Abstract
Wearable alcohol biosensors have emerged as a valuable tool for noninvasive, objective and continuous monitoring of alcohol consumption. However, to date their research and clinical applications have been limited by several factors including large size, high cost, and social stigma. In contrast, recently developed wrist-worn alcohol biosensors are smaller, less expensive, and may be more acceptable for daily use. However, these devices are at the prototype phase and have just begun to be tested for research applications. In this paper, we describe our experiences with two prototypes of these new wrist-worn alcohol biosensors (i.e., Quantac Tally and BACtrack Skyn) and their associated smartphone applications in both a controlled laboratory setting and the real-world environment. Our preliminary experiences with these devices highlight their advantages including comfort, high participant acceptability, and good compliance. However, there are various limitations that should be addressed prior to future research applications of these biosensors, including large interpersonal variations in transdermal alcohol readings, lack of immediately applicable data analysis/interpretation software, and poor battery life after a few months. More research is also needed to further validate the new biosensors, and investigate individual (e.g., skin thickness, gender differences) and environmental factors (e.g., humidity, temperature) contributing to the variations in transdermal alcohol readings measured by wrist-worn alcohol biosensors.
Keywords: wearable alcohol biosensor, transdermal alcohol sensor, behavioral monitoring, heavy drinking
Introduction
Recent advancements in wearable biosensors are rapidly changing the landscape of healthcare. Wearable biosensors have garnered considerable interest in the past decade owing to their tremendous research and clinical potential in facilitating real-world behavioral and physiological measurement, aiding the diagnosis and treatment of various diseases (e.g., diabetes, heart diseases), as well as providing ongoing monitoring and intervention for health behaviors (Coughlin & Stewart, 2017; Gao et al., 2016; Harrison et al., 2011; Marschollek et al., 2012; Patel, Park, Bonato, Chan, & Rodgers, 2012; Steinhubl, Muse, & Topol, 2015).
In the field of alcohol research, wearable alcohol biosensors have emerged as an important tool for alcohol use monitoring and potentially intervention (Barnett, 2015; Leffingwell et al., 2013). These biosensors provide a powerful tool to monitor alcohol use in real-time with minimal participant burden (Barnett, 2015; Barnett, Meade, & Glynn, 2014; Bond, Greenfield, Patterson, & Kerr, 2014; Dougherty et al., 2015; Greenfield, Bond, & Kerr, 2014; Sakai, Mikulich-Gilbertson, Long, & Crowley, 2006; Simons, Wills, Emery, & Marks, 2015). They can provide fine-grained data regarding alcohol use patterns in a noninvasive, objective, and continuous manner outside of the laboratory in participants’ natural daily lives. Data collected from these sensors can be used to corroborate participants’ self-reported drinking behavior and provide data for more accurate assessment of alcohol use disorder (AUD) (Simons et al., 2015). Given that a “gold standard” for accurately measuring real-world alcohol use has not yet been established, the convergence of different measures such as self-report, biomarker, and biosensor data can greatly increase researchers’ confidence in characterizing participants’ drinking behaviors (Allen, Sillanaukee, Strid, & Litten, 2004; Miller, 2009; Wang et al., 2018). In addition, long-term objective data collected from alcohol biosensors has the potential to inform more personalized feedback and adaptive interventions to enhance the efficacy of treatment for AUD (Barnett, 2015; Leffingwell et al., 2013; Neville, Williams, Goodall, Murer, & Donnelly, 2013).
The purpose of this paper is to review and to discuss new developments in wrist-worn alcohol biosensors and their potential applications in the alcohol research field. To keep the paper relatively focused, we only reviewed wearable sensors that collect data passively. We briefly reviewed underlying technology and current status of wearable alcohol biosensors, and presented our preliminary experiences with prototypes of two recently-developed wrist-worn alcohol biosensors (the Quantac Tally and BACtrack Skyn) in both a controlled laboratory setting and the real-world environment. We also discussed the potential benefits and limitations of these devices for alcohol researchers, and identify some areas for future research and development.
Technology underlying existing and emerging wearable alcohol biosensors
Wearable alcohol biosensors that are currently available and under development generally rely on a small number of underlying technologies including fuel cell- and enzyme-based sensors. Fuel cell-based sensors are the most common approach. These utilize a small fuel cell that produces measurable electric current as they interact with alcohol. Fuel cell approaches are by far the most mature, having been implemented previously in the Secure Continuous Remote Alcohol Monitor Continuous Alcohol Monitoring (SCRAM CAM) anklet (Alcohol Monitoring Systems, Inc.). The previous implementation of fuel cell technology requires an active flow of air across the sensor. In the case of the SCRAM CAM, a mechanical pump produces air flow across the sensor. Fuel cell in the device interacts with the small fraction (approximately 1%) of ingested alcohol that is excreted through the skin via sweat glands and diffusion (Swift, 1993) to measure transdermal alcohol concentration (TAC). Transdermal sensors such as the SCRAM CAM use active flow across the fuel cell sensor to facilitate frequent measurement of TAC, but this increases both size and power demands, resulting in bulkier devices. Additionally, previously-available transdermal devices use intermittent measurement such as the SCRAM CAM (e.g., sampling TAC once per 30 minutes), as opposed to continuous or quasi continuous measurement. Thus, these devices are better suited for detecting whether any alcohol drinking has occurred, and less well suited for finer-grained analysis of fluctuations in TAC as a consequence of alcohol use over a prolonged drinking episode.
