Abstract
The terminal complement complex C5b-9 plays an important role in acute ischemic stroke (AIS) and carotid atherosclerosis. However, the associations between serum C5b-9, the severity and outcome of AIS, and the stability of carotid plaques have not been well investigated. In this clinical study, 70 patients with AIS and 70 healthy controls were enrolled. Serum C5b-9 levels at 72 h after stroke onset were measured by enzyme-linked immunosorbent assay (ELISA). Infarct size, the National Institutes of Health Stroke Scale (NIHSS), the 90-day modified Rankin Scale (mRS), and carotid plaque and stenosis were evaluated. Serum C5b-9 levels were significantly higher in AIS patients than in healthy controls (p < 0.001) and were correlated with infarction sizes (p = 0.045) and the NIHSS (P = 0.035). Furthermore, 90-day mRS analysis demonstrated that the patients with poor outcomes had higher serum C5b-9 levels than those with good outcomes (P < 0.001). Moreover, serum C5b-9 levels in AIS patients with unstable carotid plaques were much higher than in those with stable carotid plaques (P = 0.009). Multivariate logistic regression indicated that C5b-9 could be an independent risk factor for AIS (P < 0.001) and unstable carotid plaques (P = 0.015). Therefore, complement complex C5b-9 may be a potential biomarker in predicting the severity and outcome, as well as the stability of carotid plaques, in AIS patients.
Keywords: Acute ischemic stroke, Terminal complement complex C5b-9, Carotid atherosclerosis, mRS
Introduction
Acute ischemic stroke (AIS) is one of the major causes of disability and death in elderly people, with approximately 2.5 million new cases per year in China alone [1]. Carotid atherosclerosis is a risk factor and accounts for approximate 15–20% of AIS [2]. Atherosclerosis is achronic inflammatory disease, and recent studies have indicated that the complement system plays an important role in the pathogenesis of atherosclerotic plaque. Therefore, it is of significance to clarify the role and mechanisms of the complement system in carotid atherosclerosis.
As an important component of the innate immune system, the complement system is crucial for clearing invasive microorganisms and is involved in a variety of chronic inflammatory diseases [3]. The complement system is activated by the classical pathway, alternative pathway, lectin pathway, and coagulation system pathway. All these pathways generate the terminal complement complex C5b-9, also known as the membrane attack complex, which consists of C5b, C6, C7, C8, and several C9 molecules, leading to the release of chemokines and growth factors as well as the mobilization of P selection [4].
Studies have shown that cerebral ischemia induces a series of inflammatory responses which in turn aggravate cerebral ischemic injury [5, 6]. The C5b-9 is associated with inflammatory responses and participates in the pathophysiology of cerebral infarction [7]. The complement system also plays an important role in the development and progression of atherosclerosis [8]. However, the role of C5b-9 in clinical severity and outcomes in AIS and carotid atherosclerosis has been under-investigated. In this controlled clinical study, we investigated whether serum C5b-9 levels are correlated with the severity and the 90-day outcomes of AIS. We also investigated whether C5b-9 levels affect the stability and number of carotid atherosclerotic plaques as well as degree of stenosis.
Methods
Patient Enrollment
Seventy AIS patients were enrolled from the neurology ward of the Affiliated Shengjing Hospital of China Medical University (Shenyang, China) from December 2015 to June 2016.
AIS was diagnosed according to the World Health Organization criteria [9]. Patients 18~80 years old who had focal neurological deficit onset within 72 h but without cerebral hemorrhage based on computed tomography were enrolled. AIS patients with the following conditions/diseases were excluded from the study: cerebral infarction caused by arteritis, hypercoagulable state, polycythemia vera, and Moyamoya disease; acute or chronic neurological diseases; severe infections; autoimmune diseases (systemic lupus erythematosus, rheumatoid, etc.); malignant tumors; severe cardiac dysfunction (acute coronary syndrome, heart failure, intractable arrhythmias, etc.); and severe liver or renal dysfunction (liver failure, renal failure, acute glomerulonephritis, etc.). Patients who had surgery or trauma during the prior 3 months or took anti-inflammatory drugs or hormones were also excluded. Healthy controls were recruited from the Medical Examination Center of Shengjing Hospital during the same period.
Clinical Evaluation
The following history/clinical data were collected: age, gender, smoking history, alcohol use, hypertension, and diabetes mellitus. Patients were subjected to systematic neurological examination on the day of admission and assessed with the National Institutes of Health Stroke Scale (NIHSS) score. A short-term outcome assessment was performed using the 90-day modified Rankin Scale (mRS), with mRS ≤ 2 defined as a good outcome and mRS > 2 as a poor outcome.
