Abstract
PURPOSE:
To establish a cohort of high-risk women undergoing intensive surveillance for breast cancer.
METHODS:
We performed dynamic contrast-enhanced magnetic resonance imaging (MRI) every 6 months in conjunction with annual mammography (MG). Eligible participants had a cumulative lifetime breast cancer risk ≥ 20% and/or tested positive for a pathogenic mutation in a known breast cancer susceptibility gene.
RESULTS:
Between 2004–2016, we prospectively enrolled 295 women, including 157 mutation carriers (75 BRCA1, 61 BRCA2); participants’ mean age at entry was 43.3 years. Seventeen cancers were later diagnosed: four ductal carcinoma in situ (DCIS) and thirteen early stage invasive breast cancers. Fifteen cancers occurred in mutation carriers (11 BRCA1, 3 BRCA2, 1 CDH1). Median size of the invasive cancers was 0.61 cm. No patients had lymph node metastasis at time of diagnosis and no interval invasive cancers occurred. The sensitivity of bi-annual MRI alone was 88.2% and annual MG plus bi-annual MRI was 94.1%. The cancer detection rate of bi-annual MRI alone was 0.7% per 100 screening episodes, which is similar to the cancer detection rate of 0.7% per 100 screening episodes for annual MG plus bi-annual MRI. The number of recalls and biopsies needed to detect one cancer by bi-annual MRI were 2.8 and 1.7 in BRCA1 carriers, 12.0 and 8.0 in BRCA2 carriers, and 11.7 and 5.0 in non-BRCA1/2 carriers, respectively.
CONCLUSIONS:
Bi-annual MRI performed well for early detection of invasive breast cancer in genomically stratified high-risk women. No benefit was associated with annual MG screening plus bi-annual MRI screening.
Keywords: BRCA, breast cancer, genetic testing, magnetic resonance imaging, mammography, screening
INTRODUCTION
Among women at high genetic risk of breast cancer, current options for prevention and early detection include prophylactic mastectomy, prophylactic bilateral salpingo-oophorectomy (BSO), chemoprevention, and heightened imaging surveillance(1–3). As an alternative to prophylactic mastectomy, intensive imaging surveillance using dynamic contrast-enhanced magnetic resonance imaging (MRI) is more sensitive than mammography (MG) alone and detects breast cancer at an earlier stage, resulting in a more favorable prognosis(4–16). The American Cancer Society and other organizations have published guidelines that recommend annual MRI in conjunction with annual MG for a well-defined category of high-risk women including: carriers of damaging mutations in breast cancer susceptibility genes and their untested first-degree relatives, women with a lifetime breast cancer risk >20% as defined by risk-prediction models, and women with prior history of chest radiation between the ages of 10 and 30 years(1, 17, 18). However, meta-analysis of the pivotal studies using this intense imaging surveillance demonstrated that a few of the participants were still diagnosed with tumors larger than 1 cm, with node positive disease, and with interval invasive breast cancers (detected between rounds of stacked annual MRI/MG examinations) (19).
While these guidelines appear to have changed clinical practice, there remain unanswered questions including optimal length of screening interval, ideal ages of initiation and completion of screening, the best combination of screening modalities, and limitations of risk-prediction models to identify ideal candidates for intensive surveillance. There is also concern for overdiagnosis of indolent DCIS leading to overtreatment of women at moderate risk(20). The potential harms from MG in young women include radiation exposure for BRCA1/2 carriers(21), anxiety associated with false-positive findings(22), and costs associated with additional procedures(23, 24). While potential risk from gadolinium exists, MRI poses no risk of radiation, has high specificity, and the aggressive behavior and natural history of BRCA1/2 associated breast cancers support the use of MRI as an effective alternative to prophylactic mastectomies(25–28). In this study, we established a novel imaging surveillance program to evaluate the performance of bi-annual MRI in conjunction with annual MG in genomically stratified, high-risk women. The results of this imaging-rich study provide a framework for optimizing MRI screening for early detection and cancer interception in women at high risk of inherited breast cancer.
METHODS
Study Population
Between 2004 and 2016, we established a prospective registry of women at high risk of breast cancer at the University of Chicago Cancer Risk Clinic (Trial Registration: NCT00989638). The targeted enrollment was 300 patients for the psychosocial pre-specified endpoints of adherence and quality of life but the Screening Registry would continue to enroll and follow an indefinite number of those at high-risk. To allow recruitment of women at different levels of risk, the eligibility criteria were as follows. Age was ≥ 25 years or, if < 25 years, was within 5 years of the youngest breast cancer diagnosis in the family. Women with prior history of cancer were eligible if at least one breast had not been previously irradiated for cancer. Finally, one or more of the following pertained: 1) carrier of a pathogenic mutation in breast cancer susceptibility genes as described in BROCA panel testing below; 2) previous breast cancer at age < 35 years, with chemotherapy completed and disease-free for at least two years; 3) previous chest irradiation at age < 30 years; 4) previous ductal carcinoma in situ (DCIS) at age < 35 years and a mother or sister with breast cancer diagnosed < 50 years or a mother or sister with ovarian cancer at any age; 5) no previous breast cancer but with probability of being a BRCA1 or BRCA2 carrier of 20% or greater based on BRCAPRO analysis or ≥ 25% risk of being a mutation carrier by Couch model in addition to a lifetime breast cancer risk ≥20% by Gail or Claus model; 6) of African ancestry with family history of breast cancer at age <40 in a mother, sister, paternal aunt, or paternal grandmother. This final criterion was included to increase participation by African American women (29) who suffer a disproportionate burden of aggressive triple-negative breast cancer. Exclusion criteria were current pregnancy, history of kidney disease, presence of any implanted metallic foreign object, breast surgery within two weeks. The study was approved by the University of Chicago Institutional Review Board and in accordance with the precepts established by the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Screening Protocol
Following initial evaluation by a physician and genetic counselor, the screening protocol consisted of bi-annual clinical breast examinations, bi-annual MRI using dedicated breast coil and techniques as described previously (30), and annual standard screening MG (Supplementary Figure S1). Every screening episode was considered a screening round. The interval round was defined as the screening episode that was performed at the 6 months time point with MRI alone. Each imaging exam had independent reading per round. Whenever possible, clinical breast exam and MG were scheduled on the same day as the MRI. Dedicated breast radiologists using the ACR Breast Imaging Reporting and Data Systems (BI-RADS) independently interpreted the MRI and MG. MRI technique evolved during the study period, specifics are fully described in Supplementary Table S1. Upon completion of 5 years of study protocol, mutation carriers were offered the opportunity to continue screening indefinitely.