Recently, manufacturers have developed smaller, wrist-worn alcohol biosensors that rely upon passive diffusion of alcohol vapor to the sensor. This approach has the advantage of being smaller and much more power efficient as it does not employ mechanical devices to generate air flow. Additionally, this approach also allows for a nearly continuous measurement as the devices are not dependent upon the initiation of a secondary device (e.g., air pump) at each measurement cycle. Current implementations of wrist-worn fuel-cell devices use a semipermeable membrane between the skin and the sensor to allow for the diffusion of alcohol from the skin while preventing undesired compounds, such as dirt and water from reaching the sensor. It is unclear what the lifespan of a fuel-cell based sensor will be, but in preliminary testing it appears to be at least on the order of months. Devices that have used this approach include the Quantac Tally (Quantac Co.) and the BACtrack Skyn (BACtrack, Inc.). Of note, Quantac ceased business operations in 2017, and as of mid-2018, the BACtrack Skyn device is currently in the prototype testing phase and is not yet available for purchase by consumers. It should also be noted that the fuel-cell based technology has been used in the earliest wrist-worn transdermal alcohol biosensor, the WrisTAS (described in detail in the next section), for decades. However, the newer generation of wrist-worn alcohol biosensors such as Quantac Tally and BACtrack Skyn features improvements to make the devices smaller, more durable, and more energy-efficient, and thus is more suitable for consumer use.
A less common approach involves the use of an enzyme-based sensor. Less information has been made available about enzyme-based approaches for the measurement of TAC, and these may prove a promising avenue. However, currently-under-development wrist-worn implementations of this technology require that the enzyme-based sensor be embedded in a replaceable cartridge that must be changed at least once a day. This requirement increases participant burden, potentially making these devices inappropriate for certain populations. It is also worth considering that a consumable TAC device may result in increased costs. To date the only wrist-worn device that we are aware of implementing this approach is the PROOF by Milo Sensors, Inc, which is also at the prototype phase and not available for purchase by consumers.
Currently validated alcohol biosensors and their limitations
Currently, the SCRAM CAM anklet is the most widely used and validated transdermal biosensor (Leffingwell et al., 2013; Sakai et al., 2006). Research shows that using the SCRAM CAM anklet as an objective monitoring tool can improve prediction of alcohol dependence, increase treatment compliance and enhance the efficacy of alcohol intervention (Barnett et al., 2017; Barnett et al., 2014; Dougherty et al., 2014; Dougherty et al., 2015; Neville et al., 2013; Simons et al., 2015). However, the SCRAM CAM anklet is designed primarily for law enforcement purposes (e.g., monitoring abstinence from alcohol for those who have been convicted of alcohol-related offenses), and wider application of this device in clinical and intervention settings is limited. Several limitations of the SCRAM CAM anklet restrict its application in such settings, including: (1) relatively large size and weight (164.4g/5.8oz) for long-term daily use; (2) high device cost ($1500) with daily service fee ($5–8); (3) a large sampling interval (30 minutes), which may be insufficient to capture the complex dynamic of drinking behaviors; (4) likely to have social stigma in some contexts and settings due to its application in law enforcement; and (5) requirement of regular data uploading via a proprietary base station with no readings accessible at real time or to the participants (Barnett, 2015; Greenfield et al., 2014; Leffingwell et al., 2013).
Unlike large ankle bracelet devices, wrist-worn alcohol biosensors are smaller and may be more acceptable for daily use. The WrisTAS was the first wrist-worn transdermal alcohol sensor made available to the research market. Produced by Giner Lab (Newton, MA), WrisTAS utilizes an electrochemical (fuel cell-based) sensor that detects ethanol via oxidation of alcohol vapor to acetic acid (Greenfield et al., 2014; Marques & McKnight, 2009; Swift, 2000). It samples at predetermined intervals, ranging from 30 seconds to 10 minutes. Data is stored in memory and can be downloaded to a computer for analysis and graphing. Validation studies found that TAC indices generated from WrisTAS data, such as peak TAC and total area under the curve, had a high correlation with corresponding breath alcohol concentration (BrAC) (Swift, 2003; Swift, Martin, Swette, Laconti, & Kackley, 1992). However, previous studies showed that many WrisTAS devices had a relatively high fail rate in the field with missing data potentially caused by problems in voltage regulation or chipset failure (Greenfield et al., 2014; Marques & McKnight, 2009). Also, its application has been restricted to a small group of researchers, and it has never been marketed to consumers (Dumett et al., 2008; Leffingwell et al., 2013; Marques & McKnight, 2009).
The newest generation of wrist-worn alcohol biosensors
The newest generation of wrist biosensors (e.g., Quantac Tally, BACtrack Skyn) is designed for consumer use, resembles popular fitness trackers or smartwatch devices, and addresses many limitations of the SCRAM anklet (see Figure 1). These devices are light, easy to wear, and relatively inexpensive (anticipated price: $200–300/device). They sample at a much higher frequency (e.g., every second) than the SCRAM anklet, can transmit data in real time, and can display data via a smartphone app, as well as in an online database available to researchers or clinicians. These advantages make the new wrist-worn biosensors a potentially powerful tool for daily alcohol monitoring and alcohol treatment outside of clinic settings. Despite the promise for wider application, these new wrist-worn biosensors are still at the prototype phase and have just begun to be tested by researchers for validation and evaluation of usability and acceptability. Recently, our group obtained prototypes of the Quantac Tally and BACtrack Skyn devices from their respective manufacturers and have been using those devices to collect data in a controlled laboratory setting and in naturalistic (real-world) environments. Below, we discussed our preliminary experiences with these devices from a research perspective. Of note, our studies with these new biosensors are still ongoing and have not achieved adequate sample size to report any aggregated findings.
Figure 1.

