Abstract
Background:
In addition to N-methyl D-aspartate receptor antagonism, ketamine produces opioid system activation. The objective of the study was to determine if opioid receptor antagonism prior to administration of intravenous ketamine attenuates its acute antidepressant and/or dissociative effects.
Methods:
In a proposed double-blind, cross-over study of 30 adults with treatment-resistant depression, we performed a planned interim analysis after studying 14 participants, 12 of whom completed both conditions in randomized order: 50mg naltrexone preceding 0.5mg/kg ketamine or placebo preceding 0.5mg/kg ketamine.
Results:
In the interim analysis, 7 of 12 adults with treatment-resistant depression met responder criteria during the ketamine + placebo condition, defined as a ≥50% reduction on the 17-item Hamilton Depression Scale score at Day 1. The subjects’ reductions in Hamilton Depression Rating Scale 6 and 17-item ratings in the ketamine + naloxone condition were significantly lower than the ratings in the ketamine + placebo condition at post-infusion Days 1 and 3. Secondary analysis of all participants completing both placebo and naloxone conditions, regardless of the robustness of response to ketamine, showed similar results. There were no differences in ketamine-induced dissociation between conditions. Because naltrexone dramatically blocked the antidepressant but not the dissociative effects of ketamine, the trial was halted at the interim analysis.
Discussion:
Ketamine’s acute antidepressant effect appears to require opioid system activation. Dissociative effects of ketamine in humans are not mediated by the opioid system, nor do they appear sufficient without the opioid effect to produce the acute antidepressant effects of ketamine in adults with treatment-resistant depression.
Keywords: Treatment-resistant depression, mu opioid receptor, ketamine, naltrexone
Introduction
Depression is the leading cause of disability worldwide (1), yet novel antidepressant development has stalled (2). While traditional antidepressant medications remain the staple for treating major depressive disorder, a significant proportion of patients fail to achieve clinical response with standard treatments (3) and require interventional approaches such as intravenous ketamine infusions (4). With 40–60% of patients meeting clinical criteria for an antidepressant response after infusion, ketamine has demonstrated impressive efficacy in patients who have failed to respond to traditional antidepressant therapies (5).
Although the specific mechanisms of action responsible for the acute antidepressant effects of ketamine have yet to be determined (5), they have generally been conceptualized to be due to N-methyl-D-aspartate receptor antagonism (5, 6). However, other candidate N-methyl D-aspartate receptor antagonists have not been proven to be effective antidepressants (5). More recently, a pre-clinical study reported antidepressant effects of ketamine are independent of N-methyl-D-aspartate receptor antagonism and were due to modulation at other receptors such as that for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (7).
Beyond the glutamate system, ketamine interacts with several additional neurotransmitter systems including mu, delta and kappa opioid receptors and is currently used as an anti-nociceptive agent for acute and chronic pain (8). Ketamine’s analgesic effects are blocked by mu and delta opioid receptor antagonists but not by kappa opioid receptor antagonists, indicating a mu or delta opioid mechanism in ketamine’s anti-nociceptive effects (9). We and others have hypothesized that ketamine’s antidepressant mechanism of action may in fact be related to intrinsic opioid receptor properties of ketamine (10) and have previously proposed that co-administration of an opioid receptor antagonist with ketamine could be employed to test this hypothesis (11). Yet, no study to date has probed the role that opioid properties of ketamine play in its antidepressant effects (12).
As a dissociative anesthetic (13, 14), ketamine is capable of producing dramatic psychotomimetic effects (15–17), and these effects have been correlated to its antidepressant efficacy (18). Here too specific receptor system(s) responsible for mediating dissociative effects of ketamine are also unknown. Some, but not all, N-methyl-D-aspartate receptor antagonists cause dissociation (19). The pure kappa opioid receptor agonist, salvinorin A, does produce dissociative effects similar to ketamine (20, 21). A low (25mg) dose of the opioid receptor antagonist, naltrexone, can augment the psychoactive effects of lower (~0.4 mg/kg/h) subanesthetic doses of ketamine, but not higher (~0.6 mg/kg/h) sub-anesthetic doses of ketamine in healthy humans (22). However, opioid receptor antagonists have not been previously used to probe the role opioid receptors play in ketamine’s dissociative effects in adults with treatment-resistant depression, and the 25mg dose of naltrexone does not completely block opioid receptors (23).
The intent of this study was not to assess ketamine’s antidepressant efficacy but rather to determine the role of the opioid system in its antidepressant and dissociative effects in adult humans with treatment-resistant depression. We conducted a randomized, double-blind, crossover trial in which intravenous ketamine was infused twice across both conditions, with participants receiving pre-treatment with either the opioid receptor antagonist, naltrexone, before one of their ketamine infusions (ketamine + naltrexone), or placebo before the other ketamine infusion (ketamine + placebo) in a counterbalanced manner. Through this mechanistic clinical trial design, we tested if pre-treatment with an opioid receptor antagonist (ketamine + naltrexone) is able to attenuate the acute antidepressant and/or dissociative effects of ketamine.
Methods
Participants
Potential study participants were brought into the clinic for a screening visit to determine eligibility. All study participants were outpatients. Inclusion criteria included a current diagnosis of a non-psychotic, non-atypical major depressive episode as part of either bipolar II disorder or major depressive disorder, defined by DSM-5 criteria (24). For the initial enrollment, all participants were required to have a score ≥20 on the 17-item Hamilton Depression Rating Scale (25). Each participant was also required to have not benefited sufficiently from trials of at least 4 different antidepressant medications or other somatic treatments as defined by the Massachusetts General Hospital Antidepressant Treatment History Questionnaire criteria (26), as well as a minimum of 6 weeks of prior psychotherapy during any major depressive episode prior to intervention.
All eligible participants provided full written informed consent. The study protocol was approved by the Stanford University Institutional Review Board. All participants were required to hold any stimulant/amphetamine drugs documented during the screening phase for the 24 hours prior to ketamine administration and could resume these medications Day 1 post-infusion after the completion of the ratings. Participants were required to withhold taking any benzodiazepine for the 8 hours prior to (or any hypnotic drugs the night prior to) ketamine administration and could resume these medications Day 1 post-infusion after the completion of the ratings. Furthermore, any medical marijuana use was held for two weeks in order to allow for proper washout prior to the baseline/randomization visit (e.g., at least 5 half-lives of the drug). We excluded individuals on opiates in order to avoid naltrexone precipitating opioid withdrawal along with eliminating the confound of ketamine-opioid interactions. If a washout period was necessary prior to study participation, the study physician maintained ongoing contact with the participant to ensure safety during this time. Those medications deemed likely not to interact with ketamine (selective serotonin reuptake inhibitor, selective norepinephrine reuptake inhibitor, monoamine oxidase inhibitor, tricyclic antidepressant, buproprion) and some adjuncts (antipsychotics, antiepileptics, and thyroid hormone) were maintained at a constant dose for at least 4 weeks. After eligibility was confirmed, participant demographic and medical data were collected.
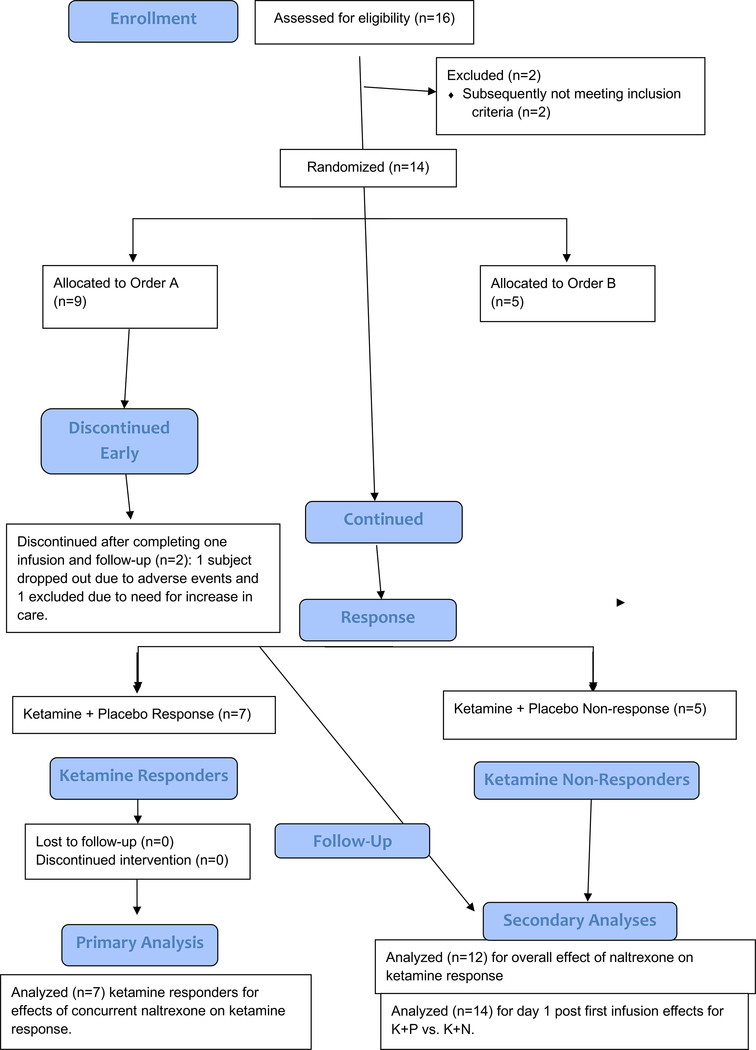
Sixteen participants consented for this study. Two participants were withdrawn: one who was found to be positive for methamphetamine and one who was found to have an unreported medical illness. The patient flow is summarized in Figure 1. Fourteen participants received at least one intravenous ketamine infusion, and 12 participants crossed over and completed both infusions. Table 1 summarizes the sample characteristics. The mean age was 41.3 (±11.8) years at baseline. Of the 12 participants who completed both conditions, all were diagnosed with recurrent major depressive disorder, 6 were women, 5 were unemployed, and 2 were receiving disability.
