Abstract
This meta-analysis aimed to investigate the protective effects of bovine colostrum against childhood infectious diarrhea. A systematic search was conducted using PubMed, Cochrane Library databases and clinicaltrial.gov. Among 166 research articles, only five RCTs were included into final analysis. Review manager (version 5.2) was used to pool the effect-size across studies. Sensitivity and risk of bias were estimated accordingly. Under a pooled analysis, bovine colostrum consumption correlated with a significant reduction in stool frequency of infectious diarrhea, by 1.42 times per day (95% CI: −2.70, −0.14). Bovine colostrum intervention also reduced occurrence of diarrhea by 71% (pooled OR = 0.29, 95%CI 0.16, 0.52). The OR of positive detection of pathogen in the stool was 0.29 (95%CI 0.08, 0.71) in bovine colostrum treated group, compared with placebo group. In the sensitivity analysis of studies with low risk of biases, bovine colostrum significantly reduced stool frequency, occurrence of diarrhea and pathogen detection. BC and related products have a significant benefit in reducing the frequency and relieving the symptoms of childhood infectious diarrhea.
Introduction
Acute diarrhea is one of the most severe diseases with high health care costs and high mortality among children, especially in developing countries. Each year, more than 700 million children under five years are affected by acute diarrhea worldwide1. Approximately two to three million deaths (mainly in young children) are caused by diarrhea in developing countries annually2. In China, the prevalence of acute diarrhea was 5% among children younger than 5 years, with an annual incidence of 1430/100,000 per person-year3. About 30% of children with diarrhea were rotavirus positive4. Among the children with infectious diarrhea, the proportion of Rotavirus, Salmonella, Vibrio parahaemolyticus and Escherichia coli was 92%, 3%, 2% and 1%, respectively5.
Bovine colostrum (BC) is the first form of milk produced by a lactating dairy cow immediately following delivery of newborn calves. BC is rich in immunoglobulin, which can protect the neonatal bovine against environmental pathogens. BC is found to be effective in the prophylaxis of recurrent respiratory tract infection and diarrhea in children6. Infants received formula supplemented with BC products had a decreased stool frequency than those with control7. In contrast, another randomized control trial (RCT) presented a non-significant effect of BC supplementation on stool frequency8. Since diarrhea is a serious disease burden in children, while BC products show heterogeneous effects on this disease, we designed a meta-analysis using RCTs, to investigate whether BC products exert a beneficial effect against infectious diarrhea among children.
Results
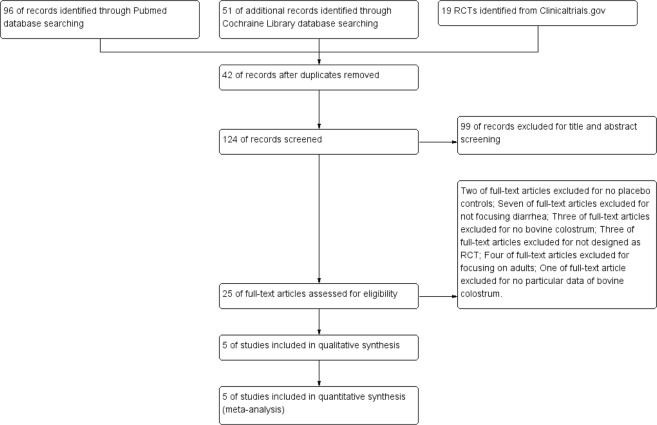
96, 51 papers and 19 trials, respectively, were searched from PubMed, Cochraine Library databases and clinicaltrials.gov (Fig. 1). 42 duplicates were removed and 99 papers were excluded after double-check on the titles and abstracts (Fig. 1). Based on detailed full-text reading, 2 articles without controls, 7 articles not focusing on diarrhea, 3 articles without using BC, 3 articles not designed as RCT, 4 articles recruiting adults, and 1 study not providing the data of BC were excluded from final analysis (Fig. 1). Finally, 5 articles in the design of RCT were included into the analysis (Fig. 1).