Visual comparison of SCRAM CAM, Quantac Tally and BACtrack Skyn devices Note. The pictures of the wrist-worn devices were prototypes we obtained for testing as of early 2018. We placed a coin (i.e., a quarter) in the Top view picture as a reference for the actual size of these new wrist biosensors.
Preliminary experiences using two new wrist-worn alcohol biosensors in the research environment
Description of the user interface
Data from both the Quantac Tally and BACtrack Skyn prototypes are transmitted wirelessly via Bluetooth to an Apple iOS-based device, where the data are presented in a specialized application (app) developed by their respective manufacturers. Pre-release apps for both the Quantac and BACtrack devices provide real-time visualizations of the current alcohol sensor reading, skin temperature, data connection status, and battery status of the device. The Quantac Tally app includes additional features, including data on humidity and an account settings function, where participants could create their own account using an email address and record basic information including gender, height, and weight. The app also includes the ability for users to manually enter drinking behavior, allowing the logging of time, volume and type of alcohol consumed using a simple graphical user interface. This user-provided data do not impact the TAC readings, but could be used as convergent evidence of drinking behavior. The Skyn app does not currently include account setting or manual drinking input functions. However, it allowed selection of sampling interval for the alcohol sensor (i.e., every 1 second, 10 seconds, 30 seconds, 1 minute, or 5 minutes). In contrast, the Tally app has a default sampling interval and displayed data at an interval of 30 seconds. Both apps included an option to enter breathalyzer readings and anecdotal notes (See Figure 2).
Figure 2.

User interface of Quantac Tally and BACtrack Skyn Note. The screenshot on the left is from the Quantac Tally app. The one on the right is from the BACtrack Skyn app. These two screenshots were taken in different drinking episodes when one of the authors was testing these devices.
Using wrist-worn alcohol biosensors during a laboratory alcohol challenge
Recently, a member of our group (DJF) began a study examining the association between transdermal alcohol response recorded using the Tally or Skyn wrist-worn biosensors and conventional breath alcohol concentration (BrAC) using Alco-Sensor IV (Intoximeters of St. Louis, MO), measured during a controlled laboratory challenge paradigm. Data collection is ongoing at this time, and participants in the study (expected n = 20) were recruited as part of a larger study (expected N = 85) of subjective alcohol responses in the laboratory and natural environment. All participants were healthy, young adults (ages 21–29 years) who met criteria for heavy drinking. “Heavy drinkers” were defined as individuals who consume 14 or more drinks per week for men, 7 or more for women, with 1–4 heavy drinking episodes (defined as consuming 5 or more drinks for men or 4 or more drinks for women over a two-hour period), but who endorsed no more than 3 AUD criteria (i.e., individuals meeting DSM-5 criteria for moderate or severe AUD are excluded from participation).
The protocol for the laboratory challenge portion of this study was based upon previous work in this area (Fridberg, Cao, & King, 2015; King, de Wit, McNamara, & Cao, 2011). Informed consent was obtained from all participants and the study protocol was approved by the University of Chicago. Briefly, upon arrival to the laboratory, participants provided a breath and urine sample to verify recent abstinence from alcohol and other drugs and consumed a standard snack. They were informed that the wrist biosensor (Quantac Tally or BACtrack Skyn) was designed to measure alcohol use and provided consent to wear the device for the duration of the laboratory session. Participants were randomly assigned to wear one of the two biosensors. They removed any jewelry from both wrists and washed and dried their hands and wrists thoroughly before attaching the biosensor snugly to their wrist. Participants wore the device for approximately 20 minutes prior to alcohol administration as they completed pre-study questionnaires. Participants then consumed an alcoholic beverage (190-proof alcohol with water, flavored drink mix, and sucralose in two drink portions over 15 min with a 5-min break in between. The alcohol dose for the beverage was 0.8g/kg of body weight, calculated according to the Widmark formula, with female participants receiving 85% of this amount to account for sex differences in body water (Watson, Watson, & Batt, 1980). BrAC was measured at pre-drink baseline and at various intervals (15–195 min) after drinking, as in previous research (King et al., 2011; Fridberg et al., 2015). Participants also provided information on subjective alcohol response at each time point. These data were collected for the parent study and are not presented here. Participants read magazines or watched DVDs between assessments, and were discharged home via car service after participation when their BrAC was 0.04 g/210L or lower. Data from the Tally or Skyn prototype were collected during the session using the pre-release app for each respective device.
Figure 3 shows BrAC and transdermal alcohol response data from a Quantac Tally prototype recorded from a 24-year-old male participant (weight = 96.2 kg/212 lbs). Biosensor data represent deviations from the average sensor response from the 20-minute pre-drinking baseline period prior to the participant’s first sip of alcohol, and are expressed as a percentage deflection of the alcohol sensor, where 100% is the maximum threshold of alcohol detectable by the sensor. As shown in the figure, BrAC rose steeply and peaked at approximately .09g/210L of breath 60 minutes after drinking started. In contrast, TAC as measured by the Tally prototype peaked at approximately 115 minutes after drinking onset with a more gradual slope to peak. The participant’s skin temperature was approximately 28.6°C (83.5°F), and humidity at skin was approximately 62% during the session. Mean ambient room temperature and relative humidity during the session were 20.6°C (69°F) and 44.4%, respectively.
Figure 3.
