Abstract
Leprosy is an infectious disease caused by Mycobacterium leprae affecting the skin and nerves. Despite decades of availability of adequate treatment, transmission is unabated and transmission routes are not completely understood. Despite the general assumption that untreated M. leprae infected humans represent the major source of transmission, scarce reports indicate that environmental sources could also play a role as a reservoir. We investigated whether M. leprae DNA is present in soil of regions where leprosy is endemic or areas with possible animal reservoirs (armadillos and red squirrels). Soil samples (n = 73) were collected in Bangladesh, Suriname and the British Isles. Presence of M. leprae DNA was determined by RLEP PCR and genotypes were further identified by Sanger sequencing. M. leprae DNA was identified in 16.0% of soil from houses of leprosy patients (Bangladesh), in 10.7% from armadillos’ holes (Suriname) and in 5% from the habitat of lepromatous red squirrels (British Isles). Genotype 1 was found in Bangladesh whilst in Suriname the genotype was 1 or 2. M. leprae DNA can be detected in soil near human and animal sources, suggesting that environmental sources represent (temporary) reservoirs for M. leprae.
Introduction
Leprosy is a debilitating infectious disease caused by Mycobacterium leprae and Mycobacterium lepromatosis that is still considered a major threat in developing countries by WHO, remaining persistently endemic in regions in Africa, South America and Asia. Every year more than 200,000 new patients are still diagnosed and this new case detection rate has been virtually stable over the last decade1. These facts indicate that multidrug therapy (MDT), although effective to treat leprosy, is insufficient to prevent transmission2.
Granting M. leprae transmission is not completely understood, risk factors for development of leprosy have been identified including close contact with untreated, multibacillary patients3, human susceptibility genes4,5, infection with soil transmitted helminths6, as well as food shortage7.
The mechanism by which bacteria are transmitted from one organism to another has not been unequivocally demonstrated8. However, based on existing evidence, skin-to-skin contact, aerosols as well as shedding of bacteria into the environment subsequently followed by infection of other individuals remain the most obvious options for human leprosy8,9. Still these routes provide no explanation for the occurrence of leprosy in individuals without known contact to leprosy patients or in areas without any reported new cases9,10.
Through PCR amplification of M. leprae DNA, its presence has been detected in environmental samples such as soil11–16 and water17,18 in areas inhabited by leprosy patients in Brazil and India. The viability of M. leprae was assessed by its multiplication in footpads of wild type mice and showed that M. leprae can remain alive in wet soil for 46 days19. Moreover, viability of M. leprae bacilli in soil from India has been studied by 16S ribosomal RNA gene analysis20. This study showed that 25% of the soil samples collected from patients’ areas contained M. leprae 16S ribosomal RNA, suggesting the presence of viable M. leprae in the soil. Additionally, if environment–free living amoebic cysts cultured in the laboratory are artificially infected with M. leprae (bacilli:amoebae ratio of 5–10:1), the bacteria can survive up to 8 months21.
Recently, M. leprae and M. lepromatosis were identified in red squirrels from the British Isles causing lepromatous disease in several animals22,23. Phylogenetic analyses determined that the M. leprae strain in squirrels (3I) was related to the lineage circulating in Medieval England, suggesting the red squirrels as a contemporary reservoir of the bacilli.
Zoonotic transmission of M. leprae from armadillos has been detected in the southeastern United States where wild armadillos and patients were infected with the same genotype (3I-2-v1)24.
Furthermore, although the prevalence of leprosy in nonhuman primates (NHP) seems to be quite low, M. leprae infections have also been reported in NHP25 carrying M. leprae strains closely related to the human strains, suggesting that NHPs transmission can occur from human (or human sources like trash), but also among NHPs25.
In this study, we aimed to explore whether besides humans and animals, environmental sources may function as a reservoir of M. leprae. For this purpose, we investigated the presence of M. leprae DNA in soil from regions with varying human leprosy endemicity in Bangladesh, Suriname, Brownsea Island and the Isle of Arran22.
Materials and Methods
DNA extraction from soil
Moist soil samples from 3 regions (Supplementary Table 1) were collected at a depth of 2 cm (Bangladesh and Suriname) or 8 cm (British Isles) in areas without sun light and stored in 50 ml tubes (Greiner Bio-One, Kremsmünster, Austria): i) in Bangladesh in front of the bedroom (right on the doorstep) in the houses of leprosy patients (n = 25) and from areas without known leprosy patients (n = 2); ii) in Suriname (Batavia and Groot Chatillon (former leprosy colonies), Pikin Slee and Gujaba) from areas known to be inhabited by nine-banded armadillos (n = 28) (samples Suriname 2, 3 and 6 from Batavia and Groot Chatillon were previously described (van Dissel et al. submitted) and are presented here for reference purposes); iii) in the British Isles in the habitat of Eurasian red squirrels carrying M. leprae (Brownsea Island, n = 10) and M. lepromatosis (Isle of Arran, n = 10).
