Abstract
Cancer treatment still represents a formidable challenge, despite substantial advancements in available therapies being made over the past decade. One major issue is poor therapeutic efficacy due to a lack of specificity and low bioavailability. The progress of nanotechnology and the development of a variety of nanoplatforms have had a significant impact in improving the therapeutic outcome of chemotherapeutics. Nanoparticles can overcome various biological barriers and localize at tumor site, while simultaneously protecting a therapeutic cargo and increasing its circulation time. Despite this, due to their synthetic origin, nanoparticles are often detected by the immune system and preferentially sequestered by filtering organs. Exosomes have recently been investigated as suitable substitutes for the shortcomings of nanoparticles due to their biological compatibility and particularly small size (i.e., 30–150 nm). In addition, exosomes have been found to play important roles in cell communication, acting as natural carriers of biological cargoes throughout the body. This review aims to highlight the use of exosomes as drug delivery vehicles for cancer and showcases the various attempts used to exploit exosomes with a focus on the delivery of chemotherapeutics and nucleic acids.
Keywords: Chemotherapy, Drug Delivery, Exosomes, Extracellular Vesicles, Gene Therapy, Nanoparticles, Nanotechnology, Surface Modifications
1-. INTRODUCTION
Cancer is considered a multifactorial disease that causes millions of global deaths per year. The identification of selective treatment able to kill only tumor cells is hard to find, since cancer arises from an organism’s own healthy cells that have developed abnormal properties, resulting in uncontrolled cell growth [1]. Currently, cancer treatment strategies are based on chemotherapy using cytotoxic drugs. Systemic administration of these agents exhibited several issues, including poor specificity, low efficacy, high toxicity and induction of drug resistance. Pharmacokinetics, physiochemical properties, and poor selectivity of chemotherapeutics contribute to reduced therapeutic efficacy. Moreover, the limited biological barrier penetration of cytotoxic agents prevents successful site-specific accumulation [2], requiring repeated administration which may lead to the acquisition of drug resistance. Furthermore, recent studies have demonstrated that the interaction between heterogeneous tumor cell populations and the microenvironment can induce therapeutic resistance [3].
Consequently, new approaches have been explored to increase the effectiveness of anti-tumor therapeutics systemically administered by enhancing therapeutic agent’s permeability and selectivity [4]. Among these, nanotechnology has been employed for drug delivery to improve therapeutic potency to cancerous cells while sparing healthy tissues. Accordingly, recent investigations have demonstrated the use of nanoparticles (NPs) as a promising platform for the delivery of therapeutic agents. NPs provide better therapeutic efficacy through the improvement of loading and release parameters [5,6], biocompatibility and drug circulation-time [7,8,9] and, finally, the controlled and targeted delivery of drugs to target sites [10–14]. In this manner, NPs significantly decrease adverse health effects and simultaneously increase tumor selectivity [15]. Efficient delivery of NPs is also the result of the enhanced permeability and retention effect (EPR) [16]. Indeed, due to high permeability and poor lymphatic drainage, the tumor vasculature is highly abnormal thus promoting the extravasation of NPs into tumors. Unfortunately, despite promising therapeutic applications, nanotechnology has provided only modest improvements in patient survival.
NPs have showed several limitations due to their suboptimal properties, including premature drug release during NPs synthesis, storage or circulation in blood and lack of specificity for the tumor. These issues result in an inability to reach and effectively penetrate tumors [17]. Additionally, after administration, NPs can interact with the immune system (i.e. uptake by macrophages known as Mononuclear Phagocytic System (MPS)) resulting in a strong adverse response to the treatment [18,19]. Currently, only a small number of NPs such as polyethylene glycol (PEG)-conjugated liposomal doxorubicin (i.e., Doxil/Caelyx) and liposomal irinotecan (i.e., Onivyde) have seen Food and Drug Administration-approval for cancer therapy [20]. PEG-conjugation of NPs prevents MPS recognition and exploits EPR-mediated tumor accumulation [21]. Nevertheless, Gabizon A.A. et al. have demonstrated that repeated injections of PEGylated liposomes are associated with the production of anti-PEG antibodies, which extend blood clearance and reduce the efficacy of these formulations [22]. In addition, biological barriers can hinder NP penetration reducing bioavailability and limiting therapeutic efficacy [2]. In conclusion, the improvement of the therapeutic index of injectable nanocarriers is strictly connected to their ability to: 1) circulate in the bloodstream while avoiding the opsonization process; 2) escape immune surveillance; 3) preserve their cargo; 4) deliver drug into tissue desired sites; 5) overcome the biological barriers; 6) penetrate target cell membranes and 7) minimize accumulation at undesired sites.
Recently, to overcome these NP limitations, exosomes have emerged as a promising platform for cancer treatment, providing a viable alternative to NPs [23]. In this review, we describe exosomes and their application in drug delivery. We will pay special attention to the use of exosome-like NPs as drug delivery systems for anti-cancer treatment.
2-. EXOSOMES
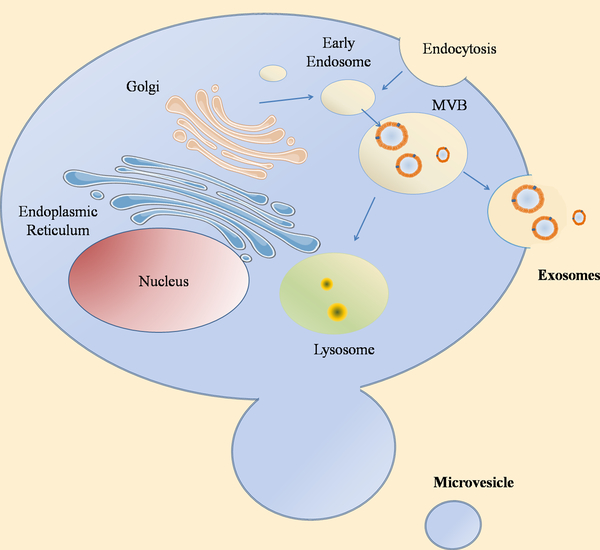
Exosomes are extracellular vesicles in the range of 30–150 nm, secreted by almost all cell types in both physiological and pathological conditions. Their origin begins from multi vesicular bodies (MVB) and their release occurs upon MVB fusion with plasma membrane (Figure 1) [24]. Exosomes are highly heterogeneous with diverse molecular compositions and contain a variety of cargoes. However, although their heterogeneity can often be dependent on the origin cell type, exosomes derived from the same cell type have also expressed different molecular compositions. The composition of the exosome surface is decorated by several cell-specific antigens, including fusion proteins, adhesion molecules, and integrins which address the selective targeting of those cells [25–27].
Figure 1. Exosomes biogenesis.
Schematic representation of the origin and release of exosomes. Exosomes originate by the fusion of intracellular vesicles and early endosomes, heading to the origin of MVBs. MVBs can either fuse with lysosomes or with the membrane ending to the release of their content. There are other types of vesicles that are generated directly from the plasma membrane: microvesicles.
Exosomes circulate in the bio-fluids and transport messages from one cell to another; these messages include several molecules, such as lipids, proteins, DNA, mRNA, non-coding RNA and various metabolites [28] that can modify the behavior of recipient cells [29] both at short- and long-distance cell communication. The cargo internalization in recipient cells occurs mainly by endocytosis but it has also been demonstrated that exosomes are able to transfer their payload by fusion with the plasma membrane of the recipient cells. [30]. It is known that exosome fusion with recipient cells occurs preferentially in acidic conditions, explaining the reason why tumors may have a better uptake than normal cells [31].
