Abstract
Orexin neurons (Orx; also referred to as hypocretin) are found exclusively in the hypothalamus, and release the neuropeptides orexin A and orexin B (also referred to as hypocretin 1 and 2) throughout the CNS. With its widespread targets, the orexin system is involved in a number of functions including, but not limited to stress, reward, wakefulness, and food seeking. Our laboratory has previously proposed that the dorsomedial hypothalamus (DMH) and perifornical (PFA) orexin neurons function in stress and arousal whereas those in lateral hypothalamus (LH) participate in reward processes (Harris and Aston-Jones 2006). In the current study, we compared Fos activation in orexin neurons located in medial hypothalamus (DMH and PFA) to those in LH during a Go/No-Go task for a highly palatable food reward, a task that would likely activate regions for arousal/attention as well as reward. The Go/No-Go paradigm is a useful behavioral tool to measure behavioral inhibition, impulsivity, learning, and reaction time. Our results revealed increased activation of medial hypothalamic orexin neurons correlated with greater accuracy on the Go/No-Go task. No correlation was found between Go/No-Go accuracy and activation of lateral hypothalamic orexin neurons. This study supports a functional dichotomy of medial vs lateral orexin neurons, and indicates a role for medial orexin neurons in behavioral performance that requires response inhibition.
Keywords: Orexin, Go/No-Go, response inhibition, palatable food, reward
1. Introduction
Orexin neurons (also referred to as hypocretin) are found exclusively in the hypothalamus, and release the neuropeptides orexin A and orexin B (also referred to as hypocretin 1 + 2) from their terminals throughout the CNS. Because it has such widespread targets, it seems possible that the orexin system is involved in multiple functions. Early experiments revealed that intracerebral administration of orexin lengthened periods of wakefulness and also produced feeding (Sakurai, Amemiya et al. 1998, de Lecea and Sutcliffe 1999, Siegel 1999, Peyron, Faraco et al. 2000, de Lecea and Sutcliffe 2005, Sakurai 2007). Further studies advanced our understanding of orexin’s role in sleep and wakefulness; for example, the loss of orexin peptide, neurons or receptors is associated with narcolepsy/cataplexy (NC) in rodents, dogs and humans (Mignot 2004, de Lecea and Sutcliffe 2005, Sakurai 2007, Mahler, Moorman et al. 2014). Previous experiments have shown that orexin’s role in feeding is specific for seeking and consuming palatable foods, and food seeking elicited by Pavlovian cues rather than simple feeding per se (Borgland, Chang et al. 2009, Cason, Smith et al. 2010, Berthoud and Munzberg 2011, Mahler, Smith et al. 2012, Mahler, Moorman et al. 2014). Orexin has been implicated in other functions including: drug seeking, responding to stress, homeostatic regulation, cognition, and motivational activation (Mahler, Moorman et al. 2014, James, Mahler et al. 2017). The current study was aimed at testing a possible role of orexin in attention required to seek palatable food rewards using a Go/No-Go task (Winstanley 2011).
There is also considerable evidence for heterogeneity amongst orexin neurons. Specifically, our laboratory proposed that the dorsomedial hypothalamus (DMH) and perifornical (PFA) orexin neurons function in stress and arousal, whereas those in lateral hypothalamus (LH) participate in reward processes (Harris and Aston-Jones 2006). In the current study, we compared Fos activation in medial hypothalamus (DMH and PFA) to LH during a Go/No-Go task for a highly palatable food reward, a task that would likely activate regions for arousal/attention as well as reward.