As a negative control soil was obtained from the surroundings of the Leiden University Medical Centre (The Netherlands) and spiked with 108 cells of M. leprae NHPD-63 as positive control.
DNA was extracted from 10 g of soil using DNeasy PowerMax Soil (Qiagen, Valencia, CA) as per manufacturer’s instructions.
PCR amplification of RLEP and LPM244
To detect the presence of M. leprae DNA in soil, a PCR amplifying an M. leprae-specific repetitive sequence (RLEP) was performed. PCR amplification of a 129 bp sequence of RLEP26 was carried by addition of 10 µl 5x Gotaq® Flexi buffer (Promega, Madison, WI), 5 µl MgCl2 (25 mM), 2 µl dNTP mix (5 mM), 0.25 µl Gotaq® G2 Flexi DNA Polymerase (5 u/µl), 5 µl (2 µM) forward and reverse primers (Supplementary Table 2) and 5 µl template DNA in a final volume of 50 µl. DNA from M. bovis BCG P3 and M. tuberculosis H37Rv were used to assess PCR-specificity. As PCR positive controls DNA from M. leprae Br4923 and Thai-53 were used.
To detect inhibition of PCR due to remaining soil components, 1 µl of M. leprae DNA was added to the aforementioned PCR mixes together with 5 µl template DNA. In samples presenting PCR inhibition, 5 µl (2 mM) Bovine Serum Albumin (BSA) Fraction V (Roche Diagnostics, Indianapolis, IN) were added to the PCR mixes.
PCR mixes were denatured for 2 min at 95 °C followed by 40 cycles of 30 s at 95 °C, 30 s at 65 °C and 30 s at 72 °C and a final extension of 10 min at 72 °C. PCR products (15 µl) were used for electrophoresis in a 3.5% agarose gel at 130 V. Amplified DNA was visualized by Midori Green Advance staining (Nippon Genetics Europe, Dueren, Germany) using a Gel Doc System (Bio-Rad Laboratories, Hercules, CA).
PCR to detect M. lepromatosis was performed for soil from the British Isles. The primers (LPM244) amplify a 244 bp region of the hemN gene not present in M. leprae or other mycobacteria27. PCR was performed as explained above with LMP244 primers (Supplementary Table 2) and an annealing temperature of 53 °C. M. lepromatosis DNA was used as a positive control.
Genotyping
To determine the genotype (1, 2, 3 or 4) of M. leprae, SNP-14676 (locus 1), SNP-1642875 (locus 2) and SNP-2935685 (locus 3) were amplified and sequenced as described28 with minor modifications: PCRs were performed with 5 µl of template DNA using the aforementioned PCR mixes and forward and reverse primers for loci 1–3 (Supplementary Table 2) in a final volume of 50 µl. DNA was denatured for 2 minutes at 95 °C, following 45 cycles of 30 s at 95 °C, 30 s at 58 °C and 30 s at 72 °C and a final extension cycle of 10 min at 72 °C. PCR products were resolved by agarose gel electrophoresis as explained above. PCR products showing a band were purified prior to sequencing using the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI). Sequencing was performed on the ABI3730xl system (Applied Biosystems, Foster City, CA) using the BigDye Terminator Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA).
Results
Detection of M. leprae DNA in soil
To determine whether M. leprae DNA is present in the environment surrounding leprosy patients, the habitat of armadillos and red squirrels with leprosy-like disease, soil was collected in each area. PCR amplification of a 129 bp sequence of the RLEP region from M. leprae was performed in a total of 75 soil samples from 3 different regions (Supplementary Table 1). Control soil samples did not show amplification of the fragment in RLEP PCR, whereas the same sample spiked with M. leprae bacilli presented a clear band confirming the applicability of the method to isolate, purify and detect M. leprae in soil. PCR amplification of 5 µl of M. bovis BCG P3 and M. tuberculosis H37Rv DNA did not show amplification of RLEP showing specificity of the PCR for M. leprae DNA.