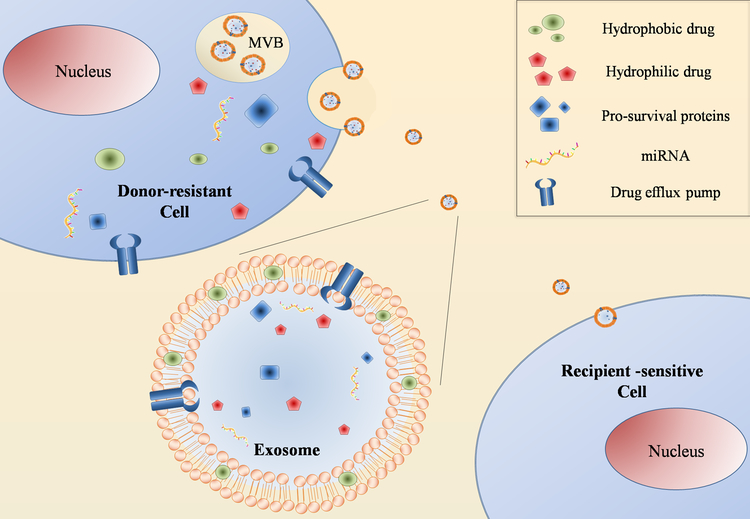
In addition, exosomes have a wide variety of functions that certainly play a key role in both physiological and pathological processes. Tumor-derived (TD) exosomes are able to modulate tumor microenvironment components and affect immune system functions. TD exosomes have an immunosuppressive behavior, helping tumor cells to avoid immune system clearance [32]. Moreover, they play a key role in the cross-talk between tumor cells and the microenvironment, converting it into a tumor prone environment. Conversely, the stroma itself can release exosomes supporting tumor growth [33]. Another important function of exosomes is the contribution in cell motility and dissemination. In fact, they can modulate the extracellular matrix, drive hematopoietic cells towards an inflammatory phenotype and stimulate epithelial to mesenchymal transition in non-metastatic cells [34]. Exosomes are also involved in chemoresistance through various mechanisms such as: 1) actively export the drug out of the cells, reducing its concentration into the cytoplasm [35]; 2) transport drug efflux pumps; 3) modulate the sensitivity of other cells [36]; 4) deliver molecules (i.e. miRNA, pro-survival proteins, etc.) to other tumor cells or to the stroma; 5) induce drug resistance mechanisms increasing drug expulsion (Figure 2) [37].
Figure 2. Exosomes role in chemoresistance.
Schematic representation of exosomes role in drug resistance acquisition. Exosomes can contribute to drug resistance by actively exporting drugs out of the cells transferring drug efflux pumps or delivering molecules (i.e. miRNA and prosurvival proteins) to sensitive recipient cells. Particularly, exosomes released by drug-resistant cells expressed high level of P-gp and are able to transfer P-gp to sensitive cells [37]. Moreover, exosomes containing miRNA expelled from drug-resistant cells can modify chemo-sensitivity in recipient cells by modulating cell cycle distribution and drug-induced apoptosis [37].
Exosomes have high versatility in translational medicine. Indeed, they can be used for diagnosis, prognosis and treatment of cancer. TD exosomes represent non-invasive biomarker because they carry tumor biomarkers and antigens and can be isolated from different body fluids of cancer patients, such as saliva, plasma and urine [38]. Thanks to their ability to activate immune cell response and the major histocompatibility complex on their surface, exosomes are a favorable strategy for cancer vaccination, inducing a potent anti-tumor response. Moreover, immunogenicity of exosomes can be artificially increased by genetic modification or fusion with specific antigens [39].
3-. USE OF EXOSOMES IN NANOMEDICINE
Exosomes represent an efficient drug delivery platform, due to their good biodistribution, biocompatibility and low immunogenicity. Exosomes present very good permeability and can cross most biological membranes [24]. Recent studies have reported that they can pass through the blood–brain barrier, demonstrating their potential in brain cancer treatment [40]. Moreover, a recent study showed that exosomes derived from fruit can efficiently deliver curcumin, a drug able to interfere with colon carcinogenesis. A phase I clinical trial has been undertaken to study the effects of curcumin delivered by fruit-derived exosomes fruit on treatment of colon cancer [41]. For all these reasons, exosomes are promising candidates for cancer treatment delivery. Currently, there are 33 clinical trials that involve exosomes as diagnostic/prognostic factors [clinicaltrials.gov].
Since interest in exosomes as nanocarriers has intensified, many techniques for their isolation and consequent loading have been developed [42–44]. Among the purification techniques, the most commonly employed protocols are based on: centrifugation [45,46], microfiltration [47,48], density gradient separation [45], immunoaffinity capture using antibodies specific for exosome surface proteins [45,49,50], and microfluidic [51,52]. On the other hand, the available methods to load drugs into exosomes include, but are not limited to, passive loading by electroporation [53,54], saponin membrane permeabilization [55], freeze/thaw cycles [56], sonication [57], and extrusion [58,59]. Alternatively, to keep the loading process as natural as possible, cells can be induced to incorporate the payload during exosome formation [60]. This approach has been employed to develop Mesenchymal Stromal Cell (MSC)-derived exosomes loaded with paclitaxel [60]. MSCs have been shown to possess innate tumor targeting abilities that successfully transfer to released exosome vesicles. Furthermore, once loaded with an anti-cancer drug, MSC-derived exosomes exhibited inhibited tumor growth, thus demonstrating the potential of this platform [60]. Recently, a new endogenous method to produce labeled exosomes, has been described by Monopoli M.P. et al. This method allows the production of labeled exosomes presenting endogenous fluorescent molecules, previously internalized by the cells. This approach can be used also to produce drug-loaded exosomes [61].
Exosomes produced by many different cells have been explored for use in clinical applications [62–64]. TD exosomes have gained the interest of many groups for delivery of anticancer drugs [65] and for their use in immunotherapy [66]. The message contained in TD exosomes can be used not only to exchange information among cells, but also as a diagnostic and prognostic biomarker of cancer [67]. Taylor D.D. et al. have used the miRNA content of TD exosomes as a diagnostic biomarker of ovarian cancer [67], while Jin H. et al. identified early diagnostic biomarkers of pancreatic cancer by using differential proteomic analysis of TD exosomes [68]. A clear and complete characterization of the genomic and proteomic content of TD exosomes is of crucial importance and many efforts have been devoted toward this aim [69,70].
Moreover, exosomes can also be isolated from sources other than cells. Recently, it has been revealed that bovine milk-derived exosomes contain mRNAs and miRNAs [71–73]. Moreover, edible plant-derived exosomes are currently under investigation for their anti-inflammatory and anti-cancer properties [74,75].
4-. EXOSOMES-LIKE NANOCARRIERS
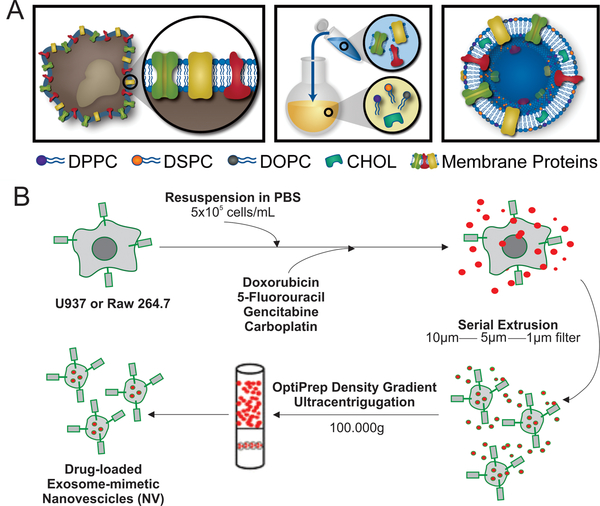
Despite the great potential of exosomes as delivery systems and the presence of clinical trials on this platform, the lack of standardized protocols for the isolation of sufficient quantities represents a major obstacle for their implementation [76]. To overcome this obstacle, synthetic extracellular nanovesicles have been recently developed. These cell-derived nanovesicles are made up of a lipid bilayer enriched with membrane-associated proteins derived from cells of interest. They are produced mainly by using common protocols developed for liposomes synthesis (Figure 3A) [10], thus allowing drug loading which is not readily achievable in naturally purified exosomes. Moreover, synthetic exosome-like nanocarriers offer excellent versatility for surface modifications with entire cell membrane patches or with only a few selected membrane proteins crucial for specific function and/or a targeting effect [22,77,78]. Using the thin layer evaporation method commonly used for liposome synthesis, our group developed a protocol for the fabrication of biomimetic exosome-like vesicles for targeting inflamed tissues [10]. These immune cell-derived nanovesicles, called leukosomes, demonstrated a natural targeting ability of immune cells toward inflamed tissues by preserving the topology of plasma membrane proteins. Specifically, we demonstrated the successful implementation of critical adhesion proteins, lymphocyte function-associated antigen 1 (LFA-1) and macrophage-1 antigen (Mac-1), along with over 300 additional proteins involved in signaling, adhesion, immunity, and transport into a lipid vesicle [79]. LFA-1 and Mac-1 have been shown to be responsible for adhesion to intracellular adhesion molecule 1, a ligand for both proteins found on endothelial cells [80]. We previously demonstrated when either LFA-1 or Mac-1 was blocked on the particle surface, a significant reduction in endothelium accumulation was observed [77].
Figure 3. Approaches for synthesis of exosome-like nanovesicles.
