Abstract
Background and Aims:
The five-year incidence of post-transplant hepatocellular carcinoma (HCC) recurrence is 8-20%. Several studies have evaluated pre-transplant risk factors for HCC recurrence, but nearly all data have treated HCC as a homogeneous condition across all etiologies of liver disease despite differences in tumor biology and baseline incidence of HCC. We sought to evaluate the impact of etiology of liver disease, maximum pre-transplant alpha fetoprotein (AFP), and the interaction of the two factors on the risk of HCC recurrence.
Methods:
We performed a retrospective cohort study of HCC transplant recipients using United Network for Organ Sharing (UNOS) data from 2002-2016. Competing risks regression was performed to identify variables associated with HCC recurrence, and an interaction term between etiology and maximum AFP category.
Results:
Among 18,406 recipients, 1,484 patients experienced HCC recurrence over 3.1 years of median follow-up time. There was a significant interaction between AFP category and etiology of liver disease (p < 0.001). Among patients with a maximum AFP <100ng/mL, those with alcoholic liver disease had the lowest risk of recurrence; by contrast, in patients with a maximum AFP of 100-499, 500-1,000, or >1,000ng/mL, those with alcoholic liver disease had the highest risk of HCC recurrence among all etiologies.
Conclusion:
Risk of HCC recurrence differs by etiology of liver disease, and the significance of elevated pre-transplant AFP varies by etiology. Patients with alcoholic liver disease and elevated maximum AFP are at uniquely high risk of HCC recurrence. These findings have potential UNOS policy implications, as the transplant selection process may ultimately benefit from etiology-specific criteria.
Keywords: alpha fetoprotein (AFP), interaction, risk factors, United Network for Organ Sharing (UNOS), competing risks regression
Introduction
Hepatocellular carcinoma (HCC) is a primary liver tumor that occurs in the setting of cirrhosis in 80-90% of cases in the United States.1,2 Among possible treatments, liver transplantation (LT) offers the highest recurrence-free survival rates.3,4 However despite strict selection criteria, the five-year post-LT recurrence risk is 8-20%,5,6,7with less than one year median survival once diagnosed.8,9,10HCC recurrence is thought to occur because of circulating tumor cells not eliminated through transplant.11,12 Numerous studies have aimed to identify predictors of HCC recurrence to improve LT patient selection and minimize adverse outcomes. Risk prediction has focused on pre-transplant laboratory criteria (e.g., alpha fetoprotein [AFP]), imaging criteria, and explant pathology.13,14,15 These findings have LT selection implications as they inform Organ Procurement and Transplantation Network (OPTN) HCC exception policies, such as those for patients with high AFP levels (>1,000ng/mL).16 Importantly, the literature on predicting HCC recurrence has treated HCC as a homogeneous condition across all liver disease etiologies, despite data suggesting otherwise.
The diagnosis and biology of HCC vary based on underlying chronic liver disease (CLD). HCC incidence differs between viral and non-viral etiologies of CLD.17,18 Furthermore, AFP serves as a marker of HCC as well as hepatic regeneration.19 Not only is AFP known to be elevated in viral CLD, even in the absence of HCC,20 but the sensitivity and specificity of AFP in diagnosing HCC varies significantly among viral and non-viral etiologies of CLD.21,22,23These disparities speak to potentially different immunological pathways leading to HCC. Indeed, recent work suggests that viral CLD results in a necroinflammatory process whereas non-viral CLD causes cell death leading to a deregulated liver immune network.24 These mechanistic differences underscore the need to evaluate risk factors for HCC recurrence through a different lens— based on etiology of liver disease.
We sought to explore whether etiology of liver disease reflects differences in HCC tumor biology and behavior, in particular its propensity for recurrence. Because the LT model entails the removal of the entire liver and that recurrence must be due to pre-transplant metastasis, we elected to operationalize our research question using national registry data. We aimed to determine: 1) whether the risk of HCC recurrence differs by etiology of liver disease, and 2) whether pre-transplant AFP predicts HCC recurrence differently based on etiology of liver disease.
Methods
Design, Patient Selection, and Variable Collection
We conducted a retrospective cohort study using United Network for Organ Sharing (UNOS) data between 2/2002 and 9/2016. Although prioritization for patients with HCC has changed over time, this can be accounted for in models and would not be expected to confound associations between etiology of liver disease, AFP, and HCC recurrence. We included patients aged ≥18 who underwent LT with standardized T2 Model for End-Stage Liver Disease (MELD) exceptions. We excluded patients without recorded AFP values and those with non-standardized HCC exceptions.
Demographic variables (age, sex, race) and body mass index (BMI) were collected, as were pre-LT MELD score, tumor characteristics including number of tumors, largest tumor diameter, adherence to the Milan criteria immediately prior to transplant, locoregional therapy prior to transplant (including embolization, ablation, and radiation-based approaches), downstaging prior to transplant, and prior surgical resection. Etiologies of liver disease were classified as hepatitis C (HCV), hepatitis B (HBV), alcoholic (EtOH), non-alcoholic fatty liver (NAFLD), autoimmune (including autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis), and other (comprised of numerous less common etiologies including hemochromatosis, sarcoidosis, alpha-1 antitrypsin, and rare inborn metabolic liver diseases). Maximum pre-transplant AFP (max AFP) was classified as a four-level categorical variable (<100ng/mL, 101-499ng/mL, 500-1,000ng/mL, and >1,000ng/mL), adapted from numerous prior studies.5,13,15,16 The max AFP variable performs similarly to AFP immediately prior to transplant (pre-transplant AFP) in HCC recurrence models,25 and reflects current OPTN policy which provisionally disallows standard exception points for patients with max AFP >1,000ng/mL.16 However, as a sensitivity analysis, pre-transplant AFP was also analyzed in models (detailed below), classified as a four-level categorical variable with the same thresholds as max AFP. Waiting time and cold ischemia time were evaluated in both continuous and categorical formats, based on prior literature.26,27
Outcome Definition
HCC recurrence was determined based on the designation of (1) post-transplant death from HCC or metastatic malignancy, or (2) post-transplant recurrence of pre-transplant malignancy. These fields are derived from LT recipient follow-up data that must be submitted annually by transplantation centers. Importantly, this HCC recurrence outcome ascertainment algorithm has been previously validated28 and utilized in numerous studies, including the recent validation of the RETREAT score.29 While UNOS does not specify imaging requirements for post-transplant HCC recurrence surveillance, it is standard to perform computed tomography (CT) or magnetic resonance imaging (MRI) on an annual or biennial basis. Furthermore, although tissue diagnosis of HCC recurrence is not available in the UNOS dataset, a recurrence diagnosis is based on imaging criteria in nearly all cases.