In Bangladesh, 4 out of 25 collected samples were positive for RLEP PCR (Fig. 1, Table 1; Supplementary Table 3), all of which were collected in houses of leprosy patients with high bacillary load (BI 5–6, Fig. 2). M. leprae DNA was not detected in the two soil samples from areas in Bangladesh without any reported leprosy cases (Supplementary Fig. 1).
Figure 1.
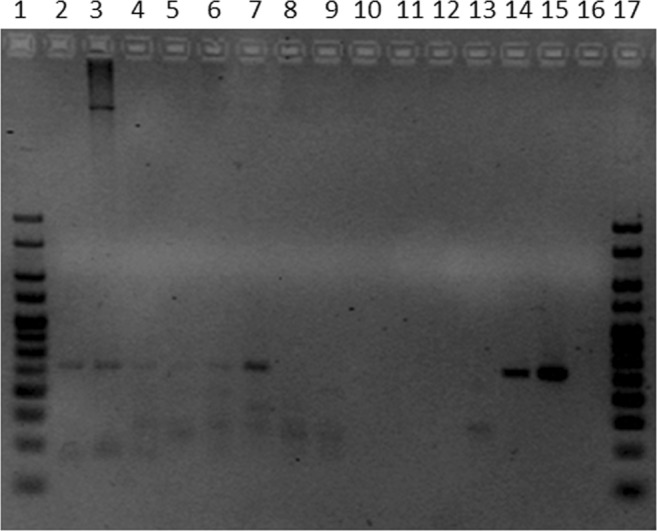
Gel of PCR for RLEP region to detect presence of M. leprae in soil samples. PCR products were electrophoresed in a 3.5% agarose gel. The size of the amplified RLEP sequence is 129 bp. Lanes 2 to 4 represent soil samples collected in Suriname (Suriname 2, 3, and 6), lanes 5 to 14 are soil samples collected in Bangladesh (01/65959/00, 01/65922/00, 01/65958/00, 02/65971/00, 02/22705/00, 01/65945/00, 01/65942/00, 01/65975/00, 01/22711/00 and 01/22723/00), lane 15 is DNA of M. leprae Thai-53 strain, lane 16 is a negative PCR control and lanes 1 and 17 are 25 bp HyperLadder (Bioline, Taunton, MA).
Table 1.
RLEP PCR results for M. leprae DNA derived from soil samples.
| Origin | Positive | Negative | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| Bangladesh | 4 | 16.0 | 21 | 84.0 |
| Suriname | 3 | 10.7 | 25 | 89.3 |
| Brownsea Island | 1 | 10.0 | 9 | 90.0 |
| Isle of Arran | 0 | 0.0 | 10 | 100.0 |
RLEP PCR result to detect M. leprae DNA in soil samples from Bangladesh, Suriname, Brownsea Island and Isle of Arran. A positive result is determined by a visible band of 129 bp in an agarose gel.
Figure 2.
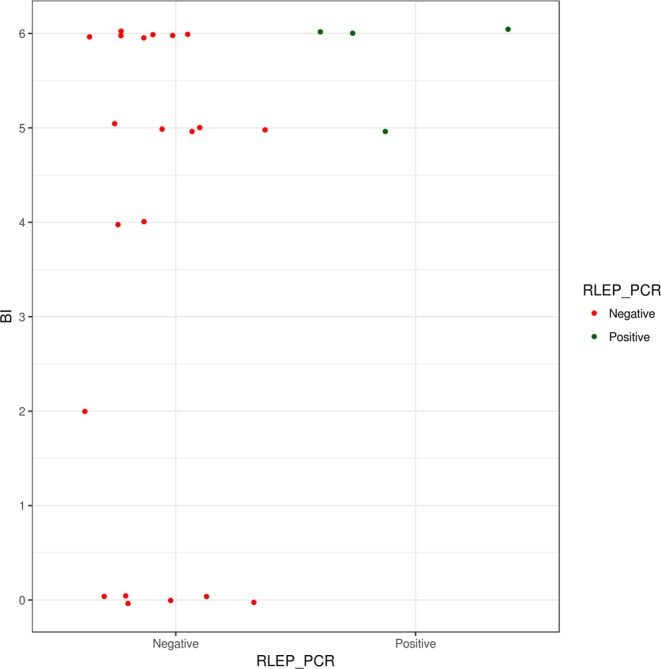
RLEP PCR positivity in soil samples from Bangladesh and bacillary load (BI) of patient. Soil samples collected in Bangladesh are represented in the graph by dots and sorted based on RLEP PCR results and bacillary load of the patient living in the household where the soil was collected.