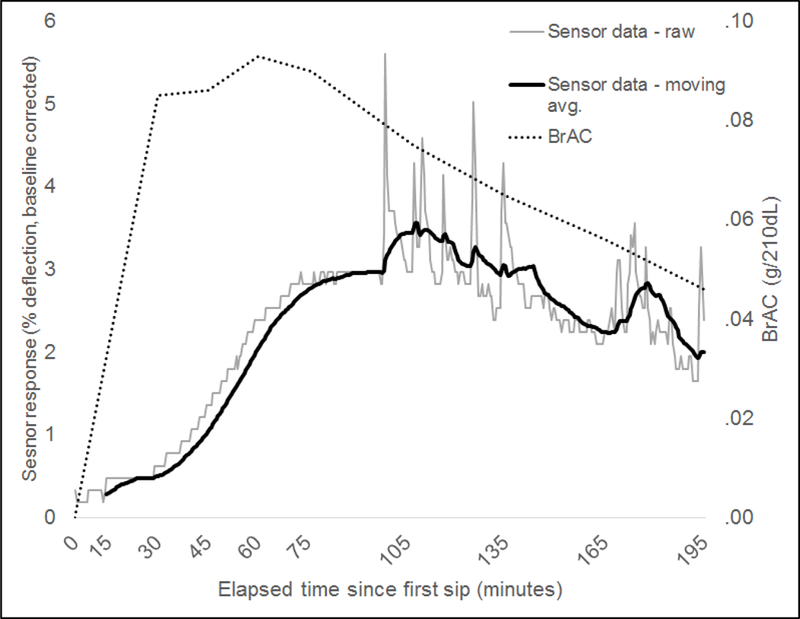
Example of BrAC and alcohol biosensor data recorded from a 24-year-old male participant using a Quantac Tally device during a laboratory alcohol challenge (alcohol dose = 0.8g/kg).
Figure 4 shows data recorded from a 23-year-old female participant (weight = 61.2 kg/135 lbs) using the prototype BACtrack Skyn device. The same alcohol administration and recording protocol was used as for the Tally prototype data depicted in Figure 1. The BACTrack Skyn device outputs data in arbitrary units that are linearly related to TAC, using a proprietary process. In this example, BrAC again peaked at approximately 60 minutes after drinking started (0.07g/210L of breath) and the biosensor response peaked approximately 135 minutes after drinking onset. The participant’s skin temperature was approximately 35.7°C (96°F) during the drinking session, and mean ambient room temperature and humidity were 28°C (82°F) and 13%, respectively.
Figure 4.
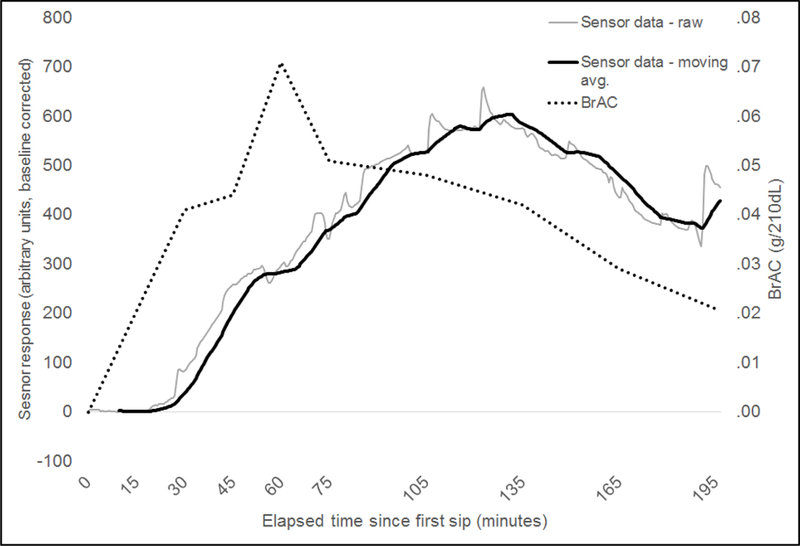
Example of BrAC and alcohol biosensor data recorded from a 23-year-old female participant using a BACtrack Skyn device during a laboratory alcohol challenge (alcohol dose = 0.8g/kg).
Using a wrist-worn alcohol biosensor in the field
Our group members (ECP & YW) also developed a protocol to use the Quantac Tally to measure alcohol use in the real-world environment over a two-week period. The study protocol included a two-week alcohol monitoring using the Quantac Tally with mobile app-based ecological momentary assessment. However, due to the fact that Quantac ceased their business and there was a significant battery life issue with the Quantac biosensors in our possession, we did not have the chance to actually complete any two-week field test as planned in the protocol. Figure 5 shows pilot data collected from a 34-year-old female participant (weight = 53.5 kg/118 lbs) using this protocol over a 16-hour period. There was clearly a drinking episode starting around 6pm as the TAC curve showed a significant elevation and then a slow declination. In the drinking log, the participant entered 1.5 standard drinks (wine) completed around 6pm. At approximately 9pm, alcohol sensor data was interrupted. This may have been the result of the sensor being taken off briefly or being misadjusted. Temporally corresponding changes in both humidity and temperature sensors appear to support this as they both abruptly fall and recover as would be expected when briefly moved from skin. This information can be used in data handling procedures, indicating data that may be deleted, interpolated or otherwise treated as noise and not a true reflection of TAC.
Figure 5.

Example of real-world data recorded from a 34-year-old female participant using a Quantac Tally device during a 16-hour field test Note. The drinking episode started around 6pm with significant elevation in TAC, followed by a peak at 9pm, and slow decline over time. The pilot participant indicated in the app that she consumed 1.5 standard drinks (wine) around 6pm. At 5pm, there was a notable spike of TAC readings that was likely to be an environmental alcohol exposure, as the slopes were too steep to reflect human alcohol metabolism. This type of spike would be identified as a non-drinking event in post processing by applying criteria such as those in the TASMAC Macro. Note that the signal dropout visible in the alcohol sensor channel around 9pm corresponds to rapid and transient change in temperature and humidity. This may indicate a period where the biosensor was removed and reapplied. It is also worth noting that there were significant fluctuations in TAC after 9pm, although these fluctuations may be common in these new wrist biosensor prototypes (also see Figure 2, 3).