Figure 1.
CONSORT Diagram of participants in a Study of Ketamine’s Antidepressant Effect after Naltrexone Pretreatment
Table 1.
Patient Characteristics in a Study of Ketamine’s Antidepressant Effect after Naltrexone Pretreatment
| All | Responder | Non-Responder | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Current Age | 41.3 | 11.8 | 39.8 | 8.2 | 44.4 | 18.2 |
| Age at MDD onset | 17.3 | 4.3 | 16.3 | 3.2 | 17.8 | 5.8 |
| Length of Illness (years) | 24.1 | 10.6 | 23.5 | 9.2 | 26.6 | 14.6 |
| Current Depressive Episode (years) | 8.6 | 7.4 | 7.7 | 8.3 | 10.2 | 6.8 |
| Total Antidepressant (primary + adjunct) in current episode | 5.7 | 5.8 | 4.0 | 3.3 | 5.0 | 1.6 |
| Antidepressant failures in current episode | 3.8 | 3.0 | 2.9 | 2.4 | 3.6 | 1.1 |
| Total number antidepressants, lifetime | 6.9 | 3.5 | 7.0 | 35 | 5.5 | 3.4 |
| N | % | N | % | N | % | |
| Gender (Female) | 6 | 42.9 | 4 | 57.1 | 1 | 20.0 |
| Diagnosis Recurrent MDD | 12 | 85.7 | 7 | 100.0 | 4 | 80.0 |
| Previous Brain Stimulation Therapies (ECT or TMS) | 6 | 42.9 | 2 | 28.6 | 2 | 40.0 |
| Past Psychotherapy | 11 | 78.6 | 6 | 85.7 | 3 | 60.0 |
| Family History of Depression | 5 | 35.7 | 3 | 42.9 | 2 | 40.0 |
| Mean | SD | Mean | SD | Mean | SD | |
| HDRS Score (17 item). Baseline | 25.9 | 4.6 | 26.0 | 4.3 | 26.6 | 5.8 |
| MADRS, Baseline | 35.3 | 4.9 | 35.1 | 3.8 | 35.6 | 4.8 |
| CGI-S, Baseline | 5.1 | 0.5 | 5.1 | 0.4 | 5.0 | 0.7 |
| BDI-II Self report. Baseline | 30.1 | 10.5 | 29.1 | 9.5 | 28.8 | 9.8 |
The average length of depressive illness was 24.1 (±10.6) years, and the average length of the current depressive episode was 8.6 (±7.4) years. Participants reported a mean of 9.8 (±6.5; mode=8) unsuccessful antidepressant treatments (primary, adjunct, somatic, psychotherapy) lifetime. Participants reported a mean of 6.9 (±3.5; mode=3) primary antidepressant medications trials lifetime. Participants reported a mean of 5.7 (±5.8; mode=3) of all antidepressant agents (adjunct and primary) during this episode (see Table 1); and a mean of 3.8 (±3.0; mode=3) primary antidepressant treatments during this episode (see Table 1). Several participants had a history of failing to respond to repetitive transcranial magnetic stimulation (6/14) and/or failing to respond to electroconvulsive therapy (1/14) (see Table 1).
Design
The study employed a crossover design comprised of two treatment conditions: oral placebo or oral naltrexone (50mg) preceding a 0.5mg/kg intravenous infusion of ketamine. Placebo and naltrexone pills were identical in appearance where the naltrexone pill was over-encapsulated. Order of treatment was randomized, and both investigators and participants were blinded to the order. Placebo or naltrexone was administered 45 minutes prior to the initiation of the ketamine infusion in order to achieve peak naltrexone levels at the initiation of the ketamine infusion (27). Ketamine 0.5mg/kg was then administered intravenously over 40 minutes.Participants were monitored with continuous 3-lead ECG, pulse oximetry, end-tidal capnography, and non-invasive blood pressure measurement every 5 minutes during the infusion. Participants were monitored by a study physician and study staff throughout the course of the infusion.
Ratings of depression were assessed on the 6-item and 17-item Hamilton Depression Scale at baseline and at Days 1, 3, 5, 7, and 14 post-infusion. The primary outcome was reduction of depressive symptoms at Day 1 post-infusion among those who met responder criterion during the ketamine + placebo condition (30). Ketamine response was defined as a ≥50% reduction in total 17-item Hamilton Depression Scale score at Day 1 post-infusion, as has been used in a number of previous ketamine studies (27). The secondary outcome measure instrument was the Clinician Administered Dissociated States Scale (28). We collected data at multiple time-points to assess for prolonged effects of ketamine and/or naltrexone including whether naltrexone blocked or delayed the antidepressant effects of ketamine since naltrexone produces a 96-hour blockade of opioid receptors in brain (23). The Clinician Administered Dissociated States Scale was collected prior to infusion and at multiple intervals up to 180 minutes post-infusion. Raters were blind to treatment condition for all assessments.
After completing their first treatment condition, participants were assessed 28 days later to evaluate for relapse, defined as having a 17-item Hamilton Depression Rating Scale score within 20% of their baseline score, and to determine eligibility for entering the second treatment condition. We selected 28 days between infusions to minimize carry-over effects. If the participant had no response at any of the time-points in the first 14 days, they could enter to the second treatment condition after Day 14. If there was a sustained antidepressant response from the first treatment, the participant was seen every two weeks until relapse occurred up to 120 days. Once relapse was determined, participants crossed over to the second treatment condition.
Data analyses
In this two-condition crossover study, we estimated a priori that 30 participants would be required to yield 15 ketamine responders as defined by a ≥50% reduction in the baseline 17item Hamilton Depression Scale score on Day 1 post-infusion in the ketamine + placebo condition (25). A power calculation indicated that analysis of 15 participants in a cross-over model would be fully powered to detect statistical significance assuming a moderate to large effect sizes and alpha of 0.05 (two-tailed). An interim analysis was planned for the midway point.
The primary endpoint evaluated the antidepressant response to ketamine + naltrexone relative to the response to ketamine + placebo in those identified as ketamine + placebo responders. A fixed-effects repeated measures model compared mean changes on the 17-item Hamilton Depression Rating Scale and the 6-item subscale scores for two time points (i.e., preinfusion day 0 and post-infusion day 1) for the two conditions. There were no missing data on the primary endpoints (i.e., 17-item and 6-item Hamilton Depression Rating Scale). Statistical comparisons at time-points after Day 1 were conditional on the primary endpoint being statistically significant (29). Paired comparisons were conducted for the Hamilton Depression Rating Scale scores measured at 3, 5, 7 and 14 days post-infusion. There were five missing Hamilton Depression Rating Scale scores across the 14-day study (3 at Day 5, 1 at Day 7, and 1 at Day 14). The secondary endpoint compared participants’ peak levels of dissociation in the two conditions, as measured by change in the Clinician Administered Dissociated States Scale at the end of the 40-minute infusion.
