DAPI (blue). Protein expression was determined by relative green or red fluorescence intensity as fold change to vehicle control (Veh). (B) UASMCs were treated with vehicle or 10 nM E2β with or without 1 μM ICI for 48 h. CBS/CSE protein was determined by immunoblotting. (C) Cells were treated with vehicle, 10 nM E2β, 10 nM of agonist of PPT ERα (PPT) or ERβ (DPN), or PPT+DPN to assess the role of ER agonists on CBS and CSE protein expressions. (D) Cells were treated with vehicle or 10nM E2β in with or without 1 μM antagonist of ERα (MPP) or ERβ (PHTPP), or MPP+PHTPP to assess the role of ER antagonists on CBS and CSE protein expressions. Cells were treated with vehicle or (E) 1 μM ICI with or without 10 nM of E2β or (F) 10 nM of PPT, DPN, or PPT+DPN to assess the role of ERα and ERβ in E2β stimulation of UASMC H2S production. Data (mean ± SEM) were collected from cells prepared from 3–5 different ewes. Bars with different letters differ significantly (P<0.05), capital letters pertain to CBS and lowercase to CSE. *** P<0.001, n.s. not statistical different. Scale bar is 50 μm.
Treatment with specific agonist of ERα (PPT, 10 nM) or ERβ (DPN, 10 nM) alone fully mimicked E2β-stimulated CSE protein expressions; however, either agonist alone only partially mimicked E2β-stimulated CBS expression, but their combination was able to fully mimic E2β-stimulated CBS protein expression (Fig. 5C). Treatment with specific antagonist for ERα (MPP, 1 μM) or ERβ (PHTPP, 1 μM) alone or in combination blocked E2β-stimulated CBS and CSE protein expressions (Fig. 5D). Either MPP or PHTPP alone or their combination did not alter baseline CBS and CSE protein levels in UASMCs.
Treatment with ICI did not alter baseline but completely blocked E2β-stimulated H2S production (Fig. 5E). Treatment with the ERα agonist PPT or ERβ agonist DPN alone provoked 2.95 ± 0.76 and 2.91 ± 0.77 fold increases (P<0.05) in H2S production, respectively. The effects of PPT or DPN alone were less effective (P<0.05) than E2β; however, the combination of PPT and DPN was as effective as E2β to stimulate H2S production in UASMCs (Fig. 5F).
DISSCUSSION
We have recently shown that exogenous and endogenous estrogens stimulate UA H2S biosynthesis by selectively upregulating SMC and EC CBS, but not CSE, mRNA and protein expressions in vivo (Lechuga et al., 2015), raising a key question as to by what mechanisms E2β selectively stimulates CBS, but not CSE, expression in vascular SMCs and ECs. In this study, we chose to focus on UASMCs to address the question because: 1) in comparison to ECs, the SMC contribution to UA hemodynamics is much less understood and 2) unlike NO that is well-documented to be mainly produced by UAEC (Govers and Rabelink, 2001), UASMCs is a major source of vascular H2S when the SMC and EC composition of UA and their cellular (SMC vs. EC) CBS and CSE expression patterns (Lechuga et al., 2015; Sheibani et al., 2017) are taken into consideration.
To accomplish our goal to address the mechanisms underlying estrogen selective stimulation of CBS expression in UASMCs, herein we have successfully developed a primary UASMC culture model from late pregnant ewes. The cells display typical spindle-shaped morphology in culture and highly express the SM marker α-SMA in culture. Quantitative analysis by flow cytometry revealed that they are >99.9% positive for α-SMA and caveolin-1. Flow cytometry analysis also detects low levels of eNOS protein in UASMCs; however, eNOS protein was not detected by immunoblotting, suggesting that the eNOS protein is background staining within the flow cytometry analysis. These cells express high levels of ERα and ERβ mRNAs (Fig. 1C) and proteins (Fig. 1D) in culture and levels of both retain comparable to that in intact UA in vivo (Liao et al., 2005), even after several passages (up to 7 tested, data not shown). Nonetheless, these findings show that UASMCs in culture possess functional ER-dependent responsiveness to E2β stimulation and thus, providing us with a suitable model for delineating the cellular and molecular mechanisms by which E2β stimulates UA H2S biosynthesis in vitro. Indeed, with this cell model we now first report that E2β stimulates UASMC H2S production in vitro, which is mediated by specific ER as this is blocked by ICI. Moreover, we show that both CBS and CSE are involved in E2β-stimulated UASMC H2S production as the stimulation was sensitive to both CBS and CSE inhibition.