Blood samples were collected from patients at 72 h after the onset of stroke. The serum was collected by centrifugation at 3000 rpm for 10 min and stored at − 80 °C. Serum C5b-9 levels were measured using a human terminal complement complex C5b-9 enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Sunred Biological Technology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. The interassay coefficient of variation was < 9%, and the intra-assay coefficient of variation was < 11%. Laboratory tests were performed for the following parameters: C-reactive protein (CRP), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and homocysteine (Hcy).
Imaging and Ultrasound Evaluation
Diameter of the infarct and location of infarct were determined by magnetic resonance imaging (MRI). Carotid intima-media thickness, numbers of plaques, and lumen diameter were measured with carotid artery color Doppler ultrasound. For carotid atherosclerosis, carotid intima-media thickness (IMT) ≥ 1.0 mm was defined as carotid intima thickening, and IMT > 1.2 mm as carotid atherosclerotic plaque formation [10].
Statistical Analysis
Statistical analysis was performed using SPSS v17.0 software (SPSS Inc., Chicago, IL, USA). Numerical data in normal distribution was expressed as mean ± SD, the independent sample t test was used for comparisons, while data with non-normal distributions were expressed as the median (interquartile range, IQR) and the Mann–Whitney U test was used for comparisons. Count data was presented as frequencies or percentages, the χ2 test was used for pairwise inter-group comparisons, and analysis of variance (ANOVA) test was used for multiple-group comparisons. Multivariate logistic regression was performed to analyze the relationship between risk factors and AIS, as well as unstable carotid plaques. p < 0.05 was defined as statistically significant.
Results
Clinical Characteristics
Seventy AIS patients including 45 males and 25 females were recruited with a mean age of 63 ± 10 years (42–85). Seventy healthy controls including 33 males and 37 females were recruited with a mean age of 61 ± 10 years (40–87). Among 70 AIS patients, 19 of them had infarction in cerebral cortex, 32 in basal ganglia, 8 in brain stem, 7 in cerebellum, and 4 in both brain stem and cerebellum. Compared with the control subjects, AIS patients had a higher incidence of hypertension and diabetes, and higher serum Hcy and CRP levels, but lower HDL (p < 0.05). No obvious differences were found between age, gender, smoking or alcohol use history, TG, TC, and LDL-C levels (p > 0.05). Serum C5b-9 levels were significantly higher in AIS patients [817.56 (737.09–927.25) ng/mL] than in the healthy control group [588.50 (535.50–696.75) ng/mL, p < 0.001] (Table 1).
Table 1.
Baseline characteristics of AIS patients and controls
| Variables | AIS patients (n = 70) | Controls (n = 70) | p |
|---|---|---|---|
| Age, years | 63 ± 10 | 61 ± 10 | 0.316 |
| Women/men | 25/45 | 37/33 | 0.061 |
| Smoking, n (%) | 33 (47.1) | 21 (30) | 0.056 |
| Alcohol use, n (%) | 18 (25.7) | 14 (20) | 0.546 |
| Hypertension, n (%) | 46 (65.7) | 32 (45.7) | 0.027 |
| Diabetes, n (%) | 22 (31.4) | 4(5.7) | < 0.001 |
| TG (mmol/L) | 1.56 (1.03–1.90) | 1.15 (0.91–1.75) | 0.114 |
| TC (mmol/L) | 4.63 ± 1.34 | 4.95 ± 0.86 | 0.100 |
| HDL-C (mmol/L) | 1.03 (0.84–1.30) | 1.210 (1.05–1.51) | < 0.001 |
| LDL-C (mmol/L) | 2.99 (2.56–3.52) | 3.13 (2.64–3.69) | 0.437 |
