Abstract
Many youth with ADHD experience peer difficulties, but the mechanisms underlying this dysfunction remain unknown. Very little work has examined neurophysiological measures of social feedback processing in relation to ADHD symptoms. The goal of this study was to examine associations of ADHD symptoms with indicators of sensitivity to social feedback in a laboratory task and self-report of rejection sensitivity. A large community sample of 10-to 15-year-old adolescents (N = 391; Mage= 12.64, 48.6% girls) participated in the study. Mothers rated youth ADHD symptoms. Youth completed the Island Getaway task, which elicits neurophysiological (i.e., event-related potentials [ERP]) measures of sensitivity to peer rejection and acceptance feedback, and also completed self-ratings of rejection sensitivity. Greater ADHD symptoms were associated with an enhanced N1 ERP component, which correlated with higher levels of self-reported rejection sensitivity. In addition, greater ADHD symptoms were associated with reduced reactivity to social acceptance, as measured by the later reward positivity ERP component. Youth with elevated ADHD symptoms exhibited enhanced sensitivity to peer rejection at the neurophysiological and self-report level, as well as reduced neurophysiological reactivity to peer acceptance. Future work including neural measures of social functioning may serve to elucidate mechanisms underlying the social dysfunction characteristic of ADHD.
Keywords: ADHD, social processes, event-related potential (ERP), peer feedback, rejection
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common disorders of childhood, and is associated with widespread peer relationship difficulties (McQuade & Hoza, 2014). Compared to youth without ADHD, youth with ADHD have fewer reciprocated friendships and are more frequently rejected by their peers (e.g., Hoza, 2007; Wiener & Mak, 2009), even within minutes of joining a new peer group (Pelham & Bender, 1982). Although there has been substantial work documenting the peer difficulties of school-aged children with ADHD, very little work has examined the core deficits underlying these difficulties (Ray, Evans, & Langberg, 2017). Peer functioning becomes increasingly salient as youth age into adolescence, and emerging work shows that the peer difficulties of school-aged children often persist or even worsen into adolescence. Marked cognitive, affective, and social changes occur in the transition to adolescence that are associated with increasing sensitivity to peer acceptance and rejection feedback (Blakemore, 2008; Crone & Dahl, 2012), and it may be that the peer difficulties of youth with ADHD may be particularly prominent at this time. Indeed, peer dysfunction among adolescents with ADHD is associated with a wide array of serious long-term negative outcomes, including depression, delinquency, and school drop-out (e.g., Humphreys et al., 2013; Mrug et al., 2012). Thus, attention to identifying mechanisms underlying the peer difficulties of youth with ADHD is of critical importance.
Examining how youth with ADHD process social cues, such as peer feedback, may serve to clarify the mechanisms underlying peer difficulties and their long-term consequences. Social information processing theory (Crick & Dodge, 1994; Dodge, 1986) provides a useful framework to examine how youth process peer feedback. Early stages of social processing involve encoding and interpretation of social cues, followed by the selection and enactment of responses to these cues. A number of studies using behavioral and self-report measures indicate that youth with ADHD demonstrate difficulty in both the early and later social processing stages, although the specific deficits vary across studies. For example, some studies show that youth with ADHD have difficulty detecting and encoding subtle social cues and attending to the most relevant social information (Andrade et al., 2012; Matthys, Cuperus, & Van Engeland, 1999; Mikami, Lee, Hinshaw, & Mullin, 2008; Sibley, Evans, & Serpell, 2010). Other studies have identified a hostile attribution bias among youth with ADHD and other youth with long histories of peer rejection (Bondu & Esser, 2009; Crick & Dodge, 1994; Will, van Lier, Crone, & Güroğlu, 2016). That is, these youth may come to anticipate rejection from peers, reacting in defensive ways (i.e., anger or anxiety), which may subsequently impede attempts to develop positive peer relationships (London, Downey, Bonica & Paltin, 2007). Several studies have shown that some youth with ADHD are overly sensitive to rejection from peers and are more likely to attribute more negative and less positive intent to peers, and, consequently, react more aggressively than their non-ADHD peers (Matthys et al., 1999; Waschbusch et al., 2002). Variability in the findings of social processing studies among youth with ADHD may be partly attributed to heterogeneity among youth with ADHD, but also to limitations of self-report and behavioral measures in assessing immediate responses to social cues.
In addition, comorbidity may partly account for variability in the effects of ADHD on processing of social cues. At least some studies suggest that comorbid psychopathology, including conduct and internalizing problems that often co-occur with ADHD (Becker, Luebbe, & Langberg, 2012), as well as high levels of peer rejection (London et al., 2007) explain alterations in social processing. For example, youth with ADHD and co-occurring conduct problems have been shown to perceive the intentions of peers as more hostile and to react in more aggressive ways to peers than youth with ADHD who do not have co-occurring conduct problems (Matthys et al., 1999; Waschbusch et al., 2002). Similarly, youth with anxiety and depression demonstrate heightened reactivity to rejection and less reactivity to peer acceptance at the neurophysiological level (Kujawa, Arfer, Klein, & Proudfit, 2014), hence some of these alterations may not be specific to ADHD.
To date, studies have primarily relied on self-report scales and direct observations of peer behavior to assess social processing, which may not be sufficiently sensitive to disentangle alterations in social feedback processing, particularly at very early stages. Indeed, self-reports are particularly limited, as prominent theories posit that the majority of social feedback processing may be automated (Crick & Dodge, 1994) and many youth with ADHD may have limited insight into their functioning (Owens, Goldfine, Evangelista, Hoza, & Kaiser, 2007). Examination of neurophysiology, particularly event-related potentials (ERPs) derived from the electroencephalogram (EEG), may provide important information beyond self-report and behavioral observations that is relevant for understanding sensitivity to social feedback in youth with ADHD. ERPs have been used in research on ADHD to identify cognitive and motivational deficits (for a review, Johnstone, Barry, & Clarke, 2013). However, they have not been applied to social feedback processing.
As a number of studies have shown that youth with ADHD demonstrate attentional difficulties in social interactions with peers, such as failure to accurately encode social cues, early ERP indicators of attentional processing, such as N1, may be altered in response to social feedback among youth with ADHD. N1 is an ERP component that is apparent across the scalp, maximal around 140 to 200 ms after a stimulus is presented, and reflects visual processing and orienting of attention (Hillyard & Anllo-Vento, 1998). Although results vary depending on task design, there is some evidence that children with ADHD show reductions in N1 magnitude during cognitive tasks, such as the continuous performance task (Lawrence et al., 2005). Importantly, in addition to cognitive tasks, N1 is also observable in studies of emotional processing and appears to be sensitive to processing of social stimuli in adolescents (DiFilipo & Grose-Fifer, 2016; Olofsson, Nordin, Sequeira, & Polich, 2008), raising the possibility that it can provide a measure of early attentional processing (i.e., encoding) of social cues in youth with ADHD.