These example data demonstrate that alcohol sensor responses may vary by device, participant, and BrAC, even in response to a fixed dose of alcohol in a well-controlled laboratory environment. Multiple individual difference factors may affect alcohol biosensor responses recorded in the field, including skin thickness, perspiration, ambient humidity, temperature, movement, and other factors. Presently, there are no established guidelines or best practices for analyzing and presenting alcohol biosensor data from these new wrist-worn biosensors. The direct translation of TAC to BAC or BrAC remains a topic of great interest to the field (Dougherty et al., 2012; Dumett et al., 2008; Gamella et al., 2014; Hill-Kapturczak et al., 2015). Luczak, Rosen, Barnett and other researchers have developed mathematical models and software (i.e., BrAC Estimator) to estimate BAC/BrAC from TAC (Dai, Rosen, Wang, Barnett, & Luczak, 2016; Luczak et al., 2018; Luczak & Rosen, 2014; Luczak, Rosen, & Wall, 2015; Rosen, Luczak, & Weiss, 2014). The BrAC Estimator includes an individualized calibration step in its modeling for more accurate estimation. However, estimated quantification of alcohol consumption or BAC from TAC signals generated from these new wrist biosensors is still under development, and not yet distributed by either Quantac or BACtrack at the time these data were collected.
Of note, the same group of researchers (Barnett et al., 2015) has developed software for Microsoft Excel (Transdermal Alcohol Sensor Data Macro, TASMAC) to translate TAC recorded via the SCRAM CAM to estimated BAC. The BrAC Estimator software mentioned above is also included as part of TASMAC. However, instead of requiring an individualized calibration step, the BrAC Estimator included in the TASMAC Macro applies a population-based approach using the mean of the parameters generated from prior lab alcohol administration studies. TASMAC may be adapted for use with these new wrist-worn biosensors; however, consideration of these wrist-worn sensors’ much higher sampling rate and the passive absorption of alcohol (as compared to SCRAM CAM’s active collection) may require specific signal processing approaches that improve TAC signal-to-noise ratio. These will likely involve the removal of high frequency signals, such as those driven by motion artifacts and other sources unrelated to the much slower physiological emission of alcohol from the skin prior to quantification. Potentially useful metrics for quantifying alcohol consumption from wrist worn biosensor TAC values include the magnitude of the signal at given time points, but other approaches are also likely to be useful including area under the curve, and time for TAC to peak and to return to baseline. These and other metrics derivable from the TAC signal may provide information previously only accessible through self-report. These potentially include drinking styles (inferable from shape and slope of ascending TAC) and alcohol elimination (inferable from descending TAC). Such metrics would allow researchers to paint a more complete picture of not only volume but pattern of alcohol consumption. Finally, much is unknown about inter and intra-individual differences in the relationship between TAC and blood alcohol concentration. Future approaches may benefit from a within subject calibration approach as well as the addition of direct measurement of perspiration using sensors of electrodermal activity.
Strengths, Limitations, and Future Directions of the New Wrist-Worn Alcohol Biosensors
While there are many advantages of these wrist-worn biosensors compared to the SCRAM CAM anklet, we identified several limitations of the prototypes as well as their associated apps from our experiences. Below, we discussed some strengths and limitations of the new wrist-worn alcohol biosensors and areas for further improvements and research on these devices.
Participant compliance
The newest generation of wrist-worn alcohol biosensors is similar in size and feels to activity monitors and or smart watches, which may increase participant compliance as they are relatively unobtrusive and may go unnoticed by participants in daily use. In our testing, observed challenges include the need for the participant to wear the band reasonably tightly across the wrist, which could result in poor participant comfort and low compliance depending on body type and device design. These biosensors claim some degree of water resistance though the manufacturers recommend that users not bathe or swim while wearing the devices, which may also limit user compliance.
Unlike the SCRAM anklet, these new wrist-worn alcohol biosensors cannot be locked onto a participant’s wrist. However, it is possible to infer participant compliance in wearing the device through other sensors included in these devices (e.g., Figure 5 as an example). For instance, the Quantac Tally has temperature, humidity and skin contact sensors (the skin contact sensor data was not provided in the Quantac app), while the BACtrack Skyn has a temperature sensor. These additional sensors can continuously track body temperature/humidity to provide useful indicators of whether the device has been properly placed on participants’ skin. As shown in the field data, it is possible to distinguish noncompliant time periods from compliant periods by examining temperature/humidity patterns. A sudden decrease or increase in temperature and/or humidity usually indicates noncompliance where the biosensor is likely removed from skin surface.
Acceptability and usability
Despite the potential concerns with participant comfort discussed above, no participant in our pilot studies complained spontaneously about wearing the biosensors and nobody asked to have the biosensor removed during the laboratory challenge. While we did not formally assess participants’ opinions about the comfort or appearance of the devices, comments from the participants included: “I didn’t even notice it was there”, “comfortable”, “unobtrusive”, and “very cool.” We also obtained qualitative data from 12 (8 males, 4 females, mean age = 32.08, SD = 5.33) current participants in another alcohol study, who engage in at least monthly heavy drinking, for their views of the new wrist-worn alcohol biosensors. When asked them to relay appealing aspects of a wrist-worn alcohol sensor, common themes that emerged including its value in decision making while drinking, particularly driving (e.g., “I like that it can detect alcohol because that’s a pretty good use for a wearable device. It can help people know when not to drive or do reckless things.”). Other appealing aspects included convenience, ability to track levels of intoxication, and preference for skin readings as opposed to breath or blood. In terms of aspects current heavy drinkers did not like, the delay between alcohol consumption and receipt of a reading from the device was the main theme. The other theme mentioned by multiple participants was that readings appear on an app rather on the device itself, but this could be potentially resolved if BACtrack delivers its smartwatch model (i.e., integrated with Apple Watch). Thus, our early experiences indicate that these new wrist-worn alcohol biosensors are highly acceptable to participants.