Our data show that E2β stimulates CBS and CSE mRNA and protein in time- and concentration-dependent manners in UASMCs. E2β stimulation of CBS mRNA and protein expressions in UASMCs in vitro is consistent with our recent in vivo findings that E2β stimulates CBS mRNA and protein expressions in UASMCs in ewes (Lechuga et al., 2015) and women (Sheibani et al., 2017). E2β also stimulates CSE mRNA and protein expressions in UASMCs in vitro. This observation is unexpected because it not only contrasts to our in vivo findings that UASMC CSE mRNA and protein are not altered by estrogens in sheep (Lechuga et al., 2015) and women (Sheibani et al., 2017), but also other reports showing that E2β does not stimulate CSE expression in mouse mesenteric artery SMCs in vitro (Li et al., 2012). Although the mechanisms underlying this discrepancy between the in vitro and in vivo findings is elusive, this raises a bell of caution when using in vitro cell models to explore the mechanisms underlying in vivo phenomena. However, estrogen stimulation of both CBS and CSE expression in UASMCs in vitro is further supported by the inhibition studies showing that both CBS and CSE are involved in E2β stimulation of H2S biosynthesis in UASMCs in vitro (Fig. 2).
Our data clearly show that E2β-stimulation of CBS and CSE mRNA and protein expressions are mediated by specific ER in UASMCs as the stimulations are completely attenuated by ICI 182, 780. UASMCs express both ERα and ERβ (Liao et al., 2005). In cells expressing both ERα and ERβ, including UAECs they can play similar or even opposite roles in mediating estrogen signaling (Zhang et al., 2012). Thus, an important question arises as to what specific roles ERα and ERβ play in mediating E2β stimulation of H2S biosynthesis in UASMCs. In studies using ER isoform-specific agonists and antagonists, we have found that both ERα and ERβ are involved in E2β stimulation of CBS and CSE mRNA and protein expressions in UASMCs in vitro; however, each seems to play a unique role in stimulating either CBS and CSE mRNA and protein in the cells. For instance, activation of either ERα or ERβ is sufficient to mediate E2β-stimulated CBS and CSE mRNA and CSE protein expressions because this stimulation can be mimicked by treatment with either specific agonist for ERα (PPT) or ERβ (DPN) alone or their combination. In contrast, E2β-stimulated CBS protein expression requires activation of both ERα and ERβ as this is fully mimicked by PPT and DPN combination and only partially by either one agonist. These findings are strengthened by studies using the specific ERα antagonist MPP and ERβ antagonist PHTPP. Consistently, we have shown that E2β stimulation of UASMC H2S production requires activation of both ERα and ERβ as this was fully mimicked by PPT and DPN combination and only partially by either agonist.
Of note, same conclusions for the roles of ERα and ERβ in E2β stimulation of CSE mRNA and protein are drawn based on findings using ERα and ERβ agonists and antagonists; however, different conclusions are obtained for the roles of ERα and ERβ in E2β stimulation of CBS mRNA and protein. These results suggest that ERα and ERβ regulate E2β stimulation of CBS and CSE expression similarly at the mRNA level but differently at the protein level. Although this study does not offer the underlying mechanisms, our current findings, nonetheless, suggest that ERα and ERβ play different roles in mediating enhanced uterine artery production of H2S in uterine hemodynamic regulation, consistent with our previous studies showing different roles of ERα and ERβ in estrogen signaling in UAEC (Zhang et al., 2012).
E2β stimulates both mRNA and protein expressions of CBS and CSE in vitro (current study) and CBS in vivo (Lechuga et al., 2015; Sheibani et al., 2017), suggesting that these stimulations occur at the level of transcription. Indeed, E2β stimulates >4-fold increases in CBS and CSE promoter activity in UASMCs in vitro (Fig. 4A). The human CBS promoter is >4k bp and encodes five distinct 5’ non-coding exons (Kraus et al., 1998), containing putative estrogen response elements (ERE) for binding ERα and ERβ, as well as other transcription factors including Sp1, Sp3 and AP-1 (Ge et al., 2001; Renga, 2011). The human CSE promoter is >1.5k bp and contains binding sites for ER, Sp1 and AP-1 (Renga, 2011; Yin et al., 2012). Thus, E2β stimulation of CBS and CSE transcription may occur through direct interactions between ERs and EREs in UASMCs. To this end, further studies are needed to delineate the specific roles of ERα and ERβ and other transcription factors, such as Sp1 and AP1, that can interact with ERα and ERβ to activate gene transcription (Schultz et al., 2005; Yang et al., 2011) during E2β stimulation of CBS and CSE transcription in UASMCs.