Patient Characteristics
Patients stratified by HCC recurrence were compared across a range of the aforementioned variables. Kaplan-Meier survival estimates were generated for 1, 3, and 5-year recurrence by max AFP category and etiology of liver disease. Descriptive statistics were computed as medians and interquartile ranges (IQR). Wilcoxon rank-sum and chi-squared tests were used to compare continuous and categorical data, respectively. For these and subsequent tests, a two-tailed alpha level = 0.05 was used as the threshold for statistical significance unless otherwise stated. All data management and computations were performed using STATA/IC version 14.2.
Cox Regression Analysis
Variables potentially associated with HCC recurrence were first analyzed with univariate Cox proportional hazards regression. Explant pathology characteristics were not included in any models, as these are not available for risk stratification in the pre-transplant setting. An alpha = 0.10 was maintained for potential inclusion in multivariable modeling. After identification of candidate variables, multivariable Cox regression was performed. Multiple selection methods were used, including researcher-driven, forward selection, and reverse selection, with a threshold alpha = 0.05 used for variable retention. As per the second study objective, an a priori interaction term between max AFP category and etiology of liver disease was included in the model. The Cox proportional hazards assumption was evaluated using log-log survival plots and plotting of Schoenfeld residuals over time. No serious violations were observed.
Competing Risks Regression Analysis
The final multivariable Cox regression model was subsequently modeled in a competing risks framework, identifying death as a competing event. The pre-specified interaction term was included, and linear combinations were used to derive subhazard ratio (SHR) estimates for each AFP-etiology level. Statistical comparisons between AFP levels, among different etiologies of liver disease, were Bonferroni-adjusted for multiple comparisons. Cumulative incidence functions were plotted for each liver disease etiology, stratified by AFP category. Of note, because the data missingness was less than 5% in the final regression models, imputation methods were not used.
Sensitivity Analyses
To determine the impact of using max AFP as opposed to pre-transplant AFP in the primary analysis, Cox regression and competing risks regression analyses were also performed using pre-transplant AFP as defined previously. Additionally, to determine if HCC recurrence dynamics have changed as a result of directly administrated antiretroviral therapy (DAART), models were produced for the pre- and post-DAART era, using January 1st, 2014 as the transition point (based on the sofosbuvir Food and Drug Administration [FDA] approval date). In both cases, tables were produced for the interaction term between AFP and etiology of liver disease, with Bonferroni-adjusted alpha thresholds used for statistical comparisons to low-AFP reference groups.
Exploratory Analysis
Explant data from the UNOS registry, catalogued since 4/2012, was utilized to perform an exploratory analysis on HCC patients with AFP ≥100ng/mL. This threshold was chosen given the smaller sample size constraints imposed by the explant dataset. Several variables were compared among etiologies based on prior literature identifying explant predictors of HCC recurrence, including number of tumors (solitary versus multiple),30,31 macrovascular invasion,32 poor tumor differentiation,33 adherence to Milan criteria on pathology, and maximum tumor size.34,35 An aggregate binary variable (termed poor prognosis) indicating the presence of multiple tumors, macrovascular invasion, or poor tumor differentiation was also included for analysis, again because of the smaller sample size. Chi-squared and Kruskal Wallis tests were performed for categorical and continuous data analysis, respectively, with a Dunn’s test used for post-hoc pairwise comparisons.
Results
Patient Characteristics
After applying selection criteria, a total of 18,406 patients were included in the analytic cohort (Supplemental Figure 1). Over median 3.1 years of follow-up, 1,484 patients were diagnosed with HCC recurrence, yielding a recurrence rate of 19.5 cases per 1,000 person-years. The characteristics of patients with HCC recurrence differed from those without recurrence across a range of demographic, laboratory, and clinical factors (Table 1). Kaplan-Meier survival estimates for HCC recurrence at 1, 3, and 5 years post-LT, stratified by max AFP category and etiology of liver disease, are presented in Supplemental Tables 1a and 1b.
