Abstract
Null models for the effect of combination therapies are widely used to evaluate synergy and antagonism of drugs. Due to the relevance of null models, their suitability is continuously discussed. Here, we contribute to the discussion by investigating the properties of five null models. Our study includes the model proposed by David J. Hand, which we refer to as Hand model. The Hand model has been introduced almost 20 years ago but hardly was used and studied. We show that the Hand model generalizes the principle of dose equivalence compared to the Loewe model and resolves the ambiguity of the Tallarida model. This provides a solution to the persisting conflict about the compatibility of two essential model properties: the sham combination principle and the principle of dose equivalence. By embedding several null models into a common framework, we shed light in their biochemical validity and provide indications that the Hand model is biochemically most plausible. We illustrate the practical implications and differences between null models by examining differences of null models on published data.
Introduction
Combination drug therapy is an advancing field of research in oncology, anaesthesiology and immunology1–6. Treatments with multiple drugs are studied to distribute side effects and minimize toxicity while attaining the full efficacy7. The discovery of synergistic combinations can enhance the development of multi-drug therapies and was ranked second place in the principles of combination therapy after decreased toxicity without decreased efficacy8. The term combination refers to the choice of pairing agents A and B, to their specific concentration ratio as well as to their explicit doses. In the following we will use the terms drug and agent interchangeable.
Synergy is detected and quantified via the comparison of an experimentally obtained effect and the mathematical reference effect of a null model. If the measured effect to a combination therapy exceeds the reference effect based on the measured effects of the individual drugs, the dose pair is considered synergistic, otherwise antagonistic. In order to quantitatively assess the level of synergy, concepts such as combination indices9 or tools of statistical analysis10,11 were introduced. A careful choice of the null model used to study synergy is important to not over-interpret results of drug combination studies12. However, this choice requires a detailed understanding of the null models and the differences between them.
Applied in combination, the single effects of an A-dose and a B-dose are expected to add up in a way the null model specifies. Although there exist cases in which the simple algebraic sum of the effects may serve as a null model13, in general this sum will exceed the maximal possible effect, e.g., when the effect is measured in percentage of bound agents. A variety of null models has been introduced in the past and each model tries to provide a plausible concept of additivity that can be biochemically justified. Conceptually, effect-based strategies and dose-effect based strategies are distinguished14. In the established Bliss model15, normalized effects are interpreted as probabilities of stochastically independent events, e.g., the event of an analgesic binding to a receptor. This effect-based strategy permits an additivity concept according to the addition rule of probabilities. Two agents which act on different components in a biochemical cascade can be modelled in this mutually non-exclusive way (e.g., the HIV-1 entry inhibitors CoRA and FI5). However, the implicit assumption of stochastic independence does not hold in case of drugs that act through a similar mechanism. For example, cetuximab and afatinib both inhibit EGFR in the pathway of non-small cell lung cancer proliferation16,17. In particular, a sham combination, i.e., a combination of an agent with itself, cannot be described by the Bliss model18.
Other null models incorporate knowledge on the functional relationship between dose concentration and effect for each single agent over a large range of doses. Such dose-effect based strategies shift the task of designing a null model towards specifying a plausible theory of addition of functions instead of a few numeric values19. This functional relationship between dose concentration and effect for the mono-therapies are frequently modelled as Hill curves, which can be fitted to experimental data by standard software20. Here, we assume, that the dose-effect curves are increasing and have their maximal numerical value at the saturation level. However, minor changes allow an analogous treatment of decreasing dose-effect curves.
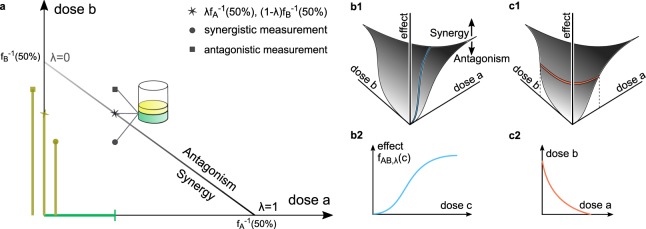
The classical dose-effect based model for mutually exclusive agents was first introduced by Loewe21,22 and popularized by Berenbaum23, Chou and Talalay9. It states that doses of drug A and drug B may be linearly converted into each other inducing no modification in effect, where the conversion rate generally depends on the current effect level. For example, if both drugs attain the same maximal effect, then one third of the half-max concentration of A, i.e., the concentration at which the half-max effect is reached, combined with two thirds of the half-max concentration of B is predicted to yield an observed effect of 50%. The set of all dose combinations in the ([a], [b])-plane, that are predicted to induce the same effect level is called an isobole, where [a] and [b] are the doses of A and B, respectively. Being an indicator of zero interaction, the isobole separates the plane into a region of synergy of points located below the isobole and a region of antagonism located above it. For the Loewe model isoboles are straight lines, whose slopes are the conversion rates as depicted by the isobologram in Fig. 1a.
Figure 1.
Loewe isobologram and the general effect surface. (a) Straight isobole at effect level 50% in the Loewe model. If the dose pairs located at star, circle, box induced 50%, the Loewe model would rate them non-interacting, synergistic and antagonistic, respectively. (b,c) visualize a general effect surface with informative cuts. (b1) Measured effects above and below the predicted effect E indicate synergy and antagonism, respectively. Vertical cuts through the effect surface along rays correspond to (b2) dose-effect curves of combined agents. (c1) Horizontal cuts through the effect surface provide (c2) isoboles.
The Loewe model is broadly accepted and used in cases of constant potency ratio. The potency ratio is the ratio of the equipotent doses of two drugs, and two dose-effect curves are said to have a constant potency ratio if they are identical up to rescaling the dose axis. This means that they are parallel in the representation with a logarithmic dose scale. We will refer to this central but rare case as the constant potency ratio case or the case of parallel dose-effect curves. If the dose-effect-relations are given by Hill curves, two curves exhibit a constant potency ratio, exactly if they coincide in their maximal effects and Hill coefficients. In the prominent cases of a full and a partial agent or different cooperativities, i.e., maximal effect or the Hill coefficient differ, the potency ratio varies across effect levels.