| Hcy (μmol/L) | 17.34 (15.36–19.12) | 14.26 (11.66–16.40) | < 0.001 |
| CRP (mg/L) | 5.63 (3.66–8.12) | 3.61 (1.85–4.69) | < 0.001 |
| C5b-9 (ng/mL) | 817.56 (737.09–927.25) | 588.50 (535.50–696.75) | < 0.001 |
Association Between C5b-9 Levels and AIS Severity
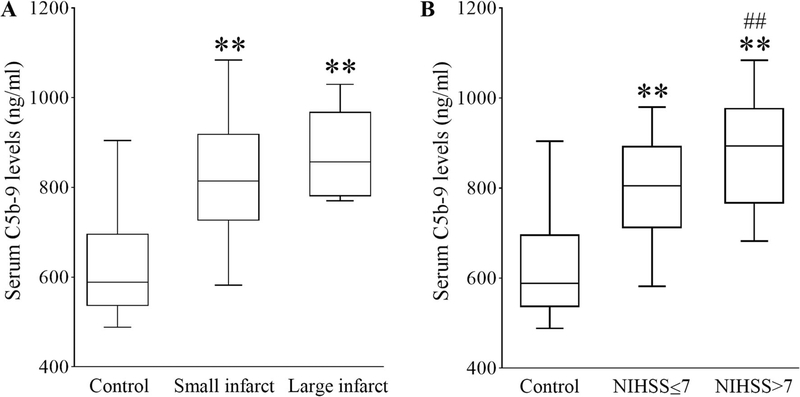
AIS patients were classified into small-infarct (≤ 4 cm) or large-infarct (> 4 cm) groups based on the longest diameter of the infarct on MRI [11]. To investigate whether C5b-9 levels were related to AIS severity, we compared the C5b-9 levels in healthy controls with stroke patients who had small and large infarcts. As shown in Fig. 1A, the range of C5b-9 in healthy controls was 588.50 (535.50–696.75) ng/mL. C5b-9 levels were markedly increased to 814.00 (725.50–919.00) ng/mL in patients with small infarct (p < 0.001) and further increased to 857.00 (780.00–968.50) ng/mL inpatients with large infarct (p < 0.001). However, there was no significant difference between patients with small and large infarct (p = 0.168).
Fig. 1.
Serum C5b-9 levels in AIS patients with different infarct sizes and NIHSS scores. The range of variation of serum C5b-9 levels in AIS patients with small and large infarct (A) or in patients with NIHSS scores ≤ 7 or > 7 (B) are displayed with box plots. IQR is denoted with an open box; maximum, minimum, and median values are denoted by top, bottom, and middle lines, respectively. **p < 0.001 versus control; ##p < 0.01 versus small infarct or NIHSS ≤ 7. n = 70 for control; n = 61 and 9 for the small and large infarct groups, respectively; n = 45 and 25 for the NIHSS ≤ 7 and NIHSS > 7 groups, respectively
We further investigated whether C5b-9 levels were related to NIHSS scores. AIS patients were divided into the NIHSS ≤ 7 group and the NIHSS > 7 group. As shown in Fig. 1B, C5b-9 levels in the NIHSS ≤ 7 group were higher than in the healthy control group [805.00 (710.50–893.50) and 588.50 (535.50–696.75) respectively, p < 0.001]. C5b-9 levels in the NIHSS > 7 group [893.00 (765.00–978.00)] were significantly higher than those of either the NIHSS ≤ 7 group or the healthy control group (p = 0.005 and p < 0.001, respectively).
Spearman analysis (Table 2) showed a positive correlation between infarct sizes and C5b-9 levels (r = 0.240 and p = 0.045). Similarly, NIHSS scores were also correlated with C5b-9 levels (r = 0.252 and p = 0.035).0.045). Similarly, NIHSS scores were also correlated with C5b-9 levels (r = 0.252 and p = 0.035).
Table 2.
The association between the levels of C5b-9 and severity of AIS
| C5b-9 | r | p |
|---|---|---|
| NIHSS | 0.252 | 0.035 |
| Infarct sizes | 0.240 | 0.045 |
Multivariate Logistic Regression Analysis of Risk Factors for AIS
As the prevalence of hypertension, diabetes, and levels of HDL-C, Hcy, CRP, and C5b-9 varied between AIS patients and controls, multivariate logistic regression analysis was further performed to exclude the effect of these factors on C5b-9 levels (Table 3). After adjusting for the effect of these risk factors, differences still existed between the two groups with respect to the prevalence of diabetes [odds ratio (OR) = 5.562, 95% CI = 1.126–27.478, p = 0.035], Hcy (OR = 1.146, 95% CI = 1.033–1.273, p = 0.010), CRP (OR = 1.177, 95% CI = 1.030–1.345, p = 0.017), and C5b-9 levels (OR = 1.011, 95% CI = 1.007–1.016, p < 0.001], indicating that C5b-9 could be an independent risk factor for AIS.
Table 3.