Along with N1, the reward positivity (RewP) may also be useful in studying social processing in youth with ADHD. RewP is a frontocentral ERP component appearing approximately 300 ms after stimuli onset. RewP has previously been referred to as the feedback negativity component, but there is growing evidence that it actually reflects a reward-related signal and is characterized by a relative positivity in the ERP wave in response to positive feedback and rewards compared to negative feedback and losses (Foti, Weinberg, Dien, & Hajcak, 2011; Gehring & Willoughby, 2002). While N1 reflects early attentional processing of salient cues, RewP reflects reward-related brain activation (Proudfit, 2015). RewP has been correlated with self-report and behavioral measures of reward responsiveness (Bress & Hajcak, 2013) and may reflect individual differences in approach motivation and reward sensitivity. There is some evidence that children with ADHD show a lack of modulation of RewP by monetary contingencies, suggesting deficits in processing motivational cues under certain conditions (van Meel, Heslenfeld, Oosterlaan, Luman, & Sergeant, 2011), but this pattern appears to change when the salience of rewards increases (Holroyd, Pakzad-Vaezi, & Krigolson, 2008). For example, Holroyd and colleagues (2008) reported that youth with ADHD showed a blunted RewP in a monetary reward task compared to youth without ADHD before receipt of the monetary reward. Once provided with the cash reward, youth with ADHD demonstrated an enhanced RewP, while the magnitude of the RewP among youth without ADHD was reduced. Although RewP is often examined in response to feedback indicating monetary reward and loss, there is growing evidence that RewP can also be reliably elicited in response to social feedback (e.g., Ethridge et al., 2017; Kujawa, Arfer, et al., 2014; van der Molen, Dekkers, Westenberg, van der Veen, & van der Molen, 2016). That is, in addition to monetary reward, social acceptance is likely another important reward domain, and one that is particularly salient in adolescence (Crone & Dahl, 2012). Examining RewP to social feedback may provide a novel neurophysiological measure of sensitivity to social reward and provide insight into the salience of positive social feedback among youth with ADHD.
To elicit neurophysiological reactivity (i.e., ERPs) to social acceptance and rejection feedback, the Island Getaway task was developed (Authors, 2014, 2017). During the task, participants play a game with simulated peers, in which they vote to reject and accept co-players, while also receiving rejection and acceptance feedback from peers. A principal component analysis (PCA) was conducted to differentiate ERPs sensitive to social feedback from the Island Getaway in early adolescence and found evidence of an early negativity (i.e., N1) maximal over central sites that was enhanced (i.e., more negative) for rejection compared to acceptance feedback. Following N1, a relative positivity (i.e., RewP), also maximal over central sites, that was enhanced for acceptance compared to rejection feedback, was identified. These findings indicate that N1 may provide a measure of very early attentional engagement with rejection cues, whereas RewP in social feedback tasks may provide a measure of sensitivity to social reward.
It is important to explore potential sex differences in social processing. Although ADHD is diagnosed more frequently in boys compared to girls, there is consistent accumulating evidence that compared to boys with ADHD, girls with ADHD experience broader and more severe social-emotional problems (Becker et al., 2013), particularly in adolescence. In addition, decades of research show that peer relationships differ by sex regardless of ADHD (Rose & Rudolph, 2006). For example, there is some evidence that girls tend to engage in more prosocial relationship behaviors, report more need for relatedness to others, and report more fear of negative peer evaluations compared to boys. This could suggest that girls with ADHD demonstrate greater sensitivity to peer feedback, and may experience greater impairments in social functioning, which may partly explain the high levels of depressive symptomology that emerge in adolescence (Rose & Rudolph, 2006). However, very little work has prioritized the study of social functioning in girls with ADHD.
The goal of the current study was to examine associations of ADHD symptoms with neurophysiological (i.e., N1 and RewP) and self-report measures of sensitivity to peer feedback in a large community sample of young adolescents completing the Island Getaway task. We examined sensitivity to social feedback across levels of analysis, as neurophysiological measures and self-report often provide distinct, but complementary information. We tested the unique effects of ADHD symptoms on social feedback processing controlling for comorbid problems. Specifically, we controlled for comorbid anxious/depressed symptoms, as internalizing problems have frequently been associated with an altered RewP (Kessel, Kujawa, Hajcak Proudfit, & Klein, 2015; Kujawa, Kessel, Carroll, Arfer, & Klein, 2017), and we controlled for aggressive behavior, which has been shown to drive some of the associations between ADHD and social processing at the behavioral level (Waschbusch et al., 2002). Additionally, we controlled for social problems that have also been shown to alter social processing at the neural level (Will et al., 2016). Primary analyses utilized a continuous measure of ADHD symptoms to maximize statistical power. Moreover, even subthreshold symptoms of ADHD are associated with social problems (e.g., Diamantopoulou, Henricsson, & Rydell, 2005), and many youth experiencing clinically significant ADHD symptoms do not meet full diagnostic criteria for ADHD (Sibley et al., 2012; Waschbusch & King, 2006). However, we also conducted parallel analyses to examine whether similar effects emerged among those with versus without an ADHD diagnosis.
Lastly, sex was explored as a moderator of the association between ADHD and the social feedback processing variables including the aforementioned covariates. Although ADHD is diagnosed more frequently in boys compared to girls, there is accumulating evidence that compared to boys with ADHD, girls with ADHD experience broader and more severe social-emotional problems (Becker, McBurnett, Hinshaw, & Pfiffner, 2013), particularly in adolescence. In addition, decades of research show that peer relationships differ by sex regardless of ADHD (Rose & Rudolph, 2006). For example, girls tend to engage in more prosocial relationship behaviors, report more need for relatedness to others, and report more fear of negative peer evaluations compared to boys, suggesting that girls with ADHD may demonstrate greater sensitivity to peer feedback (Rose & Rudolph, 2006). However, very little work has compared social processing in girls versus boys with ADHD.
Based on previous work showing greater self-reported rejection sensitivity in children with versus without ADHD (e.g., Bondu & Esser, 2015), we hypothesized that associations between ADHD and greater reactivity to peer rejection would emerge across neurophysiological measures (i.e., N1 to rejection cues) and self-report. Given previous research reporting alterations in monetary reward processing among youth with ADHD (Holroyd et al., 2008; van Meel et al., 2011), we also tested the association between ADHD and RewP to social acceptance. Based on evidence that youth with ADHD show reduced RewP to monetary rewards unless they are made particularly salient (Holroyd et al., 2008) and consistent with behavioral observations that youth with ADHD are less sensitive to some social cues (McQuade & Hoza, 2014), we hypothesized that ADHD may be associated with blunted RewP to social acceptance. We explored whether these results were specific to ADHD or were stronger for other comorbid problems, including anxiety/depression, aggression, and social problems, which have also been linked to altered reactivity to peer feedback (e.g., Matthys et al., 1999; Will et al., 2016). Lastly, based on evidence that girls are more sensitive to social feedback (Rose & Rudolph, 2006) and that girls with ADHD demonstrate greater peer difficulty than boys with the disorder (e.g., Becker et al., 2013), we hypothesized that sex would moderate the association between ADHD and social feedback, with the greatest reactivity to peer feedback demonstrated by girls with high levels of ADHD.
Method
Participants
Participants were part of a larger community sample of 609 children initially recruited using commercial mailing lists when children were 3 or 6 years old (Authors, 2014; Authors, 2010). Participants were invited to the laboratory approximately every three years to complete assessments. At the age 12 assessment, the Island Getaway task was completed while EEG data were recorded. Participants completed a battery of EEG tasks in a counterbalanced order, other results of which will be presented in a future manuscript. Mothers completed measures of child symptoms at the same assessment. Several weeks after the lab assessment, participants completed a home assessment that included a self-report measure of rejection sensitivity. The home assessment was conducted to reduce the amount of time spent on questionnaires at a single assessment. A total of 439 children completed the Island Getaway, although EEG data were lost for 7 due to technical errors and 23 were excluded for excessive noise in EEG data (Authors, 2017). Mother-reported ADHD symptoms and self-reported rejection sensitivity data were missing for 4 and 15 participants, respectively (1 was missing both measures). Therefore, the sample included 391 (46.8% female) participants (age range: 10.83–15.25 years old; Mage=12.64, SD=0.47), with 12.5% identifying as Hispanic, 89.8% as White, 6.9% Black, 2.6% Asian, 0.3% Native American, and 0.5% as other. A total of 210 (53.7%) mothers reported earning at least a 4-year college degree. Within the sample, yearly household income ranged from: less than $20K (1.8%), 20,000–39,999 (3.6%), $40,000-$59,000 (5.6%), $60,000-$79,000 (11.3%), $80,000-$99,999 (9.5%), $100,000-$119,999 (11.5%), $120,000-$149,999 (11.3%), $150,000-$179,999 (9.7%), $180,000 or more (18.9%); 7.2% of participants indicated that they preferred not to answer this question and it was missing for 9.7%. Included participants were compared to those who completed an assessment at age 6 but did not have useable age 12 data on gender, race, ethnicity, and maternal ratings on the Child Behavior Checklist (Achenbach & Rescorla, 2001) at the age 6 assessment. Demographic differences did not emerge, nor did differences in ADHD symptoms, but youth included in the current study demonstrated lower levels of social problems at age 6 (M=1.38, SD=1.81) compared to non-included youth (M=1.83, SD=1.87, p=.02). Informed consent was obtained from all parents with assent obtained from minor participants, and ethical approval for the procedures was obtained from the Stony Brook University Institutional Review Board.