A. Schematic of synthesis of leukosomes: synthetic extracellular nanovesicles composed by a lipid bilayer enriched with membrane-associated proteins derived from leukocyte. B. Exosome-like nanovesicles obtained by consecutive extrusion passages of cells through membrane filters with diminishing size, followed by density gradient ultracentrifugation. Adapted with permissions from ref [10] A, and [53] B.
Indeed, being synthetic nanovesicles, leukosomes were easily loaded with several types of payloads with different chemical compositions, demonstrating the versatility of this platform [10]. Leukosomes can also be used as an imaging tool of inflamed vasculature. Specifically, leukosomes showcased superior accumulation in in vivo models of breast cancer tumor and vascular lesions (i.e. atherosclerotic plaque), demonstrating their potential for theranostic drug delivery [81]. The leukosome’s membrane proteins, which have been characterized by proteomic-based approaches [10,82], induce also the formation of a protein corona [83–85] that is responsible for the prolonged circulation time of these nanovesicles when compared to traditional liposomes [86]. In fact, when nanoparticles encounter biological fluids, they are rapidly encased in a layer of biomolecules, creating a crown around their surface [87]. This corona has been shown to alter the biological fate of nanoparticles, with a considerable effect observed for active targeting applications [87]. Many studies have investigated the composition, structural conformation and impact of the protein corona on cellular uptake, targeting, cytotoxicity of inorganic and lipid nanoparticles (for a complete review, please refer to references [85]).
Taken further, even less is known regarding the protein corona of extracellular vesicles (e.g., exosomes) and exosome-like nanovesicles. In theory, exosomes should not have any corona other than specific receptors for their surface antigens. Exosome-like nanovesicles may or may not have a protein corona according to the method/material employed for their synthesis. To the best of our knowledge, our work on the protein corona of exosome-like nanovesicles (i.e., leukosomes) represents the only such example in literature [88]. Our synthesis process implied the use of phospholipids commonly used for liposomes. We found many common proteins between the corona of liposomes and leukosomes. However, due to the different impact of the corona on cellular uptake, we speculated that those proteins were oriented differently between the two types of nanovesicles, thus stressing the importance of considering not only the identity of proteins, but also their orientation in the corona [88]. We envision that this interesting aspect of the protein corona of exosome-like nanovesicles, so far not explored, will become of major interest very soon.
The exosome-like NPs can be easily modified to induce the over-expression of a specific membrane protein before the membrane protein extraction. Using such approach, we developed specialized leukosomes to selectively target the inflamed tissue in inflammatory bowel disease (IBD) mouse model [88]. These exosome-like NPs, are immune cells-derived nanovesicles doped with the integrin α4β7, responsible for the T-cells homing to inflamed tissue in the gastrointestinal tract. Treatment of IBD mice with these specialized nanovesicles unloaded (i.e. with no therapeutics) resulted in a reduction of inflammation and in an enhanced intestinal repair thus showing an intrinsic anti-inflammatory action of the α4β7 leukosomes [88].
With a different synthesis approach, Jang S.C. et al. have developed cell-derived nanocarriers, coined as exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to tumors [53]. In an effort to overcome the often difficult task of obtaining sufficient exosomes from traditional cell culture methods, the authors produced exosome-like nanovesicles by consecutive extrusion passages of cells through membrane filters with diminishing size followed by density gradient ultracentrifugation (Figure 3B) [53]. These immune cell-derived nanovesicles induced in vitro death of TNF-α-stimulated endothelial cell. Moreover, when loaded with chemotherapeutics and injected, these vesicles exhibited a reduction in in vivo tumor growth, demonstrating the promise of a serial extrusion approach to mitigate the potential insufficient number of exosomes obtained from traditional methods Importantly, if the plasma membrane proteins are removed by trypsinization, the nanovesicles lose their efficacy both in vitro and in vivo, thus highlighting the crucial role of plasma proteins [53]. More recently, exosome-like nanovesicles have been obtained by microfluidic approaches which represent a novel and robust method to scale-up the synthesis process [89].
5-. SURFACE MODIFICATIONS
Recent evidence indicated that changing the composition of the exosome surface permits modifications to interactions between exosomes and targeted cells. Membrane-engineering approaches represent a novel strategy for the development of advanced drug-carrier exosomes. Alvarez-Erviti L. et al. transfected dendritic cells, isolated from mice, in order to express the neuronal targeting ligand RVG coupled with the exosomal membrane protein Lamp2b. The obtained exosomes demonstrated higher cargo delivery potential to the brain in a mouse model, suggesting a possible application in the treatment of glioblastoma [90]. Ohno S.I. et al. engineered human embryonic kidney cells with a pDisplay vector encoding for GE11 peptide [91]. Compared to epidermal growth factor (EGF), this ligand showed comparable binding ability to the receptor (EGFR) but present less mitogenic and neoangiogenic activity. To increase targeting efficacy and accumulation at tumor site, the authors demonstrated exosomes purified from the transfected cells, express GE11 on their membrane and present a higher ability to target EGFR-expressing breast cancer cells both in vitro and in vivo compared to naturally purified exosomes [91]. Modification of the exosome surface using tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a so-called “death receptor”, has also been shown to provide improved targeting potential in various cancers [92]. Use of this ligand has been shown to induce apoptosis in tumor cells while not effecting normal cells. However, the non-specific accumulation has led investigators to investigate the incorporation of TRAIL into exosome-like vesicles. Evaluation of exosomes enhanced with TRAIL was found to promote apoptosis in myeloma- and melanoma-based tumors. Despite this, no significant effect on tumor volume was observed in vivo. Evaluation of the treatment of lymphoma tumor-bearing mice showed similar signs of apoptosis in vitro; however, an opposite effect was observed in vivo [93].
Next to the engineering of donor cells to produce ligand-conjugated or drug-loaded exosomes, other techniques have been developed to improve the targeting ability of exosomes by surface modification. Tian T. et al. developed an efficient method to conjugate multiple functional ligands to the surface of exosomes pre-isolated from culture medium or body fluids without affecting the exosome features or the bioactivity of the loaded cargo [40] Through a “copper-free click chemistry” reaction the authors conjugated c(RGDyK) peptide to the surface of exosomes derived from MSC [40]. Functionalized exosomes, delivering curcumin, could target the ischemic brain region in a mouse model of cerebral artery occlusion and reduce inflammation. Since c(RGDyK) is a recognized tumor-targeting ligand, the use of these synthetic exosomes could be investigated as drug carriers in cancer treatment. Another approach exploited the use of liposomes for the generation of synthetic exosomes. Using this technique, Sato Y.T. et al. developed engineered hybrid exosomes by fusing phospholipidic liposomes with tyrosin kinase receptor (HER2)-expressing exosomes purified from HER2 expressing cells [56]. These results strongly imply that not all the components of the exosome’s membrane are required for an efficient delivery to the target cells, suggesting synthetic mimetic exosomes as promising candidates for a tailored anti-cancer drug delivery approach.
6-. PROS AND CONS OF USING PURIFIED VS. SYNTHETIC EXOSOMES
TD exosomes mirror most of the molecular features of the tumor cells from which they originate, making them the perfect biomarker and drug delivery tool for the diagnosis and treatment of cancer. The low immunogenicity, negative surface charge and a surface protein composition similar to cell membrane are all characteristics that help improve TD exosome cellular internalization within target tumor cells. In addition, these features minimize degradation and clearance by the immune system, contributing to reduced side effects. As such, several attempts have been made in the use of TD exosomes as vehicles for small bioactive molecules and chemotherapeutics, i.e. doxorubicin [94] and paclitaxel [60]. Nevertheless, growing evidence has demonstrated that TD exosomes support tumor growth, progression and metastases formation. In non-small cell lung cancer [95], glioma [96] and gastric cancer cell lines [97] TD exosomes have been shown to enhance the tumor microenvironment resulting in cancer cell proliferation [98] and angiogenesis [99]. Harris D.A. et al. have demonstrated in three different breast cancer cell lines that, according to the cells of origin, exosomes promoted tumor cell invasiveness and metastatic potential through the expression of adhesion molecules and proteases such as urokinase plasminogen activator, vimentin, galectin 3-binding protein and annexin A1 [100]. In addition, it has been demonstrated that TD exosomes promote tumor escape from immune recognition. Indeed, exosomes triggered apoptosis of activated cytotoxic T cells through expression of ligands such as FASL, TRAIL and PSL2 [101], impaired dendritic cell differentiation from monocytes, and suppressed lymphoid activation signaling molecules [69]. In melanoma patients, Taylor D.D. et al. demonstrated that TD exosomes enhance the production of myeloid-derived suppressor cells, which play a key role in immune system modulation [102].