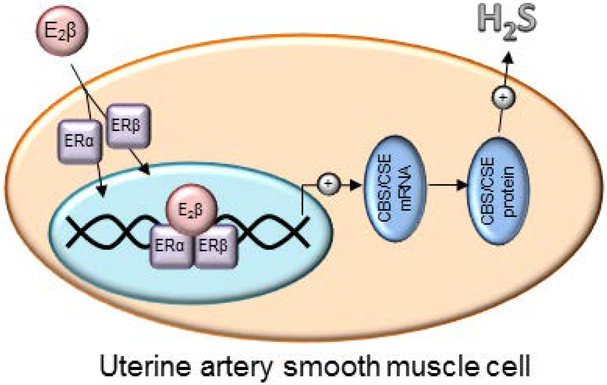
Altogether, our current study demonstrates that E2β stimulates UASMC H2S production in vitro by upregulating CBS and CSE mRNA and protein expression via specific ERα and ERβ-dependent activation of CBS and CSE transcription in UASMC in vitro (Fig. 6).
Fig. 6: ER-dependent UASMC H2S production.
PERSPECTIVES
Estrogen-induced and pregnancy-associated uterine vasodilation as measured by a rise in UBF is believed to be primarily mediated by orchestrated vasodilator networks locally produced by UA endothelium, including NO (Rosenfeld et al., 2002; Valdes et al., 2009). Endothelial NO mediates less than 65% estrogen-induced uterine vasodilation (Magness et al., 2005; Rosenfeld et al., 1996); however, the mechanisms in addition to endothelial NO have not been defined and whether UA SMCs also produces vasodilators to mediate estrogen-induced uterine vasodilation is neglected. Our current findings, together with our recent in vivo work showing H2S as a novel UA vasodilator (Lechuga et al., 2015; Sheibani et al., 2017), provide a novel mechanism for estrogen-induced uterine vasodilation via SM-derived H2S. Understanding the effects of estrogens and the mechanisms by which estrogens regulate the expression of vasodilator producing enzymes in UASM provides fundamental understanding of normal pregnancy-associated vascular adaptations and clues for the pathophysiology of preeclampsia and other cardiovascular disorders. Nonetheless, our findings necessitate the evaluation of SM H2S in the cardiovascular protective effects of estrogens as it pertains to the regulation of vascular tone in normal physiology and dysregulation in the pathophysiology of vascular diseases, such as hypertension, atherosclerosis, and gestational vascular diseases such as preeclampsia and IUGR.
Source of funding:
This study was supported in part by National Institutes of Health (NIH) grants RO1 HL70562 and R21 HL98746 (to DB Chen). TJL was an American Heart Association (AHA) Predoctoral Fellow (AHA14PRE18570033). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH and AHA.
Footnotes
Conflict of interest/Disclosure statement: The authors have no financial interests to disclose.
REFERENCES
- Barker DJ. 1995. Intrauterine programming of adult disease. Mol Med Today 1(9):418–423. [DOI] [PubMed] [Google Scholar]
- Bukovska G, Kery V, Kraus JP. 1994. Expression of human cystathionine beta-synthase in Escherichia coli: purification and characterization. Protein Expr Purif 5(5):442–448. [DOI] [PubMed] [Google Scholar]
- Byers MJ, Zangl A, Phernetton TM, Lopez G, Chen DB, Magness RR. 2005. Endothelial vasodilator production by ovine uterine and systemic arteries: ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J Physiol 565(Pt 1):85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DB, Bird IM, Zheng J, Magness RR. 2004. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology 145(1):113–125. [DOI] [PubMed] [Google Scholar]
- Erickson PF, Maxwell IH, Su LJ, Baumann M, Glode LM. 1990. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J 269(2):335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SP, Reynolds LP, Magness RR. 1982. Blood flow to the uterine and ovarian vascular beds of gilts during the estrous cycle or early pregnancy. Biol Reprod 27(4):878–885. [DOI] [PubMed] [Google Scholar]
- Gadalla MM, Snyder SH. 2010. Hydrogen sulfide as a gasotransmitter. J Neurochem 113(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Konrad MA, Matherly LH, Taub JW. 2001. Transcriptional regulation of the human cystathionine beta-synthase −1b basal promoter: synergistic transactivation by transcription factors NF-Y and Sp1/Sp3. Biochem J 357(Pt 1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers R, Rabelink TJ. 2001. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 280(2):F193–206. [DOI] [PubMed] [Google Scholar]
- Jiang CW, Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. 1991. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br J Pharmacol 104(4):1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes LE, Moore LG, Walchak SJ, Dempsey EC. 1996. Pregnancy-stimulated growth of vascular smooth muscle cells: importance of protein kinase C-dependent synergy between estrogen and platelet-derived growth factor. J Cell Physiol 166(1):22–32. [DOI] [PubMed] [Google Scholar]
- Khan LH, Rosenfeld CR, Liu XT, Magness RR. 2010. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab 298(2):E222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus JP, Oliveriusova J, Sokolova J, Kraus E, Vlcek C, de Franchis R, Maclean KN, Bao L, Bukovsk Patterson D, Paces V, Ansorge W, Kozich V. 1998. The human cystathionine beta-synthase (CBS) gene: complete sequence, alternative splicing, and polymorphisms. Genomics 52(3):312–324. [DOI] [PubMed] [Google Scholar]