Table 1 –
Patient Characteristics by Hepatocellular Carcinoma Recurrence Status
| Variable | No Recurrence (N = 16922) | HCC Recurrence (N = 1484) | p-value |
|---|---|---|---|
| Age at Listing, median (IQR) | 58.0 (53.0, 62.0) | 57.0 (53.0, 62.0) | 0.640 |
| Sex | <0.001* | ||
| Female | 3920 (23.2%) | 271 (18.3%) | |
| Male | 13002 (76.8%) | 1213 (81.7%) | |
| Race | 0.015* | ||
| White | 11202 (66.2%) | 1036 (69.8%) | |
| Black | 1609 (9.5%) | 121 (8.2%) | |
| Hispanic | 2480 (14.7%) | 180 (12.1%) | |
| Asian | 1409 (8.3%) | 131 (8.8%) | |
| Other | 222 (1.3%) | 16 (1.1%) | |
| Etiology of Chronic Liver Disease | 0.002* | ||
| HCV | 10224 (60.4%) | 921 (62.1%) | |
| HBV | 1023 (6.0%) | 96 (6.5%) | |
| EtOH | 1556 (9.2%) | 111 (7.5%) | |
| NAFLD | 1564 (9.2%) | 106 (7.1%) | |
| Autoimmune | 504 (3.0%) | 36 (2.4%) | |
| Other | 2051 (12.1%) | 214 (14.4%) | |
| MELD at Listing, median (IQR) | 12.0 (9.0, 16.0) | 11.0 (8.0, 15.0) | 0.002* |
| BMI at Listing, median (IQR) | 28.1 (25.0, 31.6) | 27.8 (25.0, 31.6) | 0.400 |
| Months on Waiting List, median (IQR) | 5.5 (2.0, 12.4) | 4.2 (1.5, 9.5) | <0.001* |
| Waiting Time <6 Months | 8894 (52.6%) | 905 (61.0%) | <0.001* |
| Waiting Time >18 Months | 2531 (15.0%) | 172 (11.6%) | <0.001* |
| Pre-transplant AFP, median (IQR) | 9.0 (5.0, 31.0) | 25.0 (7.0, 136.0) | <0.001* |
| Pre-transplant AFP Range (ng/mL) | <0.001* | ||
| <100 | 14101 (87.5%) | 1018 (71.3%) | |
| 100 – 499 | 1441 (8.9%) | 243 (17.0%) | |
| 500 – 1000 | 287 (1.8%) | 64 (4.5%) | |
| >1000 | 284 (1.8%) | 102 (7.1%) | |
| Max AFP, median (IQR) | 12.0 (5.0, 47.0) | 31.0 (9.0, 166.5) | <0.001* |
| Max AFP Range (ng/mL) | <0.001* | ||
| <100 | 14166 (83.7%) | 1001 (67.5%) | |
| 100 – 499 | 1935 (11.4%) | 293 (19.7%) | |
| 500 – 1000 | 399 (2.4%) | 74 (5.0%) | |
| >1000 | 422 (2.5%) | 116 (7.8%) | |
| Number of Viable Tumors at Transplant | <0.001* | ||
| 1 | 12775 (75.5%) | 1064 (71.7%) | |
| 2 | 2998 (17.7%) | 282 (19.0%) | |
| 3 | 1104 (6.5%) | 130 (8.8%) | |
| 4 or more | 45 (0.3%) | 8 (0.5%) | |
| Largest Tumor at Transplant (cm), median (IQR) | 2.1 (1.1, 2.8) | 2.5 (1.7, 3.3) | <0.001* |
| Within Milan Criteria at Transplant | 16397 (96.9%) | 1393 (93.9%) | <0.001* |
| Locoregional Therapy Prior to Transplant | 11973 (70.8%) | 1071 (72.2%) | 0.250 |
| Downstaged Prior to Transplant | 264 (1.6%) | 35 (2.4%) | 0.020* |
| Prior Surgical Resection | 200 (1.2%) | 21 (1.4%) | 0.430 |
| Cold Ischemia Time (Hours), median (IQR) | 6.2 (4.9, 8.0) | 6.5 (5.0, 8.1) | 0.004* |
| Cold Ischemia Time >10 Hours | 2213 (13.1%) | 233 (15.7%) | 0.004* |
Statistically significant at the alpha = 0.05 level
Cox Regression and Competing Risks Analyses
By all selection methods, the final multivariable Cox regression and competing risks regression models included etiology of liver disease, max AFP range, the etiology-AFP interaction term, and were adjusted for the following covariates: age, sex, race, waiting time, largest tumor size at transplant, adherence to Milan criteria, locoregional therapy prior to transplant, and downstaging prior to transplant (Table 2; univariate analysis available in Supplemental Table 2). The recurrence risk was higher in males, patients with increased max AFP, and patients who received locoregional therapy or who were downstaged prior to transplant; recurrence risks were lower in blacks, Hispanics, patients with alcoholic liver disease, and those within Milan criteria.
Table 2 –
Multivariable Cox Regression and Competing Risks Regression Analyses: Variables Associated with Post-transplant HCC Recurrence
| Multivariable Cox Regression |
Competing Risks Regression |
|||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value | Subhazard Ratio (95% CI) | p-value | |
| Variables of Interest | ||||
| Etiology (ref = HCV) | ||||
| HBV | 1.10 (0.83 – 1.45) | 0.514 | 1.14 (0.86 – 1.51) | 0.359 |
| EtOH | 0.65 (0.51 – 0.83) | 0.001* | 0.66 (0.52 – 0.85) | 0.001* |
| NAFLD | 0.87 (0.69 – 1.09) | 0.223 | 0.88 (0.70 – 1.10) | 0.267 |
| Autoimmune | 0.87 (0.58 – 1.31) | 0.510 | 0.90 (0.60 – 1.36) | 0.612 |
| Other | 1.10 (0.92 – 1.32) | 0.294 | 1.13 (0.94 – 1.35) | 0.202 |
| Max AFP Range (ng/mL; ref <100) | ||||
| 100 – 499 | 2.08 (1.77 – 2.45) | <0.001* | 2.02 (1.72 – 2.38) | <0.001* |
| 500 – 1000 | 2.34 (1.73 – 3.17) | <0.001* | 2.26 (1.65 – 3.08) | <0.001* |
| >1000 | 4.06 (3.20 – 5.16) | <0.001* | 3.73 (2.92 – 4.77) | <0.001* |