To more fully describe the relative effects of ketamine + placebo versus ketamine + naltrexone, we applied similar analytical methods to those reported by Zarate (30). After testing the primary mechanistic hypothesis among ketamine responders, two sets of analyses were used to more fully understand the effect of ketamine + placebo and of ketamine + naltrexone on the 17-item and 6-item Hamilton Depression Rating Scales. The first set included analyses of all 12 participants who completed the crossover and received both treatment conditions (i.e., both responders and non-responders). For these participants (n=12), a general linear model for repeated measurements tested within-subject effects of the two treatment conditions on change in Hamilton Depression Rating Scale scores from Day 0 to Day 1.
Effect sizes were also calculated using standardized mean differences between conditions for the primary endpoints (i.e., 17-item and 6-item Hamilton Depression Rating Scale pre- and post-infusion). Potential carryover effects were tested using a fixed effects model with treatment order as a between-subjects factor, and the HDRS baseline measure for each phase as the dependent variable. An alpha of 0.05 (two-tailed) was used to determine statistical significance.
Results
Fourteen participants received at least one infusion, and 12 participants completed the crossover and underwent both ketamine + placebo as well as ketamine + naltrexone conditions. The interval of time between ending the first condition and starting the second condition ranged from 14 to 63 days (M=33, SD=14.8). After unblinding, analyses indicated that 7 of the 12 participants who completed both study treatment conditions met the pre-specified criterion of responder, defined as a 50% or greater reduction from baseline to day 1 in HDRS-17 scores in the ketamine + placebo condition (see Figure 1).
Depression
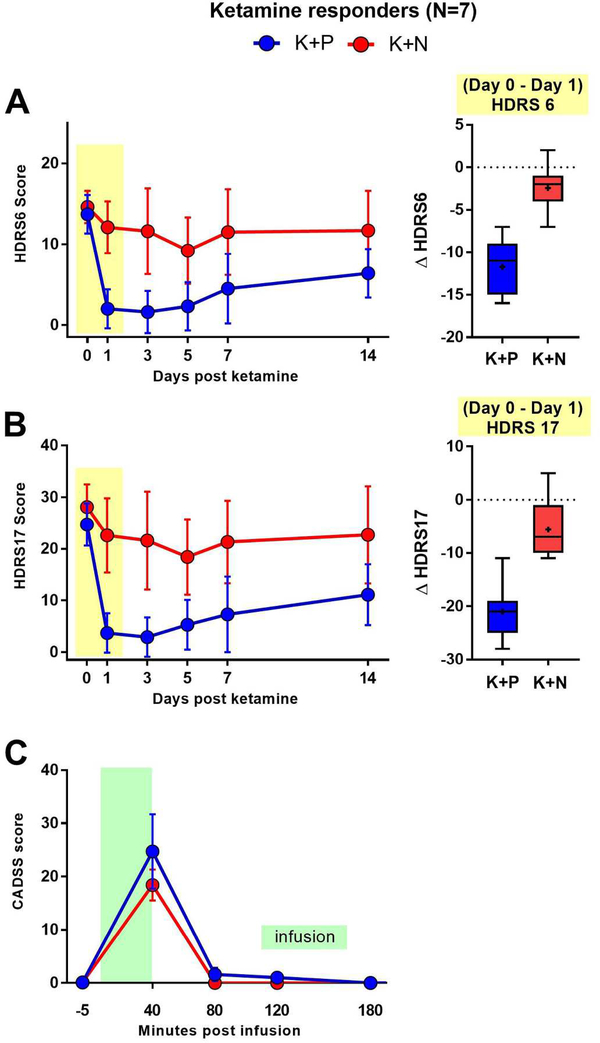
There were no significant differences in the mean baseline 17-item Hamilton Depression Rating Scale scores and the mean 6-item Hamilton Depression Rating Scale scores for ketamine + placebo (M=26.7, SD=5.4) and ketamine + naltrexone (M=28.1, SD=5.4) conditions. Robust reductions in mean 17-item Hamilton Depression Rating Scale scores were observed at 1 day post-infusion in the ketamine + placebo condition (M=−22.3, SD=3.2; F=106, p=0.000048). Significant reductions from baseline were also observed in the ketamine + naltrexone condition (M=−5.6, SD=5.7; F=6.8, p=0.04), however ketamine-induced reductions in depression symptoms were significantly attenuated when naltrexone was administered (Mean difference=16.7, SD=6.7; F=43.6, p=0.0006; effect size δ=2.5). Significant differences between ketamine + placebo and ketamine + naltrexone conditions were still evident at day 3 – but not at days 5, 7, and 14 post infusion (see Figure 2A).
Figure 2. Naltrexone pretreatment blocks ketamine’s antidepressant effect, but not dissociative symptoms.
Time-course of primary outcome measures (mean ± SD) for ketamine-responsive TRD patients (n=7) in two crossed over conditions, ketamine + naltrexone (K+N) and ketamine + placebo (K+P). Treatments delivered on Day 0 following first questionnaire. A. Left, HDRS6 time-course. Analysis of between-group HDRS6 differences on Day 1 shows that K+N group scores were significantly higher than K+P group scores, with the latter group demonstrating expected post-infusion HDRS6 score reduction. Right, Box and whisker plot illustrating the distribution of the Day 0-to-Day 1 score changes for HDRS6 in each treatment condition in ketamine responders. (Horizontal line, median; “+”, mean; box, 25–75th percentile; whiskers, minimum-maximum score). B. Left, HDRS17 time-course, demonstrating qualitatively similar results as in A. Right, Box and whisker plot for distribution of Day 0-to-Day 1 score changes for HDRS17 in each treatment condition, as in A. C. Time course of the secondary outcome measure, CADSS score, on day of infusion. Peak CADSS scores immediately following infusion (+40 minutes) did not differ between groups.
On day 1 post- ketamine + placebo infusion, 5 of 7 responders met criteria for remission (17-item Hamilton Depression Rating Scale ≤7) (31). In contrast, on day 1 of the ketamine + naltrexone infusion, none (0 of 7) of the ketamine + placebo responders met responder criteria (≥50% reduction in 17-item Hamilton Depression Rating Scale).
Using the 6-item Hamilton Depression Rating Scale, which assesses the core symptoms of depression, similar results were observed. In the ketamine + placebo condition, statistically significant reductions from baseline in mean 6-item Hamilton Depression Rating Scale scores were observed at Day 1 post-infusion (M=−11.7±3.1; F=93.8, p=0.0007). In the ketamine + naltrexone condition, mean changes from baseline on 6-item Hamilton Depression Rating Scale were observed at Day 1, although the reduction was not statistically significant (M=−2.4±2.8; F=5.4, p=0.059). Comparison of reduction between conditions indicated that the reduction in 6item Hamilton Depression Rating Scale scores observed in the ketamine + naltrexone condition was significantly lower than the reduction observed in the ketamine + placebo condition (Mean Difference=9.3, SD=4; F=29.8, p=0.002; effect size δ=2.3; see Figure 2B).
