Abstract
Proper regulation of sterol biosynthesis is critical for eukaryotic cellular homeostasis. Cholesterol and isoprenoids serve key roles in eukaryotic cells by regulating membrane fluidity and correct localization of proteins. It is becoming increasingly appreciated that dysregulated sterol metabolism engages pathways that lead to inflammation. Of particular importance are inflammasomes, which are multiplatform protein complexes that activate caspase-1 in order to process the pro-inflammatory and pyrogenic cytokines IL-1β and IL-18. In this review, we highlight recent research that links altered sterol biosynthetic pathway activity to inflammasome activation. We discuss how clues from human genetics have led to new insights into how alterations in isoprenoid biosynthesis connect to inflammation. We also discuss new mechanisms that show how macrophage cholesterol buildup can lead to inflammasome activation.
Introduction
Cholesterol and isoprenoids play crucial roles in supporting eukaryotic cellular homeostasis. Cholesterol serves as a structural component of mammalian cell membranes, regulating membrane fluidity and promoting signal transduction via the generation of lipid rafts [1]. Isoprenoids such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) are required for post-translational modification of proteins that promote their proper cellular localization [2]. The production of these two types of metabolites are controlled by the same transcriptional regulators, the sterol regulatory element binding proteins (SREBPs), which normally exist as transmembrane proteins in the endoplasmic reticulum (ER). In states of low cellular cholesterol content, SREBPs translocate to the Golgi apparatus where they are proteolytically processed; this releases the N-terminal basic helix-loop-helix (bHLH) domain to translocate to the nucleus and promote expression of genes encoding enzymes of the isoprenoid and cholesterol biosynthesis pathways [3]. Recent insights from human and mouse genetics have revealed that alterations in the biosynthesis of isoprenoids and cholesterol can lead to innate immune inflammation, particularly via production of the cytokine IL-1β.
IL-1 family cytokines are unique in their absence of a leader sequence. IL-1β and IL-18 are translated in a cytosolic pro-form and must be cleaved for activation. Cleavage of IL-1β and IL-18 is mediated by the cysteine protease caspase-1 [4]. The inflammasome, a term coined by Jurg Tschopp [5], is a multiprotein complex that functions to activate caspase-1. Inflammasome activation is initiated by triggering of a sensor protein. Bona fide Inflammasome sensor proteins include members of the Nucleotide binding domain and Leucine-rich Repeat (NLR) family (NLRPIa, NLRPIb, NLRP3, NAIP1, NAIP2, NAIP5, NAIP6), members of the Absent in Melanoma-like receptor family (AIM2), and the Pyrin protein encoded by the MEFV gene. Upon ligand binding, inflammasome sensor proteins oligomerize and recruit the adaptor protein Apoptosis-Associated Speck-like (ASC). ASC subsequently oligomerizes and recruits caspase-1. The multiple rounds of oligomerization allow many caspase-1 molecules to come into close proximity in order to promote autoproteolysis. This allows release of the active domain of caspase-1 to process IL-1 family cytokines. Herein, we discuss how altered isoprenoid and cholesterol homeostasis can lead to triggering of inflammasomes.
Loss of isoprenoid synthesis from the mevalonate pathway leads to Pyrin inflammasome activation
The rate-limiting entry step into sterol biosynthesis involves the conversion of HMG-CoA into mevalonate via HMG-CoA reductase (HMGCR), the target of statins. The outputs of the mevalonate pathway can become cyclized to initiate cholesterol biosynthesis or be converted into isoprenoids such as FPP and GGPP. Numerous cellular proteins, such as Rho and Ras family GTPases, are post-translationally modified by conjugation to FPP or GGPP, which results in membrane localization [6,7]. Initial evidence suggesting a link between the mevalonate pathway and inflammasome activation came from humans with loss-of-function mutations in mevalonate kinase (MVK) who have a condition known as Hyper-immunoglobulin D syndrome (HID). These patients have a hyper-inflammatory phenotype characterized by periodic fever, elevated production of inflammatory cytokines, lymphadenopathy, and arthritis [8,9]. This was subsequently argued to be inflammasome-dependent inflammation based on studies showing that inhibition of IL-1β with Anakinra or blocking antibodies alleviated disease [10]. Additionally, mevalonate pathway blockade using statins in macrophages phenocopied key aspects of HID, causing caspase-1 activation and IL-1β processing [11,12]. Interestingly, the inflammasome activation observed with statin-treatment could be rescued by addback of geranylgeraniol, arguing that this effect was caused by diminished isoprenoid rather than cholesterol levels. The basis for the high circulating IgD in some patients is unclear, but is unlikely due to inflammasome activity as it is not seen in other diseases driven by gain-of-function mutations in inflammasome sensor proteins, known as inflammasomopathies [13].
Initial studies argued that inflammasome activation in macrophages from HID patients was due to NLRP3 activation [14]. This was attributed to impaired mitochondrial clearance due to defective mitophagy in cells from HID patients [15]. However, these results were called into question, and there was as-of-yet no direct genetic evidence to support NLRP3 involvement in MVK deficiency [16].