| Etiology-AFP Range Interaction | 0.006* | 0.015* | ||
| Covariates | ||||
| Age (per 5 years) | 1.05 (1.01 – 1.09) | 0.008* | 1.04 (1.00 – 1.08) | 0.041* |
| Male Sex | 1.36 (1.18 – 1.55) | <0.001* | 1.36 (1.19 – 1.55) | <0.001* |
| Race (ref = white) | ||||
| Black | 0.77 (0.64 – 0.93) | 0.007* | 0.73 (0.60 – 0.88) | 0.001* |
| Hispanic | 0.82 (0.70 – 0.96) | 0.015* | 0.82 (0.70 – 0.97) | 0.017* |
| Asian | 0.84 (0.68 – 1.04) | 0.107 | 0.87 (0.70 – 1.08) | 0.211 |
| Other | 0.69 (0.42 – 1.13) | 0.136 | 0.69 (0.41 – 1.15) | 0.152 |
| Waiting Time (per 3 months) | 0.99 (0.98 – 1.00) | 0.013* | 0.99 (0.98 – 1.00) | 0.022* |
| Largest Tumor at Transplant (cm) | 1.11 (1.08 – 1.13) | <0.001* | 1.11 (1.07 – 1.15) | <0.001* |
| Within Milan Criteria at Transplant | 0.76 (0.60 – 0.96) | 0.020* | 0.77 (0.61 – 0.98) | 0.031* |
| Locoregional Therapy Prior to Transplant | 1.27 (1.13 – 1.43) | <0.001* | 1.29 (1.14 – 1.44) | <0.001* |
| Downstaged Prior to Transplant | 1.53 (1.09 – 2.14) | 0.014* | 1.53 (1.09 – 2.16) | 0.014* |
Statistically significant at the alpha = 0.05 level
MELD pre-transplant and number of viable tumors at transplant were not statistically significant at the alpha = 0.05 level and were removed from these models
Interaction between Etiology of Liver Disease and AFP
The etiology-AFP interaction term was significant in both Cox and competing risks regression models (p < 0.01 and p = 0.02, respectively). When max AFP category was stratified by liver disease, HCC patients with EtOH and NAFLD were less likely to produce AFP at the highest levels (Figure 1; p < 0.001). The HCC recurrence risk with increased max AFP differed by etiology of liver disease (Table 3). For example, among HCV patients, the recurrence risk was more than 3.5 times higher for those with AFP >1,000ng/mL versus AFP <100ng/mL (SHR: 3.73, 95% CI: 2.92 – 4.77, p < 0.001). However, for EtOH patients, those with AFP >1,000ng/mL had a more than seven times increased risk of recurrence versus those with AFP <100ng/mL (SHR: 7.20, 95% CI: 3.75 – 13.81, p < 0.001). Estimates for recurrence risk with high AFP in HBV, NAFLD, or autoimmune disease were generally lower than those for HCV or EtOH, and often there were no significant differences in risk compared to low AFP values. When stratified by max AFP category, EtOH patients had the lowest HCC recurrence risk for AFP levels <100ng/mL, but the highest risk among etiologies for elevated AFP levels (Figures 2a, b, c, d). In both sensitivity analyses, the interaction term between etiology of liver disease and max AFP was statistically significant in multivariable Cox and competing risks regression models (all p < 0.05). Similar trends to those noted above were observed using pre-transplant AFP (Supplemental Table 3) as well as using max AFP in the pre- and post-DAART eras (Supplemental Table 4).
Figure 1 –
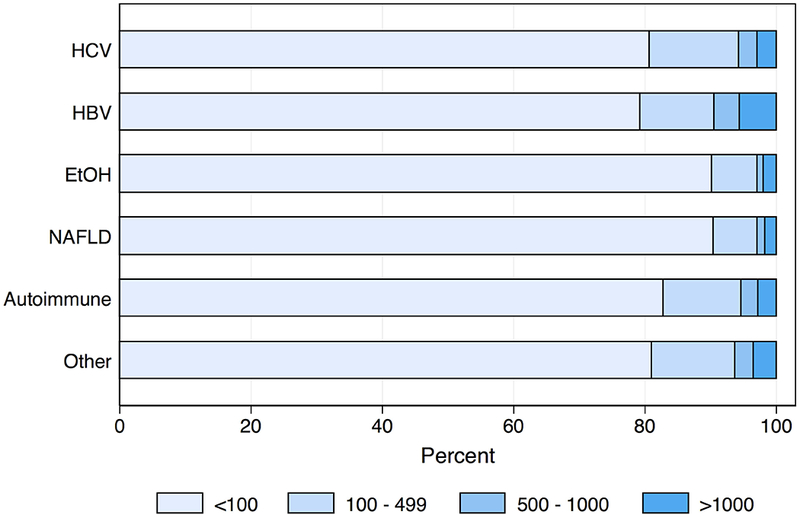
Proportional Categorizations of Pre-transplant AFP (ng/mL), Stratified by Etiology Liver Disease
Table 3 –
Multivariable Competing Risks Regression Results: Subhazard Ratios Derived from Interaction between Etiology of Liver Disease and Max AFP range (ng/mL)
| Variable | Subhazard Ratio (95% CI) | p-value |
|---|---|---|
| HCV | ||
| AFP <100 | 1 (reference) | |
| AFP 100 – 499 | 2.02 (1.72 – 2.38) | <0.001* |
| AFP 500 – 1000 | 2.26 (1.65 – 3.08) | <0.001* |
| AFP >1000 | 3.73 (2.92 – 4.77) | <0.001* |
| HBV | ||
| AFP <100 | 1 (reference) | |
| AFP 100 – 499 | 1.24 (0.67 – 2.29) | 0.489 |
| AFP 500 – 1000 | 2.62 (1.27 – 5.40) | 0.009* |
| AFP >1000 | 1.97 (0.97 – 4.03) | 0.062 |
| EtOH | ||
| AFP <100 | 1 (reference) | |
| AFP 100 – 499 | 4.69 (2.92 – 7.52) | <0.001* |
| AFP 500 – 1000 | 6.57 (2.75 – 15.68) | <0.001* |
| AFP >1000 | 7.20 (3.75 – 13.81) | <0.001* |
| NAFLD | ||
| AFP <100 | 1 (reference) | |
| AFP 100 – 499 | 1.55 (0.83 – 2.89) | 0.168 |
| AFP 500 – 1000 | 2.22 (0.76 – 6.52) | 0.145 |
| AFP >1000 | 3.26 (1.39 – 7.65) | 0.007* |
| Autoimmune | ||
| AFP <100 | 1 (reference) | |
| AFP 100 – 499 | 2.55 (1.18 – 5.50) | 0.017* |
| AFP 500 – 1000 | 1.58 (0.23 – 10.65) | 0.640 |
| AFP >1000 | 2.10 (0.52 – 8.52) | 0.300 |
| Other | ||
| AFP <100 | 1 (reference) | |
| AFP 100 – 499 | 1.90 (1.35 – 2.69) | <0.001* |
| AFP 500 – 1000 | 2.42 (1.33 – 4.40) | 0.004* |
| AFP >1000 | 1.81 (1.01 – 3.23) | 0.045 |
Statistically significant at the alpha = 0.017 level (Bonferroni corrected)
Figure 2a -.