In Suriname, samples (n = 28) were taken in three different locations inhabited by armadillos and M. leprae DNA was detected in 3 samples obtained at former leprosy colonies in Batavia and Groot-Chatillon (Fig. 1, Table 1; Supplementary Table 4).
Since all PCRs performed with UK samples were negative, we investigated whether PCRs were inhibited by compounds in the soil. DNA of M. leprae was added to the PCR mixes containing the DNA isolated from all soil samples and inhibition of PCR was determined by a negative PCR result. Inhibition was observed in 7 of the 10 soil samples from Brownsea Island, 8 out of the 10 from the Isle of Arran and 1 out of the 28 from Suriname. Since humic acid in soil can act as a PCR inhibitor29,30, 5 µl of 2 mM BSA was added to the PCRs with soil samples from the British Isles to overcome inhibition. Indeed, addition of BSA to soil-DNA spiked with M. leprae DNA (Br4923 or Thai-53), resulted in PCR-positivity for all spiked samples, indicating that BSA can prevent PCR inhibition due to undetermined soil compounds (data not shown).
Ten soil samples were collected in the surroundings of the infected red squirrels one of which was RLEP PCR positive (Tables 1 and 2). To determine whether M. lepromatosis DNA was also present in soil from the Isle of Arran with reported M. lepromatosis infection in red squirrels, PCRs were performed amplifying a 244 bp region of the hemN gene unique of M. lepromatosis27. None of the 10 soil samples collected resulted in PCR-positivity using LPM244 primers.
Table 2.
SNP typing results.
| Locus 1 | Locus 2 | Locus 3 | Genotype | |
|---|---|---|---|---|
| Tamil Nadu (reference strain) | C | G | A | 1 |
| Br4923 (reference strain) | T | T | C | 4 |
| Suriname 2 | UD | UD | A | 1 or 2 |
| Suriname 3 | UD | UD | A | 1 or 2 |
| Suriname 6 | C | UD | A | 1 or 2 |
| Bangladesh 01/65922/00 | UD | G | UD | 1 |
| Bangladesh 01/65958/00 | UD | G | UD | 1 |
| Bangladesh 01/22723/00 | C | G | A | 1 |
Polymorphic sites in the genome of M. leprae: locus 1 (SNP-14676), locus 2 (SNP-1642875) and locus 3 (SNP-2935685) and the corresponding genotype. Nucleic acid corresponding to each polymorphic site of M. leprae reference strains Tamil Nadu and Br4923 and soil samples that were successfully sequenced. When PCR amplification or sequencing of the locus was not successful it is marked as undetermined (UD).
Next, for all RLEP PCR positive samples from Bangladesh (n = 4), Suriname (n = 3) and the British Isles (n = 1) the PCR-amplified 129 bp RLEP region was sequenced. Sequence alignment with the RLEP region of M. leprae was found for all 8 samples, confirming that M. leprae specific DNA can be identified in soil using the above described procedure.
Genotyping
Genotypes of the RLEP PCR positive soil samples (n = 8) were investigated and determined according to the combination of SNPs in loci 1–3 as described by Monot et al.28 RLEP-positive soil from Bangladesh were classified as genotype 1 (Table 2) according to the polymorphism in locus 2 or loci 1–3 (01/22723/00, Fig. 3). For the soil from Suriname the genotype was narrowed down to either 1 or 2 since only sequencing of locus 3 (Suriname 2, 3 and 6) and locus 1 (Suriname 6) were identified. For the RLEP positive sample from Brownsea Island it was not possible to obtain sequence information for any of the polymorphic loci to assign a genotype. This was most likely due to the small amount of M. leprae DNA in the samples.
Figure 3.