Recent work elucidating the mechanisms underlying activation of the enigmatic Pyrin inflammasome has opened up new investigation into how isoprenoids interface with inflammation. Earlier studies had shown that the Clostridium difficile effector proteins TcdA/B and C3, inactivators of Rho and/or Rac family small GTPases, could also trigger inflammasome activation [17,18]. Extending from these observations, Xu et al. found that inflammasome activation by TcdB did not require the inflammasome sensor proteins NLRP3, NLRC4, or AIM2 [19]. A gain-of-function in vitro screen using RFP-ASC foci formation in 293T cells as a readout found that Pyrin was sufficient to confer ASC oligomerization in response to TcdB [19]. Intriguingly, Pyrin had already been implicated in inflammasome activation, as gain-of-function mutations in the Pyrin-encoding MEFVgene cause the autoinflammatory syndrome Familial Mediterranean Fever (FMF)[20]. Further experiments by Xu et al. determined that Pyrin is a sensor of Rho GTPase loss-of-function induced by bacterial effector proteins.
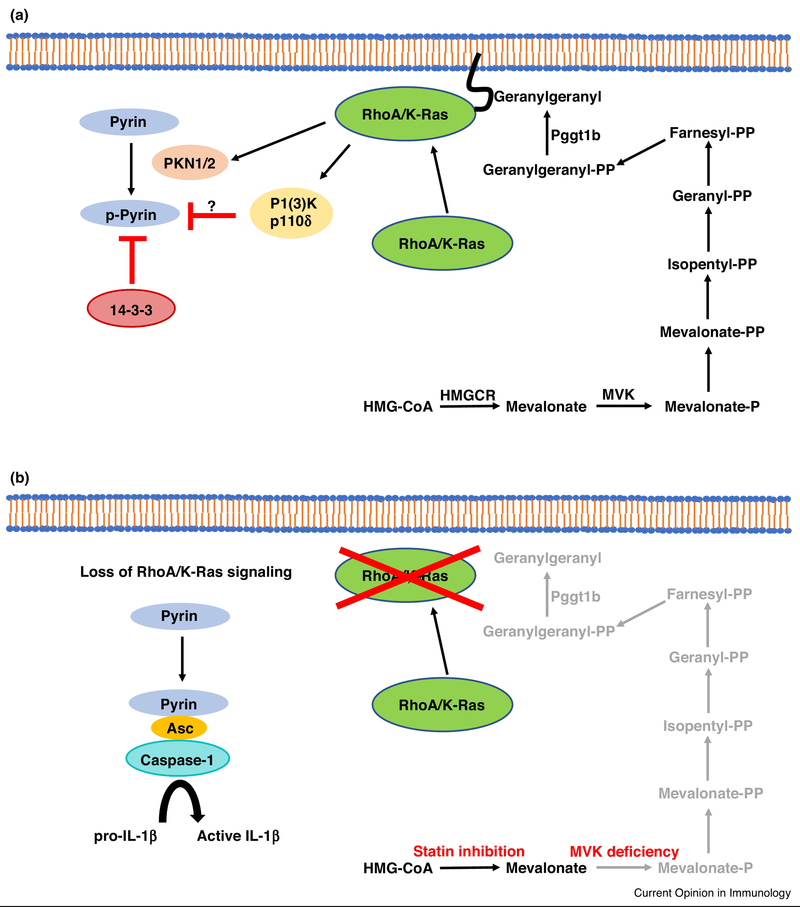
As Rho GTPases require prenylation for membrane localization and function, Park et al. tested the hypothesis that decreased flux through the mevalonate pathway, as in statin-treatment or MVK deficiency, causes Pyrin inflammasome activation via inactivation of Rho [21]. After confirming that C3 and TcdB toxins could activate the Pyrin inflammasome, the authors found that this could be reversed by pharmacologic agents or bacterial toxins that activate RhoA. They additionally found that RhoA effector kinases (PKN1 and 2) phosphorylate Pyrin directly, causing its inactivation via binding to 14-3-3 proteins. This suggests a model whereby Pyrin normally defaults towards oligomerization and interaction with ASC, but this is inhibited by constitutive RhoA signaling (Figure 1). Importantly, Park et al. found that inflammasome hyperactivation observed with statin-treatment of macrophages was rescued upon genetic deletion of MEFV, but not NLRP3, NLRC4, or AIM2. They additionally found that IL-1β release from PBMCs derived from MVK-deficient patients could be blocked by pharmacologic inhibitors of Pyrin.
Figure 1.
Regulation of the Pyrin inflammasome by prenylated signaling molecules. (A) RhoA/K-Ras both require prenylation for tethering to the plasma membrane and subsequent activation. RhoA/K-Ras activate downstream kinases, PKN1/2 and PI(3)Kδ respectively, that inactivate the inflammasome sensor protein Pyrin. PNK1/2 directly phosphorylate Pyrin, which results in its inactivation via the binding of the 14-3-3 complex. The mechanism for PI(3)Kδ-dependent Pyrin inactivation remains to be determined. (B) Mevalonate kinase deficiency in humans results in a hyper-inflammasome phenotype; this can also be observed in vitro by treating macrophages with statins. Inflammasome activation in MVK-deficient cells is a result of impaired prenylation of RhoA/K-Ras, resulting in activation of Pyrin and subsequent recruitment of the adaptor protein Asc and the protease caspase-1. Activated caspase-1 then cleaves pro-IL-1β into active IL-1β, which drives inflammatory pathology in MVK-deficient patients. HMGCR = HMG-CoA Reductase, MVK = Mevalonate Kinase, P = Phosphate, PP = Pyrophosphate
Akula et al. also looked at the contribution of decreased isoprenoid production to inflammasome activation [22]. Macrophages deficient in protein geranylgeranyl transferase-1β (Pggtlb) were found to hyper-produce IL-1β in response to LPS stimulation in a Pyrin-dependent manner. However, in contrast to Park et al., the authors attributed Pyrin activation to decreased prenylation of Kras rather than RhoA. IL-1β production in Pggtl b-knockout BMDMs could be rescued by overexpression of constitutively active PI(3)K catalytic subunit p110δ. As catalytic PI(3)K subunits have a Ras-binding domain (RBD), the authors concluded that decreased isoprenoid biosynthesis activates Pyrin via lack of Kras-dependent p110δ activation. They additionally argued that p110δ inhibits Pyrin inflammasome activity at the level of MEFV transcription. It remains to be determined whether p110δ also controls, directly or indirectly, Pyrin phosphorylation and 14-3-3 binding, as it is not clear that Pyrin activation is controlled solely at the level of transcription (Figure 1).