The Loewe model is accepted for parallel dose-effect curves because this case implies that the conversion rate does not depend on the effect level, i.e., the isoboles for all effect levels in the Loewe model are parallel. Two principles were extracted from the constant potency ratio case that seem to permit a transfer to the varying potency ratio case: The principle of dose equivalence24 and the principle of sham combinations25. The former states that each A-dose can be assigned a B-equivalent, which induces the same effect. The latter states that, a drug is not expected to synergise in a combination with itself. The sham combination principle is rather specific to mutually exclusive agents and violated by several null models, including the Highest Single Agent (HSA) model, the Bliss model and the Fisher’s dosage orthogonality26.
The attempt to base a null model on coupling both principles for curves of varying potency ratio24,27,28 fails to identify unique isoboles, as objected by Lorenzo29. The failure of this null model, which we call the Tallarida model in the following, has incorrectly been attributed to Loewe as the section indeterminate Loewe additivity solution in Geary19 indicates. In fact, the Loewe solution cannot be indeterminate, because the model, by definition, postulates a unique linear isobole. To the contrary, the Loewe additivity equation can very well be combined with existing theory on dose-effects30,31, causing no mathematical contradiction. The indetermination in the varying potency ratio case results of ambiguously combining dose equivalence and sham combination principle in the way Grabovsky and Tallarida suggest24.
While the Loewe model does not lack uniqueness, it is nonetheless justified to doubt its validity in several cases. Among the critics of its validity in the rather generic case of varying potency ratio one finds Loewe himself22. In particular, scepticism in the case of differing maximal effects when combining a full and a partial agent has led to an increasing amount of competitive models18,32–34. Experimentalists emphasize that curved rather than straight isoboles are expected19,35 if dose-effect curves are not parallel, which is confirmed by examples of mechanistic models36. To account for these deficits, Hand introduced an alternative and more general non-interaction model37, which has unfortunately been overlooked in the reception of synergy detection models. Hand suggests to construct dose-effect curves for combined agents via an ordinary differential equation (ODE) in a way that both agents contribute linearly to the instantaneous gain in effect.
The conflict about the compatibility of sham combination principle and dose equivalence has been persisting for long. In this manuscript, we show how the Hand model can be obtained as a unique limit model of the Tallarida model. In doing so, we add an argument to dissolving this conflict. We introduce effect-sensitivity curves as a visualization tool, that provides the best insight into the Hand model’s concept of additivity. This does not replace the dose-effect-visualization, but adds an alternative view, which confirms the biochemical plausibility of the Hand model in terms of the dynamical change in effect. We explore qualitative features of the Hand model that are not discussed in the original publication37. Most strikingly, its isoboles are always convex, which reveals that the Hand model systematically provides a more conservative prediction than the Loewe model. Furthermore, we demonstrate how the models by Loewe, Hand and Tallarida can be interpreted as contrasting strategies for solving to some extent an unsolvable partial differential equation characterizing the effect-surface. Finally, we compare the reference effects of different null models for a set of published dose effect data.
Mathematical Background
Dose-effect curves and drug combinations
The dose-effect curves of the mono-therapies with drugs A and B are denoted by fA and fB. Both curves are assumed to be strictly increasing and their inverses are denoted by and . In practice, Hill functions are widely used20, i.e., dose-effect curve
| 1 |
with inverse
| 2 |
for drug A with dose a, effect level x, minimal effect Emin,A, maximal effect Emax,A, the half-max dose EC50,A and Hill coefficient nA. The minimal effect Emin,A describes the untreated condition and is identical for different drugs, Emin,A = Emin,B. The maximal effects Emax,A and Emax,B can differ and the drug with the greater maximal effect is called the full agent, whereas the other is labelled as partial agent. In general Emin,A is normalized to zero, and the larger of Emax,A and Emax,B is normalized to one.
The potency ratio α(x) at effect level x quantifies how much more potent A is compared to B in generating effect x, i.e.,
If the potency ratio is constant, both curves are identical up to rescaling the dose axis, or equivalently both curves are parallel, when plotted on a logarithmic dose axis.
We denote a combination drug of A and B at fixed ratio λ ∈ [0, 1] by Cλ, i.e., one unit [c] of the agent Cλ is composed as [c] = λ[a] + (1 − λ)[b]. Accordingly, the treatment with an amount c of the combination drug Cλ corresponds to a treatment with a = λc of drug A together with b = (1 − λ)c of drug B.
Favourable properties of a null model
Given the dose-effect curves fA and fB of the single agents, a null model predicts the effect as a function E:[0, ∞) × [0, ∞) → [0, ∞),
Two cuts through the two-dimensional effect-surface {(a, b, E(a, b))|a, b ≥ 0} visualize the combined effect18: Dose-effect curves of combination drugs Cλ vertically cut the surface along a ray directed according to the fixed ratio λ, i.e., the combined curve follows
where we sometimes oppress the index λ for readability (Fig. 1b). Isoboles are horizontal cuts at level x, i.e., level sets of E, projected onto the ([a], [b])− plane (Fig. 1c).
A null model for drug combinations should ideally possess at least two properties to be biochemically plausible:
-
The combination of a drug with itself should neither result in synergy nor antagonism, meaning that it does not interact with itself and meets the sham combination principle. In the above notation E satisfies the sham combination principle if for all λ ∈ [0, 1]
Geometrically the isoboles of the effect surface are then straight lines with unit slope.