Conversely, synthetic exosomes produced by genetic modification of the exosome-producing tumor cells represent a promising tool to achieve a higher antitumor immune response. The expression induction of artificial neoantigens or neoepitopes on the surface of exosomes can evoke anti-tumor recognition by immune cells. Koyama Y. et al. previously demonstrated that early secretory antigen target-6 (ESAT-6) is a potent antigen able to induce immune response. They produced ESAT-6 carrier exosomes from genetically modified tumor cells and showed significant tumor growth reduction in mice treated with these exosomes [103]. A different approach has been exploited by Wang J. et al. [104]. These authors labeled donor cell membranes with biotin and exposed them to a potent anti-neoplastic drug. After cell functionalization with avidin, to improve the targeting efficiency, they obtained exosomes expressing both biotin and avidin on the membrane surface and encapsulated with the drug. These synthetic exosomes showed high target ability to tumor cells and receptor-mediated cellular uptake.
The production of synthetic exosomes has yielded to overcome another important aspect in the application of purified exosomes in cancer treatment. Indeed, although cells continuously produce exosomes, the recovery is often too small especially for clinical application [105]. To obtain the desired amount of material, it is necessary to begin with a large number of initial cells and effectively quantity the cell media. Moreover, biological fluids contain a mixture of exosomes produced by all a variety of cell types. Due to this aspect and the exosome’s small size, the processes of purification and isolation are still challenging. To date several techniques have been developed to isolate and purify exosomes. However, most of them require multiple, complicated and time-consuming steps of ultracentrifugation and with a lack of specificity [40]. On the contrary, the production of synthetic exosomes can be scaled up and the assembling process is easily controllable, overcoming the difficulties of using naturally derived exosomes.
7-. DELIVERY OF CHEMOTHERAPEUTICS
The ability of exosomes to enable intercellular communication through the transfer of biological molecules to a variety of cells [106] and through the presentation of important surface proteins [26] has led to significant efforts being made to investigate their potential as a drug delivery vehicle. In particular, the use of exosomes in the treatment of cancer has raised considerable interest due to their low toxicity and immunogenicity. In one instance, exosomes were evaluated for their ability to shuttle chemotherapeutic doxorubicin to tumor tissue [107]. In an effort to provide improved tumor targeting, murine immature dendritic cells were transfected with an iRGD peptide segment and collected by purifying cell supernatant [107]. iRGD peptide was selected for its ability to readily binds to αv integrins, which are commonly overexpressed on tumor cells [107]. Next, following an electroporation-mediated loading of doxorubicin into exosomes, the iRGD-modified exosomes were found to significantly decrease cancer cell proliferation 24 h following treatment with a rate closely matching free doxorubicin [107]. In this same study, a similar observation was obtained following the treatment of a MDA-MB-231 tumor-bearing mouse model. It was reported that iRGD-modified exosomes were able to decrease tumor growth by ~3.5-fold compared to free doxorubicin or non-modified exosomes [107]. This strategy demonstrates the improvement possible when exosome surface is functionalized with peptides or other proteins.
Similar strategies employing dendritic cell-derived exosomes have been increasingly explored due to their biocompatibility and have already begun to be investigated in early phase clinical trials for cancer treatments [108–110]. To elaborate, assessment of exosomes with and without granulocyte–macrophage colony-stimulating factor administered to 20 patients demonstrated the safety of exosomes as an immunotherapy tool for colorectal cancer [108]. Exosomes were further proven to be suitable tools for cancer vaccines as demonstrated in a phase I study that evaluated exosome usage in non-small cell lung carcinomas [110]. In this study, exosomes containing MAGE antigens, commonly express in a variety of cancers [111], were evaluated and found to serve as a feasible treatment option for this disease. Nevertheless, although these clinical trials represent promising therapeutic outcomes, further work is still needed to fully realize exosome’s potential in the treatment of cancer-related diseases.
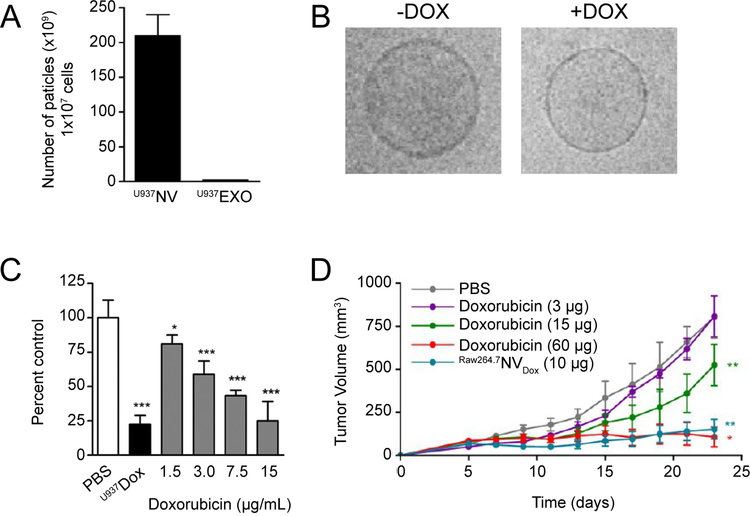
As described in Section 4, strategies aimed at significantly increasing exosome yield have also been investigated. Specifically, Jang S.C. et al. devised a multi-tiered, serial extrusion protocol to develop high-yield exosome-mimetic particles derived from monocytes (i.e. U937 or RAW264.7 – Figure 3B) [53]. This resulted in an increase of total protein and particle yielded by more than 100-fold compared to naturally-occurring exosomes (Figure 4A). In addition, the use of a leukocyte cell source allowed for the ability to efficiently target activated endothelium. When exosome-mimetic particles were loaded with doxorubicin, a significant increase in cell death was achieved for activated endothelium in vitro with over a 10-fold increase in cell-death compared to free doxorubicin (Figure 4C). Similar targeting potential was reached when exosome-mimetic particles were used in the treatment of colon carcinoma in vivo. Specifically, it was exhibited that a 6-fold increase in potency was achieved when doxorubicin was encapsulated in exosome-mimetic particles (Figure 4D) [53].
Figure 4. Evaluation of chemotherapy-loaded exosome nanoparticles.
A. Total particle yield of exosome-mimetic nanovesicles (NV) and exosomes (EXO) derived from 1 × 107 total cells. B. Cryo-transmission electron micrographs depicting NV with and without doxorubicin (DOX). C. Evaluation of TNF-α-activated HUVEC proliferation following treatment with varying doses of DOX-loaded NV. D. Tumor growth following administration of NV and NV containing DOX (2 μg or 10 μg). Reprint with permissions from ref [53].
Exosomes generated using brain endothelial and glioblastoma-astrocytoma cells (U-87) were shown to also act as favorable drug delivery vehicles for model chemotherapies doxorubicin and paclitaxel [112]. In particular, doxorubicin and paclitaxel-loaded exosomes were found to inhibit in vitro cell proliferation by up to 50%. In addition, encapsulation of chemotherapeutic payload was found to primarily localize to the site of cancer in a zebrafish model, resulting in minimal expression of tumor cells following treatment. Evaluation of this technology with a xenotransplanted brain tumor model was found to inhibit expression of cancer cells when vascular endothelial growth factor siRNA was delivered [113].
8-. DELIVERY OF NUCLEIC ACIDS
Exosomes have also been shown to provide favorable properties for effective gene therapy. Although viral vectors have shown considerable promise for gene therapy, their inherent toxicity, infectivity, and immunogenic potential limit their function as cancer therapies [114]. In a strategy to increase the efficacy of gene knockdown in a hepatocellular carcinoma mouse model, Liang G. et al. modified the surface of exosomes with an Apo-A1/CD63 complex, a known target of scavenger receptor class B type 1 receptors, that has been shown to be highly expressed in liver cancers [115]. Following encapsulation of miR-26a, a known downregulated miRNA in liver cancer, a significant reduction in proliferation of HepG2 liver carcinoma cells was observed. This was found to be attributed to the arrest of the cell cycle at the G1 phase as a result of miR-26a delivery [115].
For targeted delivery of exosomes to the brain, exosomes were generated using dendritic cells modified to express the exosome membrane protein Lamp2b fused with the neuron-specific rabies viral glycoprotein (RVG) peptide [115] or a muscle-specific peptide. This was found to result in an effective delivery strategy that could be exploited in the delivery of siRNA to the brain [90], a site that is traditionally challenging to target. To demonstrate the exosome targeting potential, murine muscle (C2C12) and neuronal (Neuro2A) cells were evaluated for their ability to bind MSP- and RVG-modified exosomes, respectively. It was reported that the exosome-mediated delivery was peptide-dependent and performed similar to transfection reagents. More so, exosomes displayed minimal nonspecific uptake in vivo with significant knockdown of being reported in the brain of RVG exosome-treated mice [90].