Cholesterol crystals and inflammasome activation
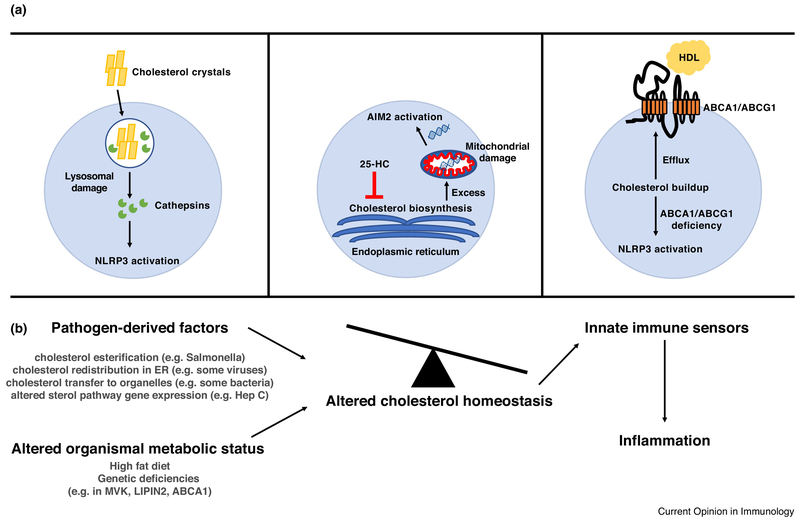
There has been longstanding interest in the intersection of cholesterol metabolism and inflammation in the context of atherosclerosis and metabolic disease. Despite this, there is little known about how cholesterol engages with innate immune sensing pathways that couple to inflammatory outputs. The first link between cholesterol and innate immune sensing came from Duewell et al. who showed that cholesterol crystals were sufficient to drive NLRP3 inflammasome activation in macrophages. The authors found that cholesterol crystals could be observed in early aortic atherosclerotic lesions in ApoE-knockout mice fed a high-cholesterol diet. Furthermore, addition of cholesterol crystals to macrophages could drive IL-1β production; this effect was lost in NLRP3- and ASC-deficient macrophages [23]. The authors found that inflammasome activation was abrogated after inhibition of actin polymerization and was also diminished in cathepsin B and cathepsin L knockout macrophages. This suggested a model whereby phagocytosis of cholesterol crystals causes lysosomal rupture, and cathepsins subsequently trigger NLRP3 activation via undefined mechanisms (Figure 2A). Importantly, the authors also found that NLRP3-, ASC-, and IL-1α/β knockout mice had diminished atherosclerosis, suggesting that inflammasome activation contributes to metabolic disease. This prediction has been borne out in human data, as a recent clinical trial reported that treatment of patients who had a previous myocardial infarction with Canakinumab, a blocking antibody against IL-1β, provided protection from recurrent cardiovascular events independently of lipid levels [24].
Figure 2.
Dysregulated cholesterol homeostasis drives inflammasome activation. (A) Left: Phagocytosis of cholesterol crystals by macrophages can cause damage/rupture of the lysosome. This releases lysosomal contents into the cytosol, and proteins such as cathepsins can subsequently cause cellular damage that is sensed by the NLRP3 inflammasome. Middle: Upon bacterial sensing, macrophages produce the oxysterol 25-HC in order to maintain inhibition of the SREBP2 cholesterol biosynthesis pathway. Macrophages that are impaired for 25-HC production have excess cholesterol, which drives mitochondrial damage and activation of the AIM2 inflammasome via mitochondrial DNA. Right: Macrophages utilize ABCA1/ABCG1 to promote cholesterol efflux to HDL. Deletion of ABCA1/ABCG1 in myeloid cells causes cholesterol buildup and subsequent triggering of the NLRP3 inflammasome. (B) Diagram suggesting possible connection between altered cholesterol homeostasis resulting from pathogen infection or altered organismal metabolic status and engagement of innate immune sensors and inflammation. HDL = High density lipoprotein, 25-HC = 25-hydroxycholesterol
Sheedy et al. extended this work by demonstrating that the scavenger receptor CD36 can promote the uptake and crystallization of soluble NLRP3 activators such as oxidized LDL (oxLDL). The authors found that treatment of macrophages with oxLDL was sufficient to drive IL-1β and that this was diminished in CD36- and NLRP3-knockout cells [25]. Imaging studies found that subsequent to uptake, oxLDL formed intracellular cholesterol crystals in macrophages. Similar to Duewell at al., they also suggested that oxLDL-dependent NLRP3 activation required crystal-induced lysosomal damage. The authors suggested that release of free cholesterol in the lysosome by lysosomal acid lipase (LIPA) could result in crystal formation; consistent with this idea, treatment of macrophages with the LIPA inhibitor lalistat diminished inflammasome activation in response to oxLDL. However, other groups have suggested that cholesterol crystal-dependent inflammasome triggering depends on generating cellular oxidative stress or activation of the complement cascade [26–28]. Moreover, the extent to which cholesterol crystals form in vivo during human disease processes remains an active area of discussion [29,30]. A very recent study has provided evidence for their appearance in the brain of aged mice exposed to a focal demyelinating agent. Exposing BMDMs to cholesterol-rich myelin debris was sufficient to engage NLRP3 and deficiency in this sensor led to enhanced re-myelination of spinal cord lesions [31].