- Kredich NM, Foote LJ, Keenan BS. 1973. The stoichiometry and kinetics of the inducible cysteine desulfhydrase from Salmonella typhimurium. J Biol Chem 248(17):6187–6196. [PubMed] [Google Scholar]
- Lechuga TJ, Zhang H, Sheibani L, Karim M, Jia J, Magness RR, Rosenfeld CR, Chen DB. 2015. Estrogen Replacement Therapy in Ovariectomized Nonpregnant Ewes Stimulates Uterine Artery Hydrogen Sulfide Biosynthesis by Selectively Upregulating Cystathionine beta Synthase Expression. Endocrinology 156(6):2288–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mani S, Cao W, Yang G, Lai C, Wu L, Wang R. 2012. Interaction of hydrogen sulfide and estrogen on the proliferation of vascular smooth muscle cells. PLoS One 7(8):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WX, Magness RR, Chen DB. 2005. Expression of estrogen receptors-alpha and -beta in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol Reprod 72(3):530–537. [DOI] [PubMed] [Google Scholar]
- Magness RR, Phernetton TM, Gibson TC, Chen DB. 2005. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol 565(Pt 1):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR. 1989. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol 256(4 Pt 1):E536–542. [DOI] [PubMed] [Google Scholar]
- Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. 2001. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x). Am J Physiol Heart Circ Physiol 280(4):H1692–1698. [DOI] [PubMed] [Google Scholar]
- Mata-Greenwood E, Liao WX, Wang W, Zheng J, Chen DB. 2010. Activation of AP-1 transcription factors differentiates FGF2 and vascular endothelial growth factor regulation of endothelial nitric-oxide synthase expression in placental artery endothelial cells. J Biol Chem 285(23):17348–17358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. 1993. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res 27(11):1939–1942. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Snyder SH. 2009. Signaling by gasotransmitters. Science signaling 2(68):re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary P, Boyne P, Flett P, Beilby J, James I. 1991. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem 37(5):667–672. [PubMed] [Google Scholar]
- Osol G, Moore LG. 2014. Maternal uterine vascular remodeling during pregnancy. Microcirculation 21(1):38–47. [DOI] [PubMed] [Google Scholar]
- Renga B 2011. Hydrogen sulfide generation in mammals: the molecular biology of cystathionine-beta- synthase (CBS) and cystathionine-gamma-lyase (CSE). Inflamm Allergy Drug Targets 10(2):85–91. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR. 1977. Distribution of cardiac output in ovine pregnancy. Am J Physiol 232(3):H231–235. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, Cox BE, Roy T, Magness RR. 1996. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest 98(9):2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CR, Killam AP, Battaglia FC, Makowski EL, Meschia G. 1973. Effect of estradiol-17, on the magnitude and distribution of uterine blood flow in nonpregnant, oophorectomized ewes. Pediatr Res 7(3):139–148. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, Roy T, Cox BE. 2002. Mechanisms modulating estrogen-induced uterine vasodilation. Vascul Pharmacol 38(2):115–125. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM. 2005. Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors alpha and beta. J Biol Chem 280(1):347–354. [DOI] [PubMed] [Google Scholar]
- Sheibani L, Lechuga TJ, Zhang H, Hameed A, Wing DA, Kumar S, Rosenfeld CR, Chen DB. 2017. Augmented H2S production via cystathionine-beta-synthase upregulation plays a role in pregnancy-associated uterine vasodilationdagger. Biol Reprod 96(3):664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J. 2009. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol 7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R 2012. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92(2):791–896. [DOI] [PubMed] [Google Scholar]
- White RE, Darkow DJ, Lang JL. 1995. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res 77(5):936–942. [DOI] [PubMed] [Google Scholar]
- Yang G, Pei Y, Teng H, Cao Q, Wang R. 2011. Specificity protein-1 as a critical regulator of human cystathionine gamma-lyase in smooth muscle cells. J Biol Chem 286(30):26450–26460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. 2008. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322(5901):587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y, Li N, Qi J, Wang L, Shi Y, Qiu S, Fan J, Zha X. 2012. Sp1 is involved in regulation of cystathionine gamma-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell Signal 24(6):1229–1240. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Feng L, Wang W, Magness RR, Chen DB. 2012. Estrogen-responsive nitroso-proteome in uterine artery endothelial cells: role of endothelial nitric oxide synthase and estrogen receptor-beta. J Cell Physiol 227(1):146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]