ADHD Measures
ADHD symptoms.
Mothers completed the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001), a 113-item measure of behavioral and emotional problems. Primary analyses focused on the attention problems raw score, which has good diagnostic accuracy in predicting ADHD diagnosis in children ages 6 to 18 years old (Hudziak, Copeland, Stanger, & Wadsworth, 2004; Warnick, Bracken, & Kasl, 2008). The attention problems score includes 10 items assessing inattention and hyperactivity/impulsivity and was used as a measure of ADHD symptoms. Items were rated as 0 (not true), 1 (somewhat or sometimes true), or 2 (very true or often true), with total scores on the attention problems scale ranging from 0 to 20. Internal consistency (Cronbach’s alpha) was .87. Raw scores on the aggressive behavior (alpha=.87), anxious/depressed (alpha=.81), and social problems subscales (alpha=.74) from the CBCL were used as covariates.
Diagnostic assessment.
To assess lifetime diagnosis of DSM-IV ADHD, one parent and the child were interviewed using the Schedule of Affective Disorders and Schizophrenia for School-Age Children (K-SADS; Axelson, Birmaher, Zelazny, Kaufman, & Gill, 2009). Advanced clinical psychology doctoral students or Master’s level clinicians administered the K-SADS first to the parent and then to the child. Lifetime diagnoses were based on a combination of parent and child reports, and if discrepancies arose, the interviewer attempted to reconcile them with both the parent and child at the end of the interview. All cases were reviewed with an expert child psychiatrist and clinical psychologist to confirm diagnoses (see Authors, 2014 for more detail on the diagnostic assessment). Diagnostic interviews were administered at two time points: the assessment wave prior to completion of the social feedback EEG task (approximately age 9) and again at the time of the EEG assessment (approximately age 12). Most (95.9%) participants were interviewed at both age 9 and 12, with diagnoses combined to yield lifetime diagnostic status. If age 9 diagnostic data were unavailable, lifetime diagnoses were obtained at the age 12 assessment.
Sixty (15.3%) participants had a lifetime ADHD diagnosis (36 inattentive, 1 hyperactive/impulsive, 10 combined subtype, and 13 not otherwise specified), and 16 were taking stimulant medication during the EEG assessment. There were 43 boys and 17 girls with a diagnosis of ADHD. With regard to other disorders, 19 (4.9%) had a lifetime diagnosis of oppositional defiant disorder (ODD) or conduct disorder, 120 (30.7%) had a lifetime anxiety disorder diagnosis, and 8 (2.0%) had a lifetime depression diagnosis. Of youth with ADHD diagnoses, 12 (20.0%) had a history of ODD or conduct disorder, 28 (46.7%) had a lifetime anxiety disorder diagnosis, and 1 (1.7%) had a history of depression.
Social Feedback Measures
Island Getaway task.
Participants were told they would be playing a game with 11 age-matched co-players in which they would be traveling in the Hawaiian Islands, and at each island, they would have to vote whether they wanted each co-player to continue on in the game and would receive feedback on how co-players voted for them. There were 11 co-players so that including the participant, the total number of players was even and balanced for gender. In the first round, participants created a profile with their photograph and background information, and reviewed profiles of computerized co-players. In the subsequent five rounds, participants responded to a poll question (e.g., “Who do you most admire?”) and reviewed co-player responses. Following review of profiles and poll responses in each round, participants completed a voting and feedback phase. Participants were prompted to vote to accept (“Keep”) or reject (“Kick out”) each co-player, and after each vote, they saw feedback indicating whether that co-player had voted to accept or reject them. Acceptance feedback was indicated by an image of a green “thumbs up” and rejection feedback was indicated by a red “thumbs down.” Each voting trial began with a co-player profile presented until participants voted. To simulate variation in co-player response speed, a co-player voting time was selected for each trial based on actual variability in participants’ voting speeds. If participants voted faster than the simulated voting time for that co-player, the message “Waiting for [co-player’s name] to vote...” was displayed. Lastly, a fixation “+” was presented for 1000 ms, followed by feedback displayed for 2000 ms. A blank screen was presented for 1500 ms before the start of the next trial. The task included a total of 51 feedback trials split evenly between acceptance and rejection, with the last trial type determined randomly. At least 20–25 trials are needed to obtain stable ERPs for rejection and acceptance conditions. After each round, participants were informed that one of the co-players had been sent home, and after completing the sixth, participants were told that they made it to the “Big Island” and were in the winning group.
During Island Getaway, participants were instructed to vote off at least one co-player per round (six kick out votes total), but were free to vote off as many co-players as they chose. The number of total kick out votes in the sample ranged from 0 to 50 (M=21.66, SD=8.79). A small proportion of participants made fewer than six kick out votes across the task, but most (97.7%) participants made six or more votes to kick out peers. ADHD symptoms in those who made at least six kick out votes (M=1.98, SD=2.97) vs. those with fewer than six kick out votes across the task (M=5.56, SD=5.41) were compared but did not reach significance, t=1.98, p =.08.
Following completion of the task, participants responded to three self-report items rated on a 5-point scale: “I really wanted to stay in the game,” “I would’ve liked to play this game again,” and “After a while I lost interest in staying in the game” (reverse scored). Scores were averaged to derive a measure of task engagement ranging from 1 to 5, with higher scores indicating greater engagement. The average self-rating of task engagement for the sample was 3.85 (SD=0.83), and Cronbach’s alpha was .57. Task engagement was not significantly associated with ADHD symptoms (r=.06, p=.27).
EEG data acquisition and processing.
Continuous EEG was recorded using a 34-electrode cap (32 channels with the addition of FCz and Iz) and a BioSemi system (BioSemi, Amsterdam, Netherlands). The electrooculogram (EOG) generated from eye movements and blinks was recorded using facial electrodes placed approximately 1 cm above and below the eye and 1 cm from the outer corners of the eyes. Electrodes were also placed on the left and right mastoids. Recordings were digitized with a sampling rate of 1024 Hz.
Offline processing was conducted using BrainVision Analyzer software (Brain Products, Munich, Germany). Data were referenced to an average of the recordings from left and right mastoids, band-pass filtered with cutoffs of 0.1 and 30 Hz, and segmented for each trial 200 ms before feedback, continuing for 1000 ms after feedback onset. Eye-blink correction (Gratton, Coles, & Donchin, 1983) and semi-automatic artifact rejection procedures were conducted. Criteria of a voltage step of 50 μV between sample points, a maximum voltage difference of 300 μV within a 200 ms interval, and minimum activity of 0.5 μV within 100ms intervals were used to automatically detect artifacts, with additional artifacts removed by visual inspection. In cases of unusable data from a specific recording site, data were interpolated from adjacent electrodes. ERPs were averaged for acceptance and rejection feedback, and baseline corrected to activity 200 ms prior to feedback. All participants had a minimum of 22 trials per condition after artifact rejection, which exceeds the number of trials needed to produce stable ERPs (Levinson, Speed, Infantolino, & Hajcak, 2017; Pontifex et al., 2010). Recent work also shows this task elicits a reliable RewP (Cronbach’s alpha=.83) (Ethridge & Weinberg, 2018).