Intrinsic control of macrophage cholesterol synthesis regulates the inflammasome
Recent work has suggested that control of macrophage cholesterol biosynthesis by cytokine signaling is an additional axis in inflammasome regulation. It has been appreciated since the 1970s that the oxysterol 25-hydroxycholesterol (25-HC) is capable of inhibiting cholesterol biosynthesis [32]. This was subsequently found to be due to the potent capacity of 25-HC to bind INSIG and inhibit SCAP-mediated chaperoning of SREBP ER-to-Golgi translocation [33]. However, deletion of cholesterol- 25-hydroxylase (Ch25h), which catalyzes the conversion of cholesterol to 25-HC, did not lead to baseline abnormalities in cholesterol homeostasis [34]. Subsequently it was found that while Ch25h is expressed at very low levels in most tissues, it is highly induced by type I interferon signaling in macrophages [35–37]. Performing RNA-seq on LPS-stimulated Ch25h-deficient macrophages, Reboldi et al. found that the most upregulated class of genes were SREBP targets in the cholesterol biosynthesis pathway, providing evidence that endogenously produced 25-HC can act as a feedback inhibitor of SREBP during inflammation [38]. The authors also observed that Ch25h-deficient macrophages had spontaneous production of IL-1β after LPS stimulation and hyper-produced IL-1β in response to a variety of inflammasome activators. These mice additionally showed signs of hyper-inflammation in the form of increased baseline IL- 17A+ lymphocytes, increased LPS sepsis susceptibility, and worsened experimental autoimmune encephalitis (EAE) clinical scores. Overexpression of INSIG1 in Ch25h-deficient macrophages rescued the overproduction of IL-1β, suggesting that increased activation of SREBP was responsible for the phenotype. Further supporting this conclusion, SCAP-deficient macrophages had decreased IL-1β production. A seperate study found that Ch25h-deficiency protected mice from EAE, perhaps by causing loss of the leukocyte chemoattractant 7α,25-HC [39]. IL-1β production was not tested and the discrepancy in EAE findings between the studies might reflect differences in the microbiome and strong influences of this compartment on development of EAE [40].
Extending from these studies, Dang et al. observed that loss of ASC reversed the IL-1β overproduction in Ch25h-deficient macrophages responding to LPS plus ATP or Listeria monocytogenes [41]. Ch25h-deficient macrophages had increased sterol pathway intermediates and higher total cholesterol content. Genetic and biochemical enforcement of increased macrophage cholesterol content triggered inflammasome activation in a crystal-independent manner. Intriguingly, while overexpression of SREBP2 could boost activation in a 293T NLRP3 inflammasome reconstitution system, NLRP3 was not genetically required for soluble cholesterol-dependent inflammasome activity. Surprisingly, it was found that deletion of the cytosolic DNA sensor Absent in melanoma 2 (Aim2) abrogated IL-1β production in response to cholesterol overproduction, with a partially redundant contribution from NLRP3. The authors found that Ch25h-deficiency results in mitochondrial cholesterol accumulation, causing cytosolic release of mitochondrial DNA that can trigger Aim2. Mitochondria normally have very low cholesterol content and while the mode of mtDNA release was not determined it is likely facilitated by cholesterol-induced reductions in mitochondrial membrane integrity (Figure 2A) [42]. These findings might help explain a number of earlier observations such as the inflammation and neurotoxicity occurring in mice exhibiting mitochondrial cholesterol accumulation due to transgenic over-expression of SREBP2 or deficiency in the intracellular cholesterol transporting Niemann-Pick type C1 (NPC1) protein [43].
As for the relevance of the Ch25h circuit in human macrophages, Gaidt et al. recently reported that AIM2 is dispensable for inflammasome activation in response to cytosolic dsDNA in human monocytes, although this depends somewhat on cell type [44]. The authors argued that cytosolic dsDNA was sensed by cGAS/STING, which triggered lysosomal damage leading to potassium efflux and NLRP3 activation. This was observed when using PBMC-derived macrophages and a B lymphoma-derived macrophage cell line (BLaERI), whereas an acute monocytic leukemia cell line (THP-1) showed canonical AIM2 dependence for dsDNA responses. AIM2 expression was very low in both the PMBC-derived macrophages and BLaERI cells, whereas it was well expressed in THP-1 cells. Therefore, these results do not exclude human AIM2 from functionally responding to dsDNA, but provide evidence that alternative pathways exist for inflammasome activation to cytosolic nucleic acids. Regardless of cell-type specificity, it would be of interest to determine whether CH25H deficiency in human cells results in activation of either the AIM2 inflammasome or the cGAS/STING/NLRP3 inflammasome upon cholesterol-dependent mtDNA release.