-
The swap of drug A and B should not change the effective results. Mathematically, this implies that E is commutative in A and B, i.e., for all λ ∈ [0, 1]
In addition, a model may satisfy an associative property, which allows to combine combination drugs:
The combination of agents Cλ and Cμ at ratio ν should be equivalent to directly combining A and B at a suitable ratio. For [cλ] = λ[a] + (1 − λ)[b] and [cμ] = μ[a] + (1 − μ)[b], the resulting combination is ν[cλ] + (1 − ν)[cμ] = (νλ + (1 − ν)μ)[a] + (ν(1 − λ) + (1 − ν)(1 − μ))[b]. E satisfies the associative principle if for any λ, μ, ν ∈ [0, 1]
The associativity principle is stronger than commutativity, because the latter is contained in the associativity property by the particular choice λ = 0, μ = 1.
Geometrically, associativity means: Cλ and Cμ define new coordinate axes in the ([a], [b])-plane with angle smaller than 90°. The model prediction along these curves are taken to be the new mono-therapies while the rest of the effect surface is ignored. Bending these axes to an angle of 90° and applying the model to this new coordinate system should then span the same effect surface as bending the null model originally obtained from A and B. The associative property guarantees that the model is in this sense compatible with a change of the coordinate axes.
Dose-effect based null models
In this section, we introduce the null models proposed by Loewe, Tallarida and Hand. These null models are based on the dose-effect strategy and rely on the knowledge of the complete dose-effect curve.
The Loewe model
The classical approach for mutually exclusive drug combinations is the Loewe model22,23. As its core, the model postulates that the isoboles are straight21. The isobole at effect level x can thus be characterized as the set of all combinations (a, b) satisfying the Loewe additivity equation
| 3 |
The effect EL(a, b) predicted by the Loewe model for an arbitrary combination (a, b) can be obtained by (numerically) solving (3) for x, i.e., EL(a, b) is the unique effect, that solves
A generic point on the isobole has the form (Fig. 1a). EL is commutative and satisfies the sham combination principle. However, it is only defined for effects x, which are attained by both fA and fB. Hence, it is not applicable for effect sizes x ≥ min{Emax,A, Emax,B}.
The potency ratio α(x), which is geometrically the slope of the isobole, precisely expresses the conversion rate of equivalent doses in the Loewe model. If A and B exhibit a constant potency ratio, then all isoboles in the Loewe model are parallel. Tallarida27, Geary19 and others argue that this property is necessary to ensure the validity of the Loewe model. Accordingly, the probable case of a varying potency ratio would prevent the use of the Loewe model.
The Tallarida model
In an attempt to transfer the principles of dose equivalence and sham combination to the case of varying potency ratio, Grabovsky and Tallarida suggested to convert a dose a into an equivalent B-dose generating the same effect fA(a) (Fig. 2a). Adding this B-equivalent to b should predict the effect via fB24, i.e.,
| 4 |
Figure 2.
The effect decomposition in the Tallarida model forms the basis for the infinitesimal approach in the Hand model. (a) Dose a of A individually achieves an effect xA = fA(a) on its lower range of doses, while dose b of B acts on its higher dose range, contributing on top of the effect achieved by A. The contribution of drug B to the effect is . (b) The doses a and b are split into N small pieces da and db, respectively. The effect level x0 is elevated by da to x1, db raises the effect from x1 to x2. (c) The combined dose-effect curve for the mixture Cλ is constructed by applying N pieces of dc = da + db. After twice applying dc, the effect level x0 is attained. Applying a third mixture of da and db successively increases the effect to x1 and finally x2.
Since the model was further popularized by Tallarida27,28, we refer to it as the Tallarida model. Conceptually, the Tallarida model decomposes the total effect x = ET,A→B(a,b) = xA + xB as in the following way: dose a of A individually achieves an effect xA = fA(a) on its lower range of doses, while dose b of B acts on its higher dose range, contributing on top of the effect achieved by A, i.e.,
| 5 |
Note that the model with altered roles of A and B is
The models ET,A→B and ET,B→A might predict a different effect because in general
Accordingly, while the Tallarida model meets the sham combination principle, it does not fulfil the commutation property. The two model predictions ET,A→B(a, b) and ET,B→A(a, b) encompass a range of effect values. In the following, we will refer to the TallaridaLB model and the TallaridaUB model,
that give a lower and an upper bound to this range.
The Hand model
It remains the problem that the Tallarida model, introduced in 2004 by Grabovsky and Tallarida, is not consistent with changing the roles of A and B. In 2000, Hand already anticipates the ambiguity in what we call the Tallarida model. He identifies as a solution to this problem that instead of adding the effect of b as an absolute quantity to the effect level reached by a in equation (4) one rather needs to account for the simultaneous nature of drug action37. This hints at formulating additivity in terms of instantaneous effect gains rather than effect levels. Hand proposes to construct E by specifying the rate at which the effect is changing. Since the rate is a one dimensional quantity, it is advantageous to assign values to the entire plane of dose pairs by going along rays at fixed ratio λ. More precisely he suggested that the combined curve fAB,λ must satisfy the differential equation
| 6 |
This characterizing equation states that at each effect level both agents contribute linearly to the instantaneous gain in effect of the combined curve. For an arbitrary dose pair (a, b), identify the ratio λ ∈ [0, 1], such that
| 7 |
then (a, b) = (λc, (1 − λ)c) is a dose c of the drug mixture Cλ. For fAB solving (6) with λ satisfying (7), we assign
Using the inverse derivative formula, we express the differential equation (6) as
| 8 |
or in integral representation
| 9 |
The Hand model was constructed to satisfy the sham combination principle and clearly it is commutative in A and B. Hand stresses that if an agent saturates at a lower level than the other, its derivative is set to zero above that level. This is a natural interpretation of (6) given that x is not in the range of either fA or fB. The Hand model thus comprises the case of a full and a partial agent. Moreover, Hand mentions that the isoboles will be straight in the constant potency ratio case. Coinciding with the Loewe model for this sub-case, it is regarded by Hand as a generalization of the Loewe model. We will demonstrate, by embedding three of the models into a common framework, that the Loewe model is justified also for cases of varying potency ratio and thus the term generalization should be used with care.