2. Results
2.1. Go/No-Go Task and Tone Test
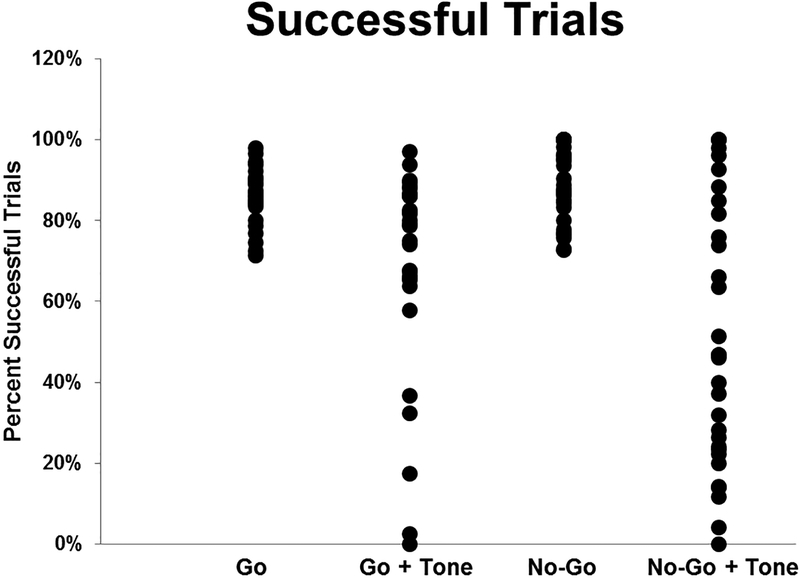
Animals (n=30) were trained on a Go/No-Go task until they met a criterion of 70% success on both Go and No-Go trials. To reach criterion, rats performed the Go/No-Go task for 17 ± 8 days. Despite the differences in the number of days to reach criterion, the number of training days did not correlate with success on Go/No-Go trials or the tone test. The reward during Go-trial training and the Go/No-Go task was a 45 mg chocolate-flavored sucrose pellet (Bio-Serv, Frenchtown, NJ, USA). After maintaining at least 70% success for at least 5 days, rats were given a tone test to evaluate distractibility and flexibility. The same discrete tone that was paired with reward during initial operant training was presented at the start of every Go/No-Go trial as a distractor. During the Tone Test, overall performance was decreased, with a significant reduction in accuracy on Go (p <0.001) and No-Go trials (Fig. 1; p<0.001). A performance ratio for each rat was calculated to quantify the change in performance due to the distractor tone, regardless of strategy. During the tone test, some animals decreased successful Go trials whereas other animals decreased successful No-Go trials. In order to collectively evaluate a change in performance, the performance ratio was calculated as: % successful Go trials/% successful No-Go trials if Go trials were affected by the distractor tone more than No-Go trials. For animals with a greater change to No-Go trials due to the tone, the performance ratio was calculated as: % successful No-Go trials/% successful Go trials. A small group of animals decreased Go success and increased No-Go success on tone trials, but most animals decreased No-Go success and maintained Go success. Therefore, animals with a significant decrease in behavioral accuracy during the tone test have a low ratio, and those animals that continued high success on both Go and No-Go trials exhibited a high ratio (closer to 1). Two groups of animals were defined based on behavior on the Tone Test: “Low Success” (performance ratio <0.5) and “High Success” (performance ratio >0.5). Further analysis of strategy is shown in Figures 3 and 4 and reviewed in the Discussion.
Figure 1. Go/No-Go Task and Tone Test.
Animals (n=30) were trained to a criterion of 70% success or higher on both Go and No-Go trials. During the Tone Test, overall performance was decreased with a significant reduction in success on No-Go trials.
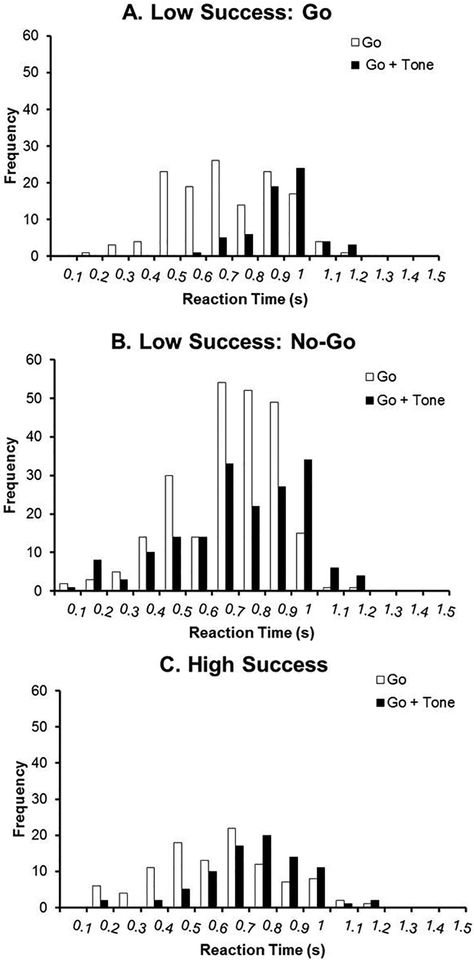
Figure 3. Reaction Times during the Go/No-Go Task and Tone Test: Go Trials.
Reaction times from Go trials are presented and separated into three groups: animals that exhibited low success specifically on Go trials during the Tone Test (Figure 3A), animals that exhibited low success specifically on No-Go trials during the Tone Test (Figure 3B), and animals that exhibited high success on both trials (Figure 3C). The frequency represents lever presses from all animals during the first 20 trials binned by 100 ms.
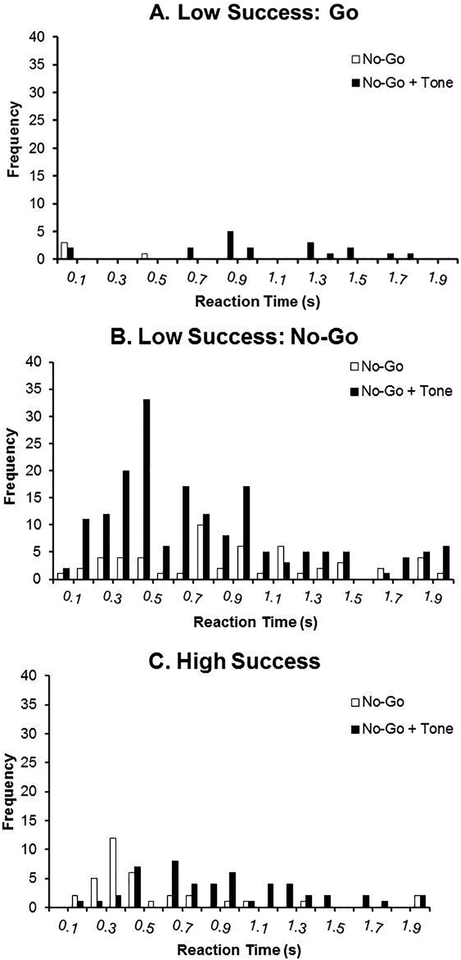
Figure 4. Reaction Times during the Go/No-Go Task and Tone Test: No-Go Trials.
Reaction times from all trials are presented for No-Go trials and separated into three groups: animals that exhibited low success specifically on Go trials during the Tone Test (Figure 4A), animals that exhibited low success specifically on No-Go trials during the Tone Test (Figure 4B), and animals that exhibited high success on both trials (Figure 4C). The frequency represents lever presses from all animals during the first 20 trials binned by 100 ms.
2.2. Fos Expression in Medial and Lateral Hypothalamic Orexin Neurons
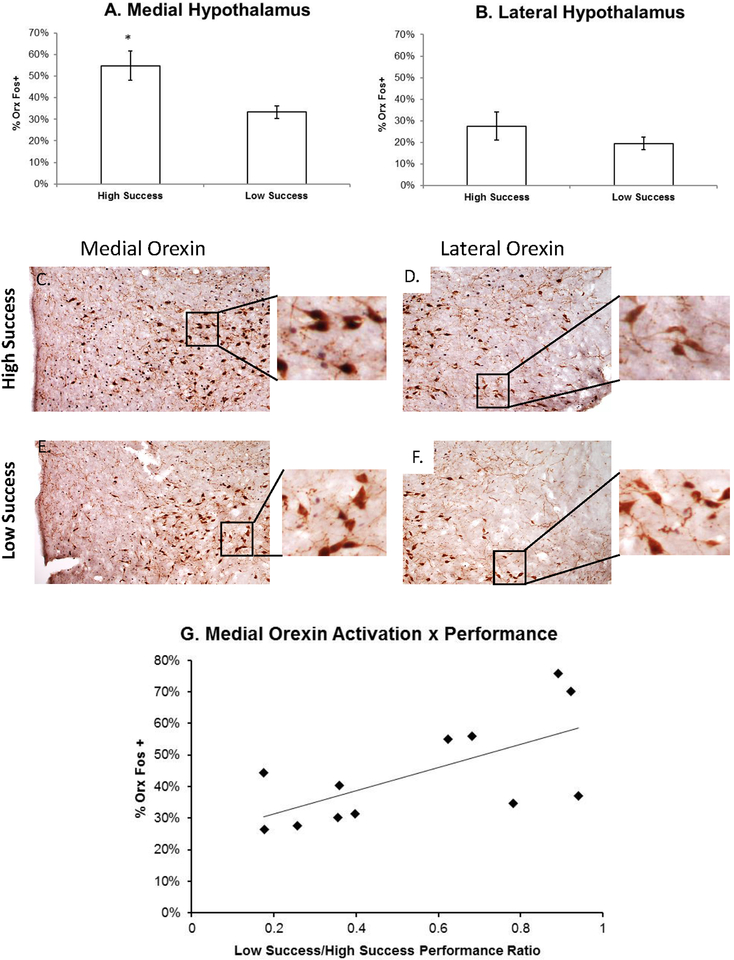
The percent of orexin neurons that were Fos-positive (%Orx Fos+) in the medial and lateral hypothalamus are presented in Fig 2 (n=12). Animals with high success on the tone test had a significantly greater percentage of Fos+ Orx neurons in the medial hypothalamus (55 ± 16.7%) compared to those with low success (33 ± 7%; p = 0.016). No significant differences were observed in the lateral hypothalamus (Low Success: 19.5 ±7% vs. High Success: 27.5 ± 16%; p = 0.29). Furthermore, % Orx Fos+ neurons was positively correlated with performance ratio (r =0.647; p = 0.023). No significant difference in the number of Orx neurons was determined between groups in the medial or lateral hypothalamus.
Figure 2. Fos Expression in Medial and Lateral Hypothalamic Orexin Neurons.
The percent of Orx neurons that were Fos-positive (%Orx Fos+) in the medial (A) and lateral hypothalamus (B) are presented. Animals with high success on the Tone Test had a significantly greater percentage of Fos+ Orx neurons in the medial hypothalamus compared to those with low success. No significant differences were observed in the lateral hypothalamus. Micrographs (C-F; 10X objective) demonstrate Orx (brown) and Fos (black) labeling in the medial and lateral orexin population (with higher power insets to reveal double-labeling). % Orx Fos+ neurons was positively correlated with performance ratio (G).
2.3. Reaction Times during the Go/No-Go Task and Tone Test
The performance ratio was calculated to evaluate a change in behavior due to the distractor tone. As previously discussed in 2.1, strategy (whether Go trials or No-Go trials were affected by the tone) was not included in the previous analysis. Here, animals are further categorized based on their specific performance in order to gain a greater understanding of changes that occurred during the tone test. Reaction time histograms are presented for the Tone Test and compared to the previous day of the Go/No-Go task (no tones) in Figures 3 and 4. Reaction times for all animals were binned by 100 ms from 0 to 1.2 seconds for Go trials and 0 to 2 seconds for No-Go trials. In Figure 3, reaction times from the first 20 trials are presented for Go trials and separated into three groups: animals that exhibited low success specifically on Go trials during the Tone Test (Figure 3A), animals that exhibited low success specifically on No-Go trials during the Tone Test (Figure 3B), and animals that exhibited high success on both trials with tones (Figure 3C). During the Go/No-Go task (no tones), the Low Success: Go group made the most lever presses 500ms −1s after the lever was extended. During the tone test, the majority of lever presses shifted to 900 ms – 1.1s. Therefore, lower success on these trials is likely due to a greater latency to press. Animals with low success on No-Go trials (and typically high success on Go trials) did not exhibit a major shift in the timing of lever presses. During the Go/No-Go experiment, the majority of lever presses occurred between 700 – 900 ms and during the tone test, the majority of lever presses occurred between 700 ms – 1 s. Animals with high success on both trial types with tones (“High Success”) exhibited a forward shift in reaction times but not to the extent of the Low success:Go group. Of note, these animals did not complete as many trials as the other groups.
In Figure 4, reaction times from all trials are presented for No-Go trials and separated into the same groups as described above. The Low Success: Go group revealed very few lever presses during the Tone Test and even fewer responses during the previous day of Go/No-Go trials. Therefore, animals earned most of their rewards by withholding responding since responses during No-Go trials are always errors. Trials were randomly presented with 70% Go trials and 30% No-Go trials; these animals earned fewer rewards overall due to this strategy. On the other hand, the Low Success: No-Go group revealed increased responding early on in the trial (400–500 ms) during the Tone Test. These reaction times were not similar to the reaction times observed during a Go trial (without the tone) and therefore, the tone was not only increasing errors, but also speeding up reaction times. The High Success group had low numbers of No-Go errors.
3. Discussion
In this study, we demonstrated increased activation of medial hypothalamic orexin neurons correlated with greater accuracy on a Go/No-Go task. No correlation was found between Go/No-Go accuracy and activation of lateral hypothalamic orexin neurons. Our laboratory has proposed a dichotomy in orexin function with medial cells affecting arousal and wakefulness and lateral cells affecting reward (Harris & Aston-Jones 2006). Previous studies from our laboratory demonstrated that LH orexin neurons that project to the VTA exhibit Fos that correlates with reward preference, whereas PFA or DMH orexin neurons that project to locus coeruleus, do not (Richardson and Aston-Jones 2012). We also found that footshock stress activates orexin neurons in DMH and PFA, but not in LH (Harris and Aston-Jones 2006). In experiments specific to a palatable food reward, mixed results for activation of medial and lateral populations of orexin neurons have been shown. For example, rats that completed conditioned place preference for a sweet cereal reward exhibited increased activation of LH orexin neurons compared to DMH/PFA orexin neurons (Harris et al. 2005). On the other hand, in a study where rats received contextual conditioning paired with a chocolate reward, both LH and PFA orexin neurons were activated compared to control rats that did not receive the chocolate pairing. In the current study, a complex task and a palatable food reward were administered. Therefore, we tested whether there was preferential activation of medial or lateral populations under these potentially conflicting circumstances. If given a complex task alone, we would hypothesize increased activation of medial orexin neurons. If given a palatable reward alone, we would hypothesize increased activation of lateral orexin neurons, based on previous studies. Our results indicate that medial orexin cells were not only more activated during this task compared to LH cells, but they also correlated with performance.
The Go/No-Go task resulted in variable performances despite rats receiving the same chocolate-flavored sucrose reward and the same training. For animals with low success on Go trials (Low Success: Go), reaction times increased to 900 ms −1 s on the tone test versus 500–700 ms on previous trials. On the other hand, animals with high success on Go trials had a slightly slower reaction time (700–900 ms), but this was still fast enough to complete a successful trial. Therefore, the Low Success: Go animals were more distracted by the tone compared to the Low Success: No-Go and High Success animals. The observed strategy for successfully completing No-Go trials was different. Here, success on the trials by the Low Success: Go and High Success groups was due to fewer responses, independent of timing. The High Success group exhibited few responses throughout the trial (100 ms – 1.9s), however, this group committed fewer errors (fewer lever presses) overall. Animals in the “Low Success: No-Go” group revealed the highest responding at 500 ms. A response at 500 ms was not a typical response during a Go trial (without the tone) and therefore, the tone was not only increasing errors, but also speeding up reaction times. During early training, the rat never experienced a tone prior to lever press and dispensing of a pellet. Instead, the rat pressed the lever and the tone and pellet were presented simultaneously. For these animals, Pavlovian-instrumental transfer (PIT) was occurring as presentation of the tone elicited increased reward seeking. These animals were more distractible and revealed less Fos+ orexin neurons in the medial hypothalamus.
Response inhibition, as measured in Go/No-Go tasks, is an important executive-control mechanism that has been proposed to be impaired in obesity (Nederkoorn, Smulders et al. 2006). A recent study in human subjects has shown decreased response inhibition which correlated with increasing BMI (Batterink, Yokum et al. 2010). Furthermore, self-reported impulsivity correlated positively with caloric intake and activation of reward circuitry in response to images of food. Interestingly, impulsivity was negatively correlated with weight loss during obesity intervention treatments (Batterink et al 2010). In our study, no significant differences in weight gain and body weights were observed between the High Success and Low Success groups. All rats were limited to 25 grams of home-cage chow per day, and while level of success on the tasks led to differences in rewards earned, it is apparent that these differences in pellets earned were not significant enough to produce significant weight differences.
This study indicates medial orexin activation is involved in successful performance of this response inhibition task. Less medial orexin activation caused greater distractibility, a possible increase in reward seeking, but less success to complete the task necessary to obtain that reward. Animals with high success on both Go and No-Go trials revealed increased medial orexin activation. Previous studies have supported a role for orexin in attention ((Lambe, Olausson et al. 2005, Fadel and Burk 2010, Mahler, Moorman et al. 2014) particularly for learning cues that predict reward (Wheeler, Wan et al. 2014). Animals with the greatest success on this Go/No-Go task initiated fewer trials but they discriminated between Go and No-Go trials with a higher frequency. Further studies will be necessary, but the correlation between increased medial orexin activation and performance suggests a role for attention. In this case, it is possible that increased orexin activity led to greater attention to learn the task in order to receive the highly palatable reward.
This study further supports the functional dichotomy of orexin neurons since we observed increased activation of medial orexin neurons correlated with Go/No-Go success but lateral orexin neurons did not. It is possible that impairment of response inhibition, as well as decreased medial orexin activation, precedes obesity. When combined with easy accessibility to high calorie foods (as found in Western populations), the epidemic proportion of obesity continues. Interestingly, higher signaling of orexin peptides at their receptors has been shown to be protective against obesity (Perez-Leighton et al. 2013). While orexin was given its name due to an important role in producing feeding, its many functions, functional dichotomy, and widespread targets must be considered, especially for orexin’s role in obesity.
4. Experimental Procedure
4.1. Animals
Male Sprague Dawley rats (~250–275 g upon arrival; Charles River Laboratories; n = 30) were single-housed and kept on a reverse 12h light schedule. Rats were fed 25 g of Harlan Teklad 8656 per day and given ad libitum access to water. All protocols and procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
4.2. Behavior
The Go/No-Go task and all behavioral training were performed in Med Associates operant chambers located in sound-attenuating cubicles (Med-Associates, St Albans, VT, USA). Each chamber contained a red house light, two retractable levers with white cue lights above them, a food hopper, and a tone generator.
First, rats were trained to lever press for a 45 mg grain-based pellet or 45 mg high fat pellet (45% fat calories; Bio-Serv, Frenchtown, NJ, USA) for a minimum of 5 days (operant conditioning). Upon lever press, a 2s discrete tone was delivered (78 dB, 2900 Hz) simultaneous with pellet delivery on a fixed ratio 1 (FR1) schedule of reinforcement. A 3s inter-trial interval (ITI) was used, and sessions lasted 30 minutes or until 60 pellets were earned. No significant differences between animals treated with grain-based pellets versus high fat pellets were observed. The independent-samples Mann-Whitney U test revealed no significant differences in the Go trials (p = 0.0164), Go + tone trials (p=0.734), No-Go trials (p=0.603), and No-Go + tone trials (p=0.667). Therefore, the groups rewarded with grain-based pellets and high fat pellets during operant conditioning were collapsed.
After completing at least five sessions of operant conditioning during which 60 pellets were earned, all animals underwent Go-trial training for 5 days. The reward during Go-trial training and the Go/No-Go task was a 45 mg chocolate-flavored sucrose pellet for all animals (Bio-Serv, Frenchtown, NJ, USA). Animals initiated trials with a lever press, after which the pressed lever was immediately retracted and the lever on the other side of the chamber was extended. After 500 ms, the cue light over the new lever was illuminated. Rats were given 1s after cue light presentation to press the lever and were rewarded with pellet delivery on a fixed ratio 1 (FR1) schedule of reinforcement. If the rat did not press the lever within 1s, the lever was retracted and no pellets were given. A 5s ITI was used for all trials, and sessions were 30 minutes long.
After five days of Go-trial training, rats were trained on the Go/No-Go task; trials were presented semi-randomly at a ratio of 70% Go trials to 30% No-Go trials. As described above, rats had to initiate trials with a lever press. After 110 ms, a cue-light was illuminated over the lever to signal a Go trial, or no cue was given to signal a No-Go trial. Rats had to respond within 1 sec on Go trials, and were rewarded with one chocolate-flavored sucrose pellet, and then given a 3s ITI. If the rat did not respond within 1s, the lever retracted, the cue light and house light were turned off, and an 8s ITI was given. For a No-Go trial, rats had to withhold responding on the extended lever for 2s. After a successful trial, the pellet was delivered and then a 3s ITI was given. For an unsuccessful No-Go trial, the lever retracted, the house light turned off and an 8s ITI was given. Sessions were 30 minutes long. Rats were trained until they met a criterion of 70% success on both Go and No-Go trials, and maintained this level of success for at least 5 days. Any rats that did not meet the minimum criteria for operant conditioning, Go-trial training, and the Go/No-Go task, as described above, were removed from the study.
Finally, rats were given a tone test to evaluate distractibility and flexibility during the Go/No-Go task. The same discrete tone (2 seconds, 78 dB, 2900 Hz) that was paired with reward during initial operant training was presented while the left lever (used to initiate trials) was extended at the start of every Go/No-Go trial as a distractor. With this tone test, we were able to observe whether performance on the Go/No-Go task was degraded by this conditioned distractor tone. Successful trials and reaction times during the tone test and the previous day were calculated for a subset of animals (n = 18).
4.3. Tissue Preparation
One hour following the tone test, animals were anesthetized with an overdose of ketamine/xylazine, perfused transcardially with 0.9% saline and 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS), and brains were collected. This time point was chosen because Fos expression is maximal at 60– 90 min after a behavioral manipulation. Brains were kept in 4% paraformaldehyde for 24h and then submerged in a 20% sucrose-azide solution. Coronal brain sections (40 μm thick) were cut through the level of the hypothalamus on a cryostat and stored in PBS-azide.
4.4. Immunohistochemistry
Sections from the hypothalamus were processed for Fos immunohistochemistry as previously described (Mahler and Aston-Jones 2012). Briefly, sections were incubated in a rabbit anti-Fos primary antibody overnight (1:10,000; Calbiochem), followed by 2 h in a donkey anti-rabbit secondary (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA), amplified with the avidin biotin complex (ABC; Vector Labs) method (1:500), and visualized with 3, 3’ diaminobenzidine (DAB) + nickel ammonium sulfate to yield a blue-black nuclear reaction product. The same sections from the hypothalamus were then processed for Orx A immunohistochemistry in a goat anti-Orx A primary antibody overnight (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA). They were incubated for 2h in a donkey anti-goat secondary antibody (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA), amplified with the avidin biotin complex (ABC; Vector Labs) method (1:500), and visualized with 3, 3’ diaminobenzidine (DAB) to yield a brown reaction product.
4.5. Quantification of Fos neurons
Photomicrographs were collected using Openlab image processing software (Improvision) and a Leica microscope (10x objective). The number of Orx-positive neurons (brown reaction product) and neurons double-labeled for Fos and Orx were quantified using a point-counter tool (ImageJ) in two or three sections from both hemispheres. Lateral hypothalamus was defined as all neurons from the lateral edge of the fornix to the cerebral peduncle and medial hypothalamus contained cells dorsal and medial to the fornix to the third ventricle (dorsomedial and perifornical hypothalamus). For all cell counting, the number of immunoreactive cells were averaged across sections for each rat so that each rat produced one mean value. Comparisons between groups were done by analyzing differences between the mean values for rats in each group.