Using a similar strategy to combat morphine addiction, exosomes were modified with the RVG peptide to localize delivery of siRNA to the brain [116,117]. To generate peptide-expressing exosomes, RVG peptide and opioid receptor Mu siRNA were co-transfected into kidney cells and collected 48 h later. RVG exosomes were found to effectively target neuron cells expressing the RVG peptide receptor, indicating its promise as a targeting tool. Similar efficacy was observed in vivo with modified exosomes displaying the ability to effectively overcome the blood brain barrier and deliver biological cargo into neural cells. Specifically, it was reported that RVG-modified exosomes were able to significantly reduce MOR mRNA and protein levels, when siRNA was encapsulated into exosomes.
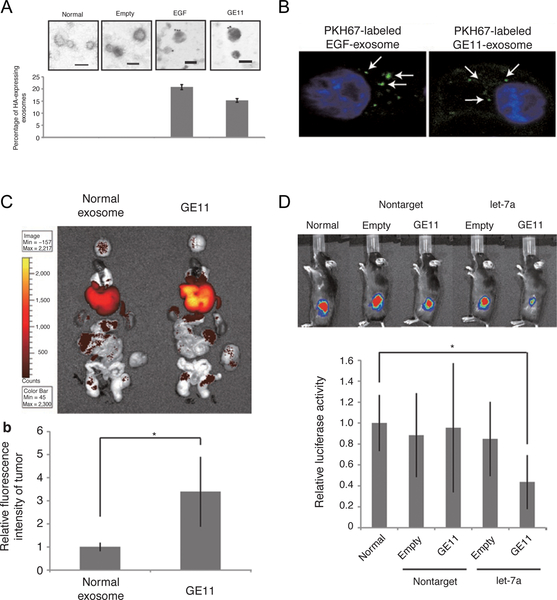
Targeting of exosomes to EGFR, expressed on a number of human tumors, was achieved similarly by incorporation of GE11 (i.e., EGFR peptide) into kidney cells (Figure 5) [91]. Following a purification step, GE11 or EGF-expressing exosomes were found to preferentially bind to EGFR-positive cells including HCC70 and MCF-7 cells. Targeting ability was further assessed in an in vivo system and displayed tumor-specific targeting with significant accumulation observed for GE11-positive exosomes compared to control (Figure 5C). Delivery of a miRNA payload was also found to be enhanced when incorporated within the GE11-expressing exosome, demonstrating exosome’s effectiveness as a drug delivery vehicle for biological cargo (Figure 5D).
Figure 5. EGFR-mediated exosome delivery of miRNA.
A. Electron microscope images depicting the presence of GE11 and EGF on the exosome surface. B. Confocal microscope images depicting PKH67 dye-loaded exosomes (green) internalized within HCC70 human breast cancer cells. C. In vivo imaging comparing normal exosome and GE11-modified exosomes accumulation within a human breast cancer tumor-bearing mouse model. D. Antitumor effect 4 week following post-administration of let7a miRNA-containing exosomes. Reprint with permission from ref [91].
The exogenous loading of siRNA into exosome-mimetic nanovesicles through electroporation, as a method to optimize encapsulation, has also been explored and found to protect the biological cargo [118]. This method was employed to knockdown c-Myc in a lymphoma cell line and to evaluate the feasibility of an exosome-mimetic delivery system. Interestingly, it was reported that shRNA incorporated within the nanovesicle were successfully internalized by the recipient cells similar to exosomes, resulted in the downregulation of c-Myc [118].
Exosomes have also been explored for their ability to deliver anti-miRNA to minimize the expression of drug resistant genes. In one case, the loading of anti-miR-214, a miRNA that correlates with multiple drug resistance, was loaded into exosomes and evaluated for its ability to prevent drug resistance in gastric cancer [119]. This analysis revealed delivery of an anti-miRNA for drug resistance resulted in a significant inhibition of gastric tumor growth when cisplatin co-administered every 4th day. This provides considerable promise in the ability of exosomes to serve as rational delivery agents for biological cargos while displaying biocompatibility with minimal immunogenic response.
9-. CONCLUSIONS
The conventional drug treatment of cancer exhibits several limitations, including reduced specificity of the target and consequently, low therapeutic index and the presence of adverse side effects. The main obstacles to achieve an efficacious therapeutic dose at the tumor site often include various biological barriers and molecular characteristics of the therapy employed. Previously, nanotechnology has made great progress in drug delivery to overcome these issues although more effort is still necessary to improve NP characteristics to obtain an efficient therapeutic effect. Exosomes represent a promising alternative to standard NPs, owing to their intrinsic advantageous features for drug delivery. In particular, they have showcased prolonged circulation time, reduced clearance levels and an ability to protect payloads from degradation or inactivation. Exosomes-like NPs have been studied for the delivery of a variety of payloads demonstrating favorable chemotherapeutic delivery and nucleic acid delivery (e.g. miRNA, shRNA). In addition, modification of the molecular composition of exosomes has been employed to improve the translational impact of exosomes. Surface modifications allow for specific recognition of the target and the achievement of increased potency for therapies at the site of disease. Nevertheless, while exosomes provide a variety of desirable traits for drug delivery, further work is still required. As described in this review, TD exosomes offer a variety of promising characteristics but as with exosome extraction, yield continues to remain a significant hurdle. As such, a variety of methods have been explored to develop exosome-like vesicles that maintain similar characteristics of TD exosomes or cell-derived exosomes. For example, discussed herein, we demonstrated the use of serial extrusion as a method for developing exosome-like vesicles with greater yield. This method incorporates key proteins into the vesicle surface, maintaining key properties of exosome function (e.g., targeting). However, while both methods demonstrate promise, further investigations are still necessary to improve exosomes properties to more effectively target and penetrate solid tumors, providing efficacious therapeutic doses and making exosomes a player in the clinic. In conclusion, we claim that exosomes have offered new possibilities in cancer treatment; in fact, they can potentially serve as drug delivery vehicles. In our point of view, exosomes will reasonably be employed for the design of innovative treatments to potentially enable translation into the clinic, but first issues into exosomes purification and/or synthesis, stabilization and loading must be addressed.
ACKNOWLEDGMENTS
This work was supported financially by Associazione Bianca Garavaglia Onlus (A/15/01O), Cancer Prevention & Research Institute of Texas (Award ID RP170466), NCI and the Office of Research on Women’s Health (1R56CA213859–01A1) and The Cullen Trust for Health Care.