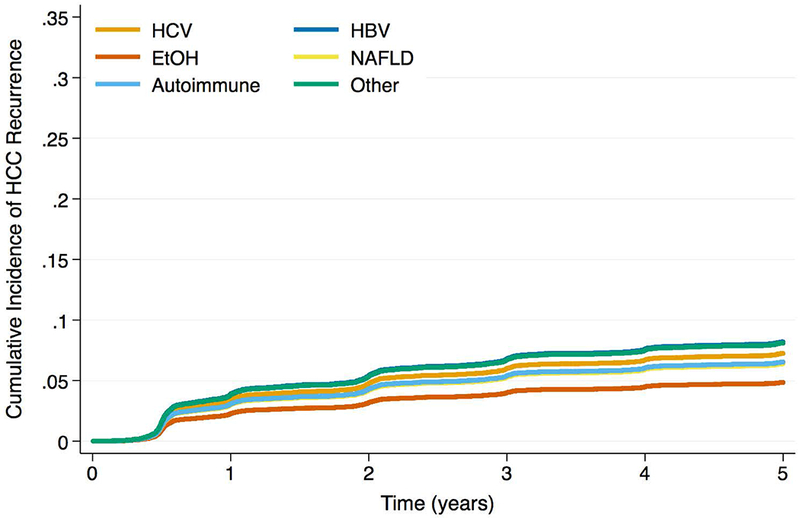
Cumulative Incidence Functions for HCC Recurrence by Max AFP Category: AFP <100ng/mL
Figure 2b -.
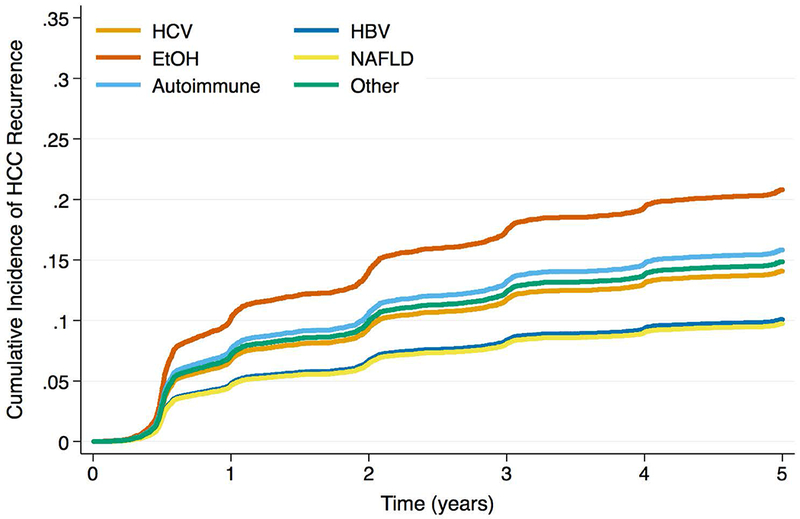
Cumulative Incidence Functions for HCC Recurrence by Max AFP Category: AFP 100 – 499ng/mL
Figure 2c -.
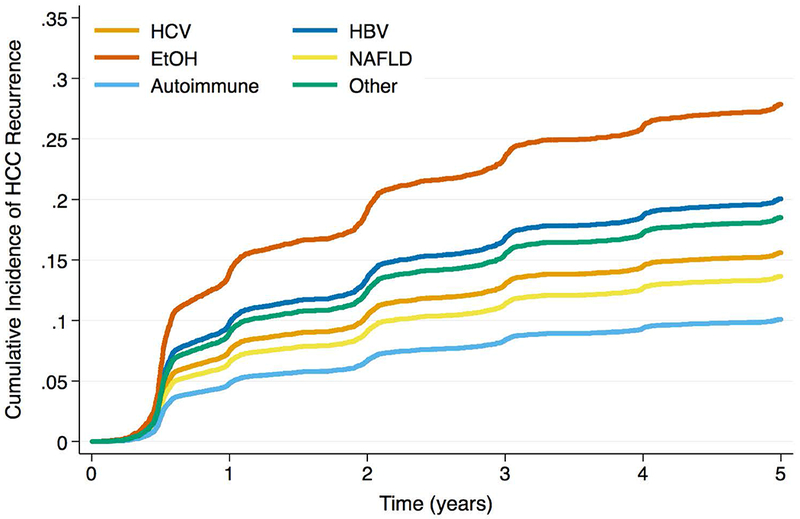
Cumulative Incidence Functions for HCC Recurrence by Max AFP Category: AFP 500 – 1000ng/mL
Figure 2d -.
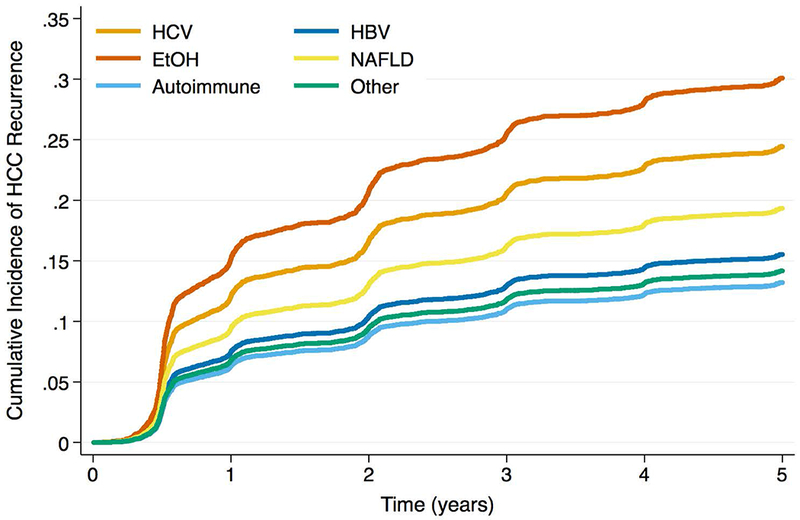
Cumulative Incidence Functions for HCC Recurrence by Max AFP Category: AFP >1000ng/mL
Exploratory Analysis
In patients with AFP ≥100ng/mL with explant data, maximum tumor size was significantly different among all etiologies (Table 4; p = 0.04). EtOH patients had the smallest maximum tumor size (median: 2.2cm, IQR: 1.7 – 3.4cm), although pairwise testing did not meet statistical significance owing to multiple comparisons (data not shown). There were significant differences in the number of tumors on explant (p = 0.04) as well as poor prognosis characteristics (p = 0.02), where EtOH patients had the highest proportions among all etiologies for both variables (64.1% multiple tumors and 76.9% poor prognosis). Again, owing to multiple comparisons, the pairwise analyses were not statistically significant (data not shown). There were no significant differences in macrovascular invasion, poorly differentiated tumors, or explant adherence to Milan criteria. Note that explant predictors based on tumor size were not included in the aggregate variable given the differences found in maximum tumor size.
