Abstract
Lupeol is a pentacyclic triterpene that shows a variety of pharmacological properties. Compared to engineering the production of sesquiterpenes and diterpenes, it is much more challenging to engineer the biosynthesis of triterpenes in microbial platforms. This study showed our efforts on engineering the triterpene pathway in Escherichia coli and Saccharomyces cerevisiae cells by recruiting the codon-optimized three lupeol pathway genes from different organisms. By comparing their activities with their respective counterparts, the squalene synthase from Thermosynechococcus elongates (tSQS), the squalene epoxidase from Rattus norvegicus (rSE) and the lupeol synthase from Olea europaea (OeLUP) were introduced into E. coli BL21(DE3), a break-through from zero was observed for lupeol biosynthesis in a prokaryotic host. We also assessed the lupeol pathway under two different yeast backgrounds-WAT11 and EPY300, and have found that the engineered strains based on EPY300, named ECHHOe, processed the best lupeol-producing ability with the maximum lupeol titer being 200.1 mg l−1 at 30 °C in a 72 h-flask culture, which so far was the highest amount of lupeol obtained by a microbial system and provides a basis for further industrial application of lupeol in the future.
Introduction
Lupeol is a commercially important pentacyclic triterpene, which exists in a variety of vegetables, fruits and medicinal plants1–3. This compound has attracted increasing attention, due to its various beneficial effects on human health (such as anti-tumor, anti-diabetes, and anti-inflammation, and medical treatment of arthritis, hepatic and renal toxicity)4–8. Its precursor squalene has also been used as an antioxidant agent and a potential biofuel9,10.
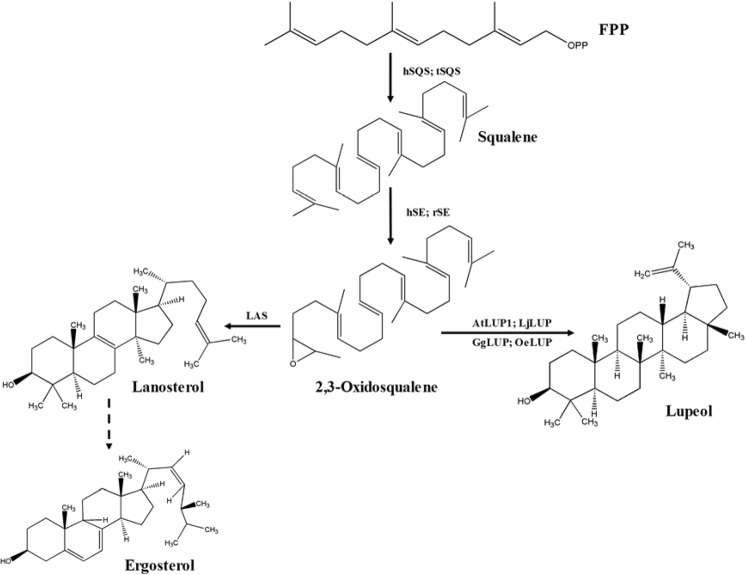
Starting from farnesyl diphosphate (FPP), the biosynthesis of lupeol begins with the condensation of two molecules of FPP to squalene by squalene synthase (EC 2.5.1.21; SQS) (Fig. 1). Squalene is then oxidized to 2,3-oxidosqualene by a membrane-bound monooxygenase, squalene epoxidase (EC 1.14.14.17; SE). After the oxidosqualene formation, there is a branch point for either sterol (e.g. ergosterol) or lupeol biosynthesis. By the action of lupeol synthase (EC 5.4.99.41; LUP), oxidosqualene is cyclized to form lupeol (Fig. 1). Genes encoding the aforementioned lupeol pathway enzymes (SQS, SE, and LUP) have been identified from various organisms. For example, the LUP-encoding genes have been cloned, and biochemically characterized from Arabidopsis thaliana11, Lotus japonicas12, Glycyrrhiza glabra13 and Olea europaea13. In many cases, lupeol naturally occurs at very low levels in plant tissues14, which has seriously limited its industrial application. For these reasons, engineering lupeol production in microbes is an attractive alternative to extraction from plant sources. The Escherichia coli and Saccharomyces cerevisiae are two well-established ‘microbial factories’, which have been widely used for generation of many types of compounds, including triterpenes15,16. Due to the lack of the SE enzyme (Fig. 1), E. coli host does not possess any sterol pathway, and the SE expression is definitely needed to engineer triterpene production in this organism. To the best of our knowledge, there are no any reports regarding the production of lupeol in E. coli. In contrast, the metabolic flux up to oxidosqualene naturally exists in S. cerevisiae, and in fact lupeol biosynthesis was ever successfully engineered in a yeast strain called NK2-LUP, however, the lupeol amount (8.2 mg l−1) yielded from that yeast strain remains pretty low17.
Figure 1.