Effect-based null models
Alternatives to the dose-effect based null models are effect-based models. These null models require only the knowledge of the effect of the single doses a and b to provide a prediction of the combined effect E(a, b). Commonly used effect-based models are the Bliss model and the HSA model. The Bliss model15 is based on a probabilistic framework, and defines the effect of a drug combination as
For this definition it is necessary, that the greater of the maximum values of the two dose-effect curves, Emax,A and Emax,B, are normalized to 1. The HSA model, often referred to as Gaddum’s non-interaction25, is defined as
The Bliss and the HSA model do not fulfil the sham and the associative principle. For a detailed comparison of the properties of all null models we refer to the corresponding section and Table 1.
Table 1.
Properties of null models.
| Loewe | Tallarida | Hand | Bliss | HSA | |
|---|---|---|---|---|---|
| uniqueness | ✓ | — | ✓ | ✓ | ✓ |
| commutativity in A and B | ✓ | — | ✓ | ✓ | ✓ |
| sham combination principle | ✓ | ✓ | ✓ | — | — |
| associative property | ✓ | — | ✓ | — | — |
| defined for full and partial agent | — | ✓ | ✓ | ✓ | ✓ |
Results
Mathematical analysis of the Hand model
In this study, we consider five previously published null models. Amongst them, the Hand model is the least well known and studied one. To facilitate an intuitive understanding, we will provide a unified derivation of the Tallarida and the Hand model; and we will introduce the novel concept of effect-sensitivity curves which confirm the additivity principle. Subsequently, we will study the isoboles of the Hand model.
Unified derivation of the Tallarida and the Hand model
In the literature, the Tallarida and the Hand model are presented and discussed separately24,37. Here, we establish a link and improve the understanding of the Tallarida and the Hand model by providing a unified derivation. By this derivation we extended the original paper’s frank justification on addition of rates.
We start from the concept of Tallarida on equivalent doses (5). However, instead of applying a entirely and then b entirely, we apply portions of both, constantly alternating (Fig. 2b,c). Formally, we split both doses a and b into N pieces and , respectively. In particular it is
with λ satisfying (7). We define dc := da + db as a small portion of the combination drug Cλ. By alternately applying portions da and db, we keep track of how the effect changes. Suppose, we have constructed the dose-effect curve fAB,λ of Cλ up to an effect level x0, induced by a mixture dose
| 10 |
Applying da elevates the effect level to and db subsequently amplifies the effect level to . By the above procedure we obtain
For N = 1, this procedure yields the Tallarida model (Fig. 2a). To study N → ∞ we take the first-order Taylor approximation, divide by dc and note that da = λ dc, db = (1 − λ)dc. This yields (Supplementary Information S1.1)
In the limit case dc → 0, i.e., an infinite amount of infinitesimal pieces dc, the difference quotient tends to the derivative on the left side in (6) and we obtain the Hand model. Note that the roles of A and B in the derivation may be switched, approaching the same differential equation in the limit. This yields a unique limit model even though each approximative model is ambiguous.
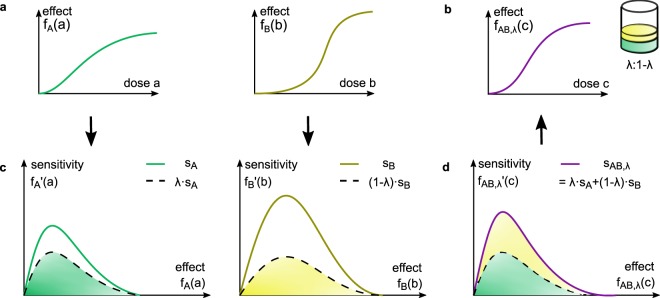
Effect-sensitivity curves and an additivity principle for the Hand model
In the Hand model (6) the instantaneous gain in effect depends on the individual dose-effect curves fA(a) and fB(b) via the functions and , respectively. We term the derivative , which is measured in effect per dose, its sensitivity. As the functions sA(x) and sB(x) describe the sensitivity of the dose-effect relation subject to the effect level, we term (x, sA(x)) and (x, sB(x)) effect-sensitivity curves. The dose-effect curve fA is then the solution to
The sensitivity is precisely the right-hand side of the autonomous ODE which guides the dynamics of the dose-effect curve, evolving over the dose range instead of time. For Hill curves as in (1) the effect-sensitivity curve is explicitly given by
for x ∈ [Emin,A, Emax,A] (Supplementary Information S2.1). Under weak conditions, it is possible to retransform effect-sensitivity into dose-effect curves, rendering them an equivalent notion (Supplementary Information S2.2). The Hand model specifies the following additivity concept
| 11 |
where the quantities, which are added, are the sensitivities; i.e. the effect-sensitivity curve of the combined agent is a weighted average of the single effect-sensitivity curves with weights according to the mixture ratio (Fig. 3). More suggestively, we say that the Hand model summates the dynamics of the single dose-effect curves.
Figure 3.
Concept of additivity according to the Hand model. (a) The Hand model specifies how to add the two dose-effect curves of drugs A and B to get the combined dose-effect curve at fixed dose ratio λ (b). (c) fA and fB are transformed to effect-sensitivity curves via , reflecting the saturation dynamics. (d) The effect-sensitivity curve sAB,λ of the mixture Cλ is their weighted algebraic sum. fAB,λ is obtained from sAB,λ by integration.
Geometric properties of the Hand model’s isoboles
The isoboles of the null model are the basis for assessing synergy and antagonism of drugs. While the Loewe model’s isoboles are easily graphically obtained and analytically given in closed form as soon as and are known, the Hand model’s isoboles are non-trivial and might often have to be computed numerically. However, we could show that the Hand isoboles share a characteristic geometric feature: they are always convex (Supplementary Information S4). As a consequence, they systematically diminish the region of synergy compared to the Loewe model.
Combination drugs for partial and full agent
In practice the maximal effect of drug A might be smaller than the maximal effect of drug B, meaning that A acts as the partial agent and B as the full agent. Since the construction in the classical Loewe model requires effect levels that are attained by both agents22, this case has motivated to a large extent the search for alternative models18,32–34. In the subsequent discussion of the Loewe and the Hand model we assume Emax,A < Emax,B.