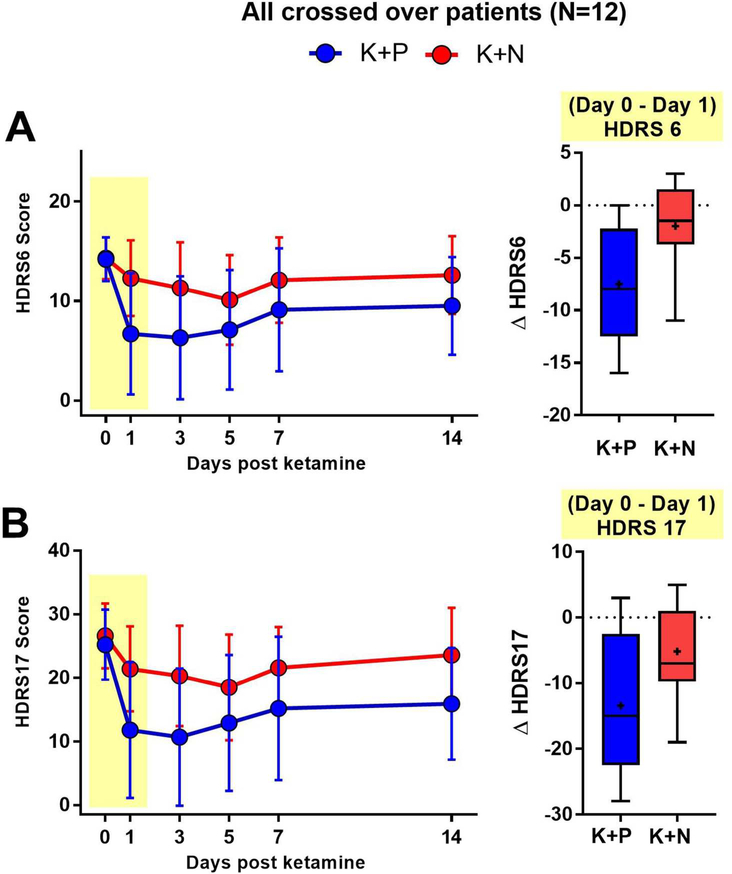
After testing the mechanistic hypothesis via assessing attenuation of response for patients who responded to ketamine + placebo (n=7), similar analyses were conducted on all participants receiving both treatment conditions (n=12), regardless of whether they met the responder criterion during the ketamine + placebo condition. These data, including mean scores on the 6 and 17-item Hamilton Depression Rating Scale, are shown in Figure 3A and 3B respectively. One day after infusion, statistically significant reductions in mean 17-item Hamilton Depression Rating Scale scores were observed in both the ketamine + placebo (M=−14.2, SD=10.7; F=19.3, p=0.0011) and ketamine + naltrexone conditions (M=−4.9, SD=6.8; F=8.7, p=0.013), with significantly smaller reduction in 17-item Hamilton Depression Rating Scale scores in the ketamine + naltrexone condition (MD=−8.4, SD=12.6; condition × time interaction F=5.4, p=0.041; effect size δ=0.7). Statistically significant reductions were also observed on the 6-item Hamilton Depression Rating Scale scores in the ketamine + placebo condition (M=−7.5, SD5.8, F=20.3, p=0.0009). In the ketamine + naltrexone condition, the mean reduction on the 6item Hamilton Depression Rating Scale scores was not statistically significant (M=−2.0, SD=3.9, F=3.0 p=0.11). The reduction in 6-item Hamilton Depression Rating Scale scores was significantly attenuated when naltrexone was administered with ketamine (Mean difference=5.5, SD=6.9, F=7.7, p=0.018; effect size δ=0.8).
Figure 3. Time-course of HDRS6 and HDRS17 in an alternate patient grouping consistently demonstrates naltrexone block of ketamine’s antidepressant effect.
A. Left, HDRS6 time-course (mean ± SD) in all patients who received both infusions in the crossed over design (N=12), including N=5 patients who did not meet the ketamine responsive criterion required for inclusion in our primary outcome analysis. A. Left, HDRS6 time-course. The difference between K+N versus K+P group scores on Day 1 after infusion is maintained with inclusion of ketamine non-responders in the cross-over analysis. Right, Box and whisker plot of the Day 0-to-Day 1 score changes for HDRS6 in each treatment condition for all crossed-over patients. (Horizontal line, median; “+”, mean; box, 25–75th percentile; whiskers, minimummaximum score). B. Left, HDRS17 time-course, demonstrating qualitatively similar results as in A. Right, Box and whisker plot for distribution of Day 0-to-Day 1 score changes for HDRS17 in each treatment condition, as in A.
Dissociation
Among the ketamine responders (n=7), mean scores on the Clinician Administered Dissociated States Scale significantly increased from pre-infusion to 40-minutes post-infusion in both conditions (ketamine + placebo: median=+23, M=+24.7, SD=18.3; ketamine + naltrexone: median=+21, M=+18.2, SD=7.6), as shown in Figure 2C. However, there was no significant difference between the ketamine + placebo and ketamine + naltrexone conditions in average levels of dissociation (Wilcoxon test, p=0.45). Among study completers (n=12), which included responders and non-responders to ketamine + placebo, dissociation scores increased in both conditions, albeit to a lesser extent in the ketamine + naltrexone condition (ketamine + placebo: median=+17.5, M=+19.1, SD=16.3; ketamine + naltrexone: median=+14.5, M=+13.8, SD=8.8). After 40 minutes, Clinician Administered Dissociated States Scale scores normalized with only 3 patients having scores ≥1 at 80 minutes.
Evaluation of Blind and Side Effects
Data were collected on a range of visual analog scales (38) that address a variety of potential psychoactive side effects at 45 min after ingestion of naltrexone or placebo and immediately before the initiation of the ketamine infusion. There were no differences in reported side effects for individuals receiving naltrexone or placebo prior to ketamine infusion on the visual analog scales for psychoactive effects (32) (see Figure S3) as has been previously demonstrated for naltrexone (23, 33–38). There was no other direct assessment of blind integrity performed. After the ketamine infusion in the 12 subjects who completed the crossover, seven participants in the naltrexone condition experienced nausea in contrast to three who developed nausea on ketamine plus placebo. Two participants experienced vomiting on either condition. At the interim analysis, it was decided to stop enrolling patients for the study rather than expose additional patients to the combination of ketamine and naltrexone.
Discussion
Ketamine has well-established rapid-onset antidepressant effects. The majority of preclinical studies investigating the mechanism of this effect have focused on N-methyl Daspartate receptor antagonism, and several clinical trials have aimed to replicate this rapid antidepressant effect with other N-methyl D-aspartate receptor antagonists, with limited success (5). We now present the first evidence in humans that opioid receptors are necessary for ketamine’s acute antidepressant effect. In ketamine-responsive treatment-resistant depression patients, pre-treatment with naltrexone profoundly attenuated ketamine’s antidepressant effect and it resulted in none of the ketamine responders meeting responder criterion at Day 1. We observed concordant effects on related measures of depression, including clinicianadministered scales the 6-item Hamilton Depression Rating Scale, and Montgomery–Åsberg Depression Rating Scale and a self-report instrument, Beck Depression Inventory Version II (see supplement), which strengthens our conclusion that ketamine’s antidepressant effects require opioid system activation. Of note, we observed a statistically significant difference at Day 1 from baseline in the 17-item Hamilton Depression Rating Scale for the ketamine + naltrexone group but not using the Montgomery–Åsberg Depression Rating Scale or the 6-item Hamilton Depression Rating Scale, scales thought to reflect core depressive symptoms.
The endogenous opioid system has been reported to play a central role in the pathophysiology and treatment of affective disorders (39–44). A robust nonhuman primate literature supports the idea that opioids are important in mediating emotions associated with depression (45, 46). Depressive disorders have been associated with dysregulation of the endogenous opioid system, particularly mu opioid receptor and kappa opioid receptor tone (40, 41). Moreover, the mu opioid receptor partial agonist and kappa opioid receptor antagonist, buprenorphine, has been shown to produce antidepressant effects (42, 47), even in individuals who have failed electroconvulsive therapy (44). In obsessive-compulsive disorder, single infusions of ketamine have been reported to produce a multi-day benefit (48), as has a single oral dose of morphine, a mu opioid receptor agonist and N-methyl D-aspartate receptor antagonist (49). These data suggest that mu opioid receptor agonists with additional N-methyl D-aspartate receptor antagonist properties may have therapeutic potential as intermittently dosed therapies for mood or anxiety disorders.
The kappa opioid receptor is also emerging as a regulator of mood and motivation (50–52) with increased kappa opioid receptor activity being associated with depression (53). As naltrexone does not have substantial selectivity for mu opioid receptor over kappa opioid receptor (54, 55), the 50mg dose of naltrexone used in this study saturate the mu opioid receptors and likely equally saturated the kappa (23, 56). Thus our data do not distinguish between the respective roles mu opioid receptor and kappa opioid receptor in mediating ketamine’s antidepressant effects. Nonetheless, given the available data implicating mu opioid receptor-based mechanisms of antidepressant efficacy, inconsistent findings regarding kappa opioid receptor antagonists in depression (57, 58), and ketamine’s putative kappa agonist mechanism (21, 59), we favor the interpretation that ketamine produces its acute antidepressant response primarily through direct and/or indirect actions at the mu opioid receptors. Naltrexone when chronically administered alone in normal healthy controls as well as individuals with mood and substance disorders has been demonstrated to either act as an antidepressant or be moodneutral across several placebo-controlled trials (23, 33–38), suggesting that naltrexone is not simply acting as a depressionogenic agent in this case, but rather providing selective blockade of the antidepressant effects produced by ketamine.