The lupeol pathway starting from FPP. The pathway enzyme variants derived from different organisms were as follows: tSQS (GenBank accession no. NP_681887), SQS from T. elongatus; hSQS (GenBank accession no. NP_004453), SQS from H. sapiens; rSE (GenBank accession no. NP_058832), SE from R. norvegicus; hSE (GenBank accession no. NP_003120), SE from H. sapiens; AtLUP1 (GenBank accession no. AEE36187), LUP from A. thaliana; LjLUP (GenBank accession no. AB181245), LUP from L. japonicas; GgLUP (GenBank accession no. AB116228), LUP from G. glabra; and OeLUP (GenBank accession no. AB025343), LUP from O. europaea; LAS, lanosterol synthase.
With an aim to improve lupeol biosynthesis in microorganisms, this study presented the following efforts: Firstly, the three lupeol pathway genes (SQS, SE and LUP) derived from different organisms were codon-optimized based on E. coli or S. cerevisiae preference and their in vivo performance with regard to their enzymatic products was evaluated; Secondly, by recruiting the better pathway candidate genes, the lupeol pathway was reconstituted, and the utility of this recruited pathway was evaluated under two different yeast strains-WAT1118 and EPY30019. For the EPY300 strain, the carbon flux up to FPP, which is the precursor for lupeol biosynthesis (Fig. 1), was genetically enhanced by overexpressing precursor genes19. Finally, a highly lupeol-producing yeast strain ECHHOe was established by this study with the lupeol titer reaching 200.1 mg l−1, which was 24.4 folds higher than that produced by the previously engineered yeast strain NK2-LUP17. Moreover, a serious bottleneck of engineering lupeol biosynthesis in E. coli was firstly discovered in this study, which would provide a valuable guidance for a prokaryotic production of lupeol in the future work.
Results
Engineering the lupeol pathway in E. coli
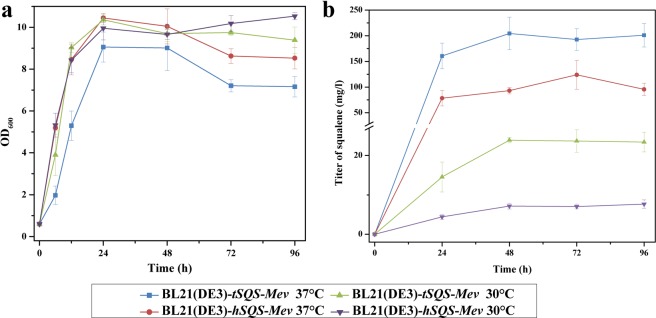
In the lupeol pathway starting from FPP, the SQS is the first enzyme (Fig. 1). The SQSs from Thermosynechococcus elongatus (tSQS) and Homo sapiens (hSQS) were chosen in this study, as they showed a relatively higher squalene-producing activity compared to the other SQSs reported so far20. The gene sequences of tSQS and hSQS were manually synthesized based on E. coli codon usage preference and individually expressed in an E. coli strain, BL21(DE3). However, both tSQS and hSQS initially led to very low levels of squalene (Fig. S1). We suspected that this low production of squalene was probably due to the limited FPP precursor supply in the transgenic E. coli cells. To increase the FPP supply, the pBbA5c-M-M plasmid, which contains eight mevalonate (MVA) pathway genes from acetyl-coA to FPP21 (see the section of Materials and Methods), was co-expressed. Apparently, the simultaneous overexpression of these eight MVA pathway genes resulted in a substantial increase in lupeol production with the tSQS generating more squalene than the hSQS (Fig. S1). The better performance of tSQS relative to hSQS was further confirmed by measuring squalene yields in a time course manner from both 30 °C- and 37 °C-incubated cultures (Fig. 2).
Figure 2.
The tSQS displayed a higher activity than the hSQS in E. coli. (a) Growth properties of the SQS-transformed strains; (b) Squalene yields produced by the SQS-transformed strains. The engineered E. coli BL21(DE3)-SQS-Mev strains were cultivated at either 30 °C or 37 °C, and cells were respectively harvested at different time intervals for the product analysis.
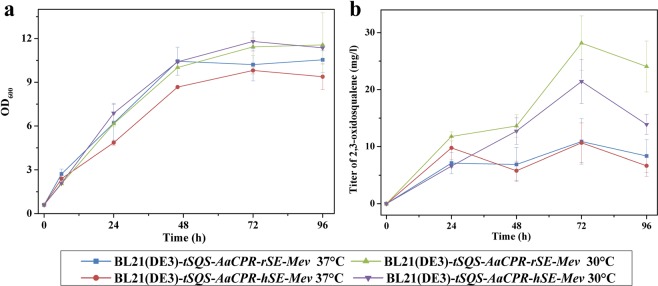
The next enzyme after squalene synthesis in the lupeol pathway is SE (Fig. 1). SE is a membrane-bound monooxygenase, which makes it difficult to obtain a soluble SE protein in E. coli cells22. Therefore, N-terminal transmembrane domain truncated version of SE was made (see the Materials and Methods section). The SEs from Rattus norvegicus (rSE)23 and H. sapiens (hSE)24 were individually co-expressed in the presence of the tSQS and the eight MVA pathway genes. Because SE is a flavin-dependent monooxygenase, a P450 reductase partner, AaCPR from Artemisia annua, was also expressed, which led to the construction of two E. coli strains of BL21(DE3)-tSQS-AaCPR-rSE-Mev and BL21(DE3)-tSQS-AaCPR-hSE-Mev. Both strains were cultured at two temperatures (30 °C and 37 °C), and their 2,3-oxidosqualene yields were compared. Generally, the 30 °C-incubated strains produced more 2,3-oxidosqualene than the 37 °C-cultured ones (Fig. 3). Between the E. coli strains containing rSE and hSE, there was no significant difference in their 2,3-oxidosqualene yields, indicating that the biochemical efficiencies of rSE and hSE are similar in this E. coli host.