Multivariate logistic regression of risk factors associated with AIS
| Variables | Wald | OR | 95% CI | p |
|---|---|---|---|---|
| Hypertension | 2.209 | 2.157 | 0.783–5.941 | 0.137 |
| Diabetes | 4.432 | 5.562 | 1.126–27.478 | 0.035 |
| HDL-C | 0.059 | 0.906 | 0.409–2.007 | 0.808 |
| Hcy | 6.557 | 1.146 | 1.033–1.273 | 0.010 |
| CRP | 5.707 | 1.177 | 1.030–1.345 | 0.017 |
| C5b-9 | 27.069 | 1.011 | 1.007–1.016 | < 0.001 |
Association Between C5b-9 Levels and Clinical Outcomes
Three cases were lost and two cases died during 90-day follow-up. The patients with poor outcomes (mRS>2) had higher serum C5b-9 levels as compared to the patients with good outcomes (mRS ≤ 2) (928.00 (857.00–962.73) vs. 773.00 (681.41–810.89) ng/mL; p < 0.05). NIHSS scores were well matched with mRS: The lower the NIHSS scores, the better the 90-day mRS outcomes (p = 0.024). Age also matched with mRS outcomes: The older the age, the worse the mRS outcomes (Table 4).
Table 4.
Baseline characteristics of AIS patients with different outcomes
| Variables | mRS ≤ 2 (n = 39) | mRS> 2 (n = 26) | p |
|---|---|---|---|
| Age, years | 60.00 (54.00–64.00) | 63.00 (55–74.25) | 0.020 |
| Women/men | 24/15 | 16/10 | 0.601 |
| Smoking, n (%) | 48.7% | 46.2% | 0.613 |
| Alcohol use, n (%) | 20.5% | 30.8% | 0.389 |
| Hypertension, n (%) | 74.4% | 53.8% | 0.112 |
| Diabetes, n (%) | 30.8% | 30.8% | 0.610 |
| TG (mmol/L) | 1.56 (0.96–1.91) | 1.47 (1.15–1.75) | 0.461 |
| TC (mmol/L) | 4.63 (3.79–5.20) | 4.75 (4.03–5.43) | 0.244 |
| HDL-C (mmol/L) | 1.03 (0.83–1.33) | 1.03 (0.85–1.18) | 0.794 |
| LDL-C (mmol/L) | 3.02 (2.58–3.59) | 3.01 (2.49–3.56) | 0.255 |
| Hcy (μmol/L) | 17.36 (15.30–19.00) | 16.87 (13.74–19.92) | 0.784 |
| CRP (mg/L) | 5.46 (3.52–8.37) | 4.57 (3.70–8.04) | 0.644 |
| C5b-9 (ng/mL) | 773.00 (681.41–810.89) | 928.00 (857.00–962.73) | 0.000 |
| NIHSS | 4.00 (2.00–7.00) | 7.50 (4.00–12.50) | 0.024 |
After adjustment for potential risk factors, a difference in C5b-9 levels between patients with poor and good outcomes still existed (OR = 1.017, 95% CI = 1.008–1.025, p < 0.001). Differences also existed in age between poor and good outcomes (OR = 1.098, 95% CI = 1.026–1.177, p = 0.007) (Table 5).
Table 5.
Multivariate logistic regression of risk factors associated with mRS
| Variables | Wald | OR | 95% CI | p |
|---|---|---|---|---|
| Age, years | 7.171 | 1.098 | 1.026–1.177 | 0.007 |
| NIHSS | 0.702 | 1.056 | 0.926–1.211 | 0.402 |
| C5b-9 | 14.528 | 1.017 | 1.008–1.025 | < 0.001 |
Association between C5b-9 Levels and Carotid Plaque Stability in AIS
AIS patients were divided into two subgroups, unstable plaque and non-unstable plaque, based on plaque texture [12]. To confirm whether complement is correlated to the formation of unstable plaques, which are a high-risk factor for ischemic stroke, we further compared the clinical manifestations, laboratory parameters, and serum C5b-9 levels between the unstable plaque group and the non-unstable plaque group. The unstable plaque group had a higher incidence of hypertension and higher levels of TG and LDL-C as compared with the non-unstable plaque group (p < 0.05), but there were no differences in the rates of diabetes and CRP levels between the two groups. Serum C5b-9 levels in the unstable plaque group were significantly higher than those in the non-unstable plaque group [875.00 (772.50–962.50) ng/mL and 786.00 (690.50–879.50) ng/mL, respectively, p < 0.05]. We investigated the relationship between carotid plaque stability and the severity of AIS (Table 6). Interestingly, we found that the unstable plaque subgroup had higher NIHSS scores than the non-unstable plaque group (p = 0.034). We further performed multivariate logistic regression analysis of risk factors for the formation of unstable plaques including hypertension, TG, LDL-C, and serum C5b-9 levels. Higher C5b-9 (OR = 1.006, 95% CI = 1.001–1.011, p = 0.015) and LDL-C levels (OR = 2.320, 95% CI = 1.051–5.040, p = 0.037) were correlated with the formation of unstable plaques (Table 7). However, there were no significant differences in TG levels between the two groups (p = 0.625).