Table 4 –
Explant Characteristics for Patients AFP ≥100ng/mL
| Variable | HCV N = 650 | HBV N = 49 | EtOH N = 39 | NAFLD N = 44 | Autoimmune N = 18 | Other N = 90 | p-value |
|---|---|---|---|---|---|---|---|
| Number of Tumors | 0.039* | ||||||
| Solitary | 286 (44.0%) | 33 (67.3%) | 14 (35.9%) | 19 (43.2%) | 9 (50.0%) | 41 (45.6%) | |
| Multiple | 364 (56.0%) | 16 (32.7%) | 25 (64.1%) | 25 (56.8%) | 9 (50.0%) | 49 (54.4%) | |
| Macrovascular Invasion | 0.240 | ||||||
| No | 630 (96.9%) | 47 (95.9%) | 37 (94.9%) | 41 (93.2%) | 18 (100.0%) | 83 (92.2%) | |
| Yes | 20 (3.1%) | 2 (4.1%) | 2 (5.1%) | 3 (6.8%) | 0 (0.0%) | 7 (7.8%) | |
| Poorly Differentiated | 0.150 | ||||||
| No | 570 (87.7%) | 45 (91.8%) | 29 (74.4%) | 37 (84.1%) | 16 (88.9%) | 75 (83.3%) | |
| Yes | 80 (12.3%) | 4 (8.2%) | 10 (25.6%) | 7 (15.9%) | 2 (11.1%) | 15 (16.7%) | |
| Poor Prognosis‡ | 0.019* | ||||||
| No | 252 (38.8%) | 29 (59.2%) | 9 (23.1%) | 18 (40.9%) | 9 (50.0%) | 34 (37.8%) | |
| Yes | 398 (61.2%) | 20 (40.8%) | 30 (76.9%) | 26 (59.1%) | 9 (50.0%) | 56 (62.2%) | |
| Within Milan Criteria | 0.620 | ||||||
| No | 213 (32.8%) | 12 (24.5%) | 12 (30.8%) | 17 (38.6%) | 7 (38.9%) | 34 (37.8%) | |
| Yes | 437 (67.2%) | 37 (75.5%) | 27 (69.2%) | 27 (61.4%) | 11 (61.1%) | 56 (62.2%) | |
| Max Tumor Size (cm), median (IQR) | 2.6 (2, 3.7) | 2.5 (1.3, 3.5) | 2.2 (1.7, 3.4) | 3.2 (2.1, 4.3) | 3.1 (2.5, 3.5) | 3 (2, 4.2) | 0.040* |
Statistically significant at the alpha = 0.05 level
Aggregate variable including the presence of: multiple tumors, macrovascular invasion, or poor tumor differentiation
Discussion
In this analysis of 15 years of HCC transplant data, we found that etiology of liver disease was significantly associated with HCC recurrence, with EtOH patients having the lowest recurrence risk overall. This finding is in opposition to other published literature. In the RETREAT study,15 etiology was not statistically significant on univariate analysis. There are several possible explanations for this difference, including the much greater power in the present study (nearly 20-fold larger sample size), as well as the relative enrichment of EtOH in our dataset. In the US Multicenter HCC Transplant Consortium, etiology was not a significant predictor of recurrence,36 but the multivariable models adjusted for explant pathology factors (where we found relevant differences in our exploratory analysis); this would be expected to abolish the association. Moreover, our finding that EtOH patients have a lower risk of HCC recurrence is not altogether unexpected given known differences in baseline risk of HCC by CLD. Indeed, the literature suggests that viral CLD confers a 20 – 25 relative risk of HCC, in contrast to 1.5 – 3 for EtOH patients.37
The key novel finding of this study was a significant interaction between AFP and etiology of liver disease. Although the risk of HCC recurrence generally increased with rising AFP for each etiology, the degree of increased risk was dramatically higher for EtOH patients. There is biological plausibility for these findings. First, we demonstrated that AFP production differs by etiology; fewer HCC patients with EtOH or NAFLD produced high levels of AFP, a finding consistent with prior literature.23 Second, our exploratory analysis revealed objective differences in explant pathology by etiology among patients with elevated AFP. While pairwise analyses were not statistically significant, the data suggest that alcoholic HCC patients with high AFP may have smaller maximum tumor sizes, poorer pathology characteristics, and more often have multiple tumors on explant. This finding implies that there may be features of the HCC tumor burden in high-AFP alcoholic patients that are unlike counterpart tumors in other liver diseases. Here it is intriguing to recall that the Milan criteria were derived from an almost exclusively viral CLD cohort which may not adequately serve HCC patients with non-viral CLD.3 Finally, immunological research supports the possibilities of different pathways to HCC, and articulates how this might occur on the basis of underlying mechanisms of disease.38 HCV and HBV cause chronic non-cytopathic damage and a necroinflammatory response, while EtOH and NAFLD cause primary hepatocyte death with production of disease-associated molecules and a deregulated innate immune system.24 Although the common result is chronic inflammation and HCC risk, the signaling and cytokine pathways that activate the inflammasome are context-specific.39 It is therefore plausible that the likelihood of pathway-specific AFP elevation and its interpretation could be different as well.