Another potential mechanism for cholesterol overload in macrophages comes from phagocytosis of apoptotic host cells, otherwise known as efferocytosis. Viaud et al. found that LIPA expression is required to release free cholesterol in order to form 25-HC from apoptotic cell-derived cholesterol, which could theoretically act as a feedback mechanism to help macrophages handle cholesterol overload [45]. The authors initially observed that treatment of LDLR-deficient mice with the LIPA inhibitor lalistat increased serum and spleen IL-1β levels. After adding apoptotic cells to THP-1 macrophages, they found that efferocytosis results in increased LIPA enzymatic activity. Mass spectrometry of THP-1 cells revealed that 25-HC levels were diminished after lalistat treatment. These results suggest that efferocytosis of apoptotic host cells is coupled to LIPA-dependent production of 25-HC, which subsequently limits inflammasome activation. In this instance it is unlikely that 25-HC is acting via SREBP inhibition, since blocking cholesterol synthesis would not be efficacious in a scenario where free cholesterol has already entered the system. 25-HC can also putatively act as a ligand for liver X receptors (LXRs) [46,47], which promotes cholesterol efflux, or as a stimulator of acyl-coenzyme A:cholesterol acyltransferases (ACATs) [48,49], which catalyze cholesterol esterification into lipid droplets. Lipid droplet formation in this context would be beneficial by preventing intracellular free cholesterol buildup. Dang et al. found that Ch25h overexpression could block inflammasome activation after free cholesterol loading of macrophages using a mechanism that did not require LXRs [41]. In the context of that result and the work from Viaud et al., it would be of future interest to determine whether anti-inflammatory actions of 25-HC require lipid droplet formation after macrophage efferocytosis of apoptotic cells.
Regulation of cholesterol export to HDL controls inflammasome activation
While the work described above provided evidence that feedback inhibition of cholesterol biosynthesis is important for preventing inflammasome activation, it is also well-appreciated that cholesterol export from cells is a highly-regulated process. Cholesterol can be released from macrophages to form HDL via the plasma membrane transporters ABCA1 and ABCG1 [50]. Tangier disease in humans is caused by loss-of-function mutations in ABCA1 and results in premature atherosclerosis, splenomegaly, and hepatomegaly [51–53]. Westerterp et al. modeled this disease by generating ABCA1/ABCG1 double knockout mice. They found that these mice developed a lupus-like phenotype characterized by glomerulonephritis and enlarged lymph nodes [54]. Interestingly, restriction of ABCA1/ABCG1-deficiency to dendritic cells, but not macrophages, recapitulated the full knockout phenotype. While dendritic cells from these mice showed no differences in ability to stimulate T cell proliferation, they did show increased capacity to prime Th1 differentiation. In keeping with this, the authors found that dendritic cells from these double knockout mice showed increased spontaneous inflammasome activity and release of IL-18, which can promote IFNγ production [55]. Deletion of NLPR3 from ABCA1/ABCG1-deficient mice resulted in a partial rescue of inflammatory phenotypes (Figure 2A).
In a follow-up study from the same group, Westerterp et al. tested whether ABCA1/ABCG1-deficiency impacted atherogenesis-associated inflammation. The authors transplanted myeloid-specific ABCA1/ABCG1-deficient bone marrow into irradiated LDLR-knockout hosts [30]. These chimeric mice were then fed a Western diet (WTD) for 4 weeks. ABCA1/ABCG1-deficiency in the hematopoietic compartment resulted in increased plasma IL-18 levels, and increased secretion of IL-1β from splenic myeloid cells, consistent with the concept that impaired cholesterol efflux can drive inflammasome activation. The authors found that concomitant NLRP3-deficiency had a partial rescue effect, whereas deletion of caspase-1/11 led to a complete reversal of the IL-18 and IL-1β overproduction, suggesting that multiple inflammasome sensor proteins may be involved in this inflammatory process. Based on the work from Dang et al., it would be of interest to test whether additional deletion of AIM2 would result in a full rescue.
If impaired cholesterol efflux to HDL causes inflammasome activation, one would predict that augmenting efflux via HDL supplementation could dampen inflammasome activity in some circumstances. Indeed, Westerterp et al. showed that HDL addback could rescue inflammasome activation in ABCA1/ABCG1-deficient macrophages, though in the absence of the efflux transporters it was unclear how cholesterol was seeded onto HDL [54]. This suggests the existence of additional cholesterol efflux pumps in macrophages. De Nardo et al. demonstrated that treatment of activated BMDMs with HDL generated an ATF3-dependent anti-inflammatory gene signature, but inflammasome activation was not directly tested in this context [56].
Dysregulated cholesterol homeostasis as a HAMP?
Although AIM2 can be directly engaged by bacterial (and possibly viral) DNA and is therefore a pathogen associated molecular pattern (PAMP) sensor, its ability to be triggered by endogenous mtDNA indicates it also serves the function of a damage-associated molecular pattern (DAMP) sensor. Beyond the now well-established paradigms for innate immune activation by PAMPs and DAMPs, a third mechanism has recently been proposed. The appreciation that Pyrin inflammasomes are activated by sensing loss of homeostatic Rho or Ras function has led to the suggestion, analogous to the plant Guard model, that Pyrin is an example of a homeostasis altering molecular pathway (HAMP) sensing system [57]. Altered cholesterol metabolism is likely to shift cells from homeostasis in multiple ways, with altered mitochondrial function being just one example. Increased ER stress may be another [58]. We speculate that as well as triggering AIM2 and NLRP3, loss of cholesterol metabolic homeostasis activates still to be defined HAMP sensors. Alterations in cholesterol biosynthesis or metabolism promoted by pathogenic microorganisms could be triggers of such HAMP sensing in macrophages (Figure 2B). Indeed numerous microbes have been shown to manipulate host cell cholesterol metabolism including, but not limited to, Hepatitis C virus [59], Mycobacterium tuberculosis [60], Coxiella burnettii [61], Salmonella typhimurium [62], and Toxoplasma gondii [63]. Also in accord with the HAMP model, cholesterol depletion may be sensed by the NLRP3 inflammasome and may contribute to Mayeed syndrome, a disorder involving IL-1β overproduction [64].