Table 6.
Baseline characteristics of AIS patients with non-unstable plaques and unstable plaques
| Variables | Non-unstable plaque | Unstable plaque | p |
|---|---|---|---|
| Age, years | 63 ± 10 | 63 ± 10 | 0.811 |
| Male sex (W/M) | 11/22 | 14/23 | 0.804 |
| Smoking, n (%) | 15/18 | 18/19 | 0.815 |
| Alcohol use, n (%) | 6 (18.2) | 12 (32.4) | 0.273 |
| Hypertension, n (%) | 17 (51.5) | 29 (78.4) | 0.024 |
| Diabetes, n (%) | 10 (30.3) | 12 (32.4) | 1.000 |
| TG (mmol/L) | 1.17 (0.83–1.69) | 1.73 (1.30–2.155) | 0.001 |
| TC (mmol/L) | 4.74 ± 1.21 | 4.42 ± 1.57 | 0.345 |
| HDL-C (mmol/L) | 1.05 (0.86–1.19) | 1.02 (0.82–1.33) | 0.925 |
| LDL-C (mmol/L) | 2.84 (1.86–3.44) | 3.17 (2.83–3.70) | 0.011 |
| Hcy (μmol/L) | 17.34 (15.62–19.41) | 17.38 (14.83–19.25) | 0.621 |
| CRP (mg/L) | 5.08 (3.70–7.85) | 5.85 (3.86–8.55) | 0.791 |
| C5b-9 (ng/mL) | 786.00 (690.5–879.5) | 875.00 (772.50–962.50) | 0.009 |
| NIHSS | 4.00 (2.00–7.50) | 6.00 (3.00–14.00) | 0.034 |
Table 7.
Multivariate logistic regression of risk factors associated with unstable plaques
| Variables | Wald | OR | 95% CI | p |
|---|---|---|---|---|
| Hypertension | 3.973 | 3.711 | 1.022–13.472 | 0.046 |
| TG | 0.240 | 1.163 | 0.636–2.127 | 0.625 |
| LDL-C | 4.345 | 2.302 | 1.051–5.040 | 0.037 |
| C5b-9 | 5.974 | 1.006 | 1.001–1.011 | 0.015 |
Correlation Between C5b-9 Levels and Numbers of Carotid Plaques
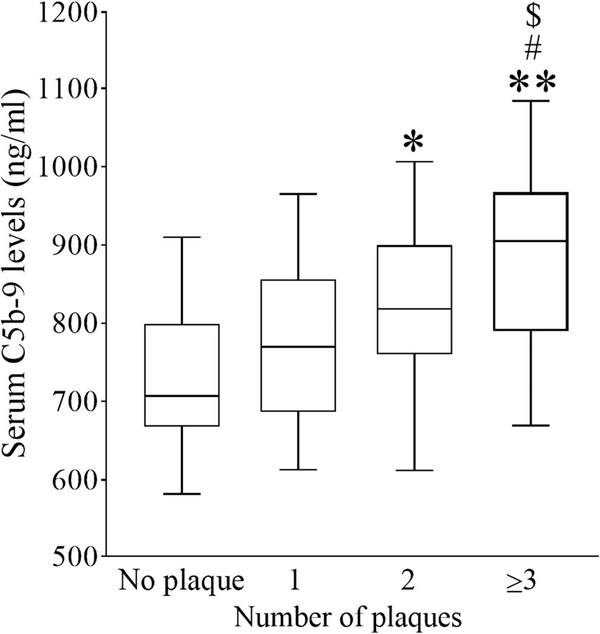
According to the number of carotid plaques detected by carotid ultrasound, patients were further divided into 0, 1, 2, and ≥ 3 subgroups, respectively. As shown in Fig. 2, the ANOVA test showed that C5b-9 levels in patients with ≥ 3 plaques [905.00 (790.00–967.00) ng/mL] were much higher than that in patients with 0, 1, and 2 plaques [707.00 (667.50–799.50), 770.00 (686.50–856.00), and 818.00 (760.50–899.50) ng/mL, respectively; p < 0.01, p < 0.05, p < 0.05, respectively]. C5b-9 levels in patients with two plaques were also significantly higher than those in patients without plaque (p < 0.05). C5b-9 levels in patients with one plaque were increased, but not significantly.
Fig. 2.