In terms of ease to use, the new wrist-worn biosensors are dependent on a smartphone app to log data, which is a consideration for research purposes (e.g., a participants’ smartphone must be compatible with the biosensor to record data, or a compatible device must be provided by the researcher). Properly operating the biosensors and their associated apps also requires some proficiency with technology. Furthermore, we noticed a shortening of battery life after a certain period of use (a few months) for both biosensors. While battery life on the new generation of devices is on the order of days, the batteries still require charging at regular intervals, which may further limit user compliance. This is especially important for real-life deployment of these biosensors, because no data will be collected if the device runs out of battery in the middle of a field test and participants may not notice the issue if they do not regularly check the status of the battery via the smartphone app.
Factors interfering with data quality
In our initial testing, high levels of perspiration seemed to be a key factor negatively impacting data quality. The incorporation of humidity and temperature sensors may be able to account for this factor to a degree, though it remains unclear if situations where perspiration is greatly increased (e.g. dancing, sex) will allow for valid data collection using these new wrist-worn alcohol biosensors. In one case, a pilot participant was heavily sweating due to exercise while she was wearing the Skyn biosensor, which resulted in no signal and apparent malfunction of the device. The biosensor returned to its normal state after a few hours when the semipermeable membrane was totally dry. It is also unclear whether the devices can produce high quality data in high humidity and high temperature environments, given that perspiration will be significantly higher in such environments. Manufacturers report active development of analytic strategies that incorporate this information and this may be a surmountable challenge.
We observed that other potentially common situations had a negative effect on data quality. For instance, the application of sunblock appeared to significantly impede permeability of the semi-permeable membrane, rendering the sensor ineffective. It may be the case that other skin treatments such as moisturizers and cosmetics will produce similar results, and consideration of this is important before these devices are deployed in real-world environments. Other sources of alcohol (not transdermal) may also negatively impact data quality. In one situation, a pilot participant spilled a small amount alcohol down their arm while preparing a beverage, an occurrence that may be unavoidable when alcohol consumption is allowed within the study design. This spill resulted in erroneous TAC readings for an extended period of time. It should be noted that Alcohol Monitoring Systems, Inc. provides a list of products/behaviors (e.g, hairspray, perfume) to avoid while wearing the SCRAM CAM bracelet to reduce the likelihood of exposure to environmental alcohol; the same list could be adapted as guidelines for users of these new wrist-worn alcohol biosensors to ensure high data quality.
Improvements needed for future research applications of wrist-worn alcohol biosensors
Wrist-worn alcohol biosensors are still in the prototype phase, and require further refinement to address some limitations prior to deployment in actual research or clinical applications. As noted above, these devices are dependent upon a smartphone or similar device for data collection and upload, which could prove challenging as researchers must ensure that all potential participants have (or are provided) a compatible device. In clinical environments where participants may be incentivized to reduce alcohol consumption or to abstain (e.g., in a contingency management intervention), the current generation of wrist-worn devices do not include functionality to ensure the device is on the desired participant. It is conceivable that a participant could remove the device and apply it to a family member or another individual, thereby circumventing the intervention.
In terms of the data collection, difficulty in pairing the biosensor to its app or poor connectivity could result in data loss for some participants even in controlled laboratory sessions (for Quantac Tally). Internet access (either via WiFi or data plan) is required for the app to stay connected with the backend data platform or upload/send data, which enables real-time data transmission to the researchers. This could create extra burden for the participants when they are sent home with the device and become responsible for these data collection/upload procedures. Significant improvements in these aspects are required before these new biosensors can be applied in real-life environment for consumers.
With regard to analysis, there is no established criteria of a “true” or “qualified” (e.g., heavy drinking) drinking episode like those established by the SCRAM company to assist with the interpretation of raw data. This is less of a problem in the laboratory setting, as researchers will know when a drinking episode starts and ends. It becomes more difficult to identify a drinking episode in the real world, where researchers have less certainty of whether and when a drinking episode happens. Therefore, certain criteria to assist in the identification of drinking episodes need to be in place before widespread research application of these biosensors occurs. Another important analysis relates to the conversion of transdermal alcohol concentration to blood alcohol level, as discussed above. The manufactures of these wrist-worn biosensors are actively working on converting the transdermal alcohol readings into estimated blood alcohol concentration. However, there is no existing solution to this challenge at this time. Future research in this area would be aided significantly by the development of a software package intended to easily import and process TAC data from these new wrist-worn biosensors, as exists currently for other psychophysiological methods such as electroencephalogram (EEG). Such software would significantly increase the accessibility of alcohol biosensors to clinical and basic researchers in the alcohol field.
Once the hardware and software of new wrist-worn alcohol biosensors become more mature and widely available, more studies will be needed to further validate these new devices for applications in research and clinical settings. Data supporting validity and reliability of these new wrist-worn biosensors are prerequisites for such applications. It is also important to investigate individual (e.g., skin thickness, gender differences, alcohol metabolism, and health conditions such as HIV infection) and environmental factors (e.g., humidity, temperature) contributing to the variations in transdermal alcohol readings measured by wrist-worn alcohol biosensors.