ABBREVIATIONS
- ESAT-6
Early secretory antigen target-6
- EGFR
EGF-receptor
- EPR
Enhanced permeability and retention
- EGF
Epidermal growth factor
- MSCs
Mesenchymal stromal cells
- MPS
Mononuclear phagocytic system
- MVBs
Multi vesicular bodies
- NPs
Nanoparticles
- PEG
Polyethylene glycol
- RVG
Rabies viral glycoprotein
- TD
Tumor derived
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Hanahan D; Weinberg RA Hallmarks of cancer: the next generation. Cell, 2011, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- [2].Blanco E; Shen H; and Ferrari M Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol, 2015, 33, 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Son B; Lee S; Youn H; Kim E; Kim W; Youn B The role of tumor microenvironment in therapeutic resistance. Oncotarget, 2017, 8, 3933–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Damia G; Garattini S The pharmacological point of view of resistance to therapy in tumors. Cancer Treat. Rev, 2014, 40, 909–16. [DOI] [PubMed] [Google Scholar]
- [5].Martinez JO; Evangelopoulos M; Bhavane R; Acciardo S; Salvatore F; Liu X; Ferrari M; Tasciotti E Multistage Nanovectors Enhance the Delivery of Free and Encapsulated Drugs. Curr. Drug Targets, 2015, 16, 1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Khaled SZ; Cevenini A; Yazdi IK; Parodi A; Evangelopoulos M; Corbo C; Scaria S; Hu Y; Haddix SG; Corradetti B; Salvatore F; Tasciotti E One-pot synthesis of pH-responsive hybrid nanogel particles for the intracellular delivery of small interfering RNA. Biomaterials, 2016, 87, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martinez JO; Evangelopoulos M; Chiappini C; Liu X; Ferrari M; Tasciotti E Degradation and biocompatibility of multistage nanovectors in physiological systems. J. Biomed. Mater. Res. A, 2014, 102, 3540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Martinez JO; Boada C; Yazdi IK; Evangelopoulos M; Brown BS; Liu X; Ferrari M; Tasciotti E Short and long term, in vitro and in vivo correlations of cellular and tissue responses to mesoporous silicon nanovectors. Small, 2013, 9, 1722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Balasubramanian K; Evangelopoulos M; Brown BS; Parodi A; Celia C;, Yazdi IK; Tasciotti E Ghee Butter as a Therapeutic Delivery System. J. Nanosci. Nanotechnol, 2017, 17, 977–82. [DOI] [PubMed] [Google Scholar]
- [10].Molinaro R; Corbo C; Livingston M; Evangelopoulos M; Parodi A; Boada C; Agostini M; Tasciotti E Inflammation and Cancer: In Medio Stat Nano. Curr. Med. Chem, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Corradetti B; Taraballi F; Martinez JO; Minardi S; Basu N; Bauza G; Evangelopoulos M; Powell S; Corbo C; Tasciotti E Hyaluronic acid coatings as a simple and efficient approach to improve MSC homing toward the site of inflammation. Sci. Rep, 2017, 7, 7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Näkki S; Martinez JO; Evangelopoulos M; Xu W; Lehto VP; Tasciotti E Chlorin e6 Functionalized Theranostic Multistage Nanovectors Transported by Stem Cells for Effective Photodynamic Therapy. ACS Appl. Mater. Interfaces, 2017, 9, 23441–23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scavo MP; Gentile E; Wolfram J; Gu J; Barone M; Evangelopoulos M; Martinez JO; Liu X; Celia C; Tasciotti E; Vilar E; Shen H Multistage vector delivery of sulindac and silymarin for prevention of colon cancer. Colloids Surf. B. Biointerfaces, 2015, 136, 694–703. [DOI] [PubMed] [Google Scholar]
- [14].Fernandez-Moure JS; Evangelopoulos M; Colvill K; Van Eps JL; Tasciotti E Nanoantibiotics: a new paradigm for the treatment of surgical infection. Nanomedicine (Lond). 2017, 11, 1319–1334. [DOI] [PubMed] [Google Scholar]
- [15].Wang AZ; Langer R; Farokhzad OC Nanoparticle delivery of cancer drugs. Annu. Rev. Med, 2012, 63, 185–198. [DOI] [PubMed] [Google Scholar]
- [16].Peters A; von Elverfeldt D; Winkler K; Pütz G Accumulating nanoparticles by EPR: A route of no return. J. Control. Release, 2016, 238, 58–70. [DOI] [PubMed] [Google Scholar]
- [17].Xu X; Saw PE; Tao W; Li Y; Ji X; Bhasin S; Liu Y; Ayyash D; Rasmussen J; Huo M;Shi J; Farokhzad OC ROS-Responsive Polyprodrug Nanoparticles for Triggered Drug Delivery and Effective Cancer Therapy. Adv. Mater, 2017, 29, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zolnik BS; González-Fernández A; Sadrieh N; Dobrovolskaia MA Nanoparticles and the immune system. Endocrinology, 2010, 151, 458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Song G; Petschauer JS; Madden AJ; Zamboni WC Nanoparticles and the mononuclear phagocyte system: pharmacokinetics and applications for inflammatory diseases. Curr. Rheumatol. Rev, 2014, 10, 22–34. [DOI] [PubMed] [Google Scholar]
- [20].Tran S; DeGiovanni PJ; Piel B; Rai P Cancer nanomedicine: a review of recent success in drug delivery. Clin. Transl. Med, 2017, 6, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Milla P, Dosio F; Cattel L PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab, 2012, 13, 105–119. [DOI] [PubMed] [Google Scholar]
- [22].Gabizon AA Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin. Cancer. Res, 2001, 7, 223–225. [PubMed] [Google Scholar]
- [23].Parodi A; Molinaro R; Sushnitha M; Evangelopoulos M; Martinez JO; Arrighetti N; Corbo C; Tasciotti E Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials., 2017, 147, 155–168. [DOI] [PubMed] [Google Scholar]
- [24].He C; Zheng S; Luo Y; Wang B Exosome Theranostics: Biology and Translational Medicine. Theranostics., 2018, 8, 237–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clayton A; Court J; Navabi H; Adams M; Mason MD; Hobot JA; Newman GR; Jasani B Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods, 2001, 247, 163–74. [DOI] [PubMed] [Google Scholar]
- [26].Raposo G; Nijman HW; Stoorvogel W; Liejendekker R; Harding CV; Melief CJ; Geuze HJ B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med, 1996, 183, 1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Clayton A; Harris CL; Court J; Mason MD; Morgan BP Antigen‐presenting cell exosomes are protected from complement‐mediated lysis by expression of CD55 and CD59. Eur. J. Immunol, 2003, 33, 522–31. [DOI] [PubMed] [Google Scholar]
- [28].Mathivanan S; Ji H; Simpson RJ Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics, 2010, 73, 1907–20. [DOI] [PubMed] [Google Scholar]
- [29].Cocucci E; Meldolesi J Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol, 2015, 25, 364–72. [DOI] [PubMed] [Google Scholar]
- [30].Mulcahy LA; Pink RC; Carter DR Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles, 2014, 3, 24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Parolini I; Federici C; Raggi C; Lugini L; Palleschi S; De Milito A; Coscia C; Iessi E; Logozzi M; Molinari A; Colone M; Tatti M; Sargiacomo M; Fais S Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem, 2009, 284, 34211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].EL Andaloussi S; Mäger I; Breakefield XO; Wood MJ Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug. Discov, 2013, 12, 347–57. [DOI] [PubMed] [Google Scholar]
- [33].Whiteside TL Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin. Immunol,2017,S1044–5323, 30010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yue S; Mu W; Erb U; Zoller M The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget, 2015, 6, 2366–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Koch R; Aung T; Vogel D; Chapuy B; Wenzel D; Becker S; Sinzig U; Venkataramani V; von Mach T; Jacob R; Truemper L; Wulf GG Nuclear Trapping through Inhibition of Exosomal Export by Indomethacin Increases Cytostatic Efficacy of Doxorubicin and Pixantrone. Clin. Cancer Res, 2016, 22, 395–404. [DOI] [PubMed] [Google Scholar]
- [36].Goler-Baron V; Assaraf YG Overcoming multidrug resistance via photodestruction of ABCG2-rich extracellular vesicles sequestering photosensitive chemotherapeutics. PLoS One, 2012, 7, e35487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sharma A Chemoresistance in cancer cells: exosomes as potential regulators of therapeutic tumor heterogeneity. Nanomedicine (Lond), 2017, 12, 2137–2148. [DOI] [PubMed] [Google Scholar]
- [38].García-Manrique P; Matos M; Gutiérrez G; Pazos C; Blanco-López MC Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J. Extracell. Vesicle, 2018, 7, 1422676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wahlund CJE; Güclüler G; Hiltbrunner S; Veerman RE; Näslund TI; Gabrielsson S Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci. Rep, 2017, 7 17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tian T; Zhang HX; He CP; Fan S; Zhu YL; Qi C; Huang NP; Xiao ZD; Lu ZH; Tannous BA; Gao J Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials, 2018, 150, 137–149. [DOI] [PubMed] [Google Scholar]
- [41].clinicaltrials.gov/ Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue.