Serum C5b-9 levels in AIS patients with different numbers of carotid plaques. Serum C5b-9 levels in AIS patients with carotid plaque numbers 1, 2, or ≥ 3 are displayed with box plots. *p < 0.05 versus plaque = 0; **p < 0.01 versus plaque = 0; #p < 0.05 versus plaque = 1; $p<0.05 versus plaque = 2; n = 9, 13, 18, and 30 for plaque = 0, 1, 2, and 3, respectively
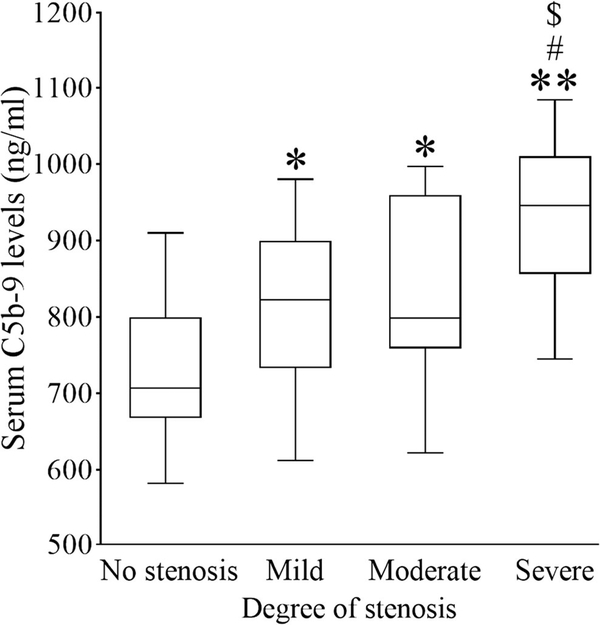
Correlation Between the Degree of Carotid Stenosis and C5b-9 Levels
AIS patients were also divided into no, mild (carotid stenosis < 50%), moderate (carotid stenosis 50–69%), and severe stenosis (carotid stenosis ≥ 70%) based on the degree of carotid stenosis [10]. As shown in Fig. 3, C5b-9 levels in the severe stenosis group [946.00 (856.00–1010.00) ng/mL] were much higher than those in the no-stenosis, mild stenosis, and moderate stenosis group [707.00 (667.50–799.50), 822.5 (732.75–899.50), and 798.50 (759.00–959.75) ng/mL, respectively; p < 0.01, p < 0.05, p < 0.05, respectively]. C5b-9 levels were significantly higher in the moderate stenosis group than those in the no-stenosis group (p < 0.05).Differences also existed between the mild stenosis and no-stenosis groups (p < 0.05). However, there were no differences between the mild stenosis and moderate stenosis groups (p > 0.05).
Fig. 3.
Serum C5b-9 levels in AIS patients with different degrees of carotid stenosis. Serum C5b-9 levels in AIS patients with no, mild, moderate, and severe stenosis are displayed with box plots. *p < 0.05 versus no stenosis; **p < 0.01 versus no stenosis; #p < 0.05 versus mild stenosis; $p < 0.05 versus moderate stenosis; n = 9, 38, 12, and 11 for no, mild, moderate, and severe stenosis, respectively
Discussion
In this study, we measured serum C5b-9 levels in AIS patients and demonstrated that serum C5b-9 levels were significantly increased following ischemic stroke and that its levels were correlated with infarct sizes, NIHSS scores, and poor outcomes. We further found that serum C5b-9 levels were significantly increased in AIS patients with unstable carotid plaques as compared to those with non-unstable carotid plaques. These data suggest that C5b-9 is an independent risk factor for AIS and unstable carotid plaques, and could be used as a biomarker in predicting the severity and outcome, as well as the stability of carotid plaque in AIS patients.
Serum C5b-9 levels change dynamically after acute cerebral infarction, and there is a controversy about their peaking timepoint. In our preliminary studies, we measured C5b-9 levels of five patients at 24 h, 72 h, and 7 days after onset and found that C5b-9 levels peaked at 72 h. This peaking timepoint is consistent with Pedersen’s report [13] showing that serum C5b-9 levels peaked at 72 h and remained high until day 12. Therefore, we chose to collect blood samples at 72 h after onset in subsequent experiments. Mocco et al. [14] also monitored the serum C5b-9 levels in AIS patients and found that C5b-9 levels decreased at days 1 and 2 but returned to normal levels after day 3 following stroke. Interestingly, Mocco’s report showed that C3a, a central complement component initiating the C5b-9 cascade, increased significantly at days 1–3. It is unclear why C3a increased significantly, while its downstream product C5b-9 decreased at days 1–3 after stroke. Race, the degree of stroke severity, and fewer numbers of cases might account for the discrepancy.