4.6. Data Analysis
Levene’s test indicated unequal variances for the Go/No-Go task (F = 14.301, p<0.001 for Go trials and F = 41.178, p<0.001 for No-Go trials). Therefore, analyses of behavior were performed using the related-samples Wilcoxon Signed-ranks test in order to evaluate Go trials vs. Go + tone trials and No-Go vs. No-Go + tone trials. Fos activation between high success and low success animals had equal variances (Levene’s test indicated equal variances; F = 2.694; p = 0.132) and was therefore analyzed with a t-test. Correlations of Fos expression with behavior were conducted with Pearson r tests, comparing the performance ratio (% low success trials/% high success trials) with Fos+ cells in the medial and lateral hypothalamus. All statistical analyses were performed using IBM SPSS Statistics (Version 21). A p- value less than 0.05 was adopted for all statistical tests.
Highlights.
In this study, a complex task and a palatable food reward were administered. Therefore, we tested whether there was preferential activation of medial or lateral populations under these potentially conflicting circumstances.
Our results revealed increased activation of medial hypothalamic orexin neurons correlated with greater accuracy on the Go/No-Go task.
No correlation was found between Go/No-Go accuracy and activation of lateral hypothalamic orexin neurons.
This study supports a role for medial orexin neurons in behavioral performance that requires response inhibition.
Acknowledgments:
Supported by PHS grants R01-DA006214 and 5T32DA7288–22.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batterink L, Yokum S and Stice E (2010). “Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study.” Neuroimage 52(4): 1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR and Munzberg H (2011). “The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics.” Physiol Behav 104(1): 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT and Bonci A (2009). “Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers.” J Neurosci 29(36): 11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC and Aston-Jones G (2010). “Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity.” Physiol Behav 100(5): 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L and Sutcliffe JG (1999). “The hypocretins/orexins: novel hypothalamic neuropeptides involved in different physiological systems.” Cell Mol Life Sci 56(5–6): 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L and Sutcliffe JG (2005). “The hypocretins and sleep.” Febs J 272(22): 5675–5688. [DOI] [PubMed] [Google Scholar]
- Fadel J and Burk JA (2010). “Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention.” Brain Res 1314: 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC and Aston-Jones G (2006). “Arousal and reward: a dichotomy in orexin function.” Trends Neurosci 29(10): 571–577. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE and Aston-Jones G (2017). “A Decade of Orexin/Hypocretin and Addiction: Where Are We Now?” Curr Top Behav Neurosci 33: 247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Olausson P, Horst NK, Taylor JR and Aghajanian GK (2005). “Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat.” J Neurosci 25(21): 5225–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV and Aston-Jones GS (2012). “Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats.” J Neurosci 32(38): 13309–13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH and Aston-Jones G (2014). “Motivational activation: a unifying hypothesis of orexin/hypocretin function.” Nat Neurosci 17(10): 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC and Aston-Jones G (2012). “Multiple roles for orexin/hypocretin in addiction.” Prog Brain Res 198: 79–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E (2004). “Sleep, sleep disorders and hypocretin (orexin).” Sleep Med 5 Suppl 1: S2–8. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders FT, Havermans RC, Roefs A and Jansen A (2006). “Impulsivity in obese women.” Appetite 47(2): 253–256. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S and Mignot E (2000). “A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains.” Nat Med 6(9): 991–997. [DOI] [PubMed] [Google Scholar]
- Sakurai T (2007). “The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness.” Nat Rev Neurosci 8(3): 171–181. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ and Yanagisawa M (1998). “Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior.” Cell 92(4): 573–585. [DOI] [PubMed] [Google Scholar]
- Siegel JM (1999). “Narcolepsy: a key role for hypocretins (orexins).” Cell 98(4): 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DS, Wan S, Miller A, Angeli N, Adileh B, Hu W and Holland PC (2014). “Role of lateral hypothalamus in two aspects of attention in associative learning.” Eur J Neurosci 40(2): 2359–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]