- [42].Taylor DD; Zacharias W; Gercel-Taylor C Exosome isolation for proteomic analyses and RNA profiling. Methods Mol. Biol, 2011, 728, 235–46. [DOI] [PubMed] [Google Scholar]
- [43].Lässer C; Eldh M; Lötvall J Isolation and characterization of RNA-containing exosomes. J. Vis. Exp, 2012, 59:e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Alvarez ML; Khosroheidari M; Ravi RK; DiStefano JK Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int, 2012, 82, 1024–32. [DOI] [PubMed] [Google Scholar]
- [45].Tauro BJ; Greening DW; Mathias RA; Ji H; Mathivanan S; Scott AM; Simpson RJ Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods, 2012, 56, 293–304. [DOI] [PubMed] [Google Scholar]
- [46].Bobrie A; Colombo M; Krumeich S; Raposo G; Théry C Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J. Extracell. Vesicles, 2012, 16, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cheruvanky A; Zhou H; Pisitkun T; Kopp JB; Knepper MA; Yuen PS; Star RA Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Renal Physiol, 2007, 292, F1657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Merchant ML; Powell DW; Wilkey DW; Cummins TD; Deegens JK; Rood IM; McAfee KJ; Fleischer C; Klein E; Klein JB Microfiltration isolation of human urinary exosomes for characterization by MS. Proteomics Clin. Appl, 2010, 4, 84–96. [DOI] [PubMed] [Google Scholar]
- [49].Yoo CE; Kim G; Kim M; Park D; Kang HJ; Lee M; Huh N A direct extraction method for microRNAs from exosomes captured by immunoaffinity beads. Anal. Biochem, 2012, 431, 96–8. [DOI] [PubMed] [Google Scholar]
- [50].Mathivanan S; Lim JW; Tauro BJ; Ji H; Moritz RL; Simpson RJ Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol. Cell. Proteomics, 2010, 9, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kanwar SS; Dunlay CJ; Simeone DM; Nagrath S Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab. Chip, 2014, 141891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liga A; Vliegenthart A; Oosthuyzen W; Dear J; Kersaudy-Kerhoas M Exosome isolation: a microfluidic road-map. Lab. Chip, 2015, 15, 2388–94. [DOI] [PubMed] [Google Scholar]
- [53].Jang SC; Kim OY; Yoon CM; Choi DS; Roh TY; Park J; Nilsson J; Lötvallm J; Kim YK; Gho YS Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano, 2013, 7, 7698–710. [DOI] [PubMed] [Google Scholar]
- [54].Lai RC; Yeo RWY; Tan KH; Lim SK Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol. Adv, 2013, 31, 543–51. [DOI] [PubMed] [Google Scholar]
- [55].Jamur MC; Oliver C Permeabilization of cell membranes. Methods Mol. Biol, 2010, 588, 63–6. [DOI] [PubMed] [Google Scholar]
- [56].Sato YT; Umezaki K; Sawada S; Mukai SA; Sasaki Y; Harada N; Shiku H; Akiyoshi K Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep, 2016, 6, 21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim MS; Haney MJ; Zhao Y; Mahajan V; Deygen I; Klyachko NL; Inskoe E; Piroyan A; Sokolsky M; Okolie O Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine, 2016, 12, 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Luan X; Sansanaphongpricha K; Myers I; Chen H; Yuan H; Sun D Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin, 2017, 38, 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fuhrmann G; Serio A; Mazo M; Nair R; Stevens MM Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release, 2015, 205, 35–44. [DOI] [PubMed] [Google Scholar]
- [60].Pascucci L; Coccè V; Bonomi A; Ami D; Ceccarelli P; Ciusani E; Viganò L; Locatelli A; Sisto F; Doglia SM Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J. Control. Release, 2014, 192, 262–70. [DOI] [PubMed] [Google Scholar]
- [61].Monopoli MP; Zendrini A; Wu D; Cheung S; Sampedro G; Ffrench B; Nolan J; Piskareva O; Stalings RL; Ducoli S; Bergese P; O’Shea DF Endogenous exosome labelling with an amphiphilic NIR-fluorescent probe. Chem. Commun. (Camb.), 2018, 54, 7219–7222. [DOI] [PubMed] [Google Scholar]
- [62].Whiteside TL Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv. Clin. Chem, 2016, 74, 103–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Romagnoli GG; Zelante BB; Toniolo PA; Migliori IK; Barbuto JAM Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front. Immunol, 2015, 5, 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Vader P; Mol EA; Pasterkamp G; Schiffelers RM Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev, 2016, 106, 148–156. [DOI] [PubMed] [Google Scholar]
- [65].Smyth T; Kullberg M; Malik N; Smith-Jones P; Graner MW; Anchordoquy TJ Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release, 2015, 199, 145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Whiteside TL The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol, 2017, 13, 2583–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Taylor DD; Gercel-Taylor C MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol, 2008, 110, 13–21. [DOI] [PubMed] [Google Scholar]
- [68].Jin H; Tan X Differential proteomic analysis of pancreatic cancer cell-derived exosomes provides new insights into cancer metastasis and novel biomarkers for early detection. Pancreatology, 2017, 17, S23. [Google Scholar]
- [69].Henderson MC; Azorsa DO The genomic and proteomic content of cancer cell-derived exosomes. Front. Oncol, 2012, 2, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kharaziha P; Ceder S; Li Q; Panaretakis T Tumor cell-derived exosomes: a message in a bottle. Biochim. Biophys. Acta, 2012, 1826, 103–11. [DOI] [PubMed] [Google Scholar]
- [71].Izumi H; Tsuda M; Sato Y; Kosaka N; Ochiya T; Iwamoto H; Namba K; Takeda Y Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci, 2015, 98, 2920–33. [DOI] [PubMed] [Google Scholar]
- [72].Yamada T; Inoshima Y; Matsuda T; Ishiguro N Comparison of methods for isolating exosomes from bovine milk. J. Vet. Med. Sci, 2012, 74, 1523–5. [DOI] [PubMed] [Google Scholar]
- [73].Hata T; Murakami K; Nakatani H; Yamamoto Y; Matsuda T; Aoki N Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem. Biophys. Res. Commun, 2010, 396, 528–33. [DOI] [PubMed] [Google Scholar]
- [74].Ju S; Mu J; Dokland T; Zhuang X; Wang Q; Jiang H; Xiang X; Deng ZB; Wang B; Zhang L Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther, 2013, 21, 1345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Raimondo S; Naselli F; Fontana S; Monteleone F; Dico AL; Saieva L; Zito G; Flugy A; Manno M; Di Bella MA Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget, 2015, 6, 19514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kooijmans SA; Vader P; van Dommelen SM; van Solinge WW; Schiffelers RM Exosome mimetics: a novel class of drug delivery systems. Int. J. Nanomedicine, 2012, 7, 1525–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Palomba R; Parodi A; Evangelopoulos M; Acciardo S; Corbo C; de Rosa E; Yazdi IK; Scaria S; Molinaro R; Furman NE; You J; Ferrari M; Salvatore F; Tasciotti E Biomimetic carriers mimicking leukocyte plasma membrane to increase tumor vasculature permeability. Sci Rep, 2016, 6, 34422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Evangelopoulos M; Parodi A; Martinez JO; Yazdi IK; Cevenini A; van de Ven AL; Quattrocchi N; Boada C; Taghipour N; Corbo C; Brown BS; Scaria S; Liu X; Ferrari M; Tasciotti E Cell source determines the immunological impact of biomimetic nanoparticles. Biomaterials, 2016, 82, 168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Molinaro R; Corbo C; Martinez JO; Taraballi F; Evangelopoulos M; Minardi S; Yazdi IK; Zhao P; De Rosa E; Sherman MB; De Vita A; Toledano Furman NE; Wang X; Parodi A; Tasciotti E Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater, 2016, 15, 1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Marlin SD; Springer TA Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell, 1987, 51, 813–9. [DOI] [PubMed] [Google Scholar]