Conclusions
The notion of embracing the revolution of wearable biosensors in alcohol research and intervention is exciting. Our experiences with two newly developed wrist-worn biosensor prototypes show the potential of these devices to be used in the laboratory and in daily life to monitor alcohol use. However, significant limitations in both hardware and software must be addressed before these devices are ready for widespread use by researchers. While the manufactures need to make improvements for a more reliable and easy to use device, data analysis software must be developed to facilitate the analysis of large alcohol biosensor data sets.
Wrist-worn alcohol biosensors may be a powerful tool for real-life alcohol monitoring.
Advantages include small size, low cost, power efficiency, & high acceptability.
Limitations include variable data quality due to personal and environmental factors.
Future directions include validation research and development of analysis software.
Acknowledgement
The studies were funded in part by grants from the National Institute on Alcohol Abuse and Alcoholism R21AA027191 (to YW), K01AA025306 (to ECP), R21AA024901 (to DJF), R21 AA023368 (to RFL) U24AA022002 (to RLC); the University of Florida, Center for Cognitive Aging and Memory and McKnight Brain Research Foundation (to ECP); the UF Health Shands Quasi-Endowment Fund (to YW); and the Southern HIV and Alcohol Research Consortium Pilot Fund (to YW & ECP). We thank Quantac, Co. and BACtrack Inc. for providing the prototypes for testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
None to declare.
References
- Allen JP, Sillanaukee P, Strid N, & Litten RZ (2004). Biomarkers of Heavy Drinking Retrieved March 23, 2017, from https://pubs.niaaa.nih.gov/publications/assessingalcohol/biomarkers.htm
- Barnett NP (2015). Alcohol sensors and their potential for improving clinical care. Addiction, 110(1), 1–3. doi: 10.1111/add.12764 [DOI] [PubMed] [Google Scholar]
- Barnett NP, Celio MA, Tidey JW, Murphy JG, Colby SM, & Swift RM (2017). A preliminary randomized controlled trial of contingency management for alcohol use reduction using a transdermal alcohol sensor. Addiction, 112(6), 1025–1035. doi: 10.1111/add.13767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Meade EB, & Glynn TR (2014). Predictors of Detection of Alcohol Use Episodes Using a Transdermal Alcohol Sensor. Experimental and Clinical Psychopharmacology, 22(1), 86–96. doi: 10.1037/a0034821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Souza T, Rosen IG, Luczak SE, Glynn TR, & Swift R (2015). Transdermal Alcohol Sensor Data Macro (Version 1.3) [software]: Brown University. [Google Scholar]
- Bond JC, Greenfield TK, Patterson D, & Kerr WC (2014). Adjustments for Drink Size and Ethanol Content: New Results from a Self-Report Diary and Transdermal Sensor Validation Study. Alcoholism-Clinical and Experimental Research, 38(12), 3060–3067. doi: 10.1111/acer.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SS, & Stewart JL (2017). Toward research-tested mobile health interventions to prevent diabetes and cardiovascular disease among persons with pre-diabetes. J Hosp Manag Health Policy, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Rosen IG, Wang CM, Barnett NP, & Luczak SE (2016). Using Drinking Data and Pharmacokinetic Modeling to Calibrate Transport Model and Blind Deconvolution Based Data Analysis Software for Transdermal Alcohol Biosensors. Mathematical Biosciences and Engineering, 13(5), 911–934. doi: 10.3034/mbe.2016023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Charles NE, Acheson A, John S, Furr RM, & Hill-Kapturczak N (2012). Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Exp Clin Psychopharmacol, 20(5), 373–381. doi: 10.1037/a0029021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang YY, Karns TE, Cates SE, Lake SL, . . . Roache JD (2014). Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug and Alcohol Dependence, 142, 301–306. doi: 10.1016/j.drugalcdep.2014.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Karns TE, Mullen J, Liang Y, Lake SL, Roache JD, & Hill-Kapturczak N (2015). Transdermal alcohol concentration data collected during a contingency management program to reduce at-risk drinking. Drug Alcohol Depend, 148, 77–84. doi: 10.1016/j.drugalcdep.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumett M, Rosen G, Sabat J, Shaman A, Tempelman L, Wang C, & Swift R (2008). Deconvolving an Estimate of Breath Measured Blood Alcohol Concentration from Biosensor Collected Transdermal Ethanol Data. Appl Math Comput, 196(2), 724–743. doi: 10.1016/j.amc.2007.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberg DJ, Cao D, & King AC (2015). Integrating alcohol response feedback in a brief intervention for young adult heavy drinkers who smoke: A pilot study. Drug Alcohol Depend, 155, 293–297. doi: 10.1016/j.drugalcdep.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamella M, Campuzano S, Manso J, Gonzalez de Rivera G, Lopez-Colino F, Reviejo AJ, & Pingarron JM (2014). A novel non-invasive electrochemical biosensing device for in situ determination of the alcohol content in blood by monitoring ethanol in sweat. Anal Chim Acta, 806, 1–7. doi: 10.1016/j.aca.2013.09.020 [DOI] [PubMed] [Google Scholar]
- Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A, . . . Javey A (2016). Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature, 529(7587), 509–514. doi: 10.1038/nature16521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield TK, Bond J, & Kerr WC (2014). Biomonitoring for Improving Alcohol Consumption Surveys: The New Gold Standard? Alcohol Res, 36(1), 39–45. [PMC free article] [PubMed] [Google Scholar]