A PCA was previously computed on these data to systematically differentiate ERPs sensitive to social feedback (Authors, 2017). ERPs in the current study were scored as mean activity where the components were identified to be maximal both by the PCA and inspection of grand averages: N1 was scored at Cz 140–180 ms after feedback and RewP was scored at Cz 300–375 ms after feedback (Figure 1). Given recent recommendations (Meyer, Lerner, De Los Reyes, Laird, & Hajcak, 2017), residual scores were computed for each component to isolate the relative variance in the ERP attributed to processing of rejection or acceptance feedback. Specifically, we evaluated residual scores for N1 to rejection adjusting for responses to acceptance, with more negative values indicating enhanced early attention towards rejection, and residual scores for RewP to acceptance adjusting for responses to rejection, with more positive values indicating enhanced reactivity to acceptance.
Figure 1.
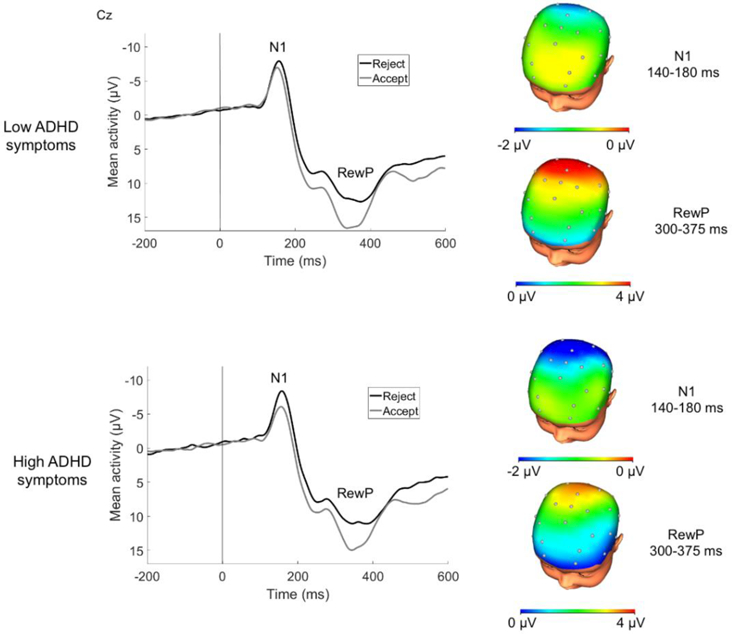
ERPs (negative up) at Cz and scalp distributions of responses to social acceptance and rejection feedback for children low and high in ADHD symptoms. Note: A median split was computed for illustrative purposes. Scalp distributions reflect the relative response to acceptance vs. rejection feedback (i.e., rejection minus acceptance difference for N1; acceptance minus rejection difference for RewP).
Self-reported rejection sensitivity.
The Children’s Rejection Sensitivity Questionnaire (Downey, Lebolt, Rincón, & Freitas, 1998) assessed self-reported rejection sensitivity using 12 vignettes depicting potential rejection situations with peers and teachers. Several vignettes were modified slightly to be age-appropriate for early adolescents.1 Youth rated how nervous they would feel in each situation from 1 (not nervous) to 6 (very, very nervous), how mad they would feel in each situation from 1 (not mad) to 6 (very, very mad), and their likelihood of being rejected in each situation from 1 (NO!!! Definitely NOT) to 6 (YES!!! Definitely). A total score, calculated by multiplying ratings for each negative affect (anxiety, anger) by the corresponding likelihood of being rejected rating and then averaging the products, was included in analyses (Cronbach’s alpha=.91). Higher scores indicate greater rejection sensitivity.
Data Analytic Plan
First, bivariate correlations were analyzed to examine associations between the CBCL Attention Problems scale, sex, and social processing variables, including N1 to rejection, RewP to acceptance, and self-reported rejection sensitivity. Next, to identify associations between ADHD symptoms and each of the social feedback processing variables accounting for co-occurring problems and covariates, separate simultaneous multiple linear regression models were calculated with N1 to rejection, RewP to acceptance, or self-reported rejection sensitivity as the dependent variables, controlling for self-reported task engagement in the Island Getaway task, stimulant medication use during the EEG assessment, and sex. We also included anxious/depressed, aggression, and social problems subscales from the CBCL as covariates to evaluate the specificity of associations between ADHD symptoms and social feedback variables when accounting for co-occurring problems. The two social feedback variables not included as the dependent variable were also included as covariates to control for associations among social feedback variables. Lastly, to explore sex a moderator of the association between ADHD symptoms and social variables, regression analyses included the interaction between ADHD symptoms and sex as an additional independent variable. Simple slopes tests were computed to probe significant interactions, and three analogous regression analyses were run replacing the dimensional ADHD symptom score with presence/absence of an ADHD diagnosis to determine whether similar results were evident for youth with a diagnosis of ADHD. Standardized regression coefficients are presented as an estimate of effect size to demonstrate the relative strength of associations and their practical meaning (Nieminen, Lehtiniemi, Vähäkangas, Huusko, & Rautio, 2013), with .10, .30, and .50 consistent with small, medium, and large effects, respectively (Cohen, 1988).
Results
Bivariate Correlations between ADHD and Sensitivity to Social Feedback
Table 1 presents means and standard deviations for ADHD symptoms, social processing variables, and co-occurring problems, along with bivariate correlations. Greater ADHD symptom severity was associated with an enhanced N1 to rejection and greater self-reported rejection sensitivity. A significant association emerged between ERP variables, with greater N1 reactivity to rejection associated with blunted RewP to acceptance. In addition, greater N1 reactivity to rejection was associated with higher levels of self-reported rejection sensitivity. When examining associations between ADHD diagnosis and social feedback variables, ADHD diagnosis was associated with an enhanced N1 to rejection.
Table 1. Descriptive statistics and bivariate correlations between ADHD symptoms and diagnosis, sex, and social processing variables.
| M | SD | Range | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. ADHD Symptoms | 2.06 | 3.09 | 0.00–17.00 | -- | ||||||||
| 2. ADHD Diagnosis | -- | -- | -- | .66** | -- | |||||||
| 3. Sex (male=0, female=1) | -- | -- | -- | −.16** | −.16** | -- | ||||||
| ERP | ||||||||||||
| 4. N1 to Rejection (residual) | 0.00 | 4.29 | −12.54–14.23 | −.10* | −.11* | −.05 | -- | |||||
| 5. RewP to Acceptance (residual) |
0.00 | 6.34 | −16.30–20.58 | −.09+ | −.03 | <.01 | −.25** | -- | ||||
| Self-report | ||||||||||||
| 6. Rejection Sensitivity | 6.93 | 2.93 | 1.13–19.38 | .14** | .08 | .04 | −.15** | .02 | -- | |||
| Co-occurring Problems | ||||||||||||
| 7. Anxious/Depressed | 1.77 | 2.65 | 0.00–13.00 | .35** | .21** | .07 | −.04 | −.04 | .22** | -- | ||
| 8. Aggressive Behavior | 2.56 | 3.62 | 0.00–20.00 | .59** | .35** | −.07 | −.01 | −.01 | .14** | .52** | -- | |
| 9. Social Problems | 1.00 | 1.78 | 0.00–14.00 | .53** | .35** | .04 | −.07 | .01 | .17** | .55** | .59** | -- |
p<.01
p<.05
p<.10
ADHD Symptoms and Sensitivity to Social Feedback
Results of the linear regression models examining the association between ADHD symptoms and social feedback variables are presented in Table 2.2 While there was no significant interaction between ADHD symptoms and sex, a modest effect emerged showing that greater ADHD symptoms were significantly associated with an enhanced N1 to rejection, considering all covariates. More blunted RewP, greater self-reported rejection sensitivity, and aggressive behavior were also associated with an enhanced N1. No other significant covariates emerged.