Our findings also have potential policy implications. In particular, they are of import to the current OPTN/UNOS HCC exception criteria, where AFP >1,000ng/mL provisionally disqualifies a patient for standardized HCC exception points.40 This policy is applied uniformly across all etiologies of liver disease, however our data suggest that etiology-specific AFP thresholds may be more appropriate. For example, because of the uniquely high HCC recurrence risks associated with elevated AFP in EtOH patients, an AFP ≥100ng/mL may be more reasonable as a provisional exclusion threshold in these patients, as the degree of increased risk relative to AFP <100ng/mL at this level was higher than any other etiology for any degree of AFP elevation. Likewise, the AFP thresholds should potentially be liberalized in patients with HBV, NAFLD, and autoimmune liver disease, because in many cases an elevated AFP did not correspond to an increased risk of HCC recurrence.
There are several limitations to our study. First, the retrospective design precludes causal inferences regarding exposure and outcome. Second, there is potential for exposure misclassification, in particular etiology of CLD. For example, some patients classified with HCV may also have alcoholic liver disease, or that EtOH patients may also have NAFLD. However, this misclassification is likely to be non-differential (i.e. not dependent on the HCC recurrence outcome). Furthermore, it is unlikely that patients labeled as having alcoholic liver disease are misclassified, and if some patients with concomitant, unlabeled alcoholic liver disease are classified under other etiologies, this would be expected to bias estimates towards the null. Third, some degree of outcome misclassification is likely. Although we employed a validated algorithm for ascertainment, there are certainly undiagnosed cases of recurrent HCC that are therefore unreported, or cases that are diagnosed but reported in a fashion not captured by our algorithm. However, because transplant centers apply uniform surveillance protocols dictated by UNOS policies, this misclassification would be non-differential and expected to underestimate HCC recurrence risks overall. Finally, our exclusion criteria may produce some selection bias. Patients without AFP values recorded in the UNOS dataset were excluded, as were those without standard HCC exception data. However, the majority of the patients without AFP data were listed prior to 5/2003, when AFP was not a required element of the HCC exception submission.41 Missing data in this case result from structural changes in submission requirements, and should be randomly distributed amongst patients with differing etiologies of liver disease. Regarding the patients excluded for non-standard HCC exceptions, this step would likely identify patients with HCC cases referred to regional review boards. These cases would be more likely to contain disease outside of the Milan criteria, and presumably a higher risk of HCC recurrence. Excluding these patients would have the effect of biasing overall regression results towards the null, making the estimates in this study more conservative than would otherwise be expected.
In conclusion, our data suggest that we should view HCC as a condition with a multitude of biological phenotypes requiring a highly tailored approach. With respect to post-LT recurrence, the significance of a high AFP differs by etiology of liver disease, and LT selection criteria may need to address these differences. Further research is undoubtedly required to validate these findings in prospective cohorts, establish acceptable AFP thresholds for etiology-specific risks, and to further elucidate the biological/immunological underpinnings of HCC that arises in the context of different chronic liver diseases.
Supplementary Material
Supplemental Figure 1 – Patient Flow Diagram
Acknowledgments
Financial Support
Nadim Mahmud is supported by a National Institutes of Health T32 Research Training Grant (2-T32-DK007740-21A1).
List of Abbreviations
- HCC
hepatocellular carcinoma
- AFP
alpha fetoprotein
- UNOS
United Network for Organ Sharing
- LT
liver transplantation
- OPTN
Organ Procurement and Transplantation Network
- CLD
chronic liver disease
- MELD
model for end-stage liver disease
- BMI
body mass index
- HCV
hepatitis C
- HBV
hepatitis B
- EtOH
alcoholic liver disease
- NAFLD
non-alcoholic fatty liver disease
- CT
computed tomography
- MRI
magnetic resonance imaging
- IQR
interquartile range
- SHR
subhazard ratio
- DAART
directly administrated antiretroviral therapy
- FDA
Food and Drug Administration
Footnotes
Conflicts of Interest
The authors of this manuscript have no conflicts of interest to disclose as described by Liver Transplantation.
References
- 1.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004. November 1;127(5):S35–50. [DOI] [PubMed] [Google Scholar]
- 2.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surgical Oncology Clinics. 2015. January 1;24(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. New England Journal of Medicine. 1996. March 14;334(11):693–700. [DOI] [PubMed] [Google Scholar]
- 4.Figueras J, Jaurrieta E, Valls C, Ramos E, Serrano T, Rafecas A, et al. Resection or transplantation for hepatocellular carcinoma in cirrhotic patients: outcomes based on indicated treatment strategy. Journal of the American College of Surgeons. 2000. May 1;190(5):580–7. [DOI] [PubMed] [Google Scholar]
- 5.Schlansky B, Chen Y, Scott DL, Austin D, Naugler WE. Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study using the United Network for Organ Sharing registry. Liver Transplantation. 2014. September 1;20(9):1045–56. [DOI] [PubMed] [Google Scholar]
- 6.Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long‐term outcome compared to tumors within Milan criteria. Hepatology. 2015. June 1;61(6):1968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transplantation. 2010. March 1;16(3):262–78. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, Busuttil RW. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Archives of surgery. 2008. February 1;143(2):182–8. [DOI] [PubMed] [Google Scholar]
- 9.Burra P, Burroughs A, Graziadei I, Pirenne J, Valdecasas JC, Muiesan P, et al. EASL clinical practice guidelines: liver transplantation. Journal of hepatology. 2016. January 1;64(2):433–85. [DOI] [PubMed] [Google Scholar]
- 10.Biggins SW. Futility and rationing in liver retransplantation: When and how can we say no?. Journal of hepatology. 2012. June 1;56(6):1404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, et al. Presence of EpCAM‐positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. International journal of cancer. 2013. November 1;133(9):2165–71. [DOI] [PubMed] [Google Scholar]