Our study showed that serum C5b-9 levels are closely related to severity and prognosis after stroke. C5b-9 levels at 72 h after stroke were significantly increased in patients with small infarct or NIHSS scores ≤ 7, and further increased in patients with large infarct or NIHSS > 7. Széplaki et al. [15] recruited 26 AIS patients and found that C5b-9 had a positive, significant association with early outcomes on day 6 after admission. We extended the follow-up time to 90 days and performed a 90-day mRS assessment. Our results indicated that C5b-9 levels are associated with 90 days of mRS, and patients with higher C5b-9 levels tended to have unfavorable outcomes. Together, our study indicated that C5b-9 may be used as a biomarker to predict the severity and outcome of AIS.
It is well known that carotid atherosclerosis is a risk factor for AIS. We found that the AIS patients with unstable plaques had more serious neurological deficits than those with non-unstable plaques. However, whether C5b-9 level is correlated with carotid plaque stability remains unclear. We found that serum C5b-9 levels were significantly increased in patients with unstable plaques as compared to patients with stable plaques. There was a positive correlation between C5b-9 levels and the numbers of carotid plaques and the degree of stenosis, two critical factors contributing to carotid damage. After excluding the effects of hypertension, increased triglyceride, and LDL-C levels, C5b-9 was still an independent risk factor for unstable plaques. In line with this, Oksjoki et al. [16] found that C5b-9 was deposited in the arterial intima breakage and was positively correlated with the severity of atherosclerosis. Lewis et al. [17] also confirmed the importance of C5b-9 in carotid atherosclerosis in a study involving Apo E−/− knockout mice. C5b-9 has been shown to encourage the infiltration of both smooth muscle and endothelial cells into the injury site of adventitia and to promote the release of growth factors, cytokines, and tumor necrosis factor [18]. C5b-9 has also been associated with carotid intimal cholesterol deposition [19].
The mechanism underlying how C5b-9 causes ischemic brain damage remains unknown. Tegla has proposed that C5b-9 could bind to nerves and vascular endothelial cells, forming hydrophilic channels which change the intra- and extra-cellular osmotic pressure and then cause cell lysis [20]. C5b-9 could also induce Ca2+ intracellular flow or promoted P selectin-mediated release of cytokines, leading to vascular leakage and inflammatory cell infiltration in the brain [4]. Studies also showed that complement-related inflammation and peripheral inflammatory cells infiltrating into the brain peaked at 72 h [21, 22]. Taken together, these findings suggest that C5b-9 either directly kills brain cells or indirectly induces the infiltration of inflammatory cells via increasing endothelial leakage. Therefore, adjusting/inhibiting complement activation may provide a new perspective on the treatment of AIS. In line with this, Alawieh et al. [23] found that inhibiting the complement activation reduced the infarct size in a mouse model of transient middle cerebral arterial occlusion. Application of a C5a inhibitor also reduced ischemic brain injury [24].
One limitation of the current study is that we did not measure the dynamic changes of C5b-9 in all 70 AIS patients; therefore, it is not clear whether C5b-9 levels could also be used as a biomarker at recovery phases of ischemic stroke. In addition, since only patients with AIS were enrolled and there were no patients with carotid plaques but without AIS in the current study, we cannot exclude the possibility that C5b-9 increase is due to ischemia alone or a synergistic effect of ischemia with carotid plaques. Future recruitments of patients with carotid plaques but without AIS will clarify this issue. Nevertheless, our data indicate that at least at the acute phase of stroke, C5b-9 could be used as a biomarker of the severity and outcomes of AIS and the stability of carotid plaques.
In conclusion, our findings suggest that C5b-9 levels could be a biomarker for the severity and outcomes of AIS. Moreover, C5b-9 is correlated with carotid atherosclerosis and plaque stability. Thus, therapy targeting C5b-9 in acute stages of AIS could alleviate the severity of AIS and improve the clinical outcomes.
Acknowledgements
We thank Carol Culver for editorial assistance.
Funding Information This project was supported by Liaoning Natural Science Fund 2012225069 (to H. Z.), Shenyang Science and Technology Project 18–014-4–82 (to H. Z.), National Institutes of Health/NINDS grant NS079345 (to G. C.), and Department of Veterans Affairs Merit Review grants BX002346 and BX003923 (to G. C.).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
The study was approved by the Ethics Committee of China Medical University.