Figure 1.
Flow chart of paper searching.
All of the included studies were designed as RCT. Three studies investigated the effects of BC or related product against diarrhea due to rotavirus and another two investigated the protective effects against E. coli (Table 1). The outcome included stool frequency, detection of pathogen in the stool and the number of patients with diarrhea at the end of the study (Table 1). Totally, 324 children were included in this meta-analysis (Table 1).
Table 1.
The characteristics of included RCT studies.
| Author | Year | Area | Participants | Intervention | Control | Outcome | Sample size |
|---|---|---|---|---|---|---|---|
| Huppertz et al. | 1999 | Germany | Children with diarrhea of E. coli | Bovine colostrum | Placebo | Stool frequency | 27 |
| Tawfeek et al. | 2003 | Iraq | Healthy infants | Immunoglobulin from hyperimmune bovine colostrum against E. coli | Placebo | Stool frequency, Detection of E. coli in the stool | 84 |
| Davidson et al. | 1989 | Australia | Children admitted into the hospital | Hyperimmune bovine colostrum against rotavirus | Placebo | Number of patients with diarrhea at the end of the study, Detection of rotavirus in the stool | 120 |
| Ebina et al. | 1985 | Japan | Children with diarrhea of rotavirus | Hyperimmune bovine colostrum against rotavirus | Placebo | Number of patients with diarrhea at the end of the study, Detection of rotavirus in the stool | 13 |
| Sarker et al. | 1998 | Sweden | Children with diarrhea of rotavirus | Immunoglobulin from hyperimmune bovine colostrum against rotavirus | Placebo | Stool frequency, Number of patients with diarrhea at the end of the study, Detection of rotavirus in the stool | 80 |
RCT: randomized control trial.
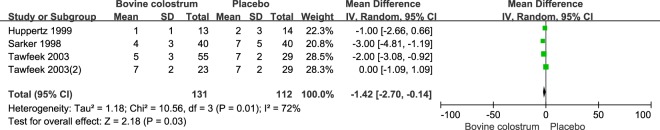
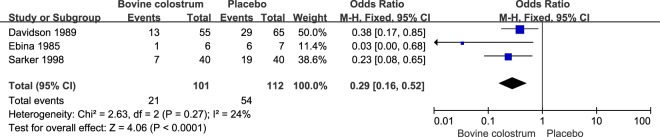
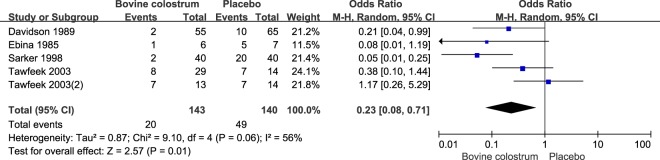
Four studies presented a protective effect from BC consumption against stool frequency per day. Stool frequency was reduced by 1.42 (95% CI: −2.70, −0.14) times per day under the random model (Fig. 2). Three studies focused the effects of BC against occurrence of diarrhea at the end of the study, with a pooled OR of 0.29 (95% CI: 0.16, 0.52) (Fig. 3). Five studies showed a protective effect of BC on the detection of pathogen in the stool (Fig. 4). Positive detection of pathogen in the stool was reduced by 77% (pooled OR = 0.23, 95% CI: 0.12, 0.43) (Fig. 4).
Figure 2.
Pooled effect of bovine colostrum on frequency of stool.
Figure 3.
Pooled effect of bovine colostrum on diarrhea after intervention.
Figure 4.
Pooled effect of bovine colostrum on positive detection of pathogen in the stool.