- 12.Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. American journal of transplantation. 2011. October 1;11(10):2031–5. [DOI] [PubMed] [Google Scholar]
- 13.Piñero F, Marciano S, Anders M, Ganem FO, Zerega A, Cagliani J, et al. Identifying patients at higher risk of hepatocellular carcinoma recurrence after liver transplantation in a multicenter cohort study from Argentina. European journal of gastroenterology & hepatology. 2016. April 1;28(4):421–7. [DOI] [PubMed] [Google Scholar]
- 14.Kashkoush S, El Moghazy W, Kawahara T, Gala‐Lopez B, Toso C, Kneteman NM. Three‐dimensional tumor volume and serum alpha‐fetoprotein are predictors of hepatocellular carcinoma recurrence after liver transplantation: refined selection criteria. Clinical transplantation. 2014. June 1;28(6):728–36. [DOI] [PubMed] [Google Scholar]
- 15.Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, et al. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. Jama oncology. 2017. April 1;3(4):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha‐fetoprotein level> 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transplantation. 2014. August 1;20(8):945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. New England journal of medicine. 1993. June 24;328(25):1797–801. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993. July 1;18(1):47–53. [PubMed] [Google Scholar]
- 19.Silver HK, Gold P, Shuster J, Javitt NB, Freedman SO, Finlayson ND. Alpha1-fetoprotein in chronic liver disease. New England Journal of Medicine. 1974. September 5;291(10):506–8. [DOI] [PubMed] [Google Scholar]
- 20.Liaw YF, Tai DI, Chen TJ, Chu CM, Huang MJ. Alpha‐fetoprotein changes in the course of chronic hepatitis: relation to bridging hepatic necrosis and hepatocellular carcinoma. Liver International. 1986. June 1;6(3):133–7. [DOI] [PubMed] [Google Scholar]
- 21.Ricco G, Cavallone D, Cosma C, Caviglia GP, Oliveri F, Biasiolo A, et al. Impact of etiology of chronic liver disease on hepatocellular carcinoma biomarkers. Cancer Biomarkers. 2017. December 15(Preprint):1–0. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Chung YH, Kim CY. Specificities of serum α‐fetoprotein in HBsAg+ and HBsAg− patients in the diagnosis of hepatocellular carcinoma. Hepatology. 1991. July 1;14(1):68–72. [DOI] [PubMed] [Google Scholar]
- 23.Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, et al. Serum α-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. Journal of hepatology. 2001. April 1;34(4):570–5. [DOI] [PubMed] [Google Scholar]
- 24.Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nature immunology. 2018. January 29:1. [DOI] [PubMed] [Google Scholar]
- 25.Berry K, Ioannou GN. Serum alpha‐fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transplantation. 2013. June 1;19(6):634–45. [DOI] [PubMed] [Google Scholar]
- 26.Mehta N, Heimbach J, Lee D, Dodge JL, Harnois D, Burns J, et al. Wait Time of Less Than 6 and Greater Than 18 Months Predicts Hepatocellular Carcinoma Recurrence After Liver Transplantation: Proposing a Wait Time “Sweet Spot”. Transplantation. 2017. September 1;101(9):2071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai S, Yoshida A, Facciuto M, Moonka D, Abouljoud MS, Schwartz ME, Florman SS. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology. 2015. March 1;61(3):895–904. [DOI] [PubMed] [Google Scholar]
- 28.Samoylova ML, Dodge JL, Vittinghoff E, Yao FY, Roberts JP. Validating posttransplant hepatocellular carcinoma recurrence data in the United Network for Organ Sharing database. Liver Transplantation. 2013. December 1;19(12):1318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta N, Dodge JL, Roberts JP, Yao FY. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. American Journal of Transplantation. 2017. October 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transplantation. 2005. September 1;11(9):1086–92. [DOI] [PubMed] [Google Scholar]
- 31.Parfitt JR, Marotta P, AlGhamdi M, Wall W, Khakhar A, Suskin NG, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver transplantation. 2007. April 1;13(4):543–51. [DOI] [PubMed] [Google Scholar]
- 32.Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. Journal of the American College of Surgeons. 2015. April 1;220(4):416–27. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Wright F, et al. Predictors of long-term outcome following liver transplantation for hepatocellular carcinoma: a single-center experience. Transplant International. 2007. September 1;20(9):747–53. [DOI] [PubMed] [Google Scholar]
- 34.Marelli L, Grasso A, Pleguezuelo M, Martines H, Stigliano R, Dhillon AP, et al. Tumour size and differentiation in predicting recurrence of hepatocellular carcinoma after liver transplantation: external validation of a new prognostic score. Annals of surgical oncology. 2008. December 1;15(12):3503. [DOI] [PubMed] [Google Scholar]
- 35.Chan EY, Larson AM, Fix OK, Yeh MM, Levy AE, Bakthavatsalam R, et al. Identifying risk for recurrent hepatocellular carcinoma after liver transplantation: implications for surveillance studies and new adjuvant therapies. Liver Transplantation. 2008. July 1;14(7):956–65. [DOI] [PubMed] [Google Scholar]
- 36.Agopian VG, Harlander-Locke MP, Ruiz RM, Klintmalm GB, Senguttuvan S, Florman SS, Haydel B, Hoteit M, Levine MH, Lee DD, Taner CB. Impact of Pretransplant Bridging Locoregional Therapy for Patients With Hepatocellular Carcinoma Within Milan Criteria Undergoing Liver Transplantation: Analysis of 3601 Patients from the US Multicenter HCC Transplant Consortium. Annals of surgery. 2017. September 1;266(3):525–35. [DOI] [PubMed] [Google Scholar]
- 37.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go?. Hepatology. 2014. November 1;60(5):1767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greten TF, Duffy AG, Korangy F. Hepatocellular carcinoma from an immunologic perspective. Clinical Cancer Research. 2013. December 15;19(24):6678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrasek J, Csak T, Ganz M, Szabo G. Differences in innate immune signaling between alcoholic and non-alcoholic steatohepatitis. Journal of gastroenterology and hepatology. 2013. August 1;28(S1):93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Procurement Organ and Network Transplantation. [2017]. OPTN/UNOS Policy Notice Clarification to Alpha-Fetoprotein (AFP) Levels for Liver Candidate Eligibility for Standardized HCC Exceptions, [online]. Available: https://optn.transplant.hrsa.gov/media/2345/executive_policynotice_afp_201712.pdf [2018, March 6]. [Google Scholar]
- 41.Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transplantation. 2013. June 1;19(6):634–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Patient Flow Diagram