- [81].Martinez JO; Molinaro R; Hartman KA; Boada C; Sukhovershin R; De Rosa E; Kirui D; Zhang S; Evangelopoulos M; Carter AM; Bibb JA; Cooke JP; Tasciotti E Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery. Theranostics, 2018, 8, 1131–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Corbo C; Parodi A; Evangelopoulos M; Engler DA; Matsunami RK; Engler AC; Molinaro R; Scaria S; Salvatore F; Tasciotti E Proteomic profiling of a biomimetic drug delivery platform. Curr. Drug. Targets, 2015, 16, 1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Corbo C; Molinaro R; Taraballi F; Toledano Furman NE; Sherman MB; Parodi A; Salvatore F; Tasciotti E Effects of the protein corona on liposome–liposome and liposome–cell interactions. Int. J. Nanomedicine, 2016, 11, 3049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Corbo C; Molinaro R; Parodi A; Toledano Furman NE; Salvatore F; Tasciotti E The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine (Lond), 2016, 11, 81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Corbo C; Molinaro R; Tabatabaei M; Farokhzad OC; Mahmoudi M Personalized protein corona on nanoparticles and its clinical implications. Biomater. Sc, 2017, 5, 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Corbo C; Molinaro R; Taraballi F; Toledano Furman NE; Hartman KA; Sherman MB; De Rosa E; Kirui DK; Salvatore F; Tasciotti E Unveiling the in vivo protein corona of circulating leukocyte-like carriers. ACS Nano, 2017, 11, 3262–3273. [DOI] [PubMed] [Google Scholar]
- [87].Salvati A;, Pitek AS; Monopoli MP; Prapainop K; Bombelli FB; Hristov DR; Kelly PM; Åberg C; Mahon E; Dawson KA Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol 2013, 8, 137–43. [DOI] [PubMed] [Google Scholar]
- [88].Corbo C; Cromer WE; Molinaro R; Toledano Furman NE; Hartman KA; De Rosa E; Boada C; Wang X; Zawieja DC; Agostini M; Salvatore F; Abraham BP; Tasciotti E Engineered biomimetic nanovesicles show intrinsic anti-inflammatory properties for the treatment of inflammatory bowel diseases. Nanoscale, 2017, 9, 14581–14591. [DOI] [PubMed] [Google Scholar]
- [89].Molinaro R; Evangelopoulos M; Hoffman JR; Corbo C; Taraballi F; Martinez JO; Hartman KA; Cosco D; Costa G; Romeo I; Sherman MB; Paolino D; Alcaro S; Tasciotti E Design and Development of Biomimetic Nanovesicles Using a Microfluidic Approach. Adv. Mater, 2018. [DOI] [PubMed] [Google Scholar]
- [90].Alvarez-Erviti L; Seow Y; Yin H; Betts C; Lakhal S; Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol, 2011, 29, 341–5. [DOI] [PubMed] [Google Scholar]
- [91].Ohno S; Takanashi M; Sudo K; Ueda S; Ishikawa A; Matsuyama N; Fujita K; Mizutani T; Ohgi T; Ochiya T; Gotoh N; Kuroda M Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther, 2013, 21, 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang S; El-Deiry WS TRAIL and apoptosis induction by TNF-family death receptors. Oncogene, 2003, 22, 8628–8633. [DOI] [PubMed] [Google Scholar]
- [93].Rivoltini L; Chiodoni C; Squarcina P; Tortoreto M; Villa A; Vergani B; Bürdek M; Botti L; Arioli I; Cova A; Mauri G; Vergani E; Bianchi B; Della Mina P; Cantone L; Bollati V; Zaffaroni N; Gianni AM; Colombo MP; Huber V TNF-Related Apoptosis-Inducing Ligand (TRAIL)-Armed Exosomes Deliver Proapoptotic Signals to Tumor Site. Clin. Cancer Res, 2016, 22, 3499–3512. [DOI] [PubMed] [Google Scholar]
- [94].Yang Y; Chen Y; Zhang F; Zhao Q; Zhong H Increased anti-tumour activity by exosomes derived from doxorubicin-treated tumour cells via heat stress. Int. J. Hyperthermia, 2015, 31, 498–506. [DOI] [PubMed] [Google Scholar]
- [95].Khalyfa A; Almendros I; Gileles-Hillel A; Akbarpour M; Trzepizur W; Mokhlesi B; Huang L; Andrade J; Farré R; Gozal D Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget, 2016, 7, 54676–54690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Skog J; Würdinger T; van Rijn S; Meijer DH; Gainche L; Sena-Esteves M; Curry WT Jr.; Carter BS; Krichevsky AM; Breakefield XO Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell. Biol, 2008, 10, 1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gu H; Ji R; Zhang X; Wang M; Zhu W; Qian H; Chen Y; Jiang P; Xu W Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol. Med. Rep, 2016, 14, 3452–8. [DOI] [PubMed] [Google Scholar]
- [98].Wang Z; Chen JQ; Liu JL; Tian L Exosomes in tumor microenvironment: novel transporters and biomarkers. J. Transl. Med, 2016, 14, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Katoh M Therapeutics targeting angiogenesis: genetics and epigenetics, extracellular miRNAs and signaling network. Int. J. Mol. Med, 2013, 32, 763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Harris DA; Patel SH; Gucek M; Hendrix A; Westbroek W; Taraska JW Exosomes Released from Breast Cancer Carcinomas Stimulate Cell Movement. PLoS One, 2015, 10, e0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kim JW; Wieckowski E; Taylor DD; Reichert TE; Watkins S; Whiteside TL Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin. Cancer Res, 2005, 11, 1010–20. [PubMed] [Google Scholar]
- [102].Taylor DD; Gercel-Taylor C Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin. Immunopathol, 2011, 33, 441–54. [DOI] [PubMed] [Google Scholar]
- [103].Koyama Y; Ito T; Hasegawa A; Eriguchi M; Inaba T; Ushigusa T; Sugiura K Exosomes derived from tumor cells genetically modified to express Mycobacterium tuberculosis antigen: a novel vaccine for cancer therapy. Biotechnol. Lett, 2016, 38, 1857–1866. [DOI] [PubMed] [Google Scholar]
- [104].Wang J; Li W; Zhang L; Ban L; Chen P; Du W; Feng X; Liu BF Chemically edited exosomes with dual ligand urified by microfluidic device for active targeted grug delivery to tumor cells. ACS Appl. Mater. Interfaces, 2017, 9, 27441–27452. [DOI] [PubMed] [Google Scholar]
- [105].Théry C; Ostrowski M; Segura E Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol, 2009, 9, 581–93. [DOI] [PubMed] [Google Scholar]
- [106].Tian Y; Li S; Song J; Ji T; Zhu M; Anderson GJ; Wei J; Nie G A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials, 2014, 35, 2383–2390. [DOI] [PubMed] [Google Scholar]
- [107].Yin H, Yang J, Zhang Q, Wang H, Xu J, Zheng J iRGD as a tumor‑penetrating peptide for cancer therapy (Review). Mol. Med. Rep, 2017, 5, 2925–2930. [DOI] [PubMed] [Google Scholar]
- [108].Dai S; Wei D; Wu Z; Zhou X; Wei X; Huang H; Li G Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther, 2008, 16, 782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Escudier B; Dorval T; Chaput N; André F; Caby MP; Novault S; Flament C; Leboulaire C; Borg C; Amigorena S; Boccaccio C; Bonnerot C; Dhellin O; Movassagh M; Piperno S; Robert C; Serra V; Valente N; Le Pecq JB; Spatz A; Lantz O; Tursz T; Angevin E; Zitvogel L Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J. Transl. Med, 2005, 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Morse MA; Garst J; Osada T; Khan S; Hobeika A; Clay TM; Valente N; Shreeniwas R; Sutton MA; Delcayre A; Hsu DH; Le Pecq JB; Lyerly HK A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med, 2005, 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zajac P; Schultz-Thater E; Tornillo L; Sadowski C; Trella E; Mengus C; Iezzi G; Spagnoli GC MAGE-A Antigens and Cancer Immunotherapy. Front. Med. (Lausanne), 2017, 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yang T; Martin P; Fogarty B; Brown A; Schurman K; Phipps R; Yin VP; Lockman P; Bai S Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res, 2015, 32, 2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yang T; Fogarty B; LaForge B; Aziz S; Pham T; Lai L; Bai S Delivery of Small Interfering RNA to Inhibit Vascular Endothelial Growth Factor in Zebrafish Using Natural Brain Endothelia Cell-Secreted Exosome Nanovesicles for the Treatment of Brain Cancer.AAPSJ, 2017, 19, 475–486. [DOI] [PubMed] [Google Scholar]
- [114].Niidome T; Huang L Gene therapy progress and prospects: nonviral vectors.Gene Ther, 2002, 9, 1647–52. [DOI] [PubMed] [Google Scholar]
- [115].Liang G; Kan S; Zhu Y; Feng S; Feng W; Gao S Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int. J. Nanomedicine, 2018, 13, 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kumar P; Wu H; McBride JL; Jung KE; Kim MH; Davidson BL; Lee SK; Shankar P; Manjunath N Transvascular delivery of small interfering RNA to the central nervous system. Nature, 2007, 448, 39–43. [DOI] [PubMed] [Google Scholar]
- [117].Liu Y; Li D; Liu Z; Zhou Y; Chu D; Li X; Jiang X; Hou D; Chen X; Chen Y; Yang Z; Jin L; Jiang W; Tian C; Zhou G; Zen K; Zhang J; Zhang Y; Li J; Zhang CY Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep, 2015, 5, 17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lunavat TR; Jang SC; Nilsson L; Park HT; Repiska G; Lässer C; Nilsson JA; Gho YS; Lötvall J RNAi delivery by exosome-mimetic nanovesicles - Implications for targeting c-Myc in cancer. Biomaterials, 2016, 102, 231–8. [DOI] [PubMed] [Google Scholar]
- [119].Wang X; Zhang H; Bai M; Ning T; Ge S; Deng T; Liu R; Zhang L; Ying G; Ba Y Exosomes Serve as Nanoparticles to Deliver Anti-miR-214 to Reverse Chemoresistance to Cisplatin in Gastric Cancer. Mol. Ther, 2018, 26, 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]