Actions to be taken following an abnormal imaging were pre-specified as follows: 1) For BI-RADS score of 4 or 5 on MRI, a percutaneous biopsy was recommended; 2) For BI-RADS score of 0 on MRI and/or BI-RADS scores of 0, 4, or 5 on MG, further investigation by imaging (e.g., ultrasonography, diagnostic MG, and/or unilateral MRI) was recommended and a biopsy was performed if clinically appropriate; 3) MRI and MG tests with BI-RADS scores of 3 were discussed case-by-case, on an individual basis in a multidisciplinary setting. The majority of cases continued screening on a short term 6 months follow-up exam in the same imaging modality as specified per protocol. In rare cases, an ultra short term follow-up MRI was performed in 4 weeks and, based on the findings, participants continued screening per protocol.
BROCA Cancer Gene Panel
Genomic DNA isolated from blood was sequenced for eleven genes known to be associated with inherited predisposition to breast cancer: ATM, BRCA1, BRCA2, TP53, PTEN, CDH1, PALB2, NBN, BRIP1, BARD1, or CHEK2. Targeted capture and multiplexed sequencing to detect all classes of mutations in these genes were carried out using BROCA(31). For some subjects, commercial testing of one or more genes (usually BRCA1 and BRCA2) had been undertaken previously. For these subjects, BROCA testing was carried out at the University of Washington or at Color Genomics (Burlingame, CA, USA) without knowledge of the prior results. In all cases, mutations previously identified were confirmed. For all 11 genes, the present analysis includes only unambiguously damaging mutations, defined as truncations, exon deletions, and splice and missense mutations shown experimentally functional. Identified mutations were validated by Sanger sequencing and real-time PCR using TaqMan probes (Life Technologies, CA, USA).
Statistical Analysis
A positive test was defined as BI-RADS score of 0, 4, or 5. Sensitivity, specificity, and predictive values of the imaging modalities were calculated. True positive findings were defined as pathologically proven invasive cancers or DCIS detected after positive screening. False negative findings were defined as symptomatic breast cancer presenting in between screening and incidental cancers detected following bilateral prophylactic mastectomy. False positive findings were defined as suspicious BI-RADS scores with a final benign diagnosis after further investigation. Finally, true negative findings included all normal studies (BI-RADS scores 1 or 2). BI-RADS 3 scores were either followed every six months per protocol or ultimately biopsied. Receiver operating characteristic (ROC) analysis was conducted on the ordinal BI-RADS scores on MRI or MG. Area under ROC curve (AUC) was estimated and compared between screening modalities using a permutation test (10000 permutations). Follow-up was calculated from the date of the study entry until the date of the last planned screening exam, detection of breast cancer, bilateral prophylactic mastectomy, or death, whichever came first. Breast cancer incidence rate was calculated per 100 person-years at risk. Biopsy rate and recall rate, which is the number of individuals asked to return for follow-up imaging or additional procedures after an anomaly is found on an imaging study, were calculated. In the calculation of sensitivity, the analysis was per patient. In the calculation of specificity, the analysis was per screen. We assumed repeat observations in the same patients were independent. We also used a bootstrapping method to account for the possible correlation within patients. We found that the 95% confidence intervals for specificity from the two methods were almost the same, suggesting that the repeat observations are independent. Thus, this finding suggests that the radiologists evaluate the “current” screening image without considering results from previous (negative) screening image. Log-rank test and Cox proportional hazard model were used to explore factors related to breast cancer risk. All statistical analyses were conducted using STATA (v.15, Stata Corp, Texas).
RESULTS
The prospective cohort study was open to enrollment in 2004 and closed to accrual in December 2016. Of 305 subjects consented, 10 were removed from further analysis because they never completed the 1st round of screening. Clinical characteristics of the remaining 295 study participants are listed in Table 1. The mean age at entry was 43.3 (±11) years; 45.8% were postmenopausal and 31.9% had prior history of BSO. 60 women (20.3%) had personal history of breast cancer. Genomic analysis was completed using BROCA panel or clinical testing in 258 participants (87.5%), 29 participants (9.8%) had testing for known familial mutation or complete BRCA1 and BRCA2 testing, and only 8 (2.7%) were not tested because they did not give a blood sample. 157 (53.2%; including two patients with mutations in two genes) carried a pathogenic mutation in at least one breast cancer susceptibility gene: 75 BRCA1, 61 BRCA2, 10 CHEK2, 4 CDH1, 3 PALB2, 2 ATM, 1 TP53, 1 PTEN, 1 NBN, and 1 BRIP1. Spectrum of pathogenic mutations is listed in Supplementary Table S2.
Table 1.
Characteristics of Study Participants
| Characteristic | Number | % |
|---|---|---|
| All participants | 295 | 100% |
| Age at entry, in years, mean (± SD) | 43.3 (±11.0) | |
| Germline deleterious mutation | ||
| BRCA1* | 75 | 25.4% |
| BRCA2** | 61 | 20.7% |
| CDH1 | 4 | 1.4% |
| PALB2 | 3 | 1.0% |
| TP53 | 1 | 0.3% |
| ATM* | 2 | 0.7% |
| NBN | 1 | 0.3% |
| BRIP1** | 1 | 0.3% |
| PTEN | 1 | 0.3% |
| CHEK2 | 10 | 3.4% |
| All tested genes wildtype | 130 | 44.1% |
| Not tested | 8 | 2.7% |
| Ancestry | ||
| Caucasian | 252 | 85.4% |
| African-American | 34 | 11.5% |
| Hispanic | 5 | 1.7% |
| Asian | 4 | 1.4% |
| Menopausal status | ||
| Pre-menopausal | 140 | 47.5% |
| Post-menopausal | ||
| Bilateral salpingo-oophorectomy (BSO) | 94 | 31.9% |
| No BSO | 41 | 13.9% |
| Missing | 20 | 6.8% |
| Mammographic breast density | ||
| Extremely or heterogeneously dense | 167 | 56.6% |
| Moderate or low density | 125 | 42.4% |
| Missing | 3 | 1.0% |
| Prior cancer history | ||
| Breast cancer | 54 | 18.3% |
| Ovarian cancer | 4 | 1.4% |
| Breast and ovarian cancer | 6 | 2.0% |
| Neither | 231 | 78.3% |
One patient has mutations in both BRCA1 and ATM genes
One patient has mutations in both BRCA2 and BRIP1 genes
Over the study period, 2111 MRI and 1223 MG were performed, representing a mean of 7.3 MRI and 4.3 MG examinations per subject. The number of screening episodes per subject ranged from 1 to 21. There were no statistically significant differences in number of screening episodes between mutation carriers, patients with previous breast cancer, and other women. Of the 1223 annual MG, 83.5% were done on the same day as MRI. Compliance rates for each screening round are shown in Supplementary Figure S2. Of subjects who had the opportunity to finish 5 years of screening, 41% did so. Change in insurance coverage, opting to have bilateral prophylactic mastectomy, moving out of town/changing care providers, and pregnancy were the top reasons why women left the study (Supplementary Table S3).