How do we reconcile these data with the large body of evidence implicating glutamate receptors in ketamine’s primary antidepressant mechanism? The majority of studies to date have focused on ketamine’s antidepressant mechanism of action as a non-competitive antagonist of the N-methyl D-aspartate receptor antagonist and subsequent activation of αamino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Recently, a preclinical study reported that a metabolite of ketamine, 2R, 6R-hydroxynorketamine, has antidepressant efficacy through stimulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor independent of N-methyl D-aspartate receptor antagonist antagonism (7). This mechanism of action has been replicated by some (60, 61) but not all (62) groups. In addition, pre-clinical studies demonstrate glutamate receptor modulation triggers downstream modulation of synthesis and release of brain-derived neurotrophic factor and enhances synaptic plasticity via activation of molecular targets such as mammalian target of rapamycin and eukaryotic elongation factor 2 (60, 61, 63–65). These glutamate system effects may in fact drive the transient maintenance of the antidepressant response through modulation of brain plasticity (66) rather than producing the actual acute antidepressant effects.
No studies to date have directly addressed the role of opioid receptors in ketamine’s antidepressant effect. However, our demonstration of an opioid system activation requirement for ketamine’s acute antidepressant effect mirrors a longstanding literature investigating the opioid mechanism of actions of ketamine’s analgesic properties. On the Montgomery–Åsberg Depression Rating Scale and the 6-item Hamilton Depression Rating Scale (which reflects the core depression symptoms (67)), our data demonstrated that the effect of ketamine was ablated by naltrexone. Meta-analyses consistently have shown that ketamine has a clinically significant opiate-sparing effect where co-administration of ketamine allows for lower doses of traditional opiates to be used in order to achieve similar anti-nociceptive effects (68). In addition to ketamine’s combined naloxone-sensitive and naloxone-insensitive analgesic effects (69), human and preclinical studies have found that ketamine 1) substantially potentiates the analgesic effect of opioids (70), 2) produces opioid receptor dependent analgesia (71–73), 3) reduces opioid tolerance and opioid-induced hyperalgesia to opioids (74), and 4) produces mu opioid receptor-dependent respiratory depression (72).
With the proviso that the scope of ketamine’s pharmacology is continually expanding (75), the available evidence suggests that ketamine-mediated analgesia involves either a direct action at mu opioid receptors (59, 76–78) or an interaction between N-methyl D-aspartate receptor antagonists and mu opioid receptors (79–81). The hypothesis that N-methyl Daspartate receptor antagonists and mu opioid receptors share subcellular co-localization and may exist as a functional complex in a crucial nociceptive brain area (the Periaqueductal Gray) (80) forms a particularly compelling explanation for apparently conflicting findings in the context of ketamine-mediated analgesia. Notably, naltrexone pretreatment did not significantly impact ketamine-induced dissociation, measured by the Clinician Administered Dissociated States Scale, nor did the Clinician Administered Dissociated States Scale correlate with ketamine’s antidepressant efficacy. Prior work has attributed ketamine’s dissociative and hypnotic properties to N-methyl D-aspartate receptor antagonist and hyperpolarization-activated cyclic nucleotide-gated cation channel 1 blockade (75), as well as activation of kappa opioid receptors (21). Our finding that the dissociative effects of ketamine persist despite naltrexone antagonism of opioid receptors suggests that opioid receptors do not play a major role in mediating ketamine’s dissociative effects.
The public health significance of ketamine’s opioid properties needs to be studied. Depression and opioid dependence are currently the two most significant public health problems facing the United States and have become leading causes of disability and death worldwide (1, 82, 83). While opioids have a history of use as antidepressants (44, 84), they pose a significant risk if used chronically (85). One-half of patients prescribed opioids have a mental health diagnosis (52, 86–88), and over half of individuals with opioid use disorders have a primary diagnosis of depression (89). There is also a significant ketamine abuse problem worldwide (90–92), and ketamine ranks highly on the list of commonly abused substances (93–97). Ketamine abusers also have high rates of depression (80) and experience significant brain dysfunction (98). While these risks have not been demonstrated in serial infusions for depressed patients (99), short-interval repetitive dosing strategies may pose greater risks (100) and there have been case reports of apparent tolerance after chronic administrations (101, 102). Ketamine tolerance has been observed in pain/anesthesia indications (103–110) as well as in animal models (111–113). The route of administration may play a role in the risk (114) along with the patient’s access to the medication (115). Thus, the abuse/dependence potential of frequent ketamine treatment in major depression needs further study, and our results provide strong justification for further caution against widespread and repeated use of ketamine before further mechanistic testing has been performed (102, 116, 117).
There are a number of strengths and weaknesses in our study. A cross-over study was the optimal method to test the study’s mechanistic hypothesis, since it can clearly identify ketamine responders post-hoc and establish, in an individual participant, that ketamine’s antidepressant effects are mediated via the opioid system. We did not employ an alternative design wherein responders would first be identified by open label pre-treatment with ketamine, which could produce an expectancy bias that they would have a similar response to the randomized treatment. Moreover, the cross-over design provides significantly greater statistical power to detect group differences with fewer subjects. Limitations of a crossover study include potential carry-over effects (118). However, because ketamine’s effects are transient, our washout period was sufficient for participants’ 17-item Hamilton Depression Rating Scale scores to return to within 20% or less of baseline, medication-related carry-over effects were limited. While we cannot completely rule out the presence of carry-over effects in our primary analysis (118), in an alternative analysis involving only the first randomized infusion (prior to crossover), we did observe a significant difference between patients receiving ketamine + placebo and ketamine + naltrexone (see supplement), further demonstrating that naltrexone blocks the antidepressant effects of ketamine.
We assessed the blind integrity post-hoc using visual analog scale assessments made just prior to ketamine infusion and 40 minutes after taking naltrexone or placebo. We could identify no item or group of items (see Figure S3), or side effect, that a subject could have reasonably used to infer their blinded condition. In any longitudinal study, regression to the mean is a possible issue. Data from Murrough et. al. indicate that initial response to ketamine is replicated by re-infusing ketamine 3 times per week for 2 weeks with repeated treatments (12). One weakness in the study was the final sample size in the interim analysis, and our findings do need to be replicated in other studies. Still, we found the same qualitative block of ketamine’s effect regardless of the depression instrument used, and with several alternate statistical analyses. We decided to stop the study because our results were both statistically and clinically significant and we were concerned about the ethics of exposing more people to a clearly ineffective and noxious combination treatment.
Future studies are necessary to expand our understanding of the opioid effects of ketamine with an emphasis on determination of the exact opioid receptors involved in mediating ketamine’s antidepressant effects using more selective opioid receptor antagonists (119), surrogate markers (120), and functional neuroimaging capable of discerning those selective effects (56). The findings presented here challenge our understanding of the mechanisms of action of ketamine that underlie its potent antidepressant properties (121, 122).
Methods
Design
We also employed the Montgomery–Åsberg Depression Rating Scale (28) and the self- rated Beck Depression Inventory Version II (29) as secondary measures of depression at Days 1, 3, 5, 7, and 14.
Data analyses
A third set of analyses included all participants who received at least one infusion (n=14). For the latter analysis, only the first infusion was considered, and treatment condition for that first infusion (ketamine + placebo [n=9] versus ketamine + naltrexone [n=5]) was a between-subjects factor. The change in 17-item and 6-item Hamilton Depression Rating Scale from pre- to post-infusion was the dependent variable. Analyses of other clinical data included descriptive statistics on the Montgomery– Åsberg Depression Rating Scale and Beck Depression Inventory Version II.
On other measures of depression, including the Montgomery–Åsberg Depression Rating Scale and Beck Depression Inventory Version II, similar attenuations of the antidepressant response in the ketamine + naltrexone condition were observed. Data from these depression measures are shown in Figures S1A and S1B. Additional data, including a between-subjects analysis of Hamilton Depression Rating Scale scores from only the first infusion (including all participants who received at least one infusion, n=14), are provided in the supplement and shown in figure S2. A fixed effects analysis of variance indicated that there was a significantly greater reduction from baseline in 17-item Hamilton Depression Rating Scale scores among those receiving ketamine + placebo as their first infusion versus reductions in the ketamine + naltrexone (F=6.1, p=0.030) as well as the 6-item scale (F=7.7 p=0.017; (Figure S3)).
Supplementary Material
Supplementary Figure 1. Naltrexone blocks ketamine’s antidepressant effect on multiple rating scales. Time-course of ancillary outcome measures (mean ± SD) for ketamineresponsive TRD patients (n=7) in two crossed over conditions, ketamine + naltrexone (K+N) and ketamine + placebo (K+P). Treatments delivered on Day 0 following first questionnaire. K+N group scores were significantly higher than K+P group scores on Day 1 post infusion for both MADRS (A), BDI-2 (B).