Loewe model for partial and full agent
The Loewe model assigns effect values to all dose pairs (a, b) with (Fig. 4a). In fact, the slope of the isoboles approaches 0 as the effect level approaches Emax,A. This phenomenon arises because the Hill curve fA saturates asymptotically. Hence the strip in the ([a], [b])-plane that is bounded by the horizontal line is covered entirely by Loewe isoboles (Supplementary Information S5). The horizontal limit isobole coincides with the HSA model’s isobole at level Emax,A, which suggests to extend the Loewe model by the HSA model.
Figure 4.
Model predictions for combination drugs of a partial agent A and full agent B. The isobolograms for the (a) Loewe model and (b) Hand model. (a) The Loewe model assigns reference effects only to dose pairs below the horizontal limit isobole at effect value Emax,A, beyond which it can be extended via the HSA model. (b) Above the partial agent A’s maximal effect value its sensitivity is set to 0. As soon as the combined curve passes the curved limit isobole (a*, b*) at effect level Emax,A it is driven by the full agent alone.
Hand model for partial and full agent
As shown in the original publication37, the Hand model comprises the case of a partial agent A and a full agent B. In terms of sensitivities, the effect-sensitivity curve sA is extended by constant zero on the effect domain [Emax,A,Emax,B) (Fig. 4b), i.e. the sensitivity of the combined curve is
If c*(λ) denotes the dose of mixture Cλ at which Emax,A is attained, then
for c ≤ c*(λ), the ODE guiding the evolution of fAB,λ is (6).
For c > c*(λ), the evolution simplifies to the initial value problem
The solution for c > c*(λ) is , , hence,
for all . Accordingly, for effect sizes greater than Emax,A only B has an effect and the dose-effect curve for Cλ in this regime corresponds to the scaled and shifted dose-effect curve fB. In contrast to the Tallarida model, the effect surface does not copy the dose-effect curve fB in the direction parallel to the B-axis.
Using the integral representation, c*(λ) is explicitly given by
Decomposing the combined dose c*(λ) into its contributions a*(λ) = λc*(λ) and b*(λ) = (1 − λ)c*(λ), the limit isobole at effect level Emax,A is parametrized by . Its B-component
is a decreasing continuous function with limλ→1b*(λ) = 0.
Let us contrast this finding with the Loewe model. Assuming Hill curves, which saturate asymptotically, as dose-effect-relations for both models, the Loewe model ignores the amount of A at the critical effect size Emax,A, while in the Hand model a higher amount of A, and thus a larger λ, reduces the amount of b*(λ) necessary to generate the effect Emax,A.
Common framework for the Loewe, the Tallarida and the Hand model
The dose-effect based null models for mutually exclusive interaction presented above pursue different approaches in constructing the effect surface E(a, b). While the Loewe model imposes a condition on the isoboles’ shape, the Tallarida model elevates the dose-effect curves to the respective initiating level of the other curve. Consequently it constructs the effect surface E(a, b) by copying and attaching the existing curves of the single agents. The Hand model specifies the evolution of effect surface E(a,b) for increasing amount of mixture dose at fixed ratio, hence constructs the surface along rays. While the models seem rather different, we could derive a novel common framework for all three, based on the concept of effect-sensitivity curves introduced in the above section.
Assuming an underlying mechanistic dynamical model for the effect of the individual drugs, it is plausible to request that the change in effect should only depend on the current effect level. If nothing but the knowledge about the single dose-effect curves is available, it is plausible that the single dynamics predict the dynamics of the combined effect. More precisely, we expect the sensitivities of fA and fB to translate changes of A- and B-doses respectively into changes in effect, i.e.,
| 12 |
For given dose-effect curves fA and fB, and the derived effect-sensitivity curves sA and sB, such a function E does in general not exist. However, if we request the equations to hold to some extent, we recover the above null models.
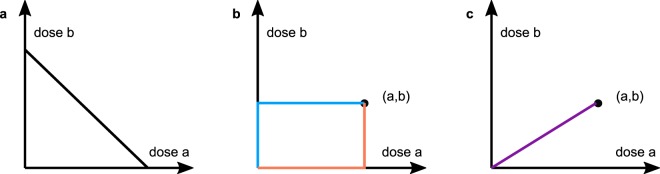
The Loewe model is recovered, if we request (12) to hold along the isoboles (Fig. 5a): Let γ = (γA, γB):[0, 1] → [0, ∞) × [0, ∞) parametrize the isobole at effect level x, then
Figure 5.
Paths for the three null models. The three null models solve equation (12) to some extent along different paths. (a) The Loewe model solves (12) along isoboles. This requirement forces the isoboles to be linear. (b) The Tallarida model solves (12) along rectangular paths. (c) The Hand model solves (12) along rays.
Using E(γ(t)) ≡ x, we obtain
| 13 |
The last equality holds because . The calculation (13) shows that the ratio does in fact not depend on t, but is constant for all t. Consequently, γ parametrizes a straight line with the slope given precisely by the potency ratio α(x). It is important to notice, that we did not request the isoboles to be straight lines in the first place, however, satisfying (12) forces them to be linear.
The Tallarida model ET,A→B is recovered by following a rectangular path from (0, 0) to (a, b) (Fig. 5b).
where ⊕ concatenates the paths, i.e. for t ∈ [0, 2]
Then along γ1 equation (12) integrates to ET,A→B(γ1(t)) = fA(ta) and along γ2 equation (12) with initial condition ET,A→B(γ2(0)) = ET,A→B(γ1(1)) = fA(a) integrates to . Analogously, the model ET,B→A is obtained by the rectangular path
The Hand model is recovered if we request (12) to hold along combined curves γ(c) = (λc, (1 − λ)c) (Fig. 5c). In this case we compute by the chain rule
and set .