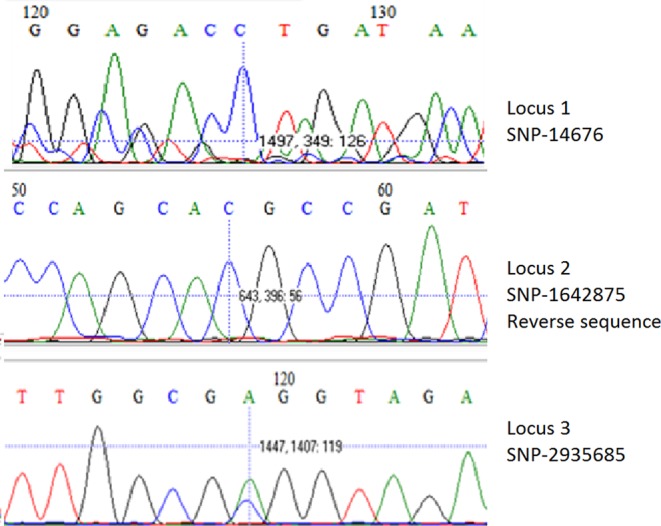
SNP analysis of loci 1, 2 and 3 from a representative M. leprae positive soil sample collected in Bangladesh. Sequencing results of locus 1 (SNP-146763) top, reverse sequence of locus 2 (SNP-1642875) middle and locus 3 (SNP-2935685) bottom, from soil sample Bangladesh 01/22723/00 used to determine the genotype of the M. leprae strain identified (genotype 1). SNP positions are based on the M. leprae TN strain. Vertical bars indicate the polymorphic base.
Discussion
Human leprosy still poses a considerable health threat in developing countries where transmission is generally assumed to take place via aerosol droplets from nasal cavities of untreated M. leprae infected individuals to their close contacts8,9. However, nonhuman animal and environmental sources have also been suggested to play a role in the pathogen’s dissemination9. As paleopathological evidence of leprosy in pre-Columbian America is lacking, leprosy was very likely introduced to the continent by European colonists or the African slave route28 also resulting in transmission to armadillos. However, nowadays infected armadillos may even be responsible for new cases in human individuals who have never had contact with leprosy patients nor travelled to leprosy endemic areas10,31. In addition, another living host that could potentially represent an environmental reservoir for M. leprae are amoebae as it has been shown that M. leprae can survive in free living amoebae21. Thus, amoebas or other protists might represent an intermediate host which would allow indirect infection with M. leprae through environmental samples.
In this study, M. leprae DNA was identified in soil collected in the houses of leprosy patients and the habitats of armadillos and red squirrels, suggesting that soil may represent a (temporary) reservoir. However, this study did not asses viability of the bacteria and since M. leprae is an obligate intracellular pathogen further investigation is needed to elucidate the role of the environment in M. leprae transmission.
Understanding how M. leprae is transmitted, and identifying sources of infection is crucial to prevent new cases and thus blocking transmission is essential to ultimately eradicate leprosy.
Although human leprosy was eradicated from the British Isles centuries ago, Eurasian red squirrels have remained a reservoir for M. leprae, containing a strain closely related to the strain present in Medieval England (3I). This indicates that M. leprae may have persisted in the environment after the human reservoir disappeared. However, M. leprae DNA was not abundantly present in soil, suggesting that the risk of environmental contamination is low.
Because the genome of M. lepromatosis contains only one copy of the hemN gene32 detected by LPM244 whereas 37 copies33 are present in the RLEP region34 of M. leprae, an equal amount of bacteria would be less well detectable by LPM244 PCR for M. lepromatosis than by RLEP PCR for M. leprae. Added to the fact that M. lepromatosis prevalence in the squirrel population is low, it is therefore possible that sensitivity was not sufficient to detect M. lepromatosis.
In Bangladesh, M. leprae was only found in soil collected in the houses of patients with high BI index (Fig. 2). At those locations more bacteria are shed and thus the likelihood of encountering bacteria in the soil is higher. However, a high BI index of the patient where the soil sample was collected was not necessarily associated with a positive RLEP PCR result. The higher percentage of RLEP positive soil in Bangladesh is likely due to a more targeted selection of the sample location in the houses of leprosy patients as well as the higher leprosy prevalence.
In previous phylogeographic analysis genotype 1 was identified as the predominant strain type in South Asia35,36 and was likely introduced to South Asia from other parts of that continent36. The genotype found in soil samples from Bangladesh (1) is therefore in accordance with previous phylogeographic data35.
In summary, this study demonstrates the presence of M. leprae DNA in soil, contributing to a OneHealth view on transmission including humans, animals and the environment. Further research is needed, however, to confirm whether M. leprae DNA in soil is derived from viable bacteria that can survive in smaller hosts such as helminths or amoebas. Thus, strategies aimed at prevention of transmission by administration of post-exposure prophylaxis to infected individuals should, besides human reservoirs of M. leprae, also consider environmental sources of (re)infection.
Supplementary information
Acknowledgements
The authors gratefully acknowledge all patients and control participants. LUMC and TLMI-B are part of the IDEAL (Initiative for Diagnostic and Epidemiological Assays for Leprosy) Consortium. We thank Dr. L. Adams (Louisiