Abstract
Background:
Cockroach allergens are an important cause of IgE-mediated sensitization in inner-city asthmatics. However, cockroach extracts used for diagnosis and immunotherapy are not standardized.
Objective:
To determine the allergen content of non-standardized German cockroach extracts and the levels of sensitization to an expanded set of cockroach allergens as determinants of in vitro extract potency for IgE reactivity.
Methods:
Twelve German cockroach extracts were compared for allergen content and for potency of IgE reactivity. Bla g 1, Bla g 2 and Bla g 5 were measured by immunoassays. IgE antibody levels to eight purified recombinant allergens from groups 1, 2, 4, 5, 6, 7, 9 and 11 were measured by ImmunoCAP. IgE antibody binding inhibition assays were performed to assess extract in vitro potencies (IC30) relative to an arbitrarily selected reference extract in five cockroach allergic subjects.
Results:
Allergen levels were highly variable. Three new major allergens (groups 6, 9 and 11), were identified among highly cockroach-sensitized subjects (CAP-class ≥ 3). Sensitization profiles were unique per subject, without immunodominant allergens. The sum of IgE to eight allergen components showed a good correlation with cockroach-specific IgE (r = 0.88; p < 0.001). In vitro potencies varied among different extracts per subject, and among subjects for each extract.
Conclusions:
The in vitro potency of German cockroach extracts for IgE reactivity depends on allergen content and allergen-specific IgE titers of the cockroach-allergic subject. These factors are relevant for selection of potent extracts to be used for immunotherapy and for the design and interpretation of data from immunotherapy trials.
Keywords: Cockroach allergy, non-standardized extracts, cockroach allergen components, immunotherapy, diagnosis
Capsule Summary:
Allergen content in non-standardized cockroach extracts and subjects’ allergen-specific sensitization profiles vary and define the in vitro extract potency for IgE reactivity. Both variables should be considered regarding extract selection for diagnosis and immunotherapy.
Introduction
Cockroach allergy is an important health problem in the U.S., especially in inner-cities, and is associated with chronic exposure and IgE sensitization to multiple allergens, which often results in the development of asthma.(1) Cockroach extracts for immunotherapy are currently not standardized. The doses of extract used for cockroach immunotherapy by the Inner-City Asthma Consortium (ICAC) were calculated based on content of the cockroach allergens Bla g 1 and Bla g 2.(2) The maintenance dose in a trial for cockroach subcutaneous immunotherapy was established as 120 μg of Bla g 1 and 6 μg of Bla g 2, based on the relevance of these two allergens.(2) Bla g 2 is one of the most important major allergens from cockroach, with a prevalence of sensitization from 54 to 72%.(3;4) Although the IgE prevalence for Bla g 1 (26–40%) was lower than that for Bla g 2, both Bla g 1 and Bla g 2 have consistently been used as markers of environmental exposure to cockroach.(4;5) However, these two allergens do not account for all the IgE reactivity against cockroach extracts.(4) Until recently, five cockroach allergens were known: Bla g 1, a gut microvilli-associated protein; Bla g 2, a gut inactive aspartic protease; Bla g 4, a lipocalin produced only in male cockroaches and excreted in the spermatophore during copulation; Bla g 5, a glutathione S-transferase and Bla g 7, a tropomyosin.(3;6–14) Satinover et al. showed that none of these five cockroach allergens was immunodominant in a U.S. population and, cumulatively, they did not account for all the IgE reactivity against cockroach extracts.(4)
The current study extends the analysis of IgE reactivity to three additional cockroach allergens (from groups 6, 9 and 11). Bla g 6 is a troponin C involved in muscle contraction.(15) An arginine kinase was identified by proteomic approaches with a 34% IgE prevalence in a Taiwanese population.(16) However, this allergen, a putative Bla g 9, had not been listed as an allergen in the World Health Organization and International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature database (www.allergen.org). α-Amylases from both B. germanica and P. americana were recently described in Korea and China as group 11, with an IgE prevalence of 41% and 83%, respectively.(17;18)
This study addresses the variability in content among German cockroach extracts prepared from different sources and using different protocols. This variability poses a challenge in terms of extract standardization, which is the difficulty of producing batches of extracts with consistent relative amounts of allergens for preparation of consistent doses for clinical use.
The main goal of the study is to compare the in vitro potencies of a group of cockroach extracts for IgE reactivity in individual cockroach allergic subjects. To achieve this goal, twelve German cockroach extracts were compared for allergen content and for potency of IgE reactivity. The extract in vitro potency for IgE reactivity was investigated by IgE antibody inhibition assays in five individual cockroach allergic subjects. Overall, this study analyzes the importance of two factors, extract content and IgE sensitization profiles of cockroach allergic subjects, on the extract in vitro potencies and implications of the results for the design and interpretation of the outcomes of cockroach immunotherapy.
Methods
Study population
A cohort of twenty-three individuals sensitized to cockroach (IgE titers > 0.35 kUA/L) were recruited from San Diego, CA, St. Louis, MO and New York, NY, according to Institutional Review Board approval (IRB protocols: VD-112–0217, 201305110 and GCO 13–0691). All had a history of allergy symptoms to cockroach, and most had asthma and/or rhinitis. All subjects enrolled in this study provided written consent. Donor information is summarized in Table E1 in the Online Repository. IgE antibody titers were determined from plasma using the ImmunoCAP system (Thermo Fisher Scientific, Uppsala, Sweden). Seventy percent of subjects were female, mean age was 39 ± 10 and cockroach-specific IgE titers were on average 16.5 ± 22.8 kUA/L (range 0.9–76.2 kUA/L).
Cockroach extracts
Twelve German cockroach extracts were acquired or prepared in-house for this study (Table I). Nine commercial extracts were purchased from Greer (Lenoir, NC). Batches from Greer were made from whole cockroach bodies and included four extracts for clinical use in humans (#1–4), two extracts for veterinarian use (#5–6) and three extracts for research use (#7–9). All extracts were formulated in 50% glycerin, except for extracts for research use which were aqueous. Extracts #1 and #7–9 used defatted cockroaches. In addition, aqueous German cockroach extracts were made in-house by different research labs. Extract #10 was made from cockroach fecal matter at Yonsei University (Seoul, South Korea), and extract #11 was made at the La Jolla Institute (La Jolla, CA, USA) from fecal matter collected at North Carolina State University (Raleigh, NC, USA). The protocol for the preparation of these two fecal extracts is described elsewhere.(17) Extract #12 was made from cockroach frass at Indoor Biotechnologies, Inc. (Charlottesville, VA, USA), as previously described with few modifications (19). The extract was prepared by stirring German cockroach frass (cockroach debris containing body parts, fecal material and egg cases) for 24–48 hours at 4°C in Phosphate Buffered Saline, pH 7.4 (PBS) (0.19 g frass/mL) and was not ether extracted.
Table I.
Content of cockroach allergen extracts
| German cockroach extract |
Bla g 1 (μg/mL) |
Bla g 2 (μg/mL) |
Bla g 5 (μg/mL) |
Protein concentration APA (mg/mL) |
Bla g 1/Prot conc (μg/mg) |
Bla g 2/Prot conc (μg/mg) |
Bla g 5/Prot conc (μg/mg) |
Three allergens/Prot conc (μg/mg) |
Endotoxin LAL (EU/mL) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Commercial - WB - Hum | 41.81 | 30.30 | 0.130 | 7.00 | 5.97 | 4.33 | 0.02 | 10.32 | 1617 |
| 2 | Commercial - WB - Hum | 10.32 | 2.86 | 0.020 | 3.14 | 3.28 | 0.91 | 0.01 | 4.20 | 10892 |
| 3 | Commercial - WB - Hum | 7.59 | 3.13 | 0.081 | 2.22 | 3.42 | 1.41 | 0.04 | 4.87 | 3175 |
| 4 | Commercial - WB - Hum | 8.06 | 3.13 | 0.023 | 2.46 | 3.28 | 1.27 | 0.01 | 4.56 | 13418 |
| 5 | Commercial - WB - Vet | 21.44 | 0.10 | <0.005 | 1.75 | 12.27 | 0.06 | 0.00 | 12.33 | 467 |
| 6 | Commercial - WB - Vet | 9.01 | 4.88 | 0.244 | 2.96 | 3.04 | 1.65 | 0.08 | 4.77 | 3069 |
| 7 | Commercial - WB - Res | 13.68 | 17.52 | 0.178 | 3.04 | 4.50 | 5.76 | 0.06 | 10.32 | 1889 |
| 8 | Commercial - WB - Res | 22.79 | 12.68 | 0.030 | 4.35 | 5.24 | 2.91 | 0.01 | 8.16 | 1546 |
| 9* | Commercial - WB - Res | 44.60 | 19.01 | 0.248 | 6.22 | 7.17 | 3.06 | 0.04 | 10.27 | 13782 |
| 10 | In-house - Fecal - Res | 127.82 | 72.80 | <0.005 | 7.71 | 16.58 | 9.44 | 0.00 | 26.02 | 342622 |
| 11 | In-house - Fecal - Res | 150.13 | 26.70 | <0.005 | 6.03 | 24.90 | 4.43 | 0.00 | 29.33 | 152713 |
| 12 | In-house - Frass - Res | 49.75 | 22.96 | <0.005 | 1.35 | 36.85 | 17.01 | 0.00 | 53.86 | 39934 |
| 13** | Negative control | <0.008 | <0.01 | <0.005 | 5.39 | 0.00 | 0.00 | 0.00 | 0.00 | 1412 |
| Average*** | 42.25 | 18.01 | 0.08 | 4.02 | 10.54 | 4.35 | 0.02 | 14.92 | 48760 | |
| Stdev*** | 47.81 | 20.11 | 0.10 | 2.18 | 10.65 | 4.74 | 0.03 | 14.74 | 101932 | |
| 20x | 728x | 12x | 6x | 733x | ||||||
| Averages: | ||||||||||
| WB | 19.92 | 10.40 | 0.11 | 3.68 | ||||||
| Hum | 16.95 | 9.86 | 0.06 | 3.71 | ||||||
| Vet | 15.23 | 2.49 | 0.12 | 2.36 | ||||||
| Res | 27.02 | 16.40 | 0.15 | 4.54 | ||||||
| Fec-Frass | 109.23 | 40.82 | 0.01 | 5.03 |
The lowest and highest values of allergen and endotoxin concentrations are underlined, and the fold difference between them is indicated in the bottom row.
Reference extract
Negative control (allergen concentrations were lower than the indicated lower limit of detection).
Negative control was excluded from the calculation of average and standard deviation (Stdev).
Extract #13 is a negative control made from food chow for cockroaches. Protein in the extracts was measured by the Advanced Protein Assay (APA) (Cytoskeleton Inc., Denver, Co). The extracts were diluted 1:5 before performing the APA to reduce the effect of glycerin on protein determination.
Expression, purification and quantification of eight recombinant cockroach allergens
German cockroach allergens Bla g 1, Bla g 2, Bla g 4, Bla g 6, Bla g 9 and Bla g 11, and American cockroach Per a 7 were expressed in Pichia pastoris, and Bla g 5 was expressed in Escherichia coli. All the allergens were expressed and purified as described in the Online Repository.
Measurement o f Bla g 1, Bla g 2, Bla g 5 and endotoxin in cockroach extracts
Bla g 1, Bla g 2 and Bla g 5 were measured by ELISA. Endotoxin levels were measured by the chromogenic Limulus Amoebocyte Lysate (LAL) assay (Lonza, Walkersville, MD). Methods are described in the Online Repository.
Measurement of IgE antibody levels by ImmunoCAP
Cockroach-specific IgE antibody binding was measured using commercially available i6 ImmunoCAPs. Allergen-specific IgE antibody levels were measured using streptavidin-CAPs optimally loaded with biotinylated purified recombinant cockroach allergens, as described in the Online Repository. Measurements of IgE antibody binding were performed in a Thermo Fisher Scientific ImmunoCAP system (Phadia™ 250 Immunoassay Analyzer) according to manufacturer’s instructions.
IgE antibody binding inhibition assays
In vitro inhibition assays were performed to compare the capacity of each extract to inhibit binding of IgE antibodies from individual subjects to an extract chosen as a reference. Commercial extract #9 was selected as reference because it contained the highest concentrations of Bla g 1 and Bla g 2 among the commercial extracts (44.60 and 19.01 μg/mL, respectively). Extract #9 also contained a relatively high amount of Bla g 1, Bla g 2 and Bla g 5 per mg of protein (10.27 μg/mg) (Table I). The window of IgE antibody binding inhibition was determined a priori with the reference extract only. Assays were performed to compare all the extracts for each subject at one time. Five subjects were selected for the inhibition assays because in a prescreening of n = 12 plasma, they showed the largest windows of IgE antibody binding inhibition at 1:4 or 1:2 dilutions compared to the other subjects. Most had high IgE titers (see Table E1 in the Online Repository; average 45.68 kUA/L, range 4.82–76.20 kUA/L).
An individual assay per each of the five subjects was performed to compare all the extracts. Microplates were coated with extract #9 at 10 μg/mL and incubated at 4°C overnight. After the plates were washed and blocked with PBS-0.05% Tween 20-1% BSA, each extract was pre-incubated with the plasma in a different mixing polypropylene plate. Extracts were prepared in micro tubes at a predetermined optimal concentration and 80 μL were added to the first wells of the mixing plate and diluted 1:4 in the consecutive wells. Plasma was then added to each well to a final dilution of 1:2–1:5, mixed and incubated for 1 hour. One hundred μL from each well of the mixing plate were then transferred to the corresponding well of the ELISA plate and incubated for 3 hours. Affinity purified peroxidase labeled goat anti-human IgE antibody (KPL, Gaithersburg, MD) was added to the plate at a dilution of 1:1,000 and incubated for 1 hour. The plate was washed and developed using ABTS in 70 mM citrate phosphate buffer, pH 4.2 and 1/1000 dilution of H2O2. Absorbance was read at 405 nm on a Bio-Tek EL800 Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT) when top standard concentration reached approximately O.D. 2.0. Extract potencies were expressed as the concentration (IC30) inhibiting 30% of the total IgE antibody binding inhibition when the same reference extract was used as inhibitor. IC30 values were normalized versus the reference extract values which had an IC30 = 1. An IC30 of 1,000 was assigned to extracts that did not reach 30% of inhibition.
Results
Content of cockroach allergens
Allergen and endotoxin levels were highly variable in the cockroach extracts analyzed (Table I, Figure 1).
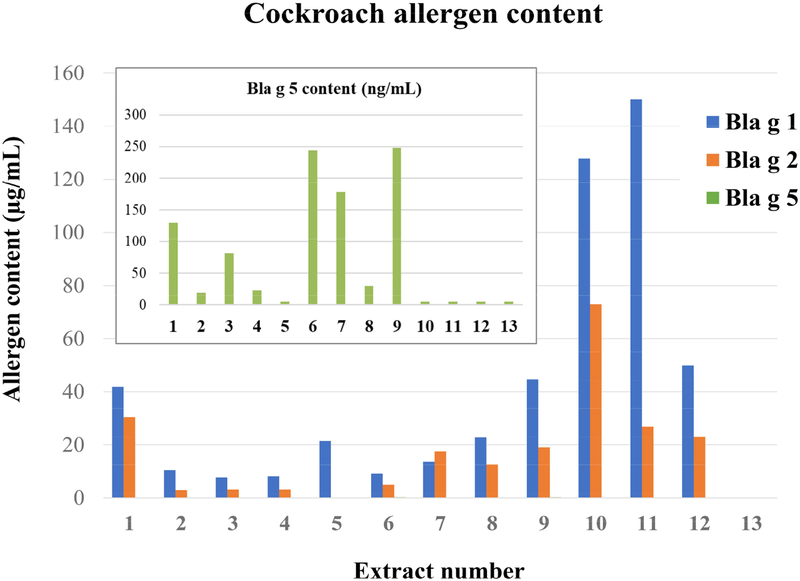
Figure 1.
Allergen levels in cockroach extracts. The concentration of Bla g 1, Bla g 2 and Bla g 5 in the extracts was measured by immunoassays (extract #13 is a negative control). The inset shows the Bla g 5 concentrations in ng/mL.
The average amount of Bla g 1 and Bla g 2 was 5.5-fold and 3.9-fold larger in fecal and frass extracts (#10–12) than in whole body extracts (#1–9) (109.2 ± 52.7 μg/mL versus 19.9 ± 14.3 μg/mL for Bla g 1, and 40.8 ± 27.8 μg/mL versus 10.4 ± 10.2 μg/mL for Bla g 2) (Table I). However, Bla g 5 was 21.3-fold larger in whole body extracts (0.11 ±0. 1 μg/mL versus 0.01 ± 0.0 μg/mL). The variability in Bla g 1, Bla g 2 and Bla g 5 was 5.5, 10.6 and 6.5-fold in commercial extracts for human use versus 2.4, 48.8 and 48.8-fold in extracts for veterinary use, respectively. On average, Bla g 1, Bla g 2 and Bla g 5 content was 70%, 30% and 0.3%, respectively, of the sum of the amount of the three allergens in the extracts. In extracts for human use, Bla g 1 content was on average 2.5-fold larger than Bla g 2, and Bla g 2 was on average 138-fold larger than Bla g 5. The in-house extracts contained the largest amount of allergen per mg of protein (26.0, 29.3 and 53.9 μg/mg for extracts #10, #11 and #12, respectively). All extracts had low amounts of Bla g 5.
IgE recognition of eight cockroach allergens
The pattern of IgE recognition of eight cockroach allergens was variable (Figure 2). The eight allergens included: a) the “traditional” cockroach allergens Bla g 1, Bla g 2, Bla g 4, Bla g 5 and Per a 7 analyzed in Satinover et al.(4), and b) three additional allergens: Bla g 6, an arginine kinase homologous to Per a 9 and Bla g 11. Based on the data presented here, the arginine kinase was proven to be an allergen and was submitted to the World Health Organization and International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature SubCommittee, which approved the assignment of the name Bla g 9 to this new allergen.
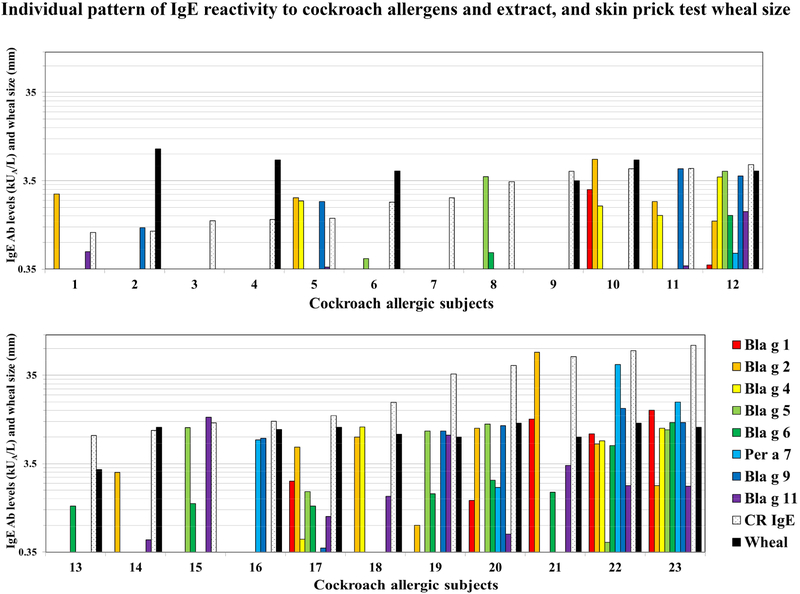
Figure 2.
Allergen-specific IgE patterns of sensitization. Patterns of IgE sensitization to 8 purified cockroach allergens in a population of 23 cockroach allergic subjects. The last two columns are cockroach-specific IgE antibody levels and skin prick test wheal size (skin prick test was not performed for patients 3, 7, 8, 11 and 15). Subjects are ordered by lowest to highest cockroach-specific IgE levels.
Different allergens were dominant for different donors. The tendency was for subjects with higher cockroach-specific IgE titers to have IgE recognizing more allergens and at higher levels. For subjects with CR-specific IgE titers < 3.5 kUA/L (CAP classes 0–2), 1 subject recognized 4 allergens, 4 recognized 1–2, and 3 did not recognize any allergen. For CAP classes 3 and above (CR-specific IgE ≥ 3.5 kUA/L), 3 subjects recognized 8 allergens, 2 recognized 7, 9 recognized 1–6 allergens and 1 did not recognize any allergen. Four subjects with cockroach-IgE values of 1.23 to 4.47 kUA/L did not recognize any of the 8 allergens tested.
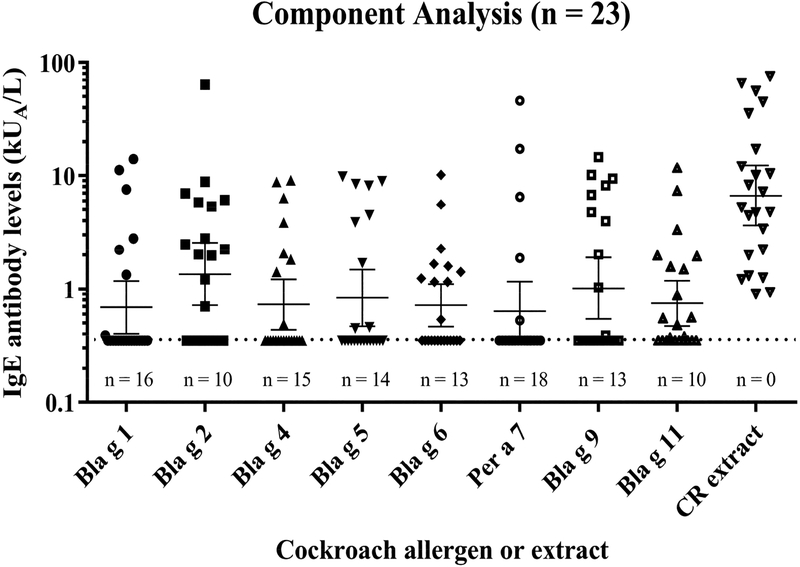
The prevalences of IgE sensitization to the 8 allergens for cockroach allergic subjects (n = 23) were: Bla g 1, 30%; Bla g 2, 57%; Bla g 4, 35%; Bla g 5, 39%; Bla g 6, 44%; Per a 7, 22%; Bla g 9, 44% and Bla g 11, 57%. For a subgroup of only n = 15 subjects with CAP class 3 or larger the prevalences were: Bla g 1, 47%; Bla g 2, 73%; Bla g 4, 47%; Bla g 5, 47%; Bla g 6, 60%; Per a 7, 33%; Bla g 9, 53% and Bla g 11, 73%. These results show that the number of major cockroach allergens increases with the cockroach-specific IgE levels in a population. In this case, there were two major allergens for n = 23 (Bla g 2 and Bla g 11), and four for a subgroup of n = 15 highly cockroach allergic subjects (Bla g 2, Bla g 6, Bla g 9 and Bla g 11). The allergen with the highest geometric mean of allergen-specific IgE for n = 23 subjects was Bla g 2 (1.36 kUA/L) (Figure 3). The average of the percentages of allergen-specific IgE was the highest for Bla g 2 (21.3 ± 20.5%), followed by Bla g 9 (15.5 ± 13.2%), Bla g 5 (14.0 ± 14.1%), Bla g 4 (11.0 ± 10.2%), Per a 7 (10.7 ± 12.8%), Bla g 11 (10.1 ± 10.3), Bla g 1 (8.8 ± 6.1%) and Bla g 6 (8.6 ± 6.4%) (see Figure E1 in the Online Repository).
Figure 3.
Component analysis of IgE reactivity to eight cockroach allergens in a U.S. population of cockroach allergic subjects. Allergen- and cockroach-specific IgE antibody levels from 23 subjects are shown. Long and short horizontal lines indicate the geometric means and 95% CI, respectively. The cut-off level for IgE quantification (0.35 kUA/L) is indicated by the horizontal dotted line. The number of negative results (below 0.35 kUA/L) is provided for each allergen under the corresponding cluster of symbols.
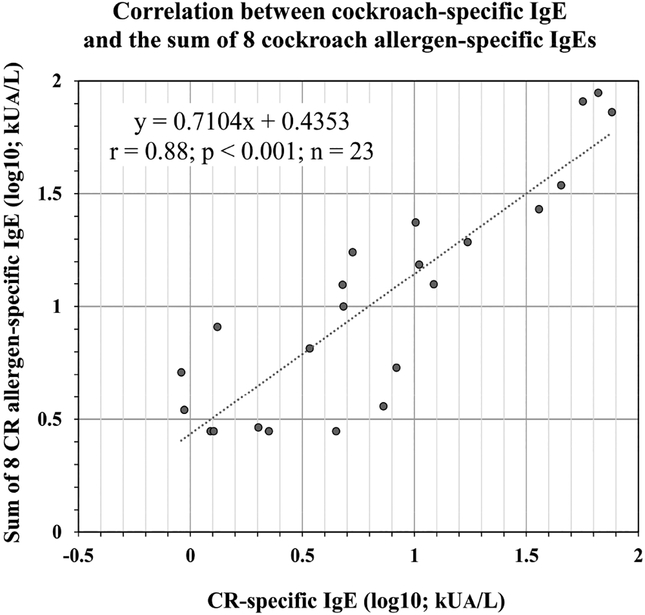
There was a highly significant correlation between cockroach specific IgE and the sum of allergen-specific IgE to 8 allergens (for log10 transformed data, r = 0.88, p < 0.001; n = 23) (Figure 4). This correlation was an improvement versus the one obtained when only 4 cockroach allergens (Bla g 1, Bla g 2, Bla g 4 and Bla g 5) (r = 0.78, p < 0.001) or only the 3 allergens that were measured in the extracts (r = 0.78, p < 0.001) were considered. A weak correlation was observed between cockroach-specific IgE and the skin prick test wheal size (n = 18; r = 0.56, p = 0.015).
Figure 4.
Correlation between cockroach-specific IgE and the sum of specific IgE to each of the eight cockroach-allergens for n = 23 subjects. Plasma cockroach-specific IgE and sum of allergen-specific IgE levels were plotted after log10 transformation for normalization of these variables.
Comparison of in vitro extract potencies by IgE inhibition assays
Cockroach extracts exhibited highly variable in vitro potency with respect to IgE recognition per subject. Differences in relative extract potencies for each subject varied up to more than 3 orders of magnitude (up to 2,800-fold). Table II shows the relative potencies (IC30) estimated from the inhibition curves displayed in Figure E2 in the Online Repository.
Table II.
in vitro IgE potencies of cockroach extracts for five cockroach allergic subjects.
| Cockroach allergic subjects | ||||||
|---|---|---|---|---|---|---|
| 1445 | 1277 | 1424 | 1425 | 1864 | ||
| Cockroach extracts | Relative extract potencies (Normalized IC30 data)* | |||||
| #1 | Commercial - WB - Hum | 3.7 | 4.3 | 1.7 | 2.0 | 1.9 |
| #2 | Commercial - WB - Hum | 0.8 | 1.2 | 1.9 | 1.3 | 1.1 |
| #3 | Commercial - WB - Hum | 0.4 | 0.6 | 1.3 | 1.6 | 1.3 |
| #4 | Commercial - WB - Hum | 0.7 | 0.9 | 1.9 | 1.7 | 1.1 |
| #5 | Commercial - WB - Vet | 944.4 | 1000.0 | 350.0 | 25.0 | 1.8 |
| #6 | Commercial - WB - Vet | 0.8 | 0.4 | 1.6 | 2.8 | 1.5 |
| #7 | Commercial - WB - Res | 9.4 | 12.5 | 2.7 | 2.9 | 2.1 |
| #8 | Commercial - WB - Res | 15.0 | 187.5 | 1.0 | 1.0 | 1.0 |
| #9 | Commercial - WB - Res | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| #10 | In-house - Fecal - Res | 444.4 | 1000.0 | 45.8 | 153.9 | 4.3 |
| #11 | In-house - Fecal - Res | 1055.6 | 1000.0 | 3.0 | 10.0 | 15.5 |
| #12 | In-house - Frass - Res | 1000.0 | 1000.0 | 22.5 | 12.2 | 1.6 |
| #13 | Negative control | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| x2375 | x2720 | x1000 | x1000 | xl000 | ||
Absolute in vitro potencies expressed in μg/mL were transformed into in vitro potencies (without units) relative to the values for extract #9 used as reference (potency of 1, underlined). 1000 was added for curves that did not reach the IC30.
Abbreviations: WB: whole body; Hum: human use; Vet: veterinary use; Res: research use.
Commercial extracts #2, #3, #4, #6 and #9 showed the highest relative potencies (IC30: 0.4–2.9). However, there was not a good correlation between the sum of three allergen levels in the extracts and the extract potencies. From the five subjects tested for extract potencies, two groups of subjects were identified since, within these groups, good correlations of the IC30s between paired subjects were observed (r > 0.9; p < 0.001) (see Table E2 in the Online Repository). The pairs of subjects showing a better in vitro potency correlation also tended to have a better correlation of specific IgE levels to eight allergens (not significant) (see Table E3 in the Online Repository).
Analyses of German cockroach extract potencies versus allergen content and versus levels of IgE sensitization to cockroach allergens
Two additional systematic analyses of extract potency data were performed. First, for each of the five patients tested for extract potencies using inhibition assays, correlations between extract potencies and allergen content of the extracts were analyzed. Only patients 1445 and 1227 showed significant correlations between both variables (r = 0.779, p = 0.0028 for 1445 and r = 0.773, p = 0.0032 for 1277, when considering the three allergens measured) (Table E4 in the Online Repository). Interestingly, the correlations were higher when Bla g 1 alone was considered (0.871; p = 0.0002 for 1445 and 0.838; p = 0.0007 for 1227), whereas there were no significant correlations when considering only Bla g 2 (see Table E4 in the Online Repository). These results might be associated to the fact that these two patients also had the highest levels of Bla g 1-specific IgE (14.06 and 7.56 kUA/L for 1445 and 1277, respectively), whereas these levels were either low or undetectable for the three other patients (see Table E4 in the Online Repository). These results also agree with the fact that the allergen content of the twelve extracts was much higher for Bla g 1 than Bla g 2 (20.1-fold in average), and very low for Bla g 5. Therefore, sensitization to Bla g 1 showed to be relevant for the positive correlations observed between potencies and allergen content (high on Bla g 1) for those two patients.
Second, for each extract, the correlations between extract potencies for five patients and the sum of allergen-specific IgE of these patients were analyzed. The allergen-specific IgE considered for analysis was either the sum of IgE to Bla g 1, Bla g 2 and Bla g 5 (the three allergens measured in the extracts), or the sum of IgE to each of the 8 allergens. The correlations were not significant for the sum of specific IgE to only three allergens, and the Pearson’s correlation coefficients increased significantly when 8 allergen-specific IgE were considered (63.2-fold in average for the twelve extracts) (see Table E5 in the Online Repository). Most extracts showed significant correlations of potencies versus the sum of IgE to 8 allergens (p < 0.05; shown in boldface in Table E5), except three extracts (#2, 4 and 6), which had the lowest levels of allergen content.
Discussion
This study addresses two factors that determine the in vitro potency of German cockroach extracts for IgE reactivity. One is the allergen content of the extract and the other is the subject’s sensitization profile to cockroach allergen components. Cockroach extracts are not standardized, and the variability of their allergen content makes it difficult to select a dose for clinical applications such as diagnostics and immunotherapy. The three allergens measured in cockroach extracts (Bla g 1, Bla g 2 and Bla g 5), which are the only ones for which immunoassays are currently available, showed variability in allergen content ranging from 5.5 to 10.6-fold among commercial extracts for human use.
Highly variable content of protein, Bla g 1 and Bla g 2 in cockroach extracts had been previously described.(20) The large variations in allergen contents of the twelve cockroach extracts are likely related to the source of the extracts and the process of extract preparation.(21) For example, fecal extracts (#10, 11) contained the highest amounts of Bla g 1 and Bla g 2, allergens that are known to be excreted in feces.(3;22) Bla g 5, an enzyme that is likely expressed in the cockroach fat body (analogous to liver), was poorly represented in fecal extracts. Other extracts made of cockroach whole body, contained less Bla g 1 and Bla g 2, but higher relative amounts of Bla g 5 than the fecal extracts. Bla g 1 and Bla g 2 were present in the extracts at levels up to 3 orders of magnitude higher than Bla g 5. Consequently, for diagnostic and immunotherapy purposes, this may suggest a severe underrepresentation of Bla g 5 in the extracts, especially considering that the IgE prevalence to Bla g 5 (39–47%) is equivalent to that of Bla g 1 (30–47%) in this study. Most importantly, these three allergens do not cover the full cockroach-specific IgE reactivity.
Early studies by Satinover et al. reported that the reactivity profile of cockroach allergic subjects to five cockroach allergens was unique, without common immunodominant allergens.(4) The five allergens tested, from groups 1, 2, 4, 5 and 7, were not recognized by 36% of cockroach-sensitized subjects, which indicated that additional cockroach allergens existed. In the current study, three more cockroach proteins were included: Bla g 6, Bla g 9 and Bla g 11. Bla g 6 showed a higher IgE prevalence (up to 60% for n=15 subjects with CAP class ≥ 3) than that reported in previous cloning studies.(15) Interestingly, the three molecules turned out to be major allergens in the current study, in a sub-population of highly allergic individuals (CAP class ≥ 3). Interestingly, four cockroach allergic subjects did not recognize the 8 allergens tested, which indicates that additional cockroach allergens still exist. Proteomic studies have reported new cockroach allergens in Asia.(16;18) The relevance of these potential allergens in a U.S. population is currently being investigated. In this study, the expansion to a set of eight cockroach allergens significantly improved the correlation between cockroach-specific IgE and the sum of allergen-specific IgE from r = 0.78 (p < 0.001), when calculated using only 3 allergens, to r = 0.88 (p < 0.001; n = 23) for 8 allergens. These results indicate that the 8 allergens account for a large proportion of cockroach sensitization.
Endotoxin, which has been reported to influence sensitization(23), was also found in variable amounts in the extracts, but the effect of endotoxin in extracts during immunotherapy remains to be investigated. The current study is part of a larger one that analyzed potencies of the same German cockroach extracts at the T cell level. Endotoxin was measured because it could be relevant for the T cell in vitro potency of the extracts, but it is not relevant for the B cell potency reported here. Flagellin, a Toll-like receptor 5 ligand from bacterial flagella that is used as adjuvant in various vaccines(24), was also measured and found undetectable in the extracts (data not shown).
The in vitro potency of twelve extracts was measured by IgE antibody binding inhibition assays in five individual subjects using an arbitrarily selected commercial extract as a reference. This approach is different from the one used by the Food and Drug Administration that measures biological potencies based on skin prick test, as previously reported for cockroach.(20;25) In one of these studies, highly variable extract biological potencies (up to 78-fold) were described using pooled allergic sera (n = 16).(20) In the other, relative potencies were measured in a competitive ELISA using a reference standard and pooled sera, which were found to parallel the biological potency of three extracts analyzed.(25) Here, the goal was to investigate if relative in vitro potencies would be different among different individuals. Therefore, relative extract potencies were obtained from experiments performed with individual, instead of pooled plasma, from five different subjects.
Inhibition assays have one limitation by the fact that potencies are dependent on a reference extract, and it is not possible to know what proteins (assuming most of the allergens) are adsorbed from the crude allergen extract onto the wells. Nevertheless, measuring relative potencies was an advantage because it allowed to consistently and easily compare the twelve extracts for each individual subject. In general, there were large differences per subject in extract potencies of up to more than three orders of magnitude, with commercial extracts being the most potent for each subject. The differences observed here are presumably due to differences in content of the extracts. However, it was not surprising to find a lack of correlation for three of the five patients tested between extract potency and the content of the only three allergens that were measured, presumably because these three allergens do not account for the total IgE reactivity to cockroach. In contrast, two of the patients who had high IgE levels to Bla g 1, showed the best significant correlations between extract potencies and Bla g 1 content. These results indicate that potency depends on levels of sensitization to allergens that are present in the extracts.
Inhibition assays were performed with individual subjects to assess the importance of the unique subject’s sensitization profile on extract potency. It was difficult to find a high number of subjects with plasma that showed a large enough window of IgE antibody binding inhibition to perform the assays. Nevertheless, the five subjects tested were sufficient to see that variability of extract potency is also dependent on the subject. Each extract showed different relative extract potencies per subject, presumably due to different subject sensitization profiles. In fact, two groups of subjects were identified, each containing individuals who showed the best correlations of extract potencies by pairs (r > 0.9; p < 0.001). The pairs of subjects showing the best potency correlations, had also a tendency to have a better correlation of allergen-specific IgE. For the five patients tested for extract potencies, most extracts (the ones with higher allergen content), showed significant correlations between extract potencies and the sum of specific IgE to 8 (but not to three) allergens. These results indicate that the subject’s levels of sensitization to a large panel of cockroach allergens is also a determinant of extract potency.
Overall, these results show that cockroach extract potency depends on a combination of two factors: a) extract allergen composition, and b) allergen-specific IgE sensitization profile. Both are relevant for the selection of potent extracts to be used for immunotherapy and the design and interpretation of data from immunotherapy trials. For example, if a subject is only allergic to Bla g 1 and Bla g 4, it would be preferable that these two allergens were present in the extract used to treat this subject. Cockroach allergy differs from cat allergy, where most cat allergic subjects are sensitized to Fel d 1, which covers most IgE reactivity to cat. The identification of new major allergens in a cockroach allergic population, shown here, also needs to be taken into consideration for B cell component analysis (allergen-specific antibody analysis), and for design and data interpretation in immunotherapy trials. The unique IgE reactivity profile per patient and lack of immunodominant allergens in a cockroach allergic population, makes it difficult to select appropriate extracts for immunotherapy that contain and cover the allergens relevant to each subject. This variability in allergen-specific reactivity profile within a cockroach allergic cohort has also been observed at the T cell level in a parallel study using the same extracts.(26)
The current study underscores the need for evaluating cockroach extracts to be used in clinical trials, and avoiding the current limitation of measuring only Bla g 1 and Bla g 2 in the doses administered. Future approaches might include the use of standardized mixes of purified natural or recombinant allergens, with known allergen concentrations, to which the patients are sensitized. Alternatively, crude cockroach extracts should carefully balance the source material to include nymphs, adults of both sexes, egg cases and feces, because different proteins are expressed by different life stages. The conclusions in this study set the stage for the imminent cockroach allergy trials that will be conducted by the Inner-City Asthma Consortium. The main recommendation is for the design of immunotherapy using extracts that are optimized for the presence of allergens relevant to the subject’s sensitization profile.
Supplementary Material
Funding:
Research in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI077653 (to AP and MDC), U19 AI135731 and U19 AI100275 (to AS), and UM1 AI114271 (ICAC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ICAC
Inner-City Asthma Consortium
- PBS
Phosphate Buffered Saline
- WB
Whole body
- WHO/IUIS
World Health Organization and International Union of Immunological Societies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest disclosure statement:
J Glesner, S Filep, LD Vailes, S Wünschmann, MD Chapman and A Pomés are employed by Indoor Biotechnologies, Inc. MD Chapman is President and CEO of the company. The remaining authors have no conflict of interests to declare.
Clinical Implications:
Allergen content, variable in non-standardized German cockroach extracts, and IgE sensitization profiles to a new expanded set of cockroach allergens determine in vitro extract potency for IgE reactivity.
References
- (1). Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997; 336(19):1356–63. [DOI] [PubMed] [Google Scholar]
- (2). Wood RA, Togias A, Wildfire J, Visness CM, Matsui EC, Gruchalla R et al. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. J Allergy Clin Immunol 2014; 133(3):846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3). Arruda LK, Vailes LD, Mann BJ, Shannon J, Fox JW, Vedvick TS et al. Molecular cloning of a major cockroach (Blattella germanica) allergen, Bla g 2. Sequence homology to the aspartic proteases. J Biol Chem 1995; 270(33):19563–8. [DOI] [PubMed] [Google Scholar]
- (4). Satinover SM, Reefer AJ, Pomés A, Chapman MD, Platts-Mills TA, Woodfolk JA. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. J Allergy Clin Immunol 2005; 115(4):803–9. [DOI] [PubMed] [Google Scholar]
- (5). Pollart SM, Smith TF, Morris EC, Gelber LE, Platts-Mills TA, Chapman MD. Environmental exposure to cockroach allergens: analysis with monoclonal antibody-based enzyme immunoassays. J Allergy Clin Immunol 1991; 87(2):505–10. [DOI] [PubMed] [Google Scholar]
- (6). Pomés A, Melén E, Vailes LD, Retief JD, Arruda LK, Chapman MD. Novel allergen structures with tandem amino acid repeats derived from German and American cockroach. J Biol Chem 1998; 273(46):30801–7. [DOI] [PubMed] [Google Scholar]
- (7). Mueller GA, Pedersen LC, Lih FB, Glesner J, Moon AF, Chapman MD et al. The novel structure of the cockroach allergen Bla g 1 has implications for allergenicity and exposure assessment. J Allergy Clin Immunol 2013; 132(6):1420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8). Pomés A, Chapman MD, Vailes LD, Blundell TL, Dhanaraj V. Cockroach allergen Bla g 2: structure, function, and implications for allergic sensitization. Am J Respir Crit Care Med 2002; 165(3):391–7. [DOI] [PubMed] [Google Scholar]
- (9). Gustchina A, Li M, Wünschmann S, Chapman MD, Pomés A, Wlodawer A. Crystal structure of cockroach allergen Bla g 2, an unusual zinc binding aspartic protease with a novel mode of self-inhibition. J Mol Biol 2005; 348(2):433–44. [DOI] [PubMed] [Google Scholar]
- (10). Arruda LK, Vailes LD, Hayden ML, Benjamin DC, Chapman MD. Cloning of cockroach allergen, Bla g 4, identifies ligand binding proteins (or calycins) as a cause of IgE antibody responses. J Biol Chem 1995; 270(52):31196–201. [DOI] [PubMed] [Google Scholar]
- (11). Fan Y, Gore JC, Redding KO, Vailes LD, Chapman MD, Schal C. Tissue localization and regulation by juvenile hormone of human allergen Bla g 4 from the German cockroach, Blattella germanica (L.). Insect Mol Biol 2005; 14(1):45–53. [DOI] [PubMed] [Google Scholar]
- (12). Arruda LK, Vailes LD, Platts-Mills TA, Hayden ML, Chapman MD. Induction of IgE antibody responses by glutathione S-transferase from the German cockroach (Blattella germanica). J Biol Chem 1997; 272(33):20907–12. [DOI] [PubMed] [Google Scholar]
- (13). Mueller GA, Pedersen LC, Glesner J, Edwards LL, Zakzuk J, London RE et al. Analysis of glutathione S-transferase allergen cross-reactivity in a North American population: Relevance for molecular diagnosis. J Allergy Clin Immunol 2015; 136(5):1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14). Jeong KY, Lee J, Lee IY, Ree HI, Hong CS, Yong TS. Allergenicity of recombinant Bla g 7, German cockroach tropomyosin. Allergy 2003; 58(10): 1059–63. [DOI] [PubMed] [Google Scholar]
- (15). Hindley J, Wünschmann S, Satinover SM, Woodfolk JA, Chew FT, Chapman MD et al. Bla g 6: a troponin C allergen from Blattella germanica with IgE binding calcium dependence. J Allergy Clin Immunol 2006; 117(6):1389–95. [DOI] [PubMed] [Google Scholar]
- (16). Chuang JG, Su SN, Chiang BL, Lee HJ, Chow LP. Proteome mining for novel IgE-binding proteins from the German cockroach (Blattella germanica) and allergen profiling of patients. Proteomics 2010; 10(21):3854–67. [DOI] [PubMed] [Google Scholar]
- (17). Jeong KY, Kim CR, Park J, Han IS, Park JW, Yong TS. Identification of novel allergenic components from German cockroach fecal extract by a proteomic approach. Int Arch Allergy Immunol 2013; 161(4):315–24. [DOI] [PubMed] [Google Scholar]
- (18). Fang Y, Long C, Bai X, Liu W, Rong M, Lai R et al. Two new types of allergens from the cockroach, Periplaneta americana. Allergy 2015; 70(12):1674–8. [DOI] [PubMed] [Google Scholar]
- (19). Pollart SM, Mullins DE, Vailes LD, Hayden ML, Platts-Mills TA, Sutherland WM et al. Identification, quantitation, and purification of cockroach allergens using monoclonal antibodies. J Allergy Clin Immunol 1991; 87(2):511–21. [DOI] [PubMed] [Google Scholar]
- (20). Patterson ML, Slater JE. Characterization and comparison of commercially available German and American cockroach allergen extracts. Clin Exp Allergy 2002; 32(5):721–7. [DOI] [PubMed] [Google Scholar]
- (21). Yun YY, KO SH, Park JW, Lee IY, Ree HI, Hong CS. Comparison of allergenic components between German cockroach whole body and fecal extracts. Ann Allergy Asthma Immunol 2001; 86(5):551–6. [DOI] [PubMed] [Google Scholar]
- (22). Gore JC, Schal C. Gene expression and tissue distribution of the major human allergen Bla g 1 in the German cockroach, Blattella germanica L. (Dictyoptera: Blattellidae).J Med Entomol 2004; 41(5):953–60. [DOI] [PubMed] [Google Scholar]
- (23). Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015; 349(6252):1106–10. [DOI] [PubMed] [Google Scholar]
- (24). Kitzmuller C, Kalser J, Mutschlechner S, Hauser M, Zlabinger GJ, Ferreira F et al. Fusion proteins of flagellin and the major birch pollen allergen Bet v 1 show enhanced immunogenicity, reduced allergenicity, and intrinsic adjuvanticity. J Allergy Clin Immunol. 2018. January;141(1):293–299.e6. [DOI] [PubMed] [Google Scholar]
- (25). Slater JE, James R, Pongracic JA, Liu AH, Sarpong S, Sampson HA et al. Biological potency of German cockroach allergen extracts determined in an inner city population. Clin Exp Allergy 2007; 37(7):1033–9. [DOI] [PubMed] [Google Scholar]
- (26). Schulten V, Birrueta G, Glesner J, Filep S, Schal C, Jeong KY et al. Variability in German cockroach extract composition has a great impact on T cell potency In cockroach-allergic donors. J Allergy Clin Immunol 2018; 141(2):AB199. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.