Figure 3.
Comparison of the in vivo performances between rSE and hSE in E. coli. (a) Growth properties of the SE-expressed strains; (b) 2,3-Oxidsqualene yields produced by the SE-expressed strains. The SEs were expressed in the strain background of BL21(DE3)-tSQS-AaCPR-Mev, and the SE-expressed strains were cultured at either 30 °C or 37 °C for four days. During the incubations, the cells were harvested at different time points and used for the analysis.
The last enzyme for lupeol synthesis is LUP (Fig. 1). To screen the LUP variants, the LUPs from A. thaliana (AtLUP1)11,25, L. japonicas (LjLUP)12, G. glabra (GgLUP)13 and O. europaea (OeLUP)13 were investigated. These LUP-encoding genes were initially codon-optimized based on the E. coli usage preference and individually expressed in the strain of BL21(DE3)-tSQS-AaCPR-rSE-Mev. However, only a trace amount of lupeol was observed in the OeLUP-expressed strain (Fig. S2), and none was detected by the others, which was probably due to a false folding of these LUPs in E. coli cells. This hypothesis could be supported by the observation that AtLUP1 and its truncated mutants were exclusively presented as inclusion bodies when they were solely expressed in BL21(DE3) cells (Fig. S3), suggesting that E. coli might not be a suitable host for functional expression of LUP enzyme.
Engineering the lupeol pathway in S. cerevisiae
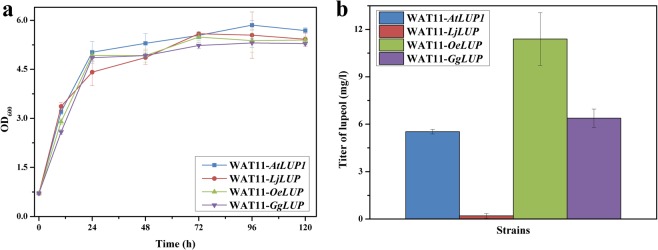
To examine whether LUP could be functionally expressed in S. cerevisiae, the aforementioned four LUP genes were re-synthesized following S. cerevisiae codon usage and initially expressed in a WAT11 yeast strain18, which naturally provides the precursor 2,3-oxidosqualene. In comparison with the controls, the expression of each of the four LUPs led to an obviously new peak which displayed the same retention time and the same mass spectrum patterns with those of authentic lupeol standard (Fig. S4), suggesting that LUP could be correctly expressed by S. cerevisiae. Next, we proceeded to assess the activity of each LUP by measuring lupeol amounts from the LUP-transformed yeast cells at different time points. As shown in Fig. 4a, the yeast strains expressing each LUP displayed similar growth rates, while with regard to lupeol yield, the OeLUP expression yielded the maximum amount and the expression of LjLUP caused the least (Fig. 4b). The functional expression of LUP enzyme in yeast allowed us to proceed to compare the contributions of the SQS and SE variants to lupeol production in this eukaryotic host.
Figure 4.
Functional analysis of different organisms-derived LUPs based on the WAT11 yeast strain. (a) Growth property; (b) Lupeol titer. The LUP-expressed strains were cultivated at 30 °C for five days, and both the cells and medium were separately harvested after the 72 h-cultivation for the product analysis.