Table 2. Regression Analyses Examining Associations between ADHD Symptoms and Social Feedback Variables.
| Unstandardized coefficient (b) |
SE | Standardized coefficient (B) |
p | |
|---|---|---|---|---|
| N1 to rejection, F(10,380)=4.750, p<.001 R2=.111 | ||||
| ADHD symptoms | −.299 | .108 | −.215 | .006 |
| Sex | −.747 | .502 | −.087 | .137 |
| ADHD medication use | .160 | 1.156 | .007 | .890 |
| Task engagement | .208 | .255 | .040 | .415 |
| Social Problems | −.053 | .163 | −.022 | .745 |
| Anxiety/Depression | −.031 | .100 | −.019 | .757 |
| Aggression | .168 | .082 | .142 | .041 |
| RewP to Acceptance | −.185 | .033 | −.273 | .000 |
| Rejection Sensitivity | −.186 | .073 | −.127 | .012 |
| ADHD symptoms × Sex | .096 | .147 | .043 | .515 |
| RewP to acceptance, F(10, 380)=4.568, p<.001, R2=.107 | ||||
| ADHD symptoms | −.581 | .158 | −.282 | .000 |
| Sex | −1.119 | .743 | −.088 | .133 |
| ADHD medication use | .804 | 1.712 | .025 | .639 |
| Task engagement | .764 | .377 | .100 | .043 |
| Social Problems | .211 | .241 | .059 | .383 |
| Anxiety/Depression | −.205 | .148 | −.085 | .168 |
| Aggression | .220 | .122 | .126 | .072 |
| N1 to Rejection | −.405 | .073 | −.274 | .000 |
| Rejection Sensitivity | −.017 | .110 | −.008 | .879 |
| ADHD symptoms x Sex | .392 | .216 | .121 | .070 |
| Rejection Sensitivity, F(10, 380)=3.350, p<.001, R2=.081 | ||||
| ADHD symptoms | .042 | .075 | .045 | .575 |
| Sex | .158 | .349 | .027 | .651 |
| ADHD medication use | −.261 | .802 | −.018 | .745 |
| Task engagement | .329 | .176 | .093 | .063 |
| Social Problems | .057 | .113 | .035 | .614 |
| Anxiety/Depression | .202 | .069 | .183 | .003 |
| Aggression | .005 | .057 | .006 | .933 |
| N1 to Rejection | −.089 | .035 | −.131 | .012 |
| RewP to Acceptance | −.004 | .024 | −.008 | .879 |
| ADHD symptoms x Sex | −.009 | .102 | −.006 | .926 |
Note. Sex was coded with 0=male and 1=female.
In the regression examining associations between ADHD symptoms and RewP to acceptance, sex did not emerge as a significant moderator. A modest effect emerged showing greater ADHD symptoms were significantly associated with more blunted RewP. More enhanced N1 was also associated with more blunted RewP, and greater self-reported task engagement was associated with enhanced RewP. No other significant covariates emerged.
In the regression model examining the association between ADHD symptoms and self-reported rejection sensitivity, sex did not emerge as a significant moderator, and a main effect of ADHD symptoms did not emerge. An enhanced N1 to rejection and greater anxiety/depression were significantly associated with self-reported rejection sensitivity.
ADHD Diagnosis and Sensitivity to Social Feedback
Results of the linear regression models examining the association between ADHD diagnosis and social feedback variables are presented in Table 3.3 In the model examining the association between ADHD and N1 to rejection, sex did not emerge as a significant moderator, although a modest effect emerged showing that ADHD diagnosis was significantly associated with an enhanced N1 to rejection, considering all covariates. More blunted RewP and greater self-reported rejection sensitivity were also associated with an enhanced N1.
Table 3. Regression Analyses Examining Associations between ADHD Diagnosis and Social Feedback Variables.
| Unstandardized coefficient (b) |
SE | Standardized coefficient (B) |
p | |
|---|---|---|---|---|
| N1 to rejection, F(10, 380)=4.425, p<.001, R2=.104 | ||||
| ADHD diagnosis | −1.707 | .792 | −.144 | .032 |
| Sex | −.593 | .456 | −.069 | .194 |
| ADHD medication use | −.060 | 1.162 | −.003 | .959 |
| Task engagement | .172 | .255 | .033 | .500 |
| Social Problems | −.108 | .159 | −.045 | .499 |
| Anxiety/Depression | −.022 | .100 | −.014 | .824 |
| Aggression | .101 | .077 | .086 | .186 |
| RewP to acceptance | −.174 | .033 | −.257 | .000 |
| Rejection Sensitivity | −.193 | .074 | −.132 | .009 |
| ADHD diagnosis x Sex | .588 | 1.288 | .028 | .648 |
| RewP to acceptance, F(10, 380)=3.695, p<.001, R2=.089 | ||||
| ADHD diagnosis | −2.271 | 1.182 | −.129 | .055 |
| Sex | −.674 | .681 | −.053 | .323 |
| ADHD medication use | −.362 | 1.732 | −.011 | .834 |
| Task engagement | .660 | .380 | .086 | .083 |
| Social Problems | .051 | .238 | .014 | .829 |
| Anxiety/Depression | −.177 | .149 | −.074 | .235 |
| Aggression | .067 | .114 | .038 | .561 |
| N1 to Rejection | −.387 | .074 | −.262 | .000 |
| Rejection Sensitivity | −.022 | .111 | −.010 | .839 |
| ADHD diagnosis x Sex | 4.005 | 1.910 | .129 | .037 |
| Rejection Sensitivity, F(10, 380)=3.343, p<.001, R2=.081 | ||||
| ADHD diagnosis | .124 | .550 | .015 | .822 |
| Sex | .168 | .316 | .029 | .595 |
| ADHD medication use | −.106 | .803 | −.007 | .895 |
| Task engagement | .341 | .176 | .096 | .053 |
| Social Problems | .079 | .110 | .048 | .471 |
| Anxiety/Depression | .201 | .068 | .182 | .003 |
| Aggression | .019 | .053 | .024 | .717 |
| N1 to Rejection | −.092 | .035 | −.135 | .009 |
| RewP to Acceptance | −.005 | .024 | −.010 | .839 |
| ADHD diagnosis x Sex | −.462 | .890 | −.032 | .604 |
Note. Sex was coded with 0=male and 1=female.
When considering the effect of the interaction between ADHD diagnosis and sex on RewP to acceptance, a significant interaction emerged. Simple slopes tests showed a trend for boys with ADHD to show more blunted RewP compared to boys without ADHD (b=−2.271, p=.055). The effect for girls was not significant (b=1.734, p=.306), and a significant main effect of ADHD did not emerge. Only an enhanced N1 to rejection was associated with a more blunted RewP to acceptance.
Sex did not moderate the association between ADHD and self-reported rejection sensitivity, and a main effect of ADHD diagnosis on self-reported rejection sensitivity did not emerge when accounting for covariates. However, greater anxiety/depression and enhanced N1 to rejection were associated with greater self-reported rejection sensitivity.
Discussion
This study is the first to examine associations between ADHD and neurophysiological measures of sensitivity to social cues. Our primary findings indicated that ADHD symptoms were associated with enhanced neurophysiological reactivity to peer rejection feedback at very early processing stages, measured by the N1 component, as well as reduced responsiveness to social reward, as measured by the later RewP component even after adjusting for sex, ADHD medication use, task engagement, anxiety/depression, aggression, social problems, and other social processing variables. Consistent with evidence of greater rejection sensitivity at the neurophysiological level, youth with elevated ADHD reported greater tendencies to perceive and overreact to social rejection. Importantly, we found that an enhanced N1 to social rejection feedback also correlated with greater self-reported rejection sensitivity in the overall sample, supporting the validity of the N1 as a measure of early attentional processing of and reactivity to rejection feedback. Taken together, these effects, although generally of modest size, indicate that greater ADHD symptoms in early adolescence are associated with alterations in sensitivity to social feedback, including enhanced attention towards social rejection cues and reduced sensitivity to positive feedback.
Previous research examining social information processing deficits among youth with ADHD has yielded mixed results, showing youth to be both under and overresponsive to social cues at the self-report and behavioral level (e.g, Andrade et al., 2012; Matthys et al., 1999; Mikami et al., 2008; Sibley et al., 2010; Waschbusch et al., 2002). Attention to early stages of neurophysiological processing of cues in our work suggests that while there may be variability in the social processing of youth with ADHD, early adolescent youth with ADHD are overly reactive to peer rejection cues, but may miss positive social cues.
An enhanced N1 to rejection among youth with ADHD in the current study using a social task contrasts with previous research showing the N1 component to be reduced among children with ADHD in cognitive tasks (Lawrence et al., 2005). Individual differences in N1 are likely modulated by task parameters, and the current results suggest that youth with ADHD may show enhanced early attentional processing of salient cues, such as rejection feedback. On the other hand, youth with greater ADHD symptoms showed a blunted RewP to acceptance, when accounting for co-occurring problems. The apparent lower levels of reactivity to social acceptance among youth with ADHD fits with previous studies demonstrating alterations in monetary reward processing among youth with ADHD at the neurophysiological level (van Meel et al., 2011) and extend these findings to suggest that youth with ADHD may have difficulty processing social contingencies. Neuroimaging studies of monetary reward processing have also demonstrated hypoactivation of the ventral striatum, a region central to reward processing and correlated with magnitude of RewP in monetary reward tasks, among individuals with ADHD (Becker, Nitsch, Miltner, & Straube, 2014; Plichta & Scheres, 2014). It is possible that the blunted RewP represents general alterations in brain reward circuits, and additional research is needed to evaluate the extent to which social and monetary reward responsiveness account for unique variance in ADHD symptoms. Alternatively, youth with greater ADHD symptoms may be less reactive to peer acceptance, partly because they attend more to negative cues (i.e., rejection) in their social environment. In turn, this blunted response to positive social cues may lead to difficulties effectively modulating social behavior. Indeed, a number of studies suggest that youth with ADHD have difficulty attending to the most relevant social cues in social settings, and as a result engage in social behavior that is frequently negative and/or inappropriate (Matthys et al., 1999; McQuade & Hoza, 2014; Mikami et al., 2008; Sibley et al., 2010). Future work is needed to examine associations between N1 and RewP to social feedback and specific social behaviors in daily life.
Associations with neurophysiological processing were specific to ADHD symptoms versus co-occurring problems and the strength of associations between ADHD and N1 and RewP were greater than associations with co-occurring problems. In contrast, the association between ADHD symptoms and self-reported rejection sensitivity was no longer statistically significant when controlling for aggressive and anxious/depressed behavior. Although ADHD symptoms and RewP to acceptance were not significantly correlated at the bivariate level, in the regression model, we found that ADHD symptoms were uniquely associated with RewP to acceptance controlling for the other covariates, including social problems, anxiety/depression, and conduct problems. Attention problems are highly correlated with co-occurring problems, such as aggressive behavior and anxiety/depression. Thus, the effect that emerges between ADHD symptoms and RewP in the regression model, controlling for variance explained by these other co-occurring difficulties, suggests that other comorbid symptoms may mask the effects of ADHD symptoms on RewP. In particular, a reduced RewP to monetary reward has often been associated with depressive symptoms in youth and adults (e.g., Proudfit, Bress, Foti, Kujawa, & Klein, 2015), and as such, the association with ADHD symptoms may only become apparent when accounting for depressive symptoms among adolescence with and without ADHD.
A modest effect of an enhanced N1 to rejection was also demonstrated among youth with a diagnosis of ADHD, suggesting that ADHD measured both dimensionally and categorically is associated with alterations in social processing, particularly early attention to peer rejection. In contrast, statistically significant effects on the RewP component and self-reported rejection sensitivity that had emerged when examining ADHD symptoms continuously no longer remained significant when comparing youth with and without a diagnosis of ADHD. Given that only 15.3% of our sample had a lifetime diagnosis of ADHD, this study may be underpowered to detect significant associations with diagnostic status.
Additional work to clarify the developmental origins of enhanced attention to peer rejection cues is needed. On the one hand, a number of studies suggest that chronic peer difficulties may lead to enhanced sensitivity to rejection cues. These deleterious effects of chronic peer dysfunction do not appear to be specific to ADHD (e.g., Rudolph, Miernicki, Troop-Gordon, Davis, & Telzer, 2016; Will et al., 2016). However, social dysfunction characteristic of youth with ADHD may elicit particularly negative interactions with peers, which could alter sensitivity to social rejection across development. On the other hand, it could also be that youth with ADHD are overly responsive to negative social cues from peers (Bondu & Esser, 2015; Matthys et al., 1999), which may contribute to difficulty modifying social behavior to appropriately fit the context, which could lead to further social dysfunction and peer rejection. Prospective research is needed to evaluate the extent to which social experiences influence N1 and RewP to social feedback across development. This work may shed light on the direction of these associations, which is crucial in order to inform intervention efforts (i.e., target peer difficulties or reactivity to social cues).
In our analyses, sex did not emerge as a significant covariate, and only one of the six exploratory regression models showed a significant interaction between ADHD and sex. Specifically, a trend emerged suggesting that ADHD diagnosis was associated with more blunted RewP for boys but not girls. While this effect contrasts with emerging work showing the social difficulties of girls with ADHD to be greater than those of boys with the disorder (e.g., Becker et al., 2013), this finding should be interpreted cautiously as there were very few girls diagnosed with ADHD, and interaction effects did not emerge in the dimensional analyses. More work sufficiently powered to examine sex differences in social processing associated with ADHD is needed. Relatedly, it may also be important to examine how sex may moderate the effects of other predictors, such as depression/anxiety, in samples of youth with ADHD.
Some additional limitations of the current study should be noted. Effects, while significant, were of modest size, which is common in large samples linking physiological and self-report measures given the lack of shared method variance (Patrick et al., 2013). Large effects in clinical neuroscience may be due, at least in part, to overestimates of effect sizes in studies with small samples (Button et al., 2013). Studies integrating multiple measures and levels of analysis of emotional and cognitive constructs of interest are essential in order to identify the clinical significance of findings. A single ERP measure may have limited clinical significance alone, but we found associations between ADHD and multiple aspects of social processing, and integrating information across levels of analysis has the potential to improve understanding of core alterations in social processing in ADHD that could be targeted in intervention. Results in our non-referred, predominantly white, sample may not generalize to other samples. However, it is likely that larger effects would be evident in clinic-referred samples. Teacher ratings of ADHD were not included, although the percentage of children meeting criteria for a diagnosis was consistent with prevalence rates documented in epidemiological studies (e.g., Rowland, Lesesne, & Abramowitz, 2002). The CBCL Attention Problems subscale used as the primary measure of ADHD does not capture all DSM ADHD symptoms, and includes some items that are not in the DSM. However, some of our major findings were also observed using ADHD diagnoses derived from semi-structured interviews. Finally, more nuanced analyses of ERPs in the Island Getaway, such as ERP responses to positive and negative feedback as a function of participant votes (e.g., more versus less desirable peers), could further inform our understanding of social processing in ADHD, but the relatively low number of trials in the task limits our ability to examine these possibilities.
Altogether, these findings indicate that youth with ADHD exhibit alterations in sensitivity to social feedback at both the neurophysiological and self-report level. While more work is needed to replicate and extend these findings to a clinical sample of youth with ADHD and to longitudinal studies, the current study suggests that N1 and RewP may be useful measures of distinct aspects of social feedback processing in youth with ADHD. Furthermore, altered neurophysiological processing of social feedback could be a potential mechanism explaining the effect of ADHD on social dysfunction. Despite substantial work on treating peer difficulties in youth with ADHD, very little progress has been made towards producing clinically meaningful improvements (McQuade & Hoza, 2014). Our findings point to the value of neurophysiological measures of social processing as markers of potentially modifiable deficits and treatment targets in youth with ADHD that may complement the existing literature on self-report and behavioral measures.
Footnotes
Dr. Antonio Freitas, one of the contributing authors to the original Children’s Rejection Sensitivity Questionnaire, assisted with modifying the vignettes.
The pattern of results remained the same when the interaction between ADHD symptoms and sex was removed.
The pattern of results remained the same when the interaction between ADHD diagnosis and sex was removed.
References
- Achenbach TM, & Rescorla L (2001). ASEBA school-age forms and profiles Burlington, VT: Aseba. [Google Scholar]
- Andrade BF, Waschbusch DA, Doucet A, King S, MacKinnon M, McGrath PJ, … Corkum P (2012). Social information processing of positive and negative hypothetical events in children with ADHD and conduct problems and controls. Journal of Attention Disorders, 16, 491–504. 10.1177/1087054711401346 [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Zelazny J, Kaufman J, & Gill MK (2009). The Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL) 2009 Working Draft Advanced Center for Intervention and Services Research, Western Psychiatric Institute and Clinic; (http://www.psychiatry.pitt.edu/sites/default/files/Documents/assessments/KSADS-PL_2009_working_draft_ full.pdf). [Google Scholar]
- Becker MPI, Nitsch AM, Miltner WHR, & Straube T (2014). A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. Journal of Neuroscience, 34, 3005–3012. 10.1523/JNEUROSCI.3684-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Luebbe AM, & Langberg JM (2012). Co-occurring mental health problems and peer functioning among youth with attention-deficit/hyperactivity disorder: a review and recommendations for future research. Clinical Child and Family Psychology Review, 15, 279–302. 10.1007/s10567-012-0122-y [DOI] [PubMed] [Google Scholar]
- Becker SP, McBurnett K, Hinshaw SP, & Pfiffner LJ (2013). Negative social preference in relation to internalizing symptoms among children with ADHD predominantly inattentive type: girls fare worse than boys. Journal of Clinical Child and Adolescent Psychology, 42, 784–795. 10.1080/15374416.2013.828298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9, 267–277. 10.1038/nrn2353 [DOI] [PubMed] [Google Scholar]
- Bondu R, & Esser G (2015). Justice and rejection sensitivity in children and adolescents with ADHD symptoms. European Child & Adolescent Psychiatry, 24, 185–198. 10.1007/s00787-014-0560-9 [DOI] [PubMed] [Google Scholar]
- Bress JN, & Hajcak G (2013). Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology, 50, 610–616. 10.1111/psyp.12053 [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek B. a, Flint J, Robinson ESJ, Munafò MR (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14, 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Crick NR & Dodge KA (1994). A review and reformulation of social information-processing mechanisms in children’s social adjustment. Psychological Bulletin, 115, 74–101. 10.1037/0033-2909.115.1.74 [DOI] [Google Scholar]
- Crone EA & Dahl RE (2012). Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience, 13, 636–650. 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Diamantopoulou S, Henricsson L, & Rydell AM (2005). ADHD symptoms and peer relations of children in a community sample: Examining associated problems, self-perceptions, and gender differences. International Journal of Behavioral Development, 29, 388–398. 10.1177/01650250500172756 [DOI] [Google Scholar]
- DiFilipo D, & Grose-Fifer J (2016). An Event-Related potential study of social information processing in adolescents. PLoS ONE, 11, 6–11. 10.1371/journal.pone.0154459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA (1986). A social information processing model of social competence in children. Perlmutter M (Ed.), Minnesota Symposium on Child Psychology (Vol. 18, pp. 77–125). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Downey G, Lebolt A, Rincón C, & Freitas AL (1998). Rejection sensitivity and children’s interpersonal difficulties. Child Development, 69, 1074–1091. 10.1111/j.1467-8624.1998.tb06161.x [DOI] [PubMed] [Google Scholar]
- Ethridge P, Kujawa A, Dirks M, Arfer K, Kessel EM, Klein DN, & Weinberg A (2017). Neural responses to social and monetary reward in early adolescence and emerging adulthood. Psychophysiology, 54, 1786–1799. 10.1111/psyp.12957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge P & Weinberg A (2018). Psychometric properties of neural responses to monetary and social rewards across development. International Journal of Psychophysiology [DOI] [PubMed]
- Foti D, Weinberg A, Dien J, & Hajcak G (2011). Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping, 32, 2207–2216. 10.1002/hbm.21182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, & Willoughby AR (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science, 295, 2279–2282. 10.1126/science.1066893 [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology, 55, 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Hajcak Proudfit G, Bress JN, Foti D, Kujawa A, & Klein DN (2015). Depression and event-related potentials: emotional disengagement and reward insensitivity. Current Opinion in Psychology, 4, 110–113. 10.1016/j.copsyc.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, & Anllo-Vento L (1998). Event-related brain potentials in the study of visual selective attention. Proceedings of the National Academy of Sciences of the United States of America, 95, 781–787. 10.1073/pnas.95.3.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, & Krigolson OE (2008). The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology, 45, 688–697. 10.1111/j.1469-8986.2008.00668.x [DOI] [PubMed] [Google Scholar]
- Hoza B (2007). Peer functioning in children with ADHD: why are peer relationships important? Journal of Pediatric Psychology, 32, 655–663. 10.1093/jpepsy/jsm024 [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Copeland W, Stanger C, & Wadsworth M (2004). Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: a receiver-operating characteristic analysis. Journal of Child Psychology and Psychiatry, 45, 1299–1307. 10.1111/j.1469-7610.2004.00314.x [DOI] [PubMed] [Google Scholar]
- Humphreys KL, Katz SJ, Lee SS, Hammen C, Brennan PA, & Najman JM (2013). The association of ADHD and depression: Mediation by peer problems and parent–child difficulties in two complementary samples. Journal of Abnormal Psychology, 122, 854–867. 10.1037/a0033895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, & Clarke AR (2013). Ten years on: A follow-up review of ERP research in attention-deficit/hyperactivity disorder. Clinical Neurophysiology, 124, 644–657. 10.1016/j.clinph.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Kessel EM, Kujawa A, Hajcak Proudfit G, & Klein DN (2015). Neural reactivity to monetary rewards and losses differentiates social from generalized anxiety in children. Journal of Child Psychology and Psychiatry, 56, 792–800. 10.1111/jcpp.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Arfer KB, Klein DN, & Proudfit GH (2014). Electrocortical reactivity to social feedback in youth: A pilot study of the Island Getaway task. Developmental Cognitive Neuroscience, 10, 140–147. 10.1016/j.dcn.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Kessel EM, Carroll A, Arfer KB, & Klein DN (2017). Social processing in early adolescence: Associations between neurophysiological, self-report, and behavioral measures. Biological Psychology, 128, 55–62. 10.1016/j.biopsycho.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, & Klein DN (2014). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology, 123, 287–297. 10.1037/a0036285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CA, Barry RJ, Clarke AR, Johnstone SJ, McCarthy R, Selikowitz M, & Broyd SJ (2005). Methylphenidate effects in attention deficit/hyperactivity disorder: Electrodermal and ERP measures during a continuous performance task. Psychopharmacology, 183, 81–91. 10.1007/s00213-005-0144-y [DOI] [PubMed] [Google Scholar]
- Levinson AR, Speed BC, Infantolino ZP,, & Hajcak G (2017). Reliability of the electrocortical response to gains and losses in the doors task. Psychophysiology, 54, 601–607. 10.1111/psyp.12813 [DOI] [PubMed] [Google Scholar]
- London B, Downey G, Bonica C, & Paltin I (2007). Social causes and consequences of rejection sensitivity. Journal of Research on Adolescence, 17, 481–506. 10.1111/j.1532-7795.2007.00531.x [DOI] [Google Scholar]
- Matthys W, Cuperus JM, & Van Engeland H (1999). Deficient social problem-solving in boys with ODD/CD, with ADHD, and with both disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 38, 311–321. 10.1097/00004583-199903000-00019 [DOI] [PubMed] [Google Scholar]
- McQuade JD & Hoza B (2014). Peer relationships of Children with ADHD. Barkley RA (Ed.), Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment (pp. 210–222). New York, NY: The Guilford Press. [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54, 114–122. 10.1111/psyp.12664 [DOI] [PubMed] [Google Scholar]
- Mikami AY, Lee SS, Hinshaw SP, & Mullin BC (2008). Relationships between social information processing and aggression among adolescent girls with and without ADHD. Journal of Youth and Adolescence, 37, 761–771. 10.1007/s10964-007-9237-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrug S, Brooke BS, Hoza B, Gerdes AC, Hinshaw SP, Hechtman L, & Arnold LE (2012). Peer rejection and friendships in children with attention-deficit/hyperactivity disorder: Contributions to long-term outcomes. Journal of Abnormal Child Psychology, 40, 1013–1026. 10.1007/s10802-012-9610-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen P, Lehtiniemi H Vähäkangas K, Huusko A, & Rautio A (2013). Standardised regression coefficient as an effect size index in summarizing findings in epidemiological studies. Epidemiology, Biostatistics and Public Health, 10, 1–15. 10.2427/8854 [DOI] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, & Polich J (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77, 247–265. 10.1016/j.biopsycho.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JS, Goldfine ME, Evangelista NM, Hoza B, & Kaiser NM (2007). A critical review of self-perceptions and the positive illusory bias in children with ADHD. Clinical Child and Family Psychology Review, 10, 335–351. 10.1007/s10567-007-0027-3 [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, & Kramer MD (2013). A construct-network approach to bridging diagnostic and physiological domains: application to assessment of externalizing psychopathology. Journal of Abnormal Psychology, 122, 902–16. 10.1037/a0032807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, & Bender ME (1982). Peer relationships in hyperactive children: Description and treatment. Gadow K & Bialer I (Eds.), Advances in learning and behavioral disabilities (pp. 365–436). Greenwich, CT: JAI Press. [Google Scholar]
- Plichta MM & Scheres A (2014). Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neuroscience and Biobehavioral Reviews, 38, 125–134. 10.1016/j.neubiorev.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu C, Themanson JR, & Hillman CH (2010). On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology, 47, 767–773. 10.1111/j.1469-8986.2010.00974.x [DOI] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on rewards to a biomarker for depression. Psychophysiology, 52, 449–459. 10.1111/psyp.12370 [DOI] [PubMed] [Google Scholar]
- Ray AR, Evans SW, & Langberg JM (2017). Factors associated with healthy and impaired social functioning in young adolescents with ADHD. Journal of Abnormal Child Psychology, 45, 883–897. 10.1007/s10802-016-0217-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, & Rudolph KD (2006). A review of sex differences in peer relationship processes: potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin, 132, 98–131. 10.1037/0033-2909.132.1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AS, Lesesne CA, & Abramowitz AJ (2002). The epidemiology of attention-deficit/hyperactivity disorder (ADHD): A public health view. Mental Retardation and Developmental Disabilities Research Reviews, 8, 162–170. 10.1002/mrdd.10036 [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Miernicki ME, Troop-Gordon W, Davis MM, & Telzer EH (2016). Adding insult to injury: neural sensitivity to social exclusion is associated with internalizing symptoms in chronically peer-victimized girls. Social Cognitive and Affective Neuroscience, 11, 829–842. 10.1093/scan/nsw021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Evans SW, & Serpell ZN (2010). Social cognition and interpersonal impairment in young adolescents with ADHD. Journal of Psychopathology and Behavioral Assessment, 32, 193–202. 10.1007/s10862-009-9152-2 [DOI] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waschbusch DA, Garefino AC, … Karch KM (2012). Diagnosing ADHD in adolescence. Journal of Consulting and Clinical Psychology, 80, 139–150. 10.1037/a0026577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Molen MJW, Dekkers LMS, Westenberg PM, van der Veen FM, & van der Molen MW (2017). Why don’t you like me? Midfrontal theta power in response to unexpected peer rejection feedback. NeuroImage, 146, 474–483. 10.1016/j.neuroimage.2016.08.045 [DOI] [PubMed] [Google Scholar]
- van Meel CS, Heslenfeld DJ, Oosterlaan J, Luman M, & Sergeant JA (2011). ERPs associated with monitoring and evaluation of monetary reward and punishment in children with ADHD. Journal of Child Psychology and Psychiatry, 52, 942–953. 10.1111/j.1469-7610.2010.02352.x [DOI] [PubMed] [Google Scholar]
- Warnick EM, Bracken MB, & Kasl S (2008). Screening efficiency of the Child Behavior Checklist and Strengths and Difficulties Questionnaire: A systematic review. Child and Adolescent Mental Health, 13, 140–147. 10.1111/j.1275-3588.2007.00461.x [DOI] [PubMed] [Google Scholar]
- Waschbusch DA, & King S (2006). Should sex-specific norms be used to assess attention-deficit/hyperactivity disorder or oppositional defiant disorder? Journal of Consulting and Clinical Psychology, 74, 179–185. 10.1037/0022-006X.74.1.179 [DOI] [PubMed] [Google Scholar]
- Waschbusch DA, Pelham WE, Jennings JR, Greiner AR, Tarter RE, & Moss HB (2002). Reactive aggression in boys with disruptive behavior disorders: Behavior, physiology, and affect. Journal of Abnormal Child Psychology, 30, 641–656. 10.1023/A:1020867831811 [DOI] [PubMed] [Google Scholar]
- Wiener J, & Mak M (2009). Peer victimization in children with Attention-Deficit/Hyperactivity Disorder. Psychology in the Schools, 46, 116–131. 10.1002/pits.20358 [DOI] [Google Scholar]
- Will GJ, van Lier PAC, Crone EA, & Güroğlu B (2016). Chronic childhood peer rejection is associated with heightened neural responses to social exclusion during adolescence. Journal of Abnormal Child Psychology, 44, 43–55. 10.1007/s10802-015-9983-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