Supplementary Figure 2. Naltrexone consistently blocks ketamine’s antidepressant effect when removing the cross-over component of the trial. Time-course of primary outcome measures (mean ± SD) for all patients receiving at least one infusion (N=14), including 2 patients who withdrew from the study following the first infusion. This analysis differs from that shown in Fig. 2 and Fig. 3 in that only the first infusion is considered, eliminating confounds of cross-over design. On the first infusion, N=9 patients received K+P and N=5 patients received K+N. Treatments delivered on Day 0 following first questionnaire. A. HDRS6 time-course. Analysis of between-group HDRS6 differences on Day 1 shows that K+N group scores were significantly higher than K+P group scores, with the latter group demonstrating expected postinfusion HDRS6 score reduction. B. HDRS17 time-course, demonstrating qualitatively similar results as in A.
Supplementary Figure 3. Visual Analog Scale (VAS) scores after naltrexone or placebo, but before ketamine infusion, do not differ between groups. Oral placebo (PBO) or naltrexone (NAL) was given 45 minutes to 1 hour before infusion. VAS scores for a variety of subjective effects were obtained 5 minutes prior to the initiation of infusion. Data is shown for all crossed-over patients (N=12, mean + SD). VAS scores do not differentiate which pretreatment a patient received.
Acknowledgments:
Thanks to KSR for her helpful feedback.
Funding/support: This work was supported by the Stanford Clinical and Translational Science Award to Spectrum [NIH UL1 TR 001085] (NRW+AFS); the 2016 NARSAD Young Investigator Grant program (NRW); Brain and Behavior Research Foundation (CIR); the Avy L. and Robert L. Miller Foundation (NRW); and the Pritzker Family Fund (AFS).
Footnotes
Previous presentations: Society of Biological Psychiatry Meeting 2018.
Disclaimer statements: None. CIR reports being a consultant for Allergan, BlackThorn Therapeutics, and Rugen Therapeutics. AFS reports Consulting: Bracket/Clintara; Alkermes; Neuronetics; McKinsey; GLG Consulting; Avanir. Equity: Xhale; Corcept; Merck; Seattle Genetics; Gilead; Titan; Incyte Genetics; Intersect. Grants; Janssen.
Clinical Trials Registration: NCT02911597
References
- 1.Friedrich MJ. Depression Is the Leading Cause of Disability Around the World. Jama. 2017;317(15):1517. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry. 2006;163(11):1905–17. [DOI] [PubMed] [Google Scholar]
- 4.Williams NR, Taylor JJ, Kerns S, Short EB, Kantor EM, George MS. Interventional psychiatry: why now? J Clin Psychiatry. 2014;75(8):895–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams NR, Schatzberg AF. NMDA antagonist treatment of depression. Curr Opin Neurobiol. 2015;36:112–7. [DOI] [PubMed] [Google Scholar]
- 6.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77(2):357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacheco Dda F, Romero TR, Duarte ID. Central antinociception induced by ketamine is mediated by endogenous opioids and mu- and delta-opioid receptors. Brain Res. 2014;1562:6975. [DOI] [PubMed] [Google Scholar]
- 10.Schatzberg AF. A word to the wise about ketamine. Am J Psychiatry. 2014;171(3):262–4. [DOI] [PubMed] [Google Scholar]
- 11.Sanacora G, Schatzberg AF. Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(5):1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119–36. [DOI] [PubMed] [Google Scholar]
- 14.Domino EF, Chodoff P, Corssen G. Pharmacologic Effects of Ci-581, a New Dissociative Anesthetic, in Man. Clin Pharmacol Ther. 1965;6:279–91. [DOI] [PubMed] [Google Scholar]
- 15.Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1997;7(1):25–38. [DOI] [PubMed] [Google Scholar]
- 16.Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL. Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. Journal of psychiatric research. 2000;34(1):35–43. [DOI] [PubMed] [Google Scholar]
- 17.Dakwar E, Anerella C, Hart CL, Levin FR, Mathew SJ, Nunes EV. Therapeutic infusions of ketamine: do the psychoactive effects matter? Drug and alcohol dependence. 2014;136:153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects? Journal of affective disorders. 2014;159:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krystal JH, D’Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS, et al. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry. 1999;7(3):125–43. [PubMed] [Google Scholar]
- 20.Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, Griffiths RR. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug and alcohol dependence. 2011;115(1–2):150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemeth CL, Paine TA, Rittiner JE, Beguin C, Carroll FI, Roth BL, et al. Role of kappaopioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology. 2010;210(2):263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krystal JH, Madonick S, Perry E, Gueorguieva R, Brush L, Wray Y, et al. Potentiation of low dose ketamine effects by naltrexone: potential implications for the pharmacotherapy of alcoholism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31(8):1793–800. [DOI] [PubMed] [Google Scholar]
- 23.Lee MC, Wagner HN, Tanada S, Frost JJ, Bice AN, Dannals RF. DURATION OF OCCUPANCY OF OPIATE RECEPTORS BY NALTREXONE. Journal of Nuclear Medicine. 1988;29(7):1207–11. [PubMed] [Google Scholar]
- 24.American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. Washington, D.C.: American Psychiatric Publishing; 2013. xliv, 947 p. p. [Google Scholar]
- 25.Hamilton M A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandler GM, Iosifescu DV, Pollack MH, Targum SD, Fava M. RESEARCH: Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015;172(10):950–66. [DOI] [PubMed] [Google Scholar]
- 28.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). Journal of Traumatic Stress. 1998;11(1):125–36. [DOI] [PubMed] [Google Scholar]
- 29.Piantadosi S Clinical Trials: A Methodologic Perspective 2005. [Google Scholar]
- 30.Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry. 2006;63(8):856–64. [DOI] [PubMed] [Google Scholar]
- 31.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Archives of general psychiatry. 1991;48(9):851–5. [DOI] [PubMed] [Google Scholar]
- 32.Double-Blind, Placebo-Controlled Trial of Ketamine Therapy in Treatment-Resistant Depression (TRD) clinicaltrials.gov 2018. [
- 33.Jayaram-Lindstrom N, Guterstam J, Haggkvist J, Ericson M, Malmlof T, Schilstrom B, et al. Naltrexone modulates dopamine release following chronic, but not acute amphetamine administration: a translational study. Translational psychiatry. 2017;7(4):e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, et al. A doubleblind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. The American journal of psychiatry. 2010;167(6):668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamson SJ, Sellman JD, Foulds JA, Frampton CM, Deering D, Dunn A, et al. A randomized trial of combined citalopram and naltrexone for nonabstinent outpatients with cooccurring alcohol dependence and major depression. Journal of clinical psychopharmacology. 2015;35(2):143–9. [DOI] [PubMed] [Google Scholar]
- 36.Malcolm R, O’Neil PM, Von JM, Dickerson PC. Naltrexone and dysphoria: a double-blind placebo controlled trial. Biological psychiatry. 1987;22(6):710–6. [DOI] [PubMed] [Google Scholar]
- 37.Murphy BL, Ravichandran C, Babb SM, Cohen BM. Naltrexone in bipolar disorder with depression: a double-blind, placebo-controlled study. Journal of clinical psychopharmacology. 2014;34(6):749–51. [DOI] [PubMed] [Google Scholar]
- 38.Miotto K, McCann M, Basch J, Rawson R, Ling W. Naltrexone and dysphoria: fact or myth? The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2002;11(2):151–60. [DOI] [PubMed] [Google Scholar]
- 39.Samuels BA, Nautiyal KM, Kruegel AC, Levinstein MR, Magalong VM, Gassaway MM, et al. The Behavioral Effects of the Antidepressant Tianeptine Require the Mu-Opioid Receptor. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber MM, Emrich HM. Current and historical concepts of opiate treatment in psychiatric disorders. Int Clin Psychopharmacol. 1988;3(3):255–66. [DOI] [PubMed] [Google Scholar]
- 41.Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends in neurosciences. 2013;36(3):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M, et al. Opioid Modulation With Buprenorphine/Samidorphan as Adjunctive Treatment for Inadequate Response to Antidepressants: A Randomized Double-Blind Placebo-Controlled Trial. Am J Psychiatry. 2016;173(5):499–508. [DOI] [PubMed] [Google Scholar]
- 43.J YYBGMMBYBIAJLARAP. Ultra-Low-Dose Buprenorphine as a Time-Limited Treatment for Severe Suicidal Ideation: A Randomized Controlled Trial. American Journal of Psychiatry. 2015;ahead of publication. [DOI] [PubMed] [Google Scholar]
- 44.Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15(1):49–57. [DOI] [PubMed] [Google Scholar]
- 45.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243(4899):1718–21. [DOI] [PubMed] [Google Scholar]
- 46.Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440(2):285–92. [DOI] [PubMed] [Google Scholar]
- 47.Yovell Y, Bar G, Mashiah M, Baruch Y, Briskman I, Asherov J, et al. Ultra-Low-Dose Buprenorphine as a Time-Limited Treatment for Severe Suicidal Ideation: A Randomized Controlled Trial. Am J Psychiatry. 2016;173(5):491–8. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(12):2475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koran LM, Aboujaoude E, Bullock KD, Franz B, Gamel N, Elliott M. Double-blind treatment with oral morphine in treatment-resistant obsessive-compulsive disorder. The Journal of clinical psychiatry. 2005;66(3):353–9. [DOI] [PubMed] [Google Scholar]
- 50.Scarone S, Gambini O, Calabrese G, Sacerdote P, Bruni M, Carucci M, et al. Asymmetrical distribution of beta-endorphin in cerebral hemispheres of suicides: preliminary data. Psychiatry research. 1990;32(2):159–66. [DOI] [PubMed] [Google Scholar]
- 51.Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Regionally selective increases in mu opioid receptor density in the brains of suicide victims. Brain research. 1990;530(2):312–6. [DOI] [PubMed] [Google Scholar]
- 52.Gabilondo AM, Meana JJ, Garcia-Sevilla JA. Increased density of mu-opioid receptors in the postmortem brain of suicide victims. Brain research. 1995;682(1–2):245–50. [DOI] [PubMed] [Google Scholar]
- 53.Carlezon WA Jr., Beguin C, Knoll AT, Cohen BM. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacology & therapeutics. 2009;123(3):334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CODD E, SHANK R, SCHUPSKY J, RAFFA R. SEROTONIN AND NOREPINEPHRINE UPTAKE INHIBITING ACTIVITY OF CENTRALLY ACTING ANALGESICS - STRUCTURAL DETERMINANTS AND ROLE IN ANTINOCICEPTION. Journal of Pharmacology and Experimental Therapeutics. 1995;274(3):1263–70. [PubMed] [Google Scholar]
- 55.Zador F, Kiraly K, Varadi A, Balogh M, Feher A, Kocsis D, et al. New opioid receptor antagonist: Naltrexone-14-O-sulfate synthesis and pharmacology. European Journal of Pharmacology. 2017;809:111–21. [DOI] [PubMed] [Google Scholar]
- 56.Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, et al. Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexonetreated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(3):653–65. [DOI] [PubMed] [Google Scholar]
- 57.Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl). 2010;210(2):241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor GT, Manzella F. Kappa Opioids, Salvinorin A and Major Depressive Disorder. Curr Neuropharmacol. 2016;14(2):165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirota K, Okawa H, Appadu BL, Grandy DK, Devi LA, Lambert DG. Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology. 1999;90(1):174–82. [DOI] [PubMed] [Google Scholar]
- 60.Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R, 6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Molecular psychiatry. 2017. [DOI] [PubMed] [Google Scholar]
- 61.Cavalleri L, Pich EM, Millan M, Chiamulera C, Kunath T, Spano P, et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Molecular psychiatry. 2017. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546(7659):E1–E3. [DOI] [PubMed] [Google Scholar]
- 63.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science. 2010;329(5994):959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monteggia LM, Gideons E, Kavalali ET. The Role of Eukaryotic Elongation Factor 2 Kinase in Rapid Antidepressant Action of Ketamine. Biological Psychiatry. 2013;73(12):1199203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duman RS, Aghajanian GK, Sanacora G, Krysta JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nature Medicine. 2016;22(3):238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galvez V, Nikolin S, Ho KA, Alonzo A, Somogyi AA, Loo CK. Increase in PAS-induced neuroplasticity after a treatment course of intranasal ketamine for depression. Report of three cases from a placebo-controlled trial. Comprehensive psychiatry. 2017;73:31–4. [DOI] [PubMed] [Google Scholar]
- 67.Timmerby N, Andersen JH, Sondergaard S, Ostergaard SD, Bech P. A Systematic Review of the Clinimetric Properties of the 6-Item Version of the Hamilton Depression Rating Scale (HAM-D6). Psychother Psychosom. 2017;86(3):141–9. [DOI] [PubMed] [Google Scholar]
- 68.Jonkman K, Dahan A, van de Donk T, Aarts L, Niesters M, van Velzen M. Ketamine for pain. F1000Research. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hustveit O, Maurset A, Oye I. INTERACTION OF THE CHIRAL FORMS OF KETAMINE WITH OPIOID, PHENCYCLIDINE, SIGMA AND MUSCARINIC RECEPTORS. Pharmacology & Toxicology. 1995;77(6):355–9. [DOI] [PubMed] [Google Scholar]
- 70.Baker AK, Hoffmann VLH, Meert TF. Dextromethorphan and ketamine potentiate the antinociceptive effects of mu- but not delta- or kappa-opioid agonists in a mouse model of acute pain. Pharmacology Biochemistry and Behavior. 2002;74(1):73–86. [DOI] [PubMed] [Google Scholar]
- 71.Ryder S, Way WL, Trevor AJ. COMPARATIVE PHARMACOLOGY OF OPTICAL ISOMERS OF KETAMINE IN MICE. European Journal of Pharmacology. 1978;49(1):15–23. [DOI] [PubMed] [Google Scholar]
- 72.Sarton E, Teppema LJ, Olievier C, Nieuwenhuijs D, Matthes HWD, Kieffer BL, et al. The involvement of the mu-opioid receptor in ketamine-induced respiratory depression and antinociception. Anesthesia and Analgesia. 2001;93(6):1495–500. [DOI] [PubMed] [Google Scholar]
- 73.Pacheco DdF Lima Romero TR, Gama Duarte ID. Central antinociception induced by ketamine is mediated by endogenous opioids and mu- and delta-opioid receptors. Brain Research. 2014;1562:69–75. [DOI] [PubMed] [Google Scholar]
- 74.Koppert W, Sittl R, Scheuber K, Alsheimer M, Schmelz M, Schuttler J. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology. 2003;99(1):152–9. [DOI] [PubMed] [Google Scholar]
- 75.Sleigh J, Harvey M, Voss L, Denny B. Ketamine–More mechanisms of action than just NMDA blockade. Trends in anaesthesia and critical care. 2014;4(2):76–81. [Google Scholar]
- 76.Finck AD, Ngai SH. OPIATE RECEPTOR MEDIATION OF KETAMINE ANALGESIA. Anesthesiology. 1982;56(4):291–7. [DOI] [PubMed] [Google Scholar]
- 77.Smith DJ, Bouchal RL, Desanctis CA, Monroe PJ, Amedro JB, Perrotti JM, et al. PROPERTIES OF THE INTERACTION BETWEEN KETAMINE AND OPIATE BINDING-SITES INVIVO AND INVITRO. Neuropharmacology. 1987;26(9):1253–60. [DOI] [PubMed] [Google Scholar]
- 78.Salat K, Siwek A, Starowicz G, Librowski T, Nowak G, Drabik U, et al. Antidepressantlike effects of ketamine, norketamine and dehydronorketamine in forced swim test: Role of activity at NMDA receptor. Neuropharmacology. 2015;99:301–7. [DOI] [PubMed] [Google Scholar]
- 79.Commons KG, Van Bockstaele EJ, Pfaff DW. Frequent colocalization of Mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. Journal of Comparative Neurology. 1999;408(4):549–59. [PubMed] [Google Scholar]
- 80.Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Berrocoso E, Garzon J. The Mu-Opioid Receptor and the NMDA Receptor Associate in PAG Neurons: Implications in Pain Control. Neuropsychopharmacology. 2012;37(2):338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chartoff EH, Connery HS. It’s MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Frontiers in Pharmacology. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. The New England journal of medicine. 2017;377(4):391–4. [DOI] [PubMed] [Google Scholar]
- 83.Curtin SC, Warner M, Hedegaard H. Increase in Suicide in the United States, 19992014. NCHS data brief. 2016(241):1–8. [PubMed] [Google Scholar]
- 84.Karp JF, Butters MA, Begley AE, Miller MD, Lenze EJ, Blumberger DM, et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. The Journal of clinical psychiatry. 2014;75(8):e785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scherrer JF, Salas J, Sullivan MD, Schneider FD, Bucholz KK, Burroughs T, et al. The influence of prescription opioid use duration and dose on development of treatment resistant depression. Preventive medicine. 2016;91:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leander JD. Buprenorphine is a potent kappa-opioid receptor antagonist in pigeons and mice. European journal of pharmacology. 1988;151(3):457–61. [DOI] [PubMed] [Google Scholar]
- 87.Leander JD. Buprenorphine has potent kappa opioid receptor antagonist activity. Neuropharmacology. 1987;26(9):1445–7. [DOI] [PubMed] [Google Scholar]
- 88.Leander JD. Opioid agonist and antagonist behavioural effects of buprenorphine. British journal of pharmacology. 1983;78(4):607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with Mental Health Disorders in the United States. J Am Board Fam Med. 2017;30(4):407–17. [DOI] [PubMed] [Google Scholar]
- 90.Fan N, Xu K, Ning Y, Wang D, Ke X, Ding Y, et al. Development of a checklist of shortterm and long-term psychological symptoms associated with ketamine use. Shanghai Arch Psychiatry. 2015;27(3):186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalsi SS, Wood DM, Dargan PI. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J. 2011;4:7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ng SH, Tse ML, Ng HW, Lau FL. Emergency department presentation of ketamine abusers in Hong Kong: a review of 233 cases. Hong Kong Med J. 2010;16(1):6–11. [PubMed] [Google Scholar]
- 93.Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, et al. Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain : a journal of neurology. 2010;133(Pt 7):2115–22. [DOI] [PubMed] [Google Scholar]
- 94.Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M. A Review of Ketamine Abuse and Diversion. Depression and anxiety. 2016;33(8):718–27. [DOI] [PubMed] [Google Scholar]
- 95.Fang YX, Wang YB, Shi J, Liu ZM, Lu L. Recent trends in drug abuse in China. Acta Pharmacol Sin. 2006;27(2):140–4. [DOI] [PubMed] [Google Scholar]
- 96.Ahmed SN, Petchkovsky L. Abuse of ketamine. The British journal of psychiatry : the journal of mental science. 1980;137:303. [DOI] [PubMed] [Google Scholar]
- 97.Amann LC, Halene TB, Ehrlichman RS, Luminais SN, Ma N, Abel T, et al. Chronic ketamine impairs fear conditioning and produces long-lasting reductions in auditory evoked potentials. Neurobiology of disease. 2009;35(2):311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morgan CJ, Dodds CM, Furby H, Pepper F, Fam J, Freeman TP, et al. Long-Term Heavy Ketamine Use is Associated with Spatial Memory Impairment and Altered Hippocampal Activation. Frontiers in psychiatry. 2014;5:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shiroma PR, Albott CS, Johns B, Thuras P, Wels J, Lim KO. Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;17(11):1805–13. [DOI] [PubMed] [Google Scholar]
- 100.Lindefors N, Barati S, O’Connor WT. Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain research. 1997;759(2):205–12. [DOI] [PubMed] [Google Scholar]
- 101.Bonnet U Long-Term Ketamine Self-Injections in Major Depressive Disorder: Focus on Tolerance in Ketamine’s Antidepressant Response and the Development of Ketamine Addiction. J Psychoactive Drugs. 2015;47(4):276–85. [DOI] [PubMed] [Google Scholar]
- 102.Prekupec MP SR, Sher Y, Lembke A. Relapse on ketamine followed by severe and prolonged withdrawal: A cautionary case and review of potential medical therapies. Journal of Nature and Science. 2017;3(10):e450. [Google Scholar]
- 103.Byer DE, Gould AB, Jr. Development of tolerance to ketamine in an infant undergoing repeated anesthesia. Anesthesiology. 1981;54(3):255–6. [DOI] [PubMed] [Google Scholar]
- 104.Cumming JF. The development of an acute tolerance to ketamine. Anesth Analg. 1976;55(6):788–91. [DOI] [PubMed] [Google Scholar]
- 105.Stevens RW, Hain WR. Tolerance to rectal ketamine in paediatric anaesthesia. Anaesthesia. 1981;36(12):1089–93. [DOI] [PubMed] [Google Scholar]
- 106.Blachut M, Solowiow K, Janus A, Ruman J, Cekus A, Matysiakiewicz J, et al. [A case of ketamine dependence]. Psychiatr Pol. 2009;43(5):593–9. [PubMed] [Google Scholar]
- 107.Critchlow DG. A case of ketamine dependence with discontinuation symptoms. Addiction. 2006;101(8):1212–3. [DOI] [PubMed] [Google Scholar]
- 108.Florkowski A, Ferfecki L. [A case of ketamine dependence]. Psychiatr Pol. 1987;21(5):434–5. [PubMed] [Google Scholar]
- 109.Bobo WV, Miller SC. Ketamine as a preferred substance of abuse. Am J Addict. 2002;11(4):332–4. [DOI] [PubMed] [Google Scholar]
- 110.Lim DK. Ketamine associated psychedelic effects and dependence. Singapore Med J. 2003;44(1):31–4. [PubMed] [Google Scholar]
- 111.Pouget P, Wattiez N, Rivaud-Pechoux S, Gaymard B. Rapid development of tolerance to sub-anaesthetic dose of ketamine: an oculomotor study in macaque monkeys. Psychopharmacology (Berl). 2010;209(4):313–8. [DOI] [PubMed] [Google Scholar]
- 112.Livingston A, Waterman AE. The development of tolerance to ketamine in rats and the significance of hepatic metabolism. Br J Pharmacol. 1978;64(1):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65–78. [DOI] [PubMed] [Google Scholar]
- 114.Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75(2):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ryan CJ, Loo C. The practicalities and ethics of ketamine for depression. Lancet Psychiatry. 2017;4(5):354–5. [DOI] [PubMed] [Google Scholar]
- 116.Singh I, Morgan C, Curran V, Nutt D, Schlag A, McShane R. Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight. Lancet Psychiatry. 2017;4(5):419–26. [DOI] [PubMed] [Google Scholar]
- 117.Schak KM, Vande Voort JL, Johnson EK, Kung S, Leung JG, Rasmussen KG, et al. Potential Risks of Poorly Monitored Ketamine Use in Depression Treatment. The American journal of psychiatry. 2016;173(3):215–8. [DOI] [PubMed] [Google Scholar]
- 118.Kraemer HC, Blasey C. How many subjects? : statistical power analysis in research Second edition. ed. Los Angeles: Sage Publicastions, Inc.; 2016. xiii, 140 pages p. [Google Scholar]
- 119.Ziauddeen H, Chamberlain SR, Nathan PJ, Koch A, Maltby K, Bush M, et al. Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Molecular psychiatry. 2013;18(12):1287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [(11)C]ABP688 and PET imaging study in depression. Mol Psychiatry. 2018;23(4):824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Albert PR, Benkelfat C. The neurobiology of depression--revisiting the serotonin hypothesis. II. Genetic, epigenetic and clinical studies. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2013;368(1615):20120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Albert PR, Benkelfat C, Descarries L. The neurobiology of depression--revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2012;367(1601):2378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Naltrexone blocks ketamine’s antidepressant effect on multiple rating scales. Time-course of ancillary outcome measures (mean ± SD) for ketamineresponsive TRD patients (n=7) in two crossed over conditions, ketamine + naltrexone (K+N) and ketamine + placebo (K+P). Treatments delivered on Day 0 following first questionnaire. K+N group scores were significantly higher than K+P group scores on Day 1 post infusion for both MADRS (A), BDI-2 (B).
Supplementary Figure 2. Naltrexone consistently blocks ketamine’s antidepressant effect when removing the cross-over component of the trial. Time-course of primary outcome measures (mean ± SD) for all patients receiving at least one infusion (N=14), including 2 patients who withdrew from the study following the first infusion. This analysis differs from that shown in Fig. 2 and Fig. 3 in that only the first infusion is considered, eliminating confounds of cross-over design. On the first infusion, N=9 patients received K+P and N=5 patients received K+N. Treatments delivered on Day 0 following first questionnaire. A. HDRS6 time-course. Analysis of between-group HDRS6 differences on Day 1 shows that K+N group scores were significantly higher than K+P group scores, with the latter group demonstrating expected postinfusion HDRS6 score reduction. B. HDRS17 time-course, demonstrating qualitatively similar results as in A.
Supplementary Figure 3. Visual Analog Scale (VAS) scores after naltrexone or placebo, but before ketamine infusion, do not differ between groups. Oral placebo (PBO) or naltrexone (NAL) was given 45 minutes to 1 hour before infusion. VAS scores for a variety of subjective effects were obtained 5 minutes prior to the initiation of infusion. Data is shown for all crossed-over patients (N=12, mean + SD). VAS scores do not differentiate which pretreatment a patient received.