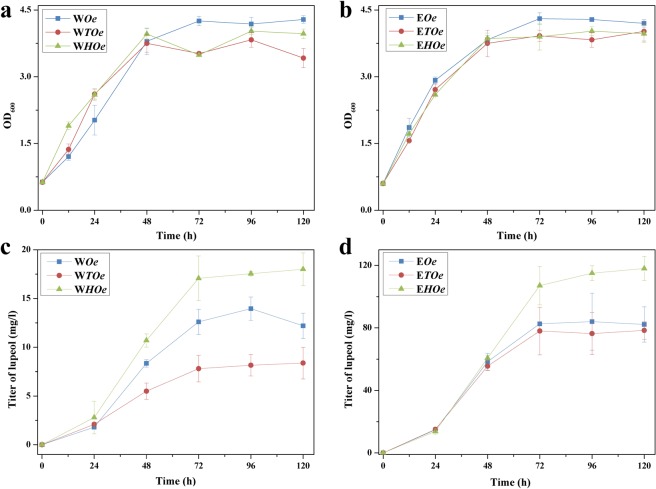
To compare the in vivo performances between tSQS and hSQS, they were individually co-expressed with OeLUP under two different yeast backgrounds (WAT11 and EPY300). As shown in Fig. S5, in either yeast background, tSQS generated a little bit higher squalene than hSQS, which trend was consistent with that obtained from the E. coli expression system (Fig. 2). Interestingly, although hSQS showed a relatively lower squalene-producing activity, it conversely generated significantly higher levels of lupeol than tSQS in both yeast backgrounds at all the time points (Fig. 5), suggesting that hSQS was more active in the flux toward lupeol production. The effects of the SQS expressions on yeast endogenous ergosterol yields were also examined using a 72 h-cultivated yeast cells. In both WAT11 and EPY300 backgrounds, the expression of either tSQS or hSQS increased ergosterol yields, this improvement was more obvious under the EPY300 background (Fig. S5).
Figure 5.
Functional analysis of different organisms-derived SQSs in the presence of the OeLUP under two different yeast strains (WAT11 and EPY300). The growth properties were shown for the WAT11-(a) and EPY300-(b) based engineered yeast strains. The lupeol yields were shown for the WAT11-(c) and EPY300-(d) based engineered yeast strains. The engineered strains were cultivated at 30 °C for five days, and both the cells and medium were separately harvested at different time intervals for the product analysis.
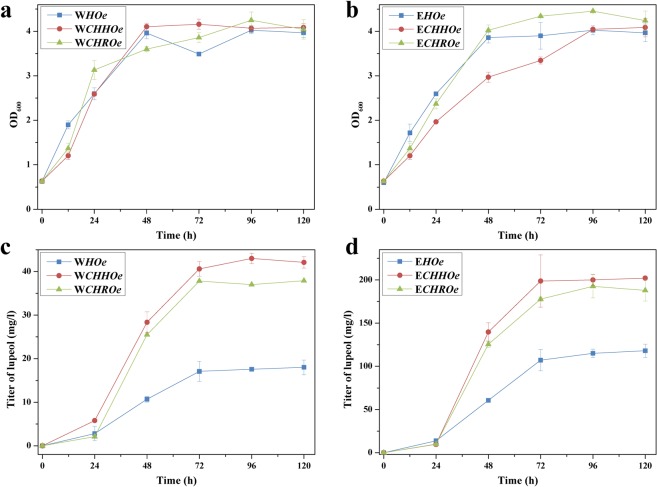
Based on the above experiments that identified hSQS and OeLUP as the better components in the lupeol pathway, they were co-expressed in both WAT11 and EPY300 backgrounds, resulting in two basis strains, named WHOe and EHOe, respectively. To evaluate the SE variants (rSE and hSE), they were individually expressed in these two basis strains. As an expression partner of SE, the reductase AaCPR was also co-expressed. It should be noted that 2,3-oxidosqualene product was not detectable in all the engineered yeasts, suggesting that it was efficiently converted to downstream end products like lupeol. Compared to the basis strains (WHOe and EHOe), the strains expressing either rSE (WCHROe and ECHROe) or hSE (WCHHOe and ECHHOe) produced 1.6–2.6 fold higher lupeol levels at all the time points after 48 h of cultivation (Fig. 6), suggesting that the transformed SEs were functional in yeast cells. The increase in lupeol production was accompanied with an obvious declining in endogenous ergosterol accumulation (Fig. S5), which indicated that the flux through the ergosterol pathway was deviated to lupeol production by the SE expression. There was no dramatic difference in lupeol yields between the rSE- and hSE-expressed yeast strains (Fig. 6), indicating of their similar activities. This data was also consistent with that generated from our E. coli experiments (Fig. 3). Overall, the lupeol amount produced by the EPY300-based yeast strains was 4.6–9.4 fold higher than that produced by the WAT11-based chassis. This data was reasonable as in the EPY300 strain the precursor flux toward FPP was strengthened19.
Figure 6.
Functional analysis of different organisms-derived SEs in the presence of both OeLUP and hSQS under the two yeast backgrounds. The growth properties were shown for the WAT11-(a) and EPY300-(b) based engineered yeast strains. The lupeol yields were shown for the WAT11-(c) and EPY300-(d) based engineered yeast strains. The engineered strains were cultivated at 30 °C for 120 h, and both the cells and medium were separately harvested at different time intervals for the product analysis.
Discussion
In general, the biosyntheses of triterpenoids and sterols compete for the same precursor 2,3-oxidosqualene, after which they are branched through different cyclization reactions by unique oxidosqualene cyclases (OSCs). Due to the importance of sterols for cell growth26, it is challenging to engineer the carbon flux predominantly transferred to triterpenoids biosynthesis in eukaryotes. On the other hand, the prokaryote E. coli does not possess any 2,3-oxidosqualene synthase and accordingly no sterols biosynthesis has been reported by this organism. The absence of sterol-producing ability of E. coli might provide a potential for high-titer triterpenoids production, hence in this study, firstly, we made efforts to engineer lupeol biosynthesis in E. coli.