Two studies had a high risk of bias due to incomplete data on clinical outcome, while two studies had a low risk of bias (Fig. 5). All of the included studies had a low risk of selection bias in random sequence generation and selective reporting (Fig. 6). A low risk of bias in allocation concealment, blinding of participants and personnel, and blinding of outcome assessment was observed in 80% of studies (Fig. 6). Attrition bias in incomplete data on outcome was showed in 50% of studies (Fig. 6). Based on the two studies with a low risk of bias in the whole research process (Huppertz 1999 and Sarker 1998), BC and related products consumption exerted a significantly pooled effect against stool frequency, diarrhea and pathogen detection in the stool (Table 2).
Figure 5.
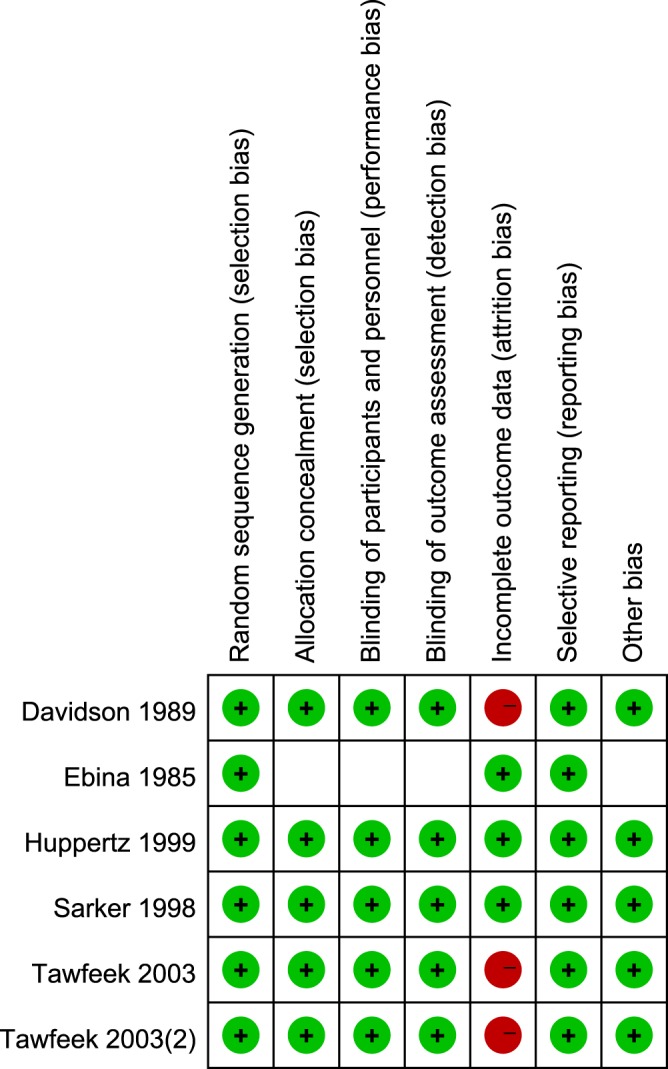
Risk biases in included studies.
Figure 6.
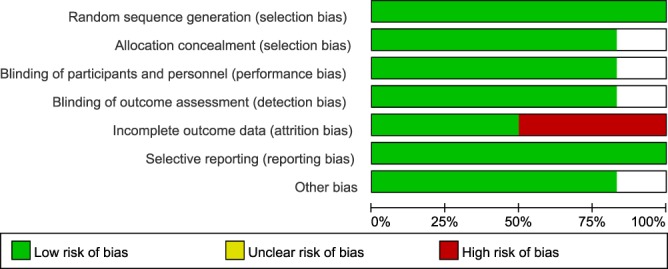
Percentage of risk biases in included studies.
Table 2.
Sensitivity analysis among studies with low risk of biases.
| Outcome | Number of studies | Overall effect | p-value for heterogeneity | Model |
|---|---|---|---|---|
| Stool frequency | 2 | −1.92 (−3.14, −0.69) | 0.11 | Fixed model |
| Diarrhea | 1 | 0.23 (0.08, 0.65) | — | Fixed model |
| Pathogen in the stool | 1 | 0.05 (0.01, 0.65) | — | Fixed model |
Discussion
Under the systematic search, five RCTs were included into this meta-analysis. 324 children were analyzed to evaluate the effects of BC (products) on the outcomes of infectious diarrhea, in terms of stool frequency, occurrence of diarrhea and detection of pathogen in the stool. Compared with placebo, BC products were effective to reduce frequency of stool, occurrence of diarrhea at the end of intervention and positive detection of rotavirus and E. coli in stool.
Children received a four-week BC treatment and presented a significant reduction in the episodes of respiratory tract infection, diarrhea and hospitalization6. Even in RCTs, BC showed a protective effect on upper respiratory tract infection9. The children with nonorganic failure to thrive received a three-month BC treatment and had a significant body weight increase10. BC has the potential to relieve infection and improve the growth of children. However, a three-week supplementation of BC to neonates with very low birth weight, failed in prophylaxis on necrotizing enterocolitis and sepsis11. BC, hyperimmune BC as well as immunoglobulin from hyperimmune BC had substantial benefits on childhood infectious diarrhea7,8,12–14. Bovine immunoglobulins are promising approches to enhance children immune function, such as phagocytosis, killing of bacteria, antigen presentation and gastrointestinal barrier function15. In addition, the hyperimmune BC was produced by vaccinating pregnant bovines with strains of particular pathogens. Hyperimmune BC was more effective than ordinary BC against diarrhea due to rotavirus16, but not the diarrhea due to shigellosis17.
Mice administered with BC showed a reduction of intestinal damages and clinical signs of colitis induced by 2,4,6 trinitrobenzene sulfonic acid. Accordingly, TLR4, IL-1β, IL-8 and IL-10 were downregulated18. Colostrum supplementation enhanced NK cell cytotoxicity and promoted immune response to primary influenza virus infection in mice19. Compared with milk-supplement, colostrum supplement treated mice had an increase in IL-6 production, as well as IgA production derived from B cells in small intestine and lung19. Even to preterm pigs, BC formula was advantageous in the prevention of gut dysfunction, necrotizing enterocolitis, and systemic infection20. There might be an interaction between BC and immunity at the intestinal epithelium.
In this meta-analysis, children with diarrhea were administered with BC in one study, hyperimmune BC in two studies, or immunoglobulin from hyperimmune BC in another two studies. Hyperimmune BC had significant effects on reduced diarrhea occurrence (OR = 0.32, 95% CI: 0.15, 0.67). Immunoglobulin from hyperimmune BC also reduced stool frequency, diarrhea occurrence and positive pathogen detection. BC was effective to reduce stool frequency by once per day (95% CI: −2.66, 0.66). In the analysis of pooled effect on stool frequency, there was a significant heterogeneity between studies and the heterogeneity was introduced by the study of Tawfeek(2). When we conducted the sensitivity analysis of excluding Tawfeek(2)’ study, BC intervention was still significant to reduce the stool frequency and the heterogeneity was non-significant.
The limited sample size was the major limitation of this study. The merge of BC, hyperimmune BC and derived immunoglobulin was another limitation in this meta-analysis.
Conclusion
BC products were effective to control clinical symptoms and pathogenic agents, in terms of stool frequency, diarrhea occurrence and positive pathogen detection, of infectious diarrhea among children. From this meta-analysis, it is meaningful to promote the application of BC products among children with infectious diarrhea.
Methods
Databases
We searched articles from PubMed Database (http://www.ncbi.nlm.nih.gov/pubmed/), Cochrane Library Database (http://onlinelibrary.wiley.com/cochranelibrary/search) and clinicaltrials.gov (https://www.clinicaltrials.gov/ct2/results?term=colostrum&Search=Apply&recrs=g&recrs=h&recrs=e&recrs=i&age_v=&age=0&gndr=&type=Intr&rslt=). Participants were “children”, the intervention was “BC or product” and the control was “placebo”. The outcome included stool frequency, diarrhea, and detection of pathogen in the stool. All included studies were designed as RCT.