Performance of the Screening Modalities
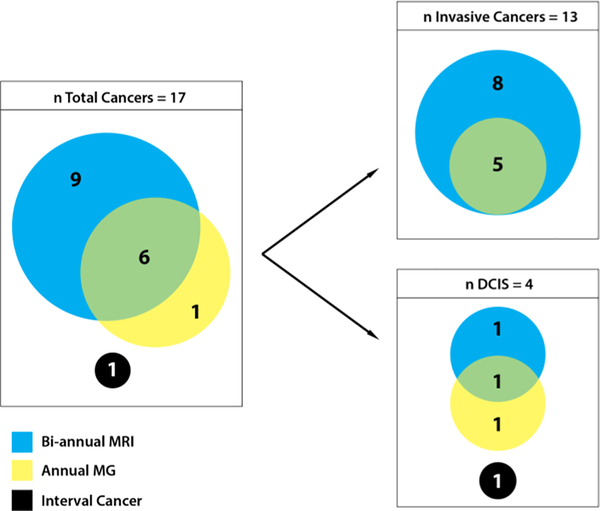
Thirteen early stage invasive breast and four DCIS were diagnosed. Fifteen of the total occurred in patients with mutations (11 BRCA1, 3 BRCA2, 1 CDH1). Eight invasive cancers and one DCIS were detected only by MRI, one DCIS was detected only by MG, and five invasive cancers and one DCIS were detected by both modalities (Figure 1). Of the nine cancers detected only by MRI, three were detected on examinations when both MRI and MG were performed and six were detected on interval rounds when only MRI was used. MRI missed one high grade DCIS in a BRCA1 mutation carrier measuring 0.5 cm that was seen on MG and one intermediate grade DCIS measuring 1.7 cm that was found incidentally (4 months after MRI and 2 months before next scheduled MRI/MG visit) in a prophylactic mastectomy specimen from a 36-year-old BRCA2 mutation carrier.
Figure 1.
Characteristics of cancers diagnosed by imaging modality
Abbreviations: DCIS, ductal carcinoma in situ; MRI, dynamic contrast-enhanced magnetic resonance imaging; MG, mammography; n, number; PMS, prophylactic mastectomy specimen.
Sensitivity, specificity, and predictive values of MRI and MG alone or combined are summarized in Table 2. The sensitivity of bi-annual MRI screening alone was 88.2% (95% confidence interval [CI] 63.6–98.5%). This was similar to the sensitivity for bi-annual MRI + annual MG screening modalities combined (94.1%, 95%CI: 71.3–99.9%) and greater than that for annual MG screening alone (41.2%, 95%CI: 18.4–67.1%). The specificity for bi-annual MRI alone, annual MG studies alone, and bi-annual MRI + annual MG screening modalities combined were 96.8% (95%CI: 95.9–97.5%), 97.8% (95%CI: 96.8–98.5%), and 96.1% (95%CI: 95.2–96.9%), respectively. The cancer detection rate of bi-annual MRI alone was 0.7% per 100 screening episodes (95% CI: 0.4%−1.2%), which is similar to the cancer detection rate of 0.7% per 100 screening episodes (95% CI: 0.4%−1.2%) for annual MG + bi-annual MRI.
Table 2.
Performance by Screening Imaging Modality and Mutation Status
| All subjects (n = 295) | BRCA1 carriers (n = 75) | BRCA2 carriers (n = 61) | Other women (n = 159) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MG | MRI | MG+MRI | MG | MRI | MG+MRI | MG | MRI | MG+MRI | MG | MRI | MG+MRI | |
| Number of screening episodes | 1223 | 2111 | 2209 | 320 | 557 | 572 | 276 | 516 | 536 | 627 | 1038 | 1101 |
| Sensitivity, % | 41.2 | 88.2 | 94.1 | 45.5 | 90.9 | 100 | 33.3 | 66.7 | 66.7 | 33.3 | 100 | 100 |
| (95% CI) | (18.4–67.1) | (63.3–98.5) | (71.3–99.9) | (16.7–76.6) | (58.7–99.8) | (71.5–100) | (0.8–90.6) | (9.4–99.2) | (9.4–99.1) | (0.8–90.6) | (29–100) | (29.2–100) |
| Specificity, % | 97.8 | 96.8 | 96.1 | 98.4 | 96.9 | 96.6 | 97.1 | 95.9 | 95.1 | 97.8 | 97.1 | 96.3 |
| (95% CI) | (96.8–98.5) | (95.9–97.5) | (95.2–96.9) | (96.3–99.5) | (95.1–98.2) | (94.8–97.9) | (94.3–98.7) | (93.8–97.4) | (92.9–96.8) | (96.3–98.8) | (95.9–98.0) | (95.0–97.3) |
| Positive predictive value, % | 20.6 | 18.1 | 15.7 | 50 | 37 | 36.7 | 11.1 | 8.7 | 7.1 | 6.7 | 9.1 | 6.8 |
| (95% CI) | (8.7–37.9) | (10.5–28.0) | (9.2–24.2) | (18.7–81.3) | (19.4–57.6) | (20.0–56.1) | (0.3–48.2) | (1.1–28.0) | (0.9–23.5) | (0.2–31.9) | (1.9–24.3) | (1.4–18.6) |
| Negative predictive value, % | 99.2 | 99.9 | 99.95 | 98.1 | 99.8 | 100 | 99.3 | 99.8 | 99.8 | 99.8 | 100 | 100 |
| (95% CI) | (98.5–99.6) | (99.6–100.0) | (99.7–100.0) | (95.8–99.3) | (99.0–100.0) | (99.3–100.0) | (97.3–99.9) | (98.9–100.0) | (98.9–100.0) | (98.8–100.0) | (99.6–100.0) | (99.7–100.0) |
| Number of recalls | 34 | 87 | 106 | 10 | 28 | 31 | 9 | 24 | 29 | 15 | 35 | 46 |
| Number of biopsies | 19 | 48 | 54 | 7 | 17 | 18 | 6 | 16 | 18 | 6 | 15 | 18 |
| Number of breast cancer detected | 7 | 15 | 16 | 5 | 10 | 11 | 1 | 2 | 2 | 1 | 3 | 3 |
| Recall rate, per 100 screening episodes | 2.8% | 4.1% | 4.8% | 3.1% | 5.0% | 5.4% | 3.3% | 4.7% | 5.4% | 2.4% | 3.4% | 4.2% |
| Biopsy rate, per 100 screening episodes | 1.6% | 2.3% | 2.4% | 2.2% | 3.1% | 3.1% | 2.2% | 3.1% | 3.4% | 1.0% | 1.4% | 1.6% |
| Breast cancer detection rate, per 100 screening episodes | 0.6% | 0.7% | 0.7% | 1.6% | 1.8% | 1.9% | 0.4% | 0.4% | 0.4% | 0.2% | 0.3% | 0.3% |
| Number of recalls needed to detect one cancer | 4.9 | 5.8 | 6.6 | 2.0 | 2.8 | 2.8 | 9.0 | 12.0 | 14.5 | 15.0 | 11.7 | 15.3 |
| Number of biopsies needed to detect one cancer | 2.7 | 3.2 | 3.4 | 1.4 | 1.7 | 1.6 | 6.0 | 8.0 | 9.0 | 6.0 | 5.0 | 6.0 |
Abbreviations: MG, annual mammography alone; MG+MRI, annual mammography combined with biannual dynamic contrast-enhanced magnetic resonance imaging; MRI, bi-annual dynamic contrast-enhanced magnetic resonance imaging alone.
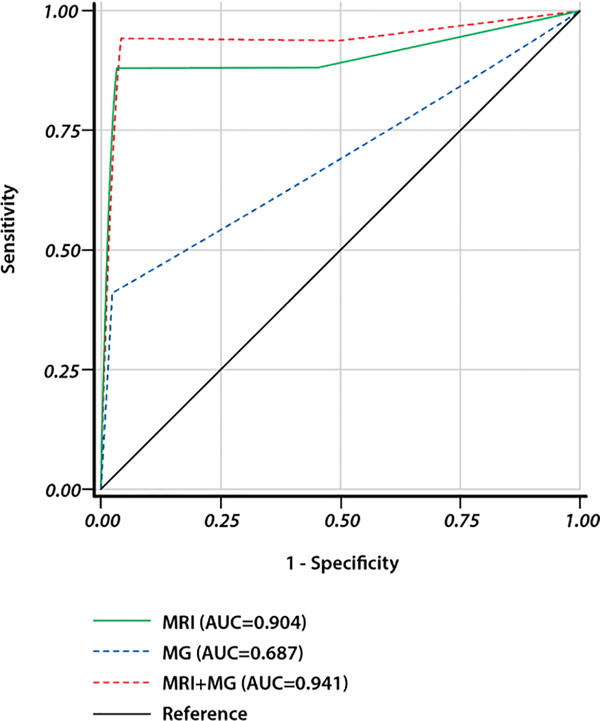
ROC curves according to BI-RADS scores were generated to evaluate the diagnostic performance of the imaging modalities (Figure 2). The AUC was 0.687 for annual MG alone, 0.904 for bi-annual MRI alone, and 0.941 for both modalities combined. There was no statistical difference in AUC between bi-annual MRI alone and bi-annual MRI + annual MG modalities combined (p=0.53), and the AUC for bi-annual MRI alone was statistically higher than that for annual MG (p=0.0052).
Figure 2.
Receiver operating characteristic (ROC) curves for DCE-MRI and MG. AUC, area under the ROC curve
The difference of the diagnostic performance employing receiver operating characteristic (ROC) analysis between MRI (AUC=0.904) and MG+MRI (AUC=0.941) was not statistically significant (p=0.53). The AUC for MRI was statistically higher than that AUC for MG (p=0.0052).
Abbreviations: MG, annual mammography alone; MRI+MG, bi-annual dynamic contrast-enhanced magnetic resonance imaging + annual mammography; MRI, bi-annual dynamic contrast-enhanced magnetic resonance imaging alone.
Clinico-pathological Features of the Screen-detected Cancers
Thirteen invasive breast cancers and three DCIS were detected with screening (Table 3). All the thirteen invasive cancers were detected by MRI and were ≤ 1 cm with a median size of 0.6 (range 0.1–1.0 cm; excluding patients receiving neoadjuvant therapy). No patients had axillary lymph node involvement. Of note, all three screening-detected, high-grade DCIS were diagnosed in BRCA1 mutation carriers. Of the thirteen invasive cancers, all but one had associated DCIS and four were triple negative breast cancer. Eleven were detected in mutation carriers (8 BRCA1, 2 BRCA2 and 1 CDH1). Except for a CDH1 mutation carrier who developed a low-grade invasive lobular carcinoma with associated LCIS, all other invasive cancers were ductal and moderate-to-high-grade.
Table 3.
Characteristics of Breast Cancers Detected
| ID | Mutant gene | Prior breast cancer | Prior BSO | Age breast ca dx | Cancer type | Grade | ER | PR | HER2 | MRI lesion (cm) | Invasive tumor (cm) | Positive nodes | Stage | Detection modality | Detection round |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BRCA1 | none | yes | 42 | DCIS only | 3 | nd | nd | nd | 0.9 (MF) | 0/4 | Stage 0 | both | 1st screening round | |
| 2 | BRCA1 | none | yes | 55 | IDC | 3 | pos | neg | neg | 0.4 | 0.3 | 0/4 | Stage 1 | both | 1st screening round |
| 3 | BRCA1 | CL | no | 27 | IDC + DCIS | 3 | pos | neg | nd | 1.2 | 0.1 | 0/2 | Stage 1 | MRI | 1st screening round |
| 4 | none | IL | no | 37 | IDC + DCIS | 3 | pos | pos | neg | 0.9 | 1.0 | 0/1 | Stage 1 | MRI | 1st screening round |
| 5 | BRCA1 | CL | yes | 46 | DCIS only | 3 | nd | nd | nd | 0.3 | 0/1 | Stage 0 | MRI | MRI interval round | |
| 6 | BRCA1 | CL | yes | 43 | IDC + DCIS | 2 | neg | neg | neg | 0.7 | 0.7 | 0/5 | Stage 1 | MRI | MRI interval round |
| 7 | none | CL | yes | 59 | IDC + DCIS | 2 | pos | pos | neg | 0.6 | 0.6 | 0/4 | Stage 1 | MRI | MRI interval round |
| 8 | BRCA1 | CL | yes | 43 | IDC + DCIS | 2 | pos | pos | neg | 1.0 | NAC (0.9) | 0/3 | Stage 1 | MRI | MRI + MG round |
| 9 | BRCA1 | none | yes | 51 | DCIS only | 3 | nd | nd | nd | 0/4 | Stage 0 | MG | MRI + MG round | ||
| 10 | CDH1 | none | no | 75 | ILC + LCIS | 1 | pos | neg | neg | 1.6 | 0.8 | 0/1 | Stage 1 | both | MRI + MG round |
| 11 | BRCA2 | CL | yes | 66 | IDC + DCIS | 3 | neg | neg | neg | 1.1 | NAC (0) | 0/3 | Stage 1 | both | MRI + MG round |
| 12 | BRCA1 | IL | yes | 61 | IDC + DCIS | 3 | pos | pos | neg | 0.9 | 0.8 | 0/2 | Stage 1 | MRI | MRI interval round |
| 13 | BRCA1 | none | no | 43 | IDC + DCIS | 3 | pos | neg | neg | 1.2 | 0.9 | 0/2 | Stage 1 | MRI | MRI interval round |
| 14 | BRCA1 | CL | yes | 50 | IDC + DCIS | 3 | neg | neg | neg | 1.3 | 0.4 | 0/2 | Stage 1 | both | MRI + MG round |
| 15 | BRCA2 | none | yes | 48 | IDC + DCIS | 3 | pos | pos | neg | 0.9 | 0.6 | 0/4 | Stage 1 | MRI | MRI interval round |
| 16 | BRCA1 | none | yes | 55 | IDC + DCIS | 3 | neg | neg | neg | 0.8 | 0.5 | 0/2 | Stage 1 | both | MRI + MG round |
| 17 | BRCA2 | none | Yes | 36 | DCIS only | 2 | pos | pos | nd | 0/0 | Stage 0 | none | PMS | ||
Abbreviations: BSO, bilateral salpingo-oophorectomy; CL, contralateral; DCIS, ductal carcinoma in situ; ER, estrogen receptor; HER2, HER2 receptor; IDC, invasive ductal cancer; ILC, invasive lobular cancer; IP, ipsilateral; LCIS, lobular carcinoma in situ; MF, multifocal (9 mm biggest); MG, mammography; MRI, dynamic contrast-enhanced magnet resonance imaging; NAC, neoadjuvant chemotherapy; nd, not done; neg, negative; PMS, prophylactic mastectomy specimen; pos, positive; PR, progesterone receptor. Symbols: +, plus.
Recall and Biopsy Rates
Ninety-one women had 106 recalls, of which nineteen were following MG alone, seventy-two following MRI alone, and fifteen following combined imaging modalities. The recall rates per 100 screening episodes were 4.1% for MRI, 2.8% for MG, and 4.8% for combined modalities. For BI-RADS 3, only four examinations were repeated with an ultra short-term MRI and then followed on study every six months per protocol. In total, 54 biopsies were performed (Table 2, Supplementary Figure S3) and 5.8 recalls and 3.4 biopsies were needed to diagnose one cancer. Of note, for BRCA1 mutation carriers, the screening yield was excellent with 2.8 recalls and 1.7 biopsies to detect one cancer using bi-annual MRI. This is in contrast to 12.0 recalls and 8.0 biopsies for one cancer detected in BRCA2 mutation carriers, and 11.7 recalls and 5.0 biopsies for one cancer detected in other women.
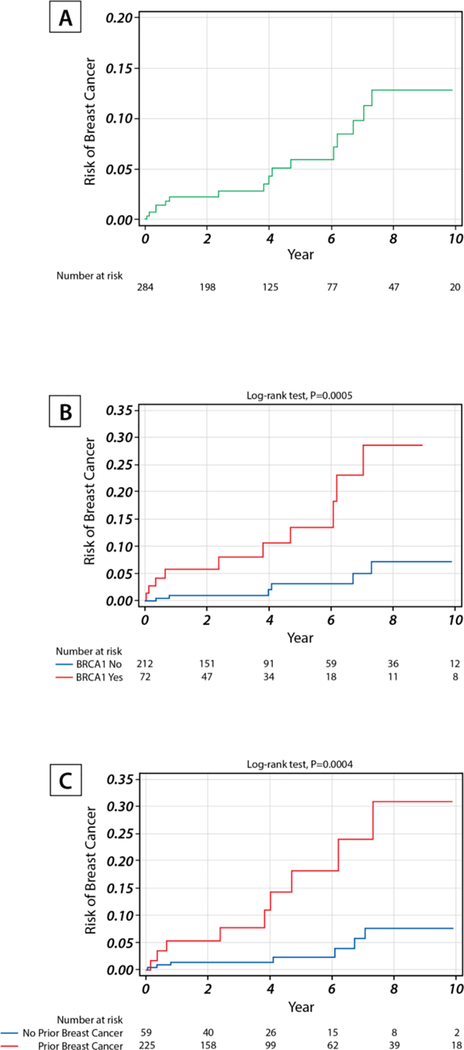
Breast Cancer Incidence Rates
Over a median follow-up of 3.1 years, the overall breast cancer incidence rate was 1.42 per 100 person-years (Figure 3A, 95% CI: 0.83–2.27). Eleven of the 75 BRCA1 mutation carriers developed breast cancers, yielding an incidence rate of 3.65 per 100 person-years (95% CI: 1.82–6.53) that was significantly higher than that in women without BRCA1 mutations (Figure 3B, p=0.0005). Women with prior history of breast cancer had higher risk of breast cancer than those without (Figure 3C, p=0.0004). In the multivariable Cox model of the two factors, both factors were predictors for breast cancer occurrence; specifically, the adjusted hazard ratio for BRCA1 was 4.86 (95% CI: 1.76–13.45) and the adjusted hazard ratio for prior breast cancer was 4.74 (95% CI: 1.76–12.78).
Figure 3.
Breast cancer incidence rate per 100 person-years in all subjects (A), by BRCA1 status (B), and by prior breast cancer status(C).
Because 11 subjects had only baseline image, 284 subjects were included in the incidence rate calculation.
DISCUSSION
This is the first report on a prospective cohort of genetically defined high-risk women undergoing intensive surveillance with MRI every 6 months in conjunction with clinical breast examinations and annual MG. Thirteen invasive cancers and four DCIS were diagnosed, predominantly in BRCA1 mutation carriers (65%). Although sensitivity and specificity of this novel approach were similar to previous studies using annual MRI and MG surveillance, the present study differs in that all invasive cancers were detected at sizes ≤ 1cm with zero nodal involvement and no interval invasive cancers. Most significantly, this prospective study demonstrates for the first time that aggressive BRCA1 associated breast cancers can be downstaged using MRI every 6 months without subjecting women to excessive recalls or biopsies. There were too few cancers in BRCA2 mutation carriers to make definitive conclusions about benefit of bi-annual MRI.
Diagnosing breast cancer at an early and treatable stage is crucial for improving outcomes for young women with breast cancer due to inherited mutations (26). In this imaging-rich study, from 3,334 imaging studies (2,111 MRI and 1.223 MG), sixteen cancers were detected with screening. Of the eight invasive cancers detected only by MRI, three were detected on examinations when both MRI and MG were performed, and five were detected on interval rounds when only MRI was used. Considering the aggressive biology of inherited breast cancers, these five invasive cancers likely represent cancers that would have been diagnosed at more advanced stages if MRI were used annually. Previous studies of combined annual MRI and MG in high-risk patients with long-term follow-up, specifically in BRCA1 and BRCA2 carriers, detected breast cancers at more advanced stages, including approximately 15% cancers with nodal involvement and 5% interval cancers(4–7, 14, 19). Few studies have evaluated a bi-annual screening approach. A retrospective single institution chart review report of alternating yearly MRI with MG in 73 BRCA1/2 carriers detected 10 invasive cancers of which 70% were > 1 cm and 10% showed lymph node involvement(15). Furthermore, considering the lack of added value of annual MG to MRI alone in surveillance of high risk women demonstrated in our study and others (16, 19, 27, 32, 33), as well as the concerns about the risk of radiation-induced breast cancer in young women(21), the routine use of MG screening for women at high genetic risk undergoing MRI screening warrants reconsideration, particularly for BRCA-mutation carriers under 40 years old.
The strengths of the study include its prospective design, genomic stratification of participants using panel sequencing, and long term follow up (34, 35). More than half of the participants were carriers of highly penetrant mutations in breast cancer susceptibility genes. The recall rate of 4.1% (87/2111) for MRI was lower than recall rates of 10–28% reported in high-risk women undergoing annual surveillance with MRI in previous studies(5, 36–39) and reached the current target rate of <7% recommended by the National Health Service Breast Cancer Screening Program in the United Kingdom (UK)(38, 40). Our study demonstrated that, with radiology reader expertise, careful clinical decision-making, and improved MRI technology, it is possible to achieve high positive predictive value and low recall rates. Most significant is the exceedingly high cancer yield in BRCA1 mutation carriers where we only needed 1.7 biopsies to diagnose 1 cancer in comparison to 8.0 and 5.0 biopsies for BRCA2 carriers and non-BRCA mutation carriers, respectively. Thus, similar sensitivity/specificity, a higher positive predictive value, and a lower false-positive biopsy rate in women with mutations in BRCA1 compared to other groups, suggest that this screening strategy may be more beneficial to BRCA1 mutation carriers. Lastly, while this screening study was not designed to provide information about overall survival, it did meet highly relevant surrogate end points of lack of interval invasive cancers and downstaging of aggressive tumors.
Study limitations include the relatively small number of events, as well as its non-randomized and single-institution design. Nonetheless, this genomic and imaging biomarker-rich study provides the framework for optimizing screening for early detection and cancer interception in high-risk populations. More than half of the incident cancers occurred in women with prior diagnosis of breast cancer but this is because genetic testing now often occurs after a diagnosis of cancer and these women are at risk for second primary cancers. These women are also highly motivated for secondary prevention opportunities to improve overall outcomes. The study also included participants who tested negative for any pathogenic mutation but had > 20% lifetime risk. These participants had a lower incidence rate of breast cancer than BRCA1 and BRCA2 mutation carriers, highlighting that better risk prediction models for women at different levels of risk, models that are molecular subtype-specific, are needed for future prevention and early detection studies (41–43).
In summary, this is the first prospective study to show that aggressive breast cancer in high-risk patients can be downstaged using bi-annual MRI in genomically stratified high-risk women. In the setting of appropriate risk stratification using BROCA panel, MRI every 6 months performed exceedingly well in BRCA1 carriers and women with prior breast cancer. Yearly MG did not increase the yield of invasive cancer diagnoses and could probably be eliminated in future studies. MG is known to lead to unnecessary biopsies and over-diagnosis of indolent lesions and DCIS(44). The goal of intensive imaging surveillance should be to downstage aggressive breast cancer as a first step towards improving overall outcomes for mutation carriers(45). In the UK, screening recommendations for young BRCA mutation carriers (< 40 years) does not include MG(46). The ongoing WISDOM Trial in the US is specifically designed to address over-diagnosis and overtreatment of indolent breast cancers by developing a population-based approach to risk stratification(20). Emerging technologies such as ultrafast and abbreviated MRI protocols and use of less contrast material have the potential to further improve performance and reduce overall costs of screening for patients at the highest risk of aggressive breast cancer without losing specificity and sensitivity (43, 47). Lastly, with improved understanding of penetrance of pathogenic mutations in breast cancer susceptibility genes such as BRCA1 and BRCA2, the cost-effectiveness of population screening to identify all mutation carriers, preferably by age 30 years, as well as the benefit of intensive surveillance coupled with primary prevention protocols deserve further evaluation.
Supplementary Material
Statement of translational relevance.
This is the first report on a prospective cohort of genomically defined high-risk women undergoing screening with bi-annual dynamic contrast-enhanced magnetic resonance imaging (MRI) in conjunction with annual mammography. This novel screening approach performed well, especially for women at high genetic risk, by detecting invasive cancers at sizes ≤ 1cm without nodal involvement and effectively avoiding interval invasive cancers with low recall rates. Annual mammography did not demonstrate a screening benefit when performed in conjunction with bi-annual MRI screening. Thus, with optimal genomic risk stratification, intensive surveillance using innovative bi-annual MRI imaging protocol has the potential to detect early stage breast cancer, especially in women at risk of aggressive BRCA1-associated breast cancer.
ACKNOWLEDGEMENTS
Special thanks to all the women who enrolled in this study as far back as 2004 and the Breast program advocates at the University of Chicago who provided much needed guidance and patient perspective for this study. We thank Dr. Gini Fleming for her service as the Data and Safety Study Monitor.
Funding: The study was supported through funding from the National Cancer Institute grant P50CA125183, the Ralph and Marian Falk Medical Research Trust, Susan G. Komen for the Cure SAC110026, Breast Cancer Research Foundation, and the Housewares Charity Foundation awarded to O.I. Olopade. M.C. King and O.I. Olopade are American Cancer Society Professors.
Footnotes
Presented at the American Society of Clinical Oncology Annual Meeting 2013 (J Clin Oncol 31, 2013 [suppl, abstract 1506]; J Clin Oncol 31, 2013 [suppl; abstr 11084]) and San Antonio Breast Cancer Symposium 2017 ([P4-02-01]; [P4-02-10]).
Disclosure of Potential Conflicts of Interest
OIO is a Co-Founder at CancerIQ.
Trial Registration: NCT00989638
REFERENCES
- 1.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 1.2018). https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf [October 12, 2017]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. [DOI] [PubMed]
- 2.Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, et al. Gene-panel sequencing and the prediction of breast-cancer risk. The New England Jjournal of medicine. 2015. June 04;372(23):2243–57. PubMed PMID: Pubmed Central PMCID: 4610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tung N, Domchek SM, Stadler Z, Nathanson KL, Couch F, Garber JE, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nature reviews Clinical oncology. 2016. September;13(9):581–8. PubMed PMID: Pubmed Central PMCID: 5513673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. The New England journal of medicine. 2004. July 29;351(5):427–37. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Warner E, Plewes DB, Hill KA, Causer PA, Zubovits JT, Jong RA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004. September 15;292(11):1317–25. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 6.Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005. November 20;23(33):8469–76. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005. May 21–27;365(9473):1769–78. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 8.Sardanelli F, Podo F, D’Agnolo G, Verdecchia A, Santaquilani M, Musumeci R, et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology. 2007. March;242(3):698–715. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 9.Lehman CD, Blume JD, Weatherall P, Thickman D, Hylton N, Warner E, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005. May 1;103(9):1898–905. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 10.Lord SJ, Lei W, Craft P, Cawson JN, Morris I, Walleser S, et al. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007. September;43(13):1905–17. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008. May 6;148(9):671–9. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 12.Weinstein SP, Localio AR, Conant EF, Rosen M, Thomas KM, Schnall MD. Multimodality screening of high-risk women: a prospective cohort study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009. December 20;27(36):6124–8. PubMed PMID: Pubmed Central PMCID: 2793033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, Konig R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010. March 20;28(9):1450–7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 14.Sardanelli F, Podo F, Santoro F, Manoukian S, Bergonzi S, Trecate G, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 study): final results. Invest Radiol. 2011. February;46(2):94–105. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 15.Le-Petross HT, Whitman GJ, Atchley DP, Yuan Y, Gutierrez-Barrera A, Hortobagyi GN, et al. Effectiveness of alternating mammography and magnetic resonance imaging for screening women with deleterious BRCA mutations at high risk of breast cancer. Cancer. 2011. September 01;117(17):3900–7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 16.Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, Tea MK, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015. April 01;33(10):1128–35. PubMed PMID: Pubmed Central PMCID: 5526626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007. Mar-Apr;57(2):75–89. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 18.Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Annals of oncology : official journal of the European Society for Medical Oncology. 2016. September;27(suppl 5):v103–v10. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 19.Phi XA, Houssami N, Obdeijn IM, Warner E, Sardanelli F, Leach MO, et al. Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age >/= 50 years: evidence from an individual patient data meta-analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015. February 01;33(4):349–56. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 20.Esserman LJ, Study W, Athena I. The WISDOM Study: breaking the deadlock in the breast cancer screening debate. NPJ breast cancer. 2017;3:34 PubMed PMID: Pubmed Central PMCID: 5597574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pijpe A, Andrieu N, Easton DF, Kesminiene A, Cardis E, Nogues C, et al. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK). BMJ. 2012;345:e5660 PubMed PMID: Pubmed Central PMCID: 3435441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegel TN, Esplen MJ, Hill KA, Wong J, Causer PA, Warner E. Psychological impact of recall on women with BRCA mutations undergoing MRI surveillance. Breast. 2011. October;20(5):424–30. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 23.Plevritis SK, Kurian AW, Sigal BM, Daniel BL, Ikeda DM, Stockdale FE, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006. May 24;295(20):2374–84. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 24.Pataky R, Armstrong L, Chia S, Coldman AJ, Kim-Sing C, McGillivray B, et al. Cost-effectiveness of MRI for breast cancer screening in BRCA1/2 mutation carriers. BMC Cancer. 2013;13:339 PubMed PMID: Pubmed Central PMCID: 3711845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phi XA, Saadatmand S, De Bock GH, Warner E, Sardanelli F, Leach MO, et al. Contribution of mammography to MRI screening in BRCA mutation carriers by BRCA status and age: individual patient data meta-analysis. British journal of cancer. 2016. March 15;114(6):631–7. PubMed PMID: Pubmed Central PMCID: 4800299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heijnsdijk EA, Warner E, Gilbert FJ, Tilanus-Linthorst MM, Evans G, Causer PA, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening-MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012. September;21(9):1458–68. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 27.Obdeijn IM, Winter-Warnars GA, Mann RM, Hooning MJ, Hunink MG, Tilanus-Linthorst MM. Should we screen BRCA1 mutation carriers only with MRI? A multicenter study. Breast cancer research and treatment. 2014. February 25 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 28.Shah P, Rosen M, Stopfer J, Siegfried J, Kaltman R, Mason B, et al. Prospective study of breast MRI in BRCA1 and BRCA2 mutation carriers: effect of mutation status on cancer incidence. Breast cancer research and treatment. 2009. December;118(3):539–46. PubMed PMID: Pubmed Central PMCID: 3342814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huo D, Senie RT, Daly M, Buys SS, Cummings S, Ogutha J, et al. Prediction of BRCA Mutations Using the BRCAPRO Model in Clinic-Based African American, Hispanic, and Other Minority Families in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009. March 10;27(8):1184–90. PubMed PMID: Pubmed Central PMCID: PMC2667822. Epub 2009/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinreb JC, Newstead G. MR imaging of the breast. Radiology. 1995. September;196(3):593–610. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 31.Walsh T, Lee MK, Casadei S, Thornton AM, Stray SM, Pennil C, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proceedings of the National Academy of Sciences of the United States of America. 2010. July 13;107(28):12629–33. PubMed PMID: Pubmed Central PMCID: 2906584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo G, Scaranelo AM, Aboras H, Ghai S, Kulkarni S, Fleming R, et al. Evaluation of the Utility of Screening Mammography for High-Risk Women Undergoing Screening Breast MR Imaging. Radiology. 2017. October;285(1):36–43. PubMed PMID: Epub 2017/06/07. [DOI] [PubMed] [Google Scholar]
- 33.van Zelst JCM, Mus RDM, Woldringh G, Rutten M, Bult P, Vreemann S, et al. Surveillance of Women with the BRCA1 or BRCA2 Mutation by Using Biannual Automated Breast US, MR Imaging, and Mammography. Radiology. 2017. November;285(2):376–88. PubMed PMID: Epub 2017/06/14. [DOI] [PubMed] [Google Scholar]
- 34.Vetter L, Keller M, Bruckner T, Golatta M, Eismann S, Evers C, et al. Adherence to the breast cancer surveillance program for women at risk for familial breast and ovarian cancer versus overscreening: a monocenter study in Germany. Breast cancer research and treatment. 2016. April;156(2):289–99. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Stout NK, Nekhlyudov L, Li L, Malin ES, Ross-Degnan D, Buist DS, et al. Rapid increase in breast magnetic resonance imaging use: trends from 2000 to 2011. JAMA internal medicine. 2014. January;174(1):114–21. PubMed PMID: Pubmed Central PMCID: 4145846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiely BE, Hossack LK, Shadbolt CL, Davis A, Cassumbhoy R, Moodie K, et al. Practicalities of developing a breast magnetic resonance imaging screening service for women at high risk for breast cancer. ANZ journal of surgery. 2011. October;81(10):688–93. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 37.Warren RM, Pointon L, Caines R, Hayes C, Thompson D, Leach MO, et al. What is the recall rate of breast MRI when used for screening asymptomatic women at high risk? Magnetic resonance imaging. 2002. September;20(7):557–65. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 38.Healy NA, O’Keeffe SA. Determination of recall rates for assessment in high-risk women undergoing annual surveillance breast MRI. Clinical radiology. 2016. November;71(11):1143–7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 39.Chiarelli AM, Prummel MV, Muradali D, Majpruz V, Horgan M, Carroll JC, et al. Effectiveness of screening with annual magnetic resonance imaging and mammography: results of the initial screen from the ontario high risk breast screening program. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014. July 20;32(21):2224–30. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 40.NHS. Technical guideline for MRI for the surveillance of women at higher risk of developing breast cancer (NHSBSP Publication No 68) Sheffield: NHS Cancer Screening Programmes; 2012. [Google Scholar]
- 41.Kuchenbaecker KB, McGuffog L, Barrowdale D, Lee A, Soucy P, Dennis J, et al. Evaluation of Polygenic Risk Scores for Breast and Ovarian Cancer Risk Prediction in BRCA1 and BRCA2 Mutation Carriers. Journal of the National Cancer Institute. 2017. July 01;109(7). PubMed PMID: Pubmed Central PMCID: 5408990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Feng B, Miron A, Chen X, Beesley J, Bimeh E, et al. Breast cancer risk prediction using a polygenic risk score in the familial setting: a prospective study from the Breast Cancer Family Registry and kConFab. Genetics in medicine : official journal of the American College of Medical Genetics. 2017. January;19(1):30–5. PubMed PMID: Pubmed Central PMCID: 5107177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ECOG-ACRIN Cancer Research Group. Abbreviated Breast MRI and Digital Tomosynthesis Mammography in Screening Women With Dense Breasts (NCT02933489). https://clinicaltrials.gov/ct2/show/NCT02933489.
- 44.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. The New England journal of medicine. 2012. November 22;367(21):1998–2005. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 45.van Luijt PA, Heijnsdijk EA, Fracheboud J, Overbeek LI, Broeders MJ, Wesseling J, et al. The distribution of ductal carcinoma in situ (DCIS) grade in 4232 women and its impact on overdiagnosis in breast cancer screening. Breast cancer research : BCR. 2016. May 10;18(1):47 PubMed PMID: Pubmed Central PMCID: 4862233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NICE 2017. Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer (CG164). https://www.nice.org.uk/guidance/cg164. [PubMed]
- 47.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated Breast Magnetic Resonance Imaging (MRI): First Postcontrast Subtracted Images and Maximum-Intensity Projection-A Novel Approach to Breast Cancer Screening With MRI. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014. August 1;32(22):2304–10. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.