Three lupeol pathway genes (SQS, SE and LUP) originally identified from different organisms were manually synthesized and their in vivo performances were compared. Consistent with their in vitro biochemical characterizations previously reported23,24, with regard to squalene production, tSQS performed better than hSQS in vivo in both E. coli and yeast systems of this study (Figs 2 and S5). SE is a membrane-localized monooxygenase, which makes it difficult to obtain a soluble SE protein in E. coli cells. Fortunately, a successful expression of SE in E. coli has previously been realized by deleting or swapping its N-terminal transmembrane domains23,24. Using the same strategy in this study, active rSE and hSE were also successfully expressed in E. coli, and a slightly higher amount of 2,3-oxidosqualene was produced by rSE compared to hSE. Further attempts to express the LUP genes in E. coli failed to produce lupeol, except that only a trace amount of lupeol was observed in the Oelup-expressed E. coli cells. We presumed that the deficiency of lupeol synthesis in E. coli could be due to the incorrect folding of LUP proteins. This hypothesis was supported by the fact that the sole expression of AtLUP1 in E. coli cells formed the LUP protein almost completely as inclusion bodies (Fig. S3). This data might explain why a successful production of lupeol in E. coli has never been reported before this study. Using an online software (http://harrier.nagahama-i-bio.ac.jp/sosui/sosui_submit.html), bioinformatics analysis predicted that several transmembrane domains exist in the N-terminus of the LUPs (Fig. S3), and the presence of membrane spanning segments probably decrease water-solubility of protein itself 27. We made efforts to get soluble LUP proteins by deleting their N-terminal transmembrane domains. Unfortunately, these deletions did not rescue the LUP expression problems in E. coli host. Further protein engineering of these LUPs is required to facilitate the functional production of lupeol in E. coli.
Because of no much success of producing lupeol in E. coli host, we switched to engineer the lupeol pathway in S. cerevisiae. Lupeol production was apparently observed by expressing each LUP enzyme in WAT11 strain, suggesting that LUP was functionally expressed. Among the LUPs tested here, OeLUP was discovered to produce the highest amount of lupeol and therefore it was selected as the LUP enzyme in the recruited pathway. The OeLUP-expressed yeast strains were then subjected to screen the SQS and SE candidates. Two different yeast strains with different backgrounds were used as the chassis for this experiment. Interestingly, relative to tSQS, hSQS, which produced the less squalene, generated more lupeol in both yeast backgrounds. A similar case was also reported for the co-expression of different SQSs with a β-amyrin synthase in yeast28. We could not explain this inconsistency, one possibility that could attribute to this disparity is that the hSQS associated with its downstream enzymes in a more tuneful context to direct the carbon flux. Compared to the SQS expression, additional expression of the SE, especially in the WAT11 background, caused a significantly larger increase in lupeol production (Fig. S5), which indicated that SE might be a rate-limiting enzyme in the lupeol pathway. With respect to yeast strain selection, EPY300 generally produced more than 4-fold higher level of squalene and 5-fold of lupeol than WAT11, which is reasonable because the carbon flux up to FPP was enhanced in EPY30019. The maximum amount (200.1 mg l−1) of lupeol obtained by the EPY300-based engineered strains (named ECHHOe) was 24.4 fold higher than that (8.2 mg l−1) produced by the previously engineered yeast strain NK2-LUP17. The difference in lupeol yields between this work and that study was most likely due to the different yeast strains used. Moreover, the rate-limiting enzyme SE was not overexpressed in the yeast strain NK2-LUP, which may partially account for the relatively lower production of lupeol as well. It was noteworthy that the ergosterol was kept at relative low levels in both engineered WAT11 and EPY300 strains. The generation of ergosterol seemed not to be significantly affected by the exogenously invaded pathway, confirming that ergosterol was essential for yeast growth and thus its metabolic flux was tightly regulated.
Conclusions
Based on E. coli and S. cerevisiae hosts, the three lupeol pathway genes (SQS, SE and LUP) derived from different organisms were systematically evaluated for their in vivo performances. The tSQS from T. elongatus, the rSE from R. norvegicus and the OeLUP from O. europaea all showed relatively higher activities than their respective counterparts, and thus were finally selected to reconstitute the lupeol pathway in this study. The reconstituted lupeol pathway was then transferred into two different yeast strains of WAT11 and EPY300 to compare their lupeol-producing abilities. EPY300 was shown to be a potentially idea strain for lupeol production, and it produced 4.6–9.4 fold higher lupeol than WAT11. Taken together, by screening the different lupeol pathway enzyme variants and assessing two different yeast hosts, a highly lupeol-producing yeast strain, named ECHHOe here, was obtained with the maximum lupeol titer after a 72 h-flask cultivation reaching 200.1 mg l−1, which was 24.4 folds higher than that produced by a previously reported strain that was the only engineered lupeol-producing yeast before this study.
Materials and Methods
Plasmids construction
All the plasmids constructed from this study were shown in Table S1. For SQS gene cloning, the tSQS from T. elongatus and hSQS from H. sapiens were chosen. For SE gene cloning, the rSE from R. norvegicus and the hSE from H. sapiens were selected and their transmembrane domains were deleted when they were synthesized by Genewiz Inc. (Suzhou, Jiangsu, China) (For the rSE, the amino acid residues from 1 to 98 were deleted with Phe223 being mutated to Ala223; for the hSE, the amino acid residues from 1 to 53 and 517 to 574 were truncated). For LUP gene cloning, the AtLUP1 from A. thaliana, the LjLUP from L. japonicas, the GgLUP from G. glabra and the OeLUP from O. europaea were selected.
For optimal expressions in E. coli host, all of the selected genes were respectively codon-optimized based on E. coli codon usage preference. The plasmid pCW-CT, which contains a cytochrome P450 reductase (CPR) encoding gene AaCPR and the costunolide pathway genes (GAS, GAO and COS), was provided by Liu’s group29 and used for SQS and SE gene cloning and expression in E. coli. Each synthesized SQS gene was cloned into pCW-CT by Nde I/Sac II digestion to replace the costunolide pathway genes which were previously engineered in it, yielding the constructs pCW-tSQS and pCW-hSQS. To construct a tSQS-SE co-expression system in E. coli, the tSQS and SE were cloned into pCW-CT through successive enzyme digestions and ligations under Xba I/Sac II and Nde I/Xba I sites with the costunolide pathway genes being replaced, resulting in the constructs of pCW-tSQS-AaCPR-rSE and pCW-tSQS-AaCPR-hSE. The plasmid pBbA5c-M-M, which contains eight MVA pathway genes in converting acetyl-CoA to FPP, was originally made by Jay Keasling’s group21. The plasmid pET-30a (Stratagene, La Jolla, CA, USA) was used for LUP gene cloning and expression in E. coli.
Similarly, for optimal expression in yeast hosts, all of the selected sequences were also individually codon-optimized according to S. cerevisiae codon preference. The plasmid pESC-Leu2d-AaCPR was obtained from Ro’s group30 and used for expressing the SQS or SE gene in yeast. The SQS was cloned into pESC-Leu2d-AaCPR under Not I/Pac I sites, yielding the constructs pESC-Leu2d-AaCPR-tSQS and pESC-Leu2d-AaCPR-hSQS. The SE was inserted into a yeast expression vector pESC-Ura (Stratagene) with BamH I and Nhe I digests to get the constructs pESC-Ura-rSE and pESC-Ura-hSE. To construct the hSQS-SE co-expression system in yeast, both pESC-Leu2d-AaCPR-hSQS and pESC-Ura-SE were digested with Pac I/Sma I, yielding the construct pESC-Leu2d-AaCPR-hSQS-rSE and pESC-Leu2d-AaCPR-hSQS-hSE. The LUP genes were individually cloned into the yeast expression vectors pESC-His (Stratagene) or pESC-Ura, leading to the constructs of pESC-His-LjLUP, pESC-His-GgLUP, pESC-His-AtLUP1 and pESC-Ura-OeLUP.
Expression in E. coli and metabolite extraction
An E. coli strain BL21(DE3) (Novagen, USA) was used as the host in this study. The constructed expression vectors were transformed into BL21(DE3) cells and the descriptions regarding to the transgenic E. coli strains are shown in Table S2. For protein expression, cells were firstly cultivated at 37 °C in terrific broth (TB) medium with appropriate concentration of antibiotics and 20 mM MgSO4 to an optical density (OD) of 0.4–0.6 at 600 nm. Expression was induced by adding a final concentration of 0.4 mM isopropyl thio-β-D-galactoside (IPTG) for 4 days at 30 °C or 37 °C. Thereafter, cells were firstly harvested by centrifugation, washed with 150 mM NaCl solution, and re-suspended in 5 ml of acetone followed by a 5 min-vortex. The crude acetone extracts were centrifuged at 10000 g for 20 min, and clear extract was then evaporated and re-suspended in 1 ml of methyl alcohol for HPLC analysis.
Expression in S. cerevisiae and metabolite extraction
Two S. cerevisiae strains, WAT1118 and EPY30019, were used as the eukaryotic hosts in this study. The constructed expression plasmids were transformed into the yeast cells and the descriptions regarding to the transgenic yeast strains are shown in Table S3. The yeast cells were respectively cultured at 30 °C for 48 h in appropriate synthetic defined dropout liquid medium containing 2% (w/v) glucose. Then cells were firstly collected by centrifugation, re-suspended using the inducible medium which contained 1.8% (w/v) galactose and 0.2% (w/v) glucose, and diluted to the OD600 reaching 0.4. At the same time, a final concentration of 100 mM 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.0–7.5) was added to the inducible medium. For metabolite extraction from the cells, yeast cells were firstly collected by centrifugation, then washed with 5 ml of 150 mM NaCl solution, and finally vacuum-dried. The dried yeast cells were mixed with 0.5 mm glass beads in acetone solution, and subsequently extracted using a SCIENTZ-48 Tissue Lyser (Scientz Biotechnology Co, Ningbo, China) for 15 min. For metabolite extraction from the medium, liquid culture was directly mixed and extracted with ethyl acetate. Finally, both the acetone extracts from the cells and ethyl acetate extracts from the medium were vacuum-evaporated and derivatized with Bis-N,O-(trimethylsilyl) trifluoroacetamide (BSTFA) at 80 °C for 30 min and subjected to Gas Chromatography-Mass Spectrometer (GC-MS) analysis.