Concluding remarks
It is now clear that dysregulation of cholesterol and isoprenoid biosynthesis can lead to inflammasome activation. However, there is still much that we do not understand about the interplay between these pathways. For example, type I interferon-dependent upregulation of Ch25h/25-HC in macrophages acts to decrease cholesterol biosynthesis and prevent AIM2 inflammasome activation, but why does this not lead to triggering of the Pyrin inflammasome through diminished isoprenoid levels? Perhaps this is simply quantitative, meaning that 25-HC does not cause enough of a decrease in isoprenoid levels to impact RhoA or Kras activity compared to MVK deficiency. Alternatively, perhaps 25-HC has poorly understood selective effects on pathway flux. It is also unclear what the consequence of overproduction of isoprenoids would be for inflammasome regulation. Ch25h-deficient macrophages have increased flux through the mevalonate pathway and the downstream cholesterol synthesis pathways, but does this also result in increased cellular isoprenoid levels? If so, does this have any contribution to inflammasome activation in addition to the effects of cholesterol? Answering these questions will require advances in tools to profile the cellular prenylated proteome, which is technically challenging at the present time. Additionally, there are many important questions at the organismal level, such as the relative contributions of crystals versus intracellular membrane dysfunction to dietary cholesterol-induced inflammation [65]. Future mechanistic insights will hopefully shed further light on the hard-wired, physiologic roles of sterol metabolism in host defense and inflammation, and how these circuits become frayed in conditions such as atherosclerosis, metabolic syndrome and neurodegenerative disease.
Highlights.
Impaired isoprenoid biosynthesis leads to Pyrin inflammasome activation
Phagocytosis of cholesterol crystals drives NLRP3 activation via lysosomal damage
25-HC prevents spurious AIM2 activation by limiting cholesterol biosynthesis
ABCA1/ABCG1 deficiency causes cholesterol accumulation and inflammasome activation
Acknowledgements
We thank Erick Lu and Michelle Mintz for critical reading of the manuscript and helpful comments. E.V.D. is supported by a National Institute of Allergy and Infectious Disease F30 grant F30AI120527 and the UCSF Medical Scientist Training Program (MSTP) National Institute for General Medical Sciences T32GM007618 , and J.G.C. is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by National Institute of Health grant AI040098 (to J.G.C.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ikonen E: Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol 2008, 9:125–138. [DOI] [PubMed] [Google Scholar]
- 2.Wang M, Casey PJ: Protein prenylation: unique fats make their mark on biology. Nat. Rev. Mol. Cell Biol 2016, 17:110–122. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JL, DeBose-Boyd RA, Brown MS: Protein sensors for membrane sterols. Cell 2006, 124:35–46. [DOI] [PubMed] [Google Scholar]
- 4.Broz P, Dixit VM: Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol 2016, 16:407–420. [DOI] [PubMed] [Google Scholar]
- 5.Martinon F, Burns K, Tschopp J: The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10:417–426. [DOI] [PubMed] [Google Scholar]
- 6.Hodge RG, Ridley AJ: Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol 2016, 17:496–510. [DOI] [PubMed] [Google Scholar]
- 7.Ahearn IM, Haigis K, Bar-Sagi D, Philips MR: Regulating the regulator: post-translational modification of RAS. Nat. Rev. Mol. Cell Biol 2011, 13:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drenth JP, Cuisset L, Grateau G, Vasseur C, van de Velde-Visser SD, de Jong JG, Beckmann JS, van der Meer JW, Delpech M: Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat. Genet 1999, 22:178–181. [DOI] [PubMed] [Google Scholar]
- 9.Houten SM, Kuis W, Duran M, de Koning TJ, van Royen-Kerkhof A, Romeijn GJ, Frenkel J, Dorland L, de Barse MM, Huijbers WA, et al. : Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat. Genet 1999, 22:175–177. [DOI] [PubMed] [Google Scholar]
- 10.Galeotti C, Meinzer U, Quartier P, Rossi-Semerano L, Bader-Meunier B, Pillet P, Koné-Paut I: Efficacy of interleukin-1-targeting drugs in mevalonate kinase deficiency. Rheumatology (Oxford) 2012, 51:1855–1859. [DOI] [PubMed] [Google Scholar]
- 11.Montero MT, Matilla J, Gómez-Mampaso E, Lasunción MA: Geranylgeraniol regulates negatively caspase-1 autoprocessing: implication in the Th1 response against Mycobacterium tuberculosis. The Journal of Immunology 2004, 173:4936–4944. [DOI] [PubMed] [Google Scholar]
- 12.Liao Y-H, Lin Y-C, Tsao S-T, Lin YC, Yang A-J, Huang C-T, Huang KC, Lin WW: HMG-CoA reductase inhibitors activate caspase-1 in human monocytes depending on ATP release and P2X7 activation. J. Leukoc. Biol 2013, 93:289–299. [DOI] [PubMed] [Google Scholar]
- 13.Masters SL, Simon A, Aksentijevich I, Kastner DL: Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu. Rev. Immunol 2009, 27:621–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pontillo A, Paoluzzi E, Crovella S: The inhibition of mevalonate pathway induces upregulation of NALP3 expression: new insight in the pathogenesis of mevalonate kinase deficiency. Eur. J. Hum. Genet 2010, 18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Burgh R, Nijhuis L, Pervolaraki K, Compeer EB, Jongeneel LH, van Gijn M, Coffer PJ, Murphy MP, Mastroberardino PG, Frenkel J, et al. : Defects in mitochondrial clearance predispose human monocytes to interleukin-1β hypersecretion. J. Biol. Chem 2014, 289:5000–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celsi F, Piscianz E, Romano M, Crovella S: Knockdown of MVK does not lead to changes in NALP3 expression or activation. J Inflamm (Lond) 2015, 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Just I, Selzer J, Wilm M, Eichel-Streiber von C, Mann M, Aktories K: Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 1995, 375:500–503. [DOI] [PubMed] [Google Scholar]
- 18.Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H, et al. : Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 2010, 139:542–52– 552.e1–3. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong Y-N, Peng X, Xi JJ, Chen S, et al. : Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 2014, 513:237–241. [DOI] [PubMed] [Google Scholar]
- 20.French FMF Consortium: A candidate gene for familial Mediterranean fever. Nat. Genet 1997, 17:25–31. [DOI] [PubMed] [Google Scholar]
- 21.Park YH, Wood G, Kastner DL, Chae JJ: Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol 2016, 17:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Here the authors provide a model to explain hyper-inflammasome activation in HIDS patients. They demonstrate that inhibition of MVK results in loss of RhoA prenylation and impaired RhoA-dependent kinase activity which is sensed by the Pyrin inflammasome.
- 22.Akula MK, Shi M, Jiang Z, Foster CE, Miao D, Li AS, Zhang X, Gavin RM, Forde SD, Germain G, et al. : Control of the innate immune response by the mevalonate pathway. Nat. Immunol 2016, 17:922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper provides additional support for the connection between loss of protein prenylation and activation of Pyrin. However, they suggest that this is due to defective Kras prenylation and subsequent loss of PI(3K)-dependent inhibition of Pyrin.
- 23.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, et al. : NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. : Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med 2017, 377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 25.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, et al. : CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol 2013, 14:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M: Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. European Journal of Immunology 2011, 41:2040–2051. [DOI] [PubMed] [Google Scholar]
- 27.Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, Lappegård KT, Brekke O-L, Lambris JD, Damås JK, et al. : Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J. Immunol 2014, 192:2837–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nymo S, Samstad EO, Niyonzima N, Aune MH, Bergseth G, Ryan L, Brekke OL, Latz E, Lambris JD, Espevik T, et al. : Cholesterol crystals activate the complement system and are phagocytosed in a complement-dependent manner. Molecular Immunology 2013, 56:246. [Google Scholar]
- 29.Tall AR, Yvan-Charvet L: Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol 2015, 15:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerterp M, Fotakis P, Ouimet M, Bochem AE, Zhang H, Molusky MM, Wang W, Abramowicz S, la Bastide-van Gemert S, Wang N, et al. : Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis and Atherogenesis. Circulation 2018, doi: 10.1161/CIRCULATIONAHA.117.032636. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Using myeloid-specific Abca1/Abcg1-deletion, the authors demonstrate that impaired cholesterol efflux results in systemic inflammasome activation and worsened atherogenesis.
- 31.Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil M-T, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lütjohann D, et al. : Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018, 359:684–688. [DOI] [PubMed] [Google Scholar]
- 32.Chen HW, Kandutsch AA, Waymouth C: Inhibition of cell growth by oxygenated derivatives of cholesterol. Nature 1974, 251:419–421. [DOI] [PubMed] [Google Scholar]
- 33.Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL: Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem 2004, 279:52772–52780. [DOI] [PubMed] [Google Scholar]
- 34.Russell DW: The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem 2003, 72:137–174. [DOI] [PubMed] [Google Scholar]
- 35.Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang G, Russell DW: 25-Hydroxycholesterol secreted by macrophages in response to Tolllike receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. U.S.A 2009, 106:16764–16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park K, Scott AL: Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol 2010, 88:1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diczfalusy U, Olofsson KE, Carlsson A-M, Gong M, Golenbock DT, Rooyackers O, Fläring U, Björkbacka H: Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res 2009, 50:2258–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG: Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 2014, 345:679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wanke F, Moos S, Croxford AL, Heinen AP, Gräf S, Kalt B, Tischner D, Zhang J, Christen I, Bruttger J, et al. : EBI2 Is Highly Expressed in Multiple Sclerosis Lesions and Promotes Early CNS Migration of Encephalitogenic CD4 T Cells. Cell Rep 2017, 18:1270–1284. [DOI] [PubMed] [Google Scholar]
- 40.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G: Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479:538–541. [DOI] [PubMed] [Google Scholar]
- 41.Dang EV, McDonald JG, Russell DW, Cyster JG: Oxysterol Restraint of Cholesterol Synthesis Prevents AIM2 Inflammasome Activation. Cell 2017, 171:1057–1071. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Using Ch25h-deficient mice, the authors demonstrate that unchecked cholesterol biosynthesis is macrophages upon pathogen encounter can drive AIM2 inflammasome activation. This is due to cholesterol-dependent mitochondrial damage and mtDNA release.