In the constant potency ratio case, (12) admits a solution and all three models coincide. Indeed, for the solution E is given by
The representation of the Loewe, the Tallarida and the Hand model as alternative paths through an underlying null model (12) provides a novel perspective. It highlights the relation of the existing dose-effect based null model and the importance of the effect-sensitivity curves. Furthermore, this common framework might facilitate the basis for the development of alternative models with novel properties.
Comparison of the mathematical properties of the null models
The null models considered in this study possess different (combinations of) properties. In this section, we collect the available information, present results on the associative principle and outline the implications of our findings. In addition, we study the reference effect values calculated by different null models and prove rigorous orderings.
Properties of the Loewe, the Tallarida and the Hand models
Given a general framework for all three null models, we studied their properties in further detail. A summary is provided in Table 1. As outlined before,
the Loewe, the Tallarida and the Hand model meet the sham combination principle (Supplementary Information S3.2), which is not the case for the Bliss and the HSA model (Supplementary Information S3.3); and
the Loewe and the Hand model meet the commutation principle (Supplementary Information S3.4). Indeed, we could also prove here that
the Loewe and the Hand model fulfil the strong property of associativity (Supplementary Information S3.6).
For drugs with a constant potency ratio, the models by Loewe, Hand and Tallarida coincide (Supplementary Information S3.1). Furthermore, the Tallarida and the Hand model are applicable to a combination of full and partial agents.
Our analysis reveals that the Hand model fulfils all the previously defined favourable properties (Supplementary Information S3 and original publication37), while all other models only fulfil some of them. As the favourable properties are biochemically motivated, this implies that the Hand model is biochemically most plausible among the considered models.
Systematic inequalities
The assessment of drug synergy or antagonism for a single dose of a combination drug is equivalent to the question: On which side of the isobole corresponding to the observed effect does the measured dose pair lie? Accordingly, the definition of drug synergy or antagonism depends directly on the isoboles, which potentially differ substantially among the null models. For the example shown in Fig. 6, the HSA, the Bliss, and the TallaridaLB model classify a dose pair to be synergistic, while the Loewe, the TallaridaUB, and the Hand model classify it as antagonistic. Note that a null model which tends to predict higher effects compared to measured values correspondingly yields an isobole closer to the origin, and less dose pairs are classified as synergistic.
Figure 6.

Assessment of synergy and antagonism using different null models. Dose-effect curves and isobologram at EC50 for different null models. A measured effect of 0.5 for a given dose pair (★) is considered as synergistic by the Bliss, the HSA and the TallaridaLB model, while it is antagonistic according to the Loewe, the Hand and the TallaridaUB model.
Mathematically, an important implication of the Hand model’s convex isoboles is that its reference effect bounds the one provided by the Loewe model from above. For a specific dose pair (a, b), the Loewe model provides a reference effect x = EL(a, b). If the dose pair is expressed in terms of a quantity c of the mixture drug with ratio , i.e., (a, b) = (λc, (1 − λ)c), then the convexity of the Hand model’s x-isobole implies that for with some . As the combined curve fλ,H(c) is an increasing function in c, we conclude
In addition, (i) TallaridaLB is by definition lower than TallaridaUB and (ii) the HSA model predicts systematically lower effects than all other null models, for instance
In summary, the following systematic inequalities hold for the reference effect values obtained by the different null models for any combination drug Cλ and combination drug concentration c:
HAS < Loewe < Hand
HSA < Bliss
HSA < TallaridaLB < TallaridaUB
Comparison of null models using experimental data
To assess the characteristic features of the different null models in practice, we considered the drug screening data collected by O’Neil et al.38. The dataset reports the effects of 39 cancer cell lines to 38 drug individual drugs and 583 drug combinations of these drugs. Each combination was measured on a 4 by 4 dosing regime. The proliferation compared to the untreated condition is taken as the readout of the effect.
For the given data, we compared the reference effects of the Loewe, the Tallarida and the Hand model, as well as the Bliss model and the HSA model. As the Tallarida model does not provide a unique solution (see the section on the Tallarida model), we evaluated both cases. The Tallarida models predicting lower and higher effects for a particular drug combination are as before denoted as the TallaridaLB and the TallaridaUB model, respectively. For the Loewe, the Tallarida and the Hand model, Hill curves and a zero-effect curves were fitted to the measurement data for the single drug treatments. The Bayesian Information Criterion (BIC)39 was used to select the more suited model (Supplementary Information S6).
For a comparison of the null models, we considered the predictions of all tested dose pairs of all tested drug combinations. For the cell line A375, the predictions are visualized in Fig. 7. Besides the systematic inequalities which have been proven in the corresponding section, we found:
Reference effects for different null models were highly correlated.
The TallaridaLB model tended to bound the references of all other models besides HSA from below, while the TallaridaUB model tended to bound the references of all other models besides the Hand model from above.
The TallaridaLB and the TallaridaUB are potentially quite different. This can lead to problems if the researcher is not aware of the ambiguity of the Tallarida model. The analysis then gives a random choice between the lower and upper prediction and no consistent and meaningful result will be obtained.
The Hand model provided relatively high reference effects, as suggested by the theory (see the section on the systematic inequalities), and lied close to the TallaridaUB model.
The Bliss model neither systematically provided lower nor higher reference effects than the Loewe, the TallaridaUB and the Hand model (Fig. 7).
Figure 7.
Distributions of reference effects. Below the diagonal a scatter plot of the null model reference effects for all drug doses tested for cell line A375 is shown. On the diagonal the distribution of the reference effects are shown. Above the diagonal a histogram of the differences of the references are given. Zero is marked by a dashed line.
Discussion
The Loewe model has persisted for long as the first choice to model additive behaviour and its validity is in general accepted in the case of constant potency ratio. Scepticism about the applicability in the case of varying potency ratio case has put forth a large variety of null models. For a full and partial agent the Loewe model is in fact incapable of providing a reference effect in a certain dose range, because the reference relies on a shared effect level of both agents. If both drugs reach the same maximal effect, a Loewe assignment is possible, even if the shapes of the saturation curves are different. This case required further arguments why to doubt the Loewe model’s validity. In this manuscript, we showed that the scepticism can be perpetuated only to some extent, see below.
It has been observed that the principle of dose equivalence and the sham combination principle very well characterize the Loewe model in the constant potency ratio case19,23,24. They seem promising if one attempts to modify the Loewe reference. The coupling of both principles has served as sufficient condition23, necessary condition14 and proof of non-validity19,24 to the Loewe model all alike. Accordingly, Berenbaum’s derivation23 of the Loewe equation ignored the shapes of dose-effect curves while assuming both principles and Foucquier14 presents them as a necessity, on which the Loewe additivity rests. We argued that the coupling is not meaningful unless the two agents have a constant potency ratio because only then the two principles are compatible at all. The approach suggested by Tallarida converts doses of different drugs by a rule of three, choosing as intermediate linking quantity an absolute effect level. Two doses which attain this effect level are considered equivalent. This notion of equivalence fails as soon as it is used for adding doses. Since one dose is applied on top of the other, it must thus be interpreted as change in dose of the former. This asymmetry in the roles as initial dose and change in dose leads to the observed non-commutativity. If one tries to resolve the ambiguity by choosing as intermediate linking quantity a change in effect rather than an effect level, one merely shifts the ambiguity. A change in dose cannot be assigned a change in effect uniquely, unless the dose-effect follow a linear relation which is rarely the case.
The above ambiguities have been addressed multiple times in the literature, but Hand’s substantial suggestion of a model that resolves them has been mostly ignored up to now. The Hand model interprets the change in effect in an instantaneous sense and allows the instantaneous change to depend on the current effect level, when constructing the combined curve. This dependency of the instantaneous change on the current quantity level is the common motive of mechanistic approaches and is appreciated in biochemical modelling because its specification is of a local instead of a global nature. The local nature of the Hand model’s additivity concept makes it biochemically plausible even though its black box approach does not incorporate knowledge on explicit molecular reactions. We showed how these instantaneous changes are best visualized when plotted subject to the effect. The resulting effect-sensitivity curves are added in the Hand model. Here, it is crucial to choose the effect as x-axis. The mechanism of the Hand model can be applied in any case in which two increasing curves must be added. Instead of summating them as functions, their dynamics are added. This not only makes the black box ansatz biochemically plausible but contributes to its versatile usage. If two dose-effect-curves exhibit a piecewise positive derivative and share a zero level, this model is applicable. For instance, this holds in the case, in which ideal data points are linearly interpolated. The extension of the Hand model to multi-drug combinations with more than two components is straightforward, as is the case with the Loewe model.
What model should be preferred? Among the five analysed models, the Loewe, Hand and Tallarida model all satisfy the sham combination principle, which is for instance not the case in Fisher’s dosage orthogonality or the Bliss model. The Tallarida model can be used if an asymmetry is justified, e.g., by a temporal delay. The Tallarida model can also be used to identify not only a curve but an area of zero-interaction. We have demonstrated by the common framework for all three models, that there is actually no reason to dismiss Loewe in the varying potency ratio case. The condition, which is provided in form of a partial differential equation and which is biochemically plausible is just as well satisfied to a limited extent by the Loewe model as it is by the Hand model. We suggest to rehabilitate the Loewe model for the case of shared maximal effect, but differing Hill coefficient. However, note that the Hand model can predict an effect for the combination of a full and a partial agent without restrictions, whereas the Loewe model fails to do so for a range of dose pairs. In the model comparison on an experimental dataset, we circumvented this handicap by extending the Loewe model via the Highest Single Agent model. Moreover, by proving the convexity of the Hand model’s isoboles we demonstrated that the Hand model guarantees a more conservative prediction of zero-interaction compared to the Loewe model.
In summary, the interest in combination drug therapy is steadily increasing and an assessment requires suitable null models. Here, we have compared several established models and have shown that the Hand model is of most versatile use, for its unique assignment, mathematical plausibility, simplicity, conservative prediction and biochemical interpretability.
Supplementary information
Author Contributions
M.S., C.L. and J.H. conceived the project. M.S. carried out the mathematical analysis of the null models. J.V. implemented the null models and analysed the differences for the considered dataset. All authors contributed to the preparation of the article. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-38907-x.
References
- 1.Mao J, Gold MS, Backonja MM. Combination drug therapy for chronic pain: A call for more clinical studies. The J. Pain. 2011;12:157–166. doi: 10.1016/j.jpain.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahl JB, et al. Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: A topical review. Acta Anaesthesiol. Scand. 2014;58:1165–1181. doi: 10.1111/aas.12382. [DOI] [PubMed] [Google Scholar]
- 3.Bansal M, et al. A community computational challenge to predict the activity of pairs of compounds. Nat. Biotech. 2014;32:1213–1221. doi: 10.1038/nbt.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Q, Sun W, Wang C, Gu Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016;98:19–34. doi: 10.1016/j.addr.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn KW, Root MJ. Complex interplay of kinetic factors governs the synergistic properties of HIV-1 entry inhibitors. The J. biological chemistry. 2017;292:16498–16510. doi: 10.1074/jbc.M117.791731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eser PÖ, Jänne PA. TGF pathway inhibition in the treatment of non-small cell lung cancer. Pharmacol. & Ther. 2018;184:112–130. doi: 10.1016/j.pharmthera.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Maguire, D. R. & France, C. P. Antinociceptive effects of mixtures of mu opioid receptor agonists and cannabinoid receptor agonists in rats: Impact of drug and fixed-dose ratio. Eur. J. Pharmacol. 819, 217–224, http://www.sciencedirect.com/science/article/pii/S0014299917307719, 10.1016/j.ejphar.2017.11.038 (2018). [DOI] [PMC free article] [PubMed]
- 8.Levin S, Harris AA. Principles of combination therapy. Bulletin of the New York Academy of Medicine. 1975;51:1020. [PMC free article] [PubMed] [Google Scholar]
- 9.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 10.Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life sciences. 1989;45:947–961. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- 11.Hennessey VG, Rosner GL, Bast RC, Chen M-Y. A Bayesian approach to dose-response assessment and synergy and its application to in vitro dose-response studies. Biom. 2010;66:1275–1283. doi: 10.1111/j.1541-0420.2010.01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 2017;171:1678–1691. doi: 10.1016/j.cell.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slinker, B. K. The statistics of synergism. J. Mol. Cell Cardiol30, 723–731, http://www.sciencedirect.com/science/article/pii/S0022282898906551, 10.1006/jmcc.1998.0655 (1998). [DOI] [PubMed]
- 14.Foucquier, J. & Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. research & perspectives3 (2015). [DOI] [PMC free article] [PubMed]
- 15.Bliss CI. The toxicity of poison applied jointly. Annals of Appl. Biol. 1939;26:585–615. doi: 10.1111/j.1744-7348.1939.tb06990.x. [DOI] [Google Scholar]
- 16.Moran C. Importance of molecular features of non–small cell lung cancer for choice of treatment. The Am. J. Pathol. 2011;178:1940–1948. doi: 10.1016/j.ajpath.2010.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janjigian YY, et al. Dual inhibition of EGFR with Afatinib and Cetuximab in kinase inhibitor–Resistant EGFR-Mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4:1036. doi: 10.1158/2159-8290.CD-14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greco WR, Bravo G, Parsons JC. The search for synergy: A critical review from a response surface perspective. Pharmacol. reviews. 1995;47:331–385. [PubMed] [Google Scholar]
- 19.Geary N. Understanding synergy. Am. J. Physiol. Endocrinol. metabolism. 2013;304:E237–53. doi: 10.1152/ajpendo.00308.2012. [DOI] [PubMed] [Google Scholar]
- 20.Motulsky, H. & Christopoulos, A. Fitting models to biological data using linear and nonlinear regression: A practical guide to curve fitting (Oxford University Press, 2004).
- 21.Loewe ST, Muischnek H. Über Kombinationswirkungen. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 1926;114:313–326. doi: 10.1007/BF01952257. [DOI] [Google Scholar]
- 22.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–290. [PubMed] [Google Scholar]
- 23.Berenbaum MC. Synergy, additivism and antagonism in immunosuppression. a critical review. Clin. Exp. Immunol. 1977;28:1–18. [PMC free article] [PubMed] [Google Scholar]
- 24.Grabovsky Y, Tallarida RJ. Isobolographic analysis for combinations of a full and partial agonist: Curved isoboles. J. Pharmacol. Exp. Ther. 2004;310:981–986. doi: 10.1124/jpet.104.067264. [DOI] [PubMed] [Google Scholar]
- 25.Berenbaum, M. C. What is synergy? Pharmacol. reviews41, 93–141, http://pharmrev.aspetjournals.org/content/41/2/93 (1989). [PubMed]
- 26.Russ, D. & Kishony, R. The null additivity of multi-drug combinations. bioRxiv, https://www.biorxiv.org/content/early/2017/12/25/239517, 10.1101/239517 (2017).
- 27.Tallarida, R. J. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther. 319, 1–7, http://jpet.aspetjournals.org/content/319/1/1 (2006). [DOI] [PubMed]
- 28.Tallarida RJ. Quantitative methods for assessing drug synergism. Genes & Cancer. 2011;2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzo, J. I. & Sánchez-Marin, P. Comments on Isobolographic analysis for combinations of a full and partial agonist: Curved isoboles. J. Pharmacol. Exp. Ther. 316, 476–478, http://jpet.aspetjournals.org/content/316/1/476, 10.1124/jpet.105.095091 (2006). [DOI] [PubMed]
- 30.Chou TC, Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur. journal biochemistry. 1981;115:207–216. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. Systems biology and combination therapy in the quest for clinical efficacy. Nat. chemical biology. 2006;2:458–466. doi: 10.1038/nchembio817. [DOI] [PubMed] [Google Scholar]
- 32.van der Borght K, et al. BIGL: Biochemically intuitive generalized Loewe null model for prediction of the expected combined effect compatible with partial agonism and antagonism. Sci. Reports. 2017;7:17935. doi: 10.1038/s41598-017-18068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twarog NR, Stewart E, Hammill CV, Shelat A. BRAID: A unifying paradigm for the analysis of combined drug action. Sci. Reports. 2016;6:25523. doi: 10.1038/srep25523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wicha SG, Chen C, Clewe O, Simonsson USH. A general pharmacodynamic interaction model identifies perpetrators and victims in drug interactions. Nat. Commun. 2017;8:2129. doi: 10.1038/s41467-017-01929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luszczki JJ. Interactions of tiagabine with ethosuximide in the mouse pentylenetetrazole-induced seizure model: An isobolographic analysis for non-parallel dose-response relationship curves. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008;378:483–492. doi: 10.1007/s00210-008-0305-8. [DOI] [PubMed] [Google Scholar]
- 36.Bosgra S, van Eijkeren JCH, Slob W. Dose addition and the isobole method as approaches for predicting the cumulative effect of non-interacting chemicals: A critical evaluation. Critical Rev. Toxicol. 2009;39:418–426. doi: 10.1080/10408440902787592. [DOI] [PubMed] [Google Scholar]
- 37.Hand, D. J. Synergy in drug combinations. In Gaul, W., Opitz, O. & Schader, M. (eds) Data Analysis: Scientific Modeling and Practical Application, 471–475, 10.1007/978-3-642-58250-9_38 (Springer Berlin Heidelberg, 2000).
- 38.O’Neil J, et al. An unbiased oncology compound screen to identify novel combination strategies. Mol. cancer therapeutics. 2016;15:1155–1162. doi: 10.1158/1535-7163.MCT-15-0843. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6:461–464. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.