High Performance Liquid Chromatography (HPLC) and GC-MS analyses
HPLC analysis was performed on an LC-20AT instrument (Shimadzu, Kyoto, Japan) using an Inersil ODS-3 C18 column (250 mm × 4.6 mm × 5 μm). The column temperature was maintained at room temperature using methyl alcohol/acetonitrile (50:50, v/v) as the mobile phase at a flow rate of 1.2 ml min−1, and the detection wavelength was set at 205 nm.
GC-MS analysis was quantified using an Agilent Technologies 5975 C gas chromatography equipped with a HP-5 MS column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies, Palo Alto, CA, USA). Nitrogen was used as the carrier gas at a flow rate of 1.2 ml min−1. The temperatures of the injector and detector were both set at 250 °C. The column temperature was programmed at an initial temperature of 80 °C for 2 min, then increased to 310 °C at a rate of 20 °C min−1, and finally held at 300 °C for 15 min. One microliter of sample was injected into the GC column, and analyzed using the SIM-Scan mode within the m/z range of 50–600.
Ethics Approval and Consent to Participate
This study does not contain any experiment with human participants or animals performed by any of the authors.
Supplementary information
Acknowledgements
The work was jointly supported by a grant from the National Key R&D Program of China (2018YFC1706200), the National Science and Technology Program of China (No. 2012AA02A704) and a grant from the Hubei Renyue Pharmaceutical Co. LTD (No. 492014-026053).
Author Contributions
Yansheng Zhang designed the project and revised the manuscript; Weibo Qiao and Zilin Zhou performed the experiments and drafted the manuscript; Qin Liang and Isidore Mosongo provided assistance in protein expression; Changfu Li helped the HPLC and GC-MS analyses, and reagent ordering.
Data Availability
All the data analyzed during this study have been included in this article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39497-4.
References
- 1.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285:109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beveridge THJ, Li TSC, Drover JCG. Phytosterol content in American ginseng seed oil. J. Agr. Food Chem. 2002;50:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 3.Lima LM, Perazzo FF, Carvalho JCT, Bastos JK. Anti-inflammatory and analgesic activities of the ethanolic extracts from Zanthoxylum riedelianum (Rutaceae) leaves and stem bark. J. Pharm. Pharmacol. 2007;59:1151–1158. doi: 10.1211/jpp.59.8.0014. [DOI] [PubMed] [Google Scholar]
- 4.Fernández MA, De lHB, García MD, Sáenz MT, Villar A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J. Pharm. Pharmacol. 2001;53:1533–1539. doi: 10.1211/0022357011777909. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita K, et al. Effect of three triterpenoids, lupeol, betulin, and betulinic acid on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta. 2002;325:91–96. doi: 10.1016/S0009-8981(02)00252-8. [DOI] [PubMed] [Google Scholar]
- 6.Setzer WN, Setzer MC. Plant-derived triterpenoids as potential antineoplastic agents. Mini-rev. Med. Chem. 2003;3:540–556. doi: 10.2174/1389557033487854. [DOI] [PubMed] [Google Scholar]
- 7.Prasad S, Kumar YV, Srivastava S, Shukla Y. Protective effects of lupeol against benzo[a]pyrene induced clastogenicity in mouse bone marrow cells. Mol. Nutr. Food Res. 2008;52:1117–1120. doi: 10.1002/mnfr.200700420. [DOI] [PubMed] [Google Scholar]
- 8.Sudharsan PT, Mythili Y, Selvakumar E, Varalakshmi P. Lupeol and its ester exhibit protective role against cyclophosphamide-induced cardiac mitochondrial toxicity. J. Cardiovasc. Pharm. 2006;47:205–210. doi: 10.1097/01.fjc.0000200658.89629.ba. [DOI] [PubMed] [Google Scholar]
- 9.Kohno Y, et al. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim. Biophys. Acta. 1995;1256:52–56. doi: 10.1016/0005-2760(95)00005-W. [DOI] [PubMed] [Google Scholar]
- 10.Englund E, et al. Production of squalene in Synechocystis sp. PCC 6803. PloS One. 2014;9:e90270. doi: 10.1371/journal.pone.0090270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera JBR, Bartel B, Wilson WK, Matsuda SPT. Cloning and characterization of the Arabidopsis thaliana lupeol synthase gene. Phytochemistry. 1998;49:1905–1911. doi: 10.1016/S0031-9422(98)00366-5. [DOI] [PubMed] [Google Scholar]
- 12.Sawai S, et al. Functional and structural analysis of genes encoding oxidosqualene cyclases of Lotus japonicus. Plant Sci. 2006;170:247–257. doi: 10.1016/j.plantsci.2005.08.027. [DOI] [Google Scholar]
- 13.Shibuya M, et al. Two branches of the lupeol synthase gene in the molecular evolution of plant oxidosqualene cyclases. Eur. J. Biochem. 1999;266:302–307. doi: 10.1046/j.1432-1327.1999.00875.x. [DOI] [PubMed] [Google Scholar]
- 14.Kosyakov DS, Ul’Yanovskii NV, Falev DI. Determination of triterpenoids from birch bark by liquid chromatography-tandem mass spectrometry. J. Anal. Chem+. 2014;69:1264–1269. doi: 10.1134/S1061934814130061. [DOI] [Google Scholar]
- 15.Xiao H, Zhong J-J. Production of useful terpenoids by higher-fungus cell factory and synthetic biology approaches. Trends Biotechnol. 2016;34:242–255. doi: 10.1016/j.tibtech.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Wang W-F, Xiao H, Zhong J-J. Biosynthesis of a ganoderic acid in Saccharomyces cerevisiae by expressing a cytochrome P450 gene from Ganoderma lucidum. Biotechnol. Bioeng. 2018;115:1842–1854. doi: 10.1002/bit.26583. [DOI] [PubMed] [Google Scholar]
- 17.Lin TT, Wang D, Dai ZB, Zhang XL, Huang LQ. Construction of cell factories for production of lupeol in Saccharomyces cerevisiae. Zhongguo Zhong Yao Za Zhi. 2016;41:1008–1015. doi: 10.4268/cjcmm20160606. [DOI] [PubMed] [Google Scholar]
- 18.Urban P, Mignotte C, Kazmaier M, Delorme F, Pompon D. Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J. Biol. Chem. 1997;272:19176–19186. doi: 10.1074/jbc.272.31.19176. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen TD, MacNevin G, Ro DK. De novo synthesis of high-value plant sesquiterpenoids in yeast. Methods Enzymol. 2012;517:261–278. doi: 10.1016/B978-0-12-404634-4.00013-9. [DOI] [PubMed] [Google Scholar]
- 20.Katabami A, et al. Production of squalene by squalene synthases and their truncated mutants in Escherichia coli. J. Biosci. Bioeng. 2015;119:165–171. doi: 10.1016/j.jbiosc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Peralta-Yahya PP, et al. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011;2:483. doi: 10.1038/ncomms1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huijbers MME, Montersino S, Westphal AH, Tischler D, van Berkel WJH. Flavin dependent monooxygenases. Arch. Biochem. Biophys. 2014;544:2–17. doi: 10.1016/j.abb.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Abe I, Abe T, Lou W, Masuoka T, Noguchi H. Site-directed mutagenesis of conserved aromatic residues in rat squalene epoxidase. Biochem. Biophys. Res. Commun. 2007;352:259–263. doi: 10.1016/j.bbrc.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Laden BP, Tang Y, Porter TD. Cloning, heterologous expression, and enzymological characterization of human squalene monooxygenase. Arch. Biochem. Biophys. 2000;374:381–388. doi: 10.1006/abbi.1999.1629. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, et al. Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus. Planta. 2012;236:1571–1581. doi: 10.1007/s00425-012-1712-0. [DOI] [PubMed] [Google Scholar]
- 26.Veen M, Stahl U, Lang C. Combined overexpression of genes of the ergosterol biosynthetic pathway leads to accumulation of sterols in Saccharomyces cerevisiae. FEMS Yeast Res. 2003;4:87–95. doi: 10.1016/S1567-1356(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 27.Sang JL, et al. N-terminal pI determines the solubility of a recombinant protein lacking an internal transmembrane-like domain in E. coli. Mol. Cells. 2010;30:127–135. doi: 10.1007/s10059-010-0097-9. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, et al. Enhancing the accumulation of β-amyrin in Saccharomyces cerevisiae by co-expression of Glycyrrhiza uralensis squalene synthase 1 and β-amyrin synthase genes. Acta Pharm. Sin. 2014;49:734–741. [PubMed] [Google Scholar]
- 29.Yin H, et al. Heterologous biosynthesis of costunolide in Escherichia coli and yield improvement. Biotechnol. Lett. 2015;37:1249–1255. doi: 10.1007/s10529-015-1784-6. [DOI] [PubMed] [Google Scholar]
- 30.Ro DK, et al. Induction of multiple pleiotropic drug resistance genes in yeast engineered to produce an increased level of anti-malarial drug precursor, artemisinic acid. BMC Biotechnol. 2008;8:83. doi: 10.1186/1472-6750-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data analyzed during this study have been included in this article.