- 42.West AP, Shadel GS: Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol 2017, 17:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernández A, Llacuna L, Fernández-Checa JC, Colell A: Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity. J. Neurosci 2009, 29:6394–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, O’Duill F, Schmid-Burgk JL, Hoss F, Buhmann R, et al. : The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell 2017, 171:1110–1124. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Here the authors make the surprising finding that human monocytes do not require AIM2 for cytosolic DNA-dependent inflammasome activation. Rather, STING-dependent DNA sensing drives lysosomal instability and subsequent triggering of NLRP3.
- 45.Viaud M, Ivanov S, Vujic N, Duta-Mare M, Aira L-E, Barouillet T, Garcia E, Orange F, Dugail I, Hainault I, et al. : Lysosomal Cholesterol Hydrolysis Couples Efferocytosis to Anti-Inflammatory Oxysterol Production. Circ. Res 2018, doi: 10.1161/CIRCRESAHA.117.312333. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Here the authors argue that LIPA-dependent release of free cholesterol upon efferocytosis is required to generate 25-HC. Blocking LIPA activity results in increased inflammasome activity.
- 46.Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW: Enzymatic Reduction of Oxysterols Impairs LXR Signaling in Cultured Cells and the Livers of Mice. Cell Metab 2007, 5:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ: An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 1996, 383:728–731. [DOI] [PubMed] [Google Scholar]
- 48.Field FJ, Mathur SN: Regulation of acyl CoA:cholesterol acyltransferase by 25-hydroxycholesterol in rabbit intestinal microsomes and absorptive cells. J. Lipid Res 1983, 24:1049–1059. [PubMed] [Google Scholar]
- 49.Gold ES, Ramsey SA, Sartain MJ, Selinummi J, Podolsky I, Rodriguez DJ, Moritz RL, Aderem A: ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation. J. Exp. Med 2012, 209:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips MC: Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem 2014, 289:24020–24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P, et al. : Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet 1999, 22:352–355. [DOI] [PubMed] [Google Scholar]
- 52.Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Ozcürümez M, et al. : The gene encoding ATPbinding cassette transporter 1 is mutated in Tangier disease. Nat. Genet 1999, 22:347–351. [DOI] [PubMed] [Google Scholar]
- 53.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, et al. : Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet 1999, 22:336–345. [DOI] [PubMed] [Google Scholar]
- 54.Westerterp M, Gautier EL, Ganda A, Molusky MM, Wang W, Fotakis P, Wang N, Randolph GJ, D’Agati VD, Yvan-Charvet L, et al. : Cholesterol Accumulation in Dendritic Cells Links the Inflammasome to Acquired Immunity. Cell Metab 2017, 25:1294–1304. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Using dendritic cell-specific ablation of Abca1/Abcg1, the authors find that impaired cholesterol efflux in antigen-presenting cells drives a lupus-like phenotype in mice. They argue that this is driven by increased dendritic cell inflammasome activation, which results in pathological IL-18-driven Th1 activation.
- 55.Dinarello CA: IL-18: A TH1 -inducing, proinflammatory cytokine and new member of the IL-1 family. Journal of Allergy and Clinical Immunology 1999, 103:11–24. [DOI] [PubMed] [Google Scholar]
- 56.De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M, Xu D, Zimmer S, Lahrmann C, Schildberg FA, et al. : High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol 2014, 15:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liston A, Masters SL: Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat. Rev. Immunol 2017, 17:208–214. [DOI] [PubMed] [Google Scholar]
- 58.Tabas I: The Role of Endoplasmic Reticulum Stress in the Progression of Atherosclerosis. Circ. Res 2010, 107:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waris G, Felmlee DJ, Negro F, Siddiqui A: Hepatitis C virus induces proteolytic cleavage of sterol regulatory element binding proteins and stimulates their phosphorylation via oxidative stress. J. Virol 2007, 81:8122–8130. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Ouimet M, Koster S, Sakowski E, Ramkhelawon B, van Solingen C, Oldebeken S, Karunakaran D, Portal-Celhay C, Sheedy FJ, Ray TD, et al. : Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol 2016, 17:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samanta D, Mulye M, Clemente TM, Justis AV, Gilk SD: Manipulation of Host Cholesterol by Obligate Intracellular Bacteria. Front Cell Infect Microbiol 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catron DM, Sylvester MD, Lange Y, Kadekoppala M, Jones BD, Monack DM, Falkow S, Haldar K: The Salmonella-containing vacuole is a major site of intracellular cholesterol accumulation and recruits the GPI-anchored protein CD55. Cellular Microbiology 2002, 4:315–328. [DOI] [PubMed] [Google Scholar]
- 63.Sonda S, Ting LM, Novak S, Kim H, Maher JJ, Farese RV, Ernst JD: Cholesterol esterification by host and parasite is essential for optimal proliferation of Toxoplasma gondii. J. Biol. Chem 2001, 276:34434–34440. [DOI] [PubMed] [Google Scholar]
- 64.Lordén G, Sanjuán-García I, de Pablo N, Meana C, Alvarez-Miguel I, Pérez-García MT, Pelegrín P, Balsinde J, Balboa MA: Lipin-2 regulates NLRP3 inflammasome by affecting P2X7 receptor activation. J. Exp. Med 2017, 214:511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Progatzky F, Sangha NJ, Yoshida N, McBrien M, Cheung J, Shia A, Scott J, Marchesi JR, Lamb JR, Bugeon L, et al. : Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat Commun 2014, 5:5864. [DOI] [PMC free article] [PubMed] [Google Scholar]