Search terms and strategies
The search term was “colostrum”. In PubMed database, the additional filters were “humans”, “clinical trial”, “age < 18 years” and “published to August 31, 2018”. In Cochrane Library database, the additional filters were “trials” and “childhood health”. In clinicaltrials.gov, the additional filters were “trials”, “children (birth-17)” and “recruitment status including suspended, completed, terminated and withdrawn”.
Included studies
The studies under the design of RCT were eligible to include in the analysis. Phase I clinical trial, observational, animal or laboratory studies were excluded from the analysis. The included studies should provide the information on stool frequency, diarrhea occurrence at the end of study, and detection of pathogen in stool. This study was focused on original RCT but not reanalysis of previous data, review, abstracts or comments.
Data extraction and quality assessment
The number of patients with diarrhea, stool frequency, pathogen detection in the stool at the end of study, publication year, sample size and research area were extracted from the included articles by two reviewers independently. The quality assessment procedure of each article was focused on random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other possible bias21. Stool frequency indicated the times of bowel movements per day. Diarrhea was defined as having four or more loose or watery stool in a 24-hour period.
Data synthesis
Reviewer Manager 5.2 (Version 5.2.9, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.) was used to estimate the overall effect and heterogeneity across included studies. Heterogeneity was estimated by Chi2-value and a p-value of <0.05 was set as significant level. For significant heterogeneity, overall effect was produced under random models; whereas for non-significant heterogeneity, under fixed models. Sensitivity was evaluated among studies with a low risk of bias.
Acknowledgements
This study was supported by Public Welfare Scientific Research Project of China (201402012), CAMS Central Public Welfare Scientific Research Institute Basal Research Expenses to HW (2016ZX310182-1), CAMS Initiative for Innovative Medicine (2016-I2M-1-008) and The Capital Health Research and Development of Special (2016-2-40114).
Author Contributions
J. Li and Q.K. Song designed this study; Y.W. Xu and J.J. Jiang collected the data from the database; J. Li and Q.K. Song analyzed the data; J. Li, Y.W. Xu, J.J. Jiang and Q.K. Song wrote, modified and approved the manuscript to be submitted. All authors agreed to account for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
All data generated or analyzed during this study are included in this published article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chaimongkol N, et al. A wide variety of diarrhea viruses circulating in pediatric patients in Thailand. Clinical laboratory. 2012;58:117–123. [PubMed] [Google Scholar]
- 2.Dennehy PH. Viral gastroenteritis in children. The Pediatric infectious disease journal. 2011;30:63–64. doi: 10.1097/INF.0b013e3182059102. [DOI] [PubMed] [Google Scholar]
- 3.Cui P, Li J, Liu N, Duan ZJ. Incidence of acute diarrheal illness in Chinese communities: a meta-analysis. BMC gastroenterology. 2018;18:114. doi: 10.1186/s12876-018-0839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu, J. et al. Prevalence of rotavirus and rapid changes in circulating rotavirus strains among children with acute diarrhea in China, 2009–2015. The Journal of infection, 10.1016/j.jinf.2018.07.004 (2018). [DOI] [PMC free article] [PubMed]
- 5.Liu HX, Zhang J. Analysis of reported infectious diarrhea (other than cholera, dysentery, typhoid and paratyphoid) in China in 2011. Zhonghua yu fang yi xue za zhi [Chinese journal of preventive medicine] 2013;47:328–332. [PubMed] [Google Scholar]