References
- 1.Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42(12):3651–4. 10.1161/STROKEAHA.111.635755. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart diease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):29–322. 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344(15):1140–4. 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 4.Bossi F, Fischetti F, Pellis V, Bulla R, Ferrero E, Mollnes TE, et al. Platelet-activating factor and kinin-dependent vascular leakage as a novel functional activity of the soluble terminal complement complex. J Immunol. 2004;173(11):6921–7. 10.4049/jimmunol.173.11.6921. [DOI] [PubMed] [Google Scholar]
- 5.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62(2):127–36. 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 6.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orsini F, De Blasio D, Zangari R, Zanier ER, De Simoni MG. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front Cell Neurosci. 2014;8: 380 10.3389/fncel.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertle E, van Greevenbroek MM, Arts IC, van der Kallen CJ, Feskens EJ, Schalkwijk CG, et al. Complement activation products C5a and sC5b-9 are associated with low-grade inflammation and endothelial dysfunction, but not with atherosclerosis in a cross-sectional analysis: the CODAM study. Int J Cardiol. 2014;174(2): 400–3. 10.1016/j.ijcard.2014.04.0579. [DOI] [PubMed] [Google Scholar]
- 9.Hatano S Experience from a multicenter stroke register: a preliminary report. Bull World Health Organ. 1976;54(5):541–53. [PMC free article] [PubMed] [Google Scholar]
- 10.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: grayscale and Doppler ultrasound diagnosis—Society of Radiologists in ultrasound consensus conference. Ultrasound Q. 2003;19(4):190–8. 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 11.Xiao D, Liu H, Zhang H, Luo Y. Impact of cystatin C levels on infarct size and hemorrhage volume in acute cerebral stroke. J Neurol. 2012;259(10):2053–9. 10.1007/s00415-012-6453-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhao D, Bi G, Feng J, Huang R, Chen X. Association of serum chemerin levels with acute ischemic stroke and carotid artery atherosclerosis in a Chinese population. Med Sci Monit. 2015;16(21): 3121–8. 10.12659/MSM.895866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen ED, Waje-Andreassen U, Vedeler CA, Aamodt G, Mollnes TE. Systemic complement activation following human acute ischaemic stroke. Clin Exp Immunol. 2004;137(1):117–22. 10.1111/j.1365-2249.2004.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mocco J, Wilson DA, Komotar RJ, Sughrue ME, Coates K, Sacco RL, et al. Alterations in plasma complement levels after human ischemic stroke. Neurosurgery. 2006;59(1):28–33. 10.1227/01.NEU.0000219221.14280.65. [DOI] [PubMed] [Google Scholar]
- 15.Széplaki G, Szegedi R, Hirschberg K, Gombos T, Varga L, Karádi I, et al. Strong complement activation after acute ischemic stroke is associated with unfavorable outcomes. Atherosclerosis. 2009;204(1):315–20. [DOI] [PubMed] [Google Scholar]
- 16.Oksjoki R, Kovanen PT, Mäyränpää MI, Laine P, Blom AM, Meri S, et al. Complement regulation in human atherosclerotic coronary lesions. Immunohistochemical evidence that C4b-binding protein negatively regulates the classical complement pathway, and that C5b-9 is formed via the alternative complement pathway. Atherosclerosis. 2007;192(1):40–8. 10.1016/j.atherosclerosis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Lewis RD, Jackson CL, Morgan BP, Hughes TR. The membrane attack complex of complement drives the progression of atherosclerosis in apolipoprotein E knockout mice. Mol Immunol. 2010;47(5):1098–105. 10.1016/j.molimm.2009.10.03519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J Thromb Haemost. 2011;9(3):428–40. 10.1111/j.1538-7836.2010.04172.x. [DOI] [PubMed] [Google Scholar]
- 19.Seifert PS, Hugo F, Hansson GK, Bhakdi S. Prelesional complement activation in experimental atherosclerosis. Terminal C5b-9 complement deposition coincides with cholesterol accumulation in the aortic intima of hypercholesterolemic rabbits. Lab Investig. 1989;60(6):747–54. [PubMed] [Google Scholar]
- 20.Tegla CA, Cudrici C, Patel S, Trippe R 3rd, Rus V, Niculescu F, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol Res. 2011;51(1):45–60. 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–57. 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 22.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–89. 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alawieh A, Elvington A, Zhu H, Yu J, Kindy MS, Atkinson C, et al. Modulation of post-stroke degenerative and regenerative processes and subacute protection by site-targeted inhibition of the alternative pathway of complement. J Neuroinflammation. 2015;12:247 10.1186/s12974-015-0464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuboly E, Futakuchi M, Varga G, Érces D, Tőkés T, Mészáros A, et al. C5a inhibitor protects against ischemia/reperfusion injury in rat small intestine. Microbiol Immunol. 2016;60(1):35–46. 10.1111/1348-0421.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]