- Harrison V, Proudfoot J, Wee PP, Parker G, Pavlovic DH, & Manicavasagar V (2011). Mobile mental health: review of the emerging field and proof of concept study. J Ment Health, 20(6), 509–524. doi: 10.3109/09638237.2011.608746 [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Roache JD, Liang Y, Karns TE, Cates SE, & Dougherty DM (2015). Accounting for sex-related differences in the estimation of breath alcohol concentrations using transdermal alcohol monitoring. Psychopharmacology (Berl), 232(1), 115–123. doi: 10.1007/s00213-014-3644-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, & Cao D (2011). Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry, 68(4), 389–399. doi: 10.1001/archgenpsychiatry.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffingwell TR, Cooney NJ, Murphy JG, Luczak S, Rosen G, Dougherty DM, & Barnett NP (2013). Continuous objective monitoring of alcohol use: twenty-first century measurement using transdermal sensors. [Review]. Alcohol Clin Exp Res, 37(1), 16–22. doi: 10.1111/j.1530-0277.2012.01869.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, Hawkins AL, Dai Z, Wichmann R, Wang C, & Rosen IG (2018). Obtaining continuous BrAC/BAC estimates in the field: A hybrid system integrating transdermal alcohol biosensor, Intellidrink smartphone app, and BrAC Estimator software tools. Addictive Behaviors, 83, 48–55. doi: 10.1016/j.addbeh.2017.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, & Rosen IG (2014). Estimating BrAC from Transdermal Alcohol Concentration Data Using the BrAC Estimator Software Program. Alcoholism-Clinical and Experimental Research, 38(8), 2243–2252. doi: 10.1111/acer.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, Rosen IG, & Wall TL (2015). Development of a Real-Time Repeated-Measures Assessment Protocol to Capture Change over the Course of a Drinking Episode. Alcohol and Alcoholism, 50(2), 180–187. doi: 10.1093/alcalc/agu100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques PR, & McKnight AS (2009). Field and laboratory alcohol detection with 2 types of transdermal devices. Alcohol Clin Exp Res, 33(4), 703–711. doi: 10.1111/j.1530-0277.2008.00887.x [DOI] [PubMed] [Google Scholar]
- Marschollek M, Gietzelt M, Schulze M, Kohlmann M, Song B, & Wolf KH (2012). Wearable sensors in healthcare and sensor-enhanced health information systems: all our tomorrows? Healthc Inform Res, 18(2), 97–104. doi: 10.4258/hir.2012.18.2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM (2009). Evidence-Based Addiction Treatment. Oxford, UK: Academic Press [Google Scholar]
- Neville FG, Williams DJ, Goodall CA, Murer JS, & Donnelly PD (2013). An Experimental Trial Exploring the Impact of Continuous Transdermal Alcohol Monitoring upon Alcohol Consumption in a Cohort of Male Students. Plos One, 8(6). doi: ARTNe6738610.1371/journal.pone.0067386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Park H, Bonato P, Chan L, & Rodgers M (2012). A review of wearable sensors and systems with application in rehabilitation. [Review]. J Neuroeng Rehabil, 9, 21. doi: 10.1186/1743-0003-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen IG, Luczak SE, & Weiss J (2014). Blind deconvolution for distributed parameter systems with unbounded input and output and determining blood alcohol concentration from transdermal biosensor data. Appl Math Comput, 231, 357–376. doi: 10.1016/j.amc.2013.12.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JT, Mikulich-Gilbertson SK, Long RJ, & Crowley TJ (2006). Validity of transdermal alcohol monitoring: Fixed and self-regulated dosing. Alcoholism-Clinical and Experimental Research, 30(1), 26–33. doi: 10.1111/j.1530.0277.2006.00004.x [DOI] [PubMed] [Google Scholar]
- Simons JS, Wills TA, Emery NN, & Marks RM (2015). Quantifying alcohol consumption: Self-report, transdermal assessment, and prediction of dependence symptoms. Addictive Behaviors, 50, 205–212. doi: 10.1016/j.addbeh.2015.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhubl SR, Muse ED, & Topol EJ (2015). The emerging field of mobile health. Sci Transl Med, 7(283), 283rv283. doi: 10.1126/scitranslmed.aaa3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift RM (1993). Transdermal Measurement of Alcohol-Consumption. Addiction, 88(8), 1037–1039. doi: DOI 10.1111/j.1360-0443.1993.tb02122.x [DOI] [PubMed] [Google Scholar]
- Swift RM (2000). Transdermal alcohol measurement for estimation of blood alcohol concentration. Alcoholism-Clinical and Experimental Research, 24(4), 422–423. doi: Doi 10.1097/00000374-200004000-00013 [DOI] [PubMed] [Google Scholar]
- Swift RM (2003). Direct measurement of alcohol and its metabolites. Addiction, 98, 73–80. doi: DOI 10.1046/j.1359-6357.2003.00605.x [DOI] [PubMed] [Google Scholar]
- Swift RM, Martin CS, Swette L, Laconti A, & Kackley N (1992). Studies on a Wearable, Electronic, Transdermal Alcohol Sensor. Alcoholism-Clinical and Experimental Research, 16(4), 721–725. doi: DOI 10.1111/j.1530-0277.1992.tb00668.x [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen X, Hahn JA, Brumback B, Zhou Z, Miguez MJ, & Cook RL (2018). Phosphatidylethanol in Comparison to Self-Reported Alcohol Consumption Among HIV-Infected Women in a Randomized Controlled Trial of Naltrexone for Reducing Hazardous Drinking. Alcoholism-Clinical and Experimental Research, 42(1), 128–134. doi: 10.1111/acer.13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE, Watson ID, & Batt RD (1980). Total-Body Water Volumes for Adult Males and Females Estimated from Simple Anthropometric Measurements. American Journal of Clinical Nutrition, 33(1), 27–39. [DOI] [PubMed] [Google Scholar]