- 6.Saad K, et al. Effects of bovine colostrum on recurrent respiratory tract infections and diarrhea in children. Medicine. 2016;95:e4560. doi: 10.1097/md.0000000000004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tawfeek HI, Najim NH, Al-Mashikhi S. Efficacy of an infant formula containing anti-Escherichia coli colostral antibodies from hyperimmunized cows in preventing diarrhea in infants and children: a field trial. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2003;7:120–128. doi: 10.1016/S1201-9712(03)90007-5. [DOI] [PubMed] [Google Scholar]
- 8.Huppertz HI, et al. Bovine colostrum ameliorates diarrhea in infection with diarrheagenic Escherichia coli, shiga toxin-producing E. Coli, and E. coli expressing intimin and hemolysin. Journal of pediatric gastroenterology and nutrition. 1999;29:452–456. doi: 10.1097/00005176-199910000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Patiroglu T, Kondolot M. The effect of bovine colostrum on viral upper respiratory tract infections in children with immunoglobulin A deficiency. The clinical respiratory journal. 2013;7:21–26. doi: 10.1111/j.1752-699X.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 10.Panahi Y, et al. Bovine colostrum in the management of nonorganic failure to thrive: a randomized clinical trial. Journal of pediatric gastroenterology and nutrition. 2010;50:551–554. doi: 10.1097/MPG.0b013e3181b91307. [DOI] [PubMed] [Google Scholar]
- 11.Balachandran B, Dutta S, Singh R, Prasad R, Kumar P. Bovine Colostrum in Prevention of Necrotizing Enterocolitis and Sepsis in Very Low Birth Weight Neonates: A Randomized, Double-blind, Placebo-controlled Pilot Trial. Journal of tropical pediatrics. 2017;63:10–17. doi: 10.1093/tropej/fmw029. [DOI] [PubMed] [Google Scholar]
- 12.Davidson GP, et al. Passive immunisation of children with bovine colostrum containing antibodies to human rotavirus. Lancet (London, England) 1989;2:709–712. doi: 10.1016/S0140-6736(89)90771-X. [DOI] [PubMed] [Google Scholar]
- 13.Ebina T, et al. Prevention of rotavirus infection by oral administration of cow colostrum containing antihumanrotavirus antibody. Medical microbiology and immunology. 1985;174:177–185. doi: 10.1007/BF02123694. [DOI] [PubMed] [Google Scholar]
- 14.Sarker SA, et al. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrum. The Pediatric infectious disease journal. 1998;17:1149–1154. doi: 10.1097/00006454-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Ulfman LH, Leusen JHW, Savelkoul HFJ, Warner JO, van Neerven RJ. J Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Frontiers in nutrition. 2018;5:52. doi: 10.3389/fnut.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitra AK, et al. Hyperimmune cow colostrum reduces diarrhoea due to rotavirus: a double-blind, controlled clinical trial. Acta paediatrica (Oslo, Norway: 1992) 1995;84:996–1001. doi: 10.1111/j.1651-2227.1995.tb13814.x. [DOI] [PubMed] [Google Scholar]
- 17.Ashraf H, Mahalanabis D, Mitra AK, Tzipori S, Fuchs GJ. Hyperimmune bovine colostrum in the treatment of shigellosis in children: a double-blind, randomized, controlled trial. Acta paediatrica (Oslo, Norway: 1992) 2001;90:1373–1378. doi: 10.1111/j.1651-2227.2001.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 18.Filipescu IE, et al. Preventive effects of bovine colostrum supplementation in TNBS-induced colitis in mice. PloS one. 2018;13:e0202929. doi: 10.1371/journal.pone.0202929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong EB, Mallet JF, Duarte J, Matar C, Ritz BW. Bovine colostrum enhances natural killer cell activity and immune response in a mouse model of influenza infection and mediates intestinal immunity through toll-like receptors 2 and 4. Nutrition research (New York, N.Y.) 2014;34:318–325. doi: 10.1016/j.nutres.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Sun, J. et al. Human Milk Fortification with Bovine Colostrum Is Superior to Formula-Based Fortifiers to Prevent Gut Dysfunction, Necrotizing Enterocolitis, and Systemic Infection in Preterm Pigs. JPEN. Journal of parenteral and enteral nutrition, 10.1002/jpen.1422 (2018). [DOI] [PubMed]
- 21.Higgins, J. P. T. & Green, S. (eds) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from, www.cochrane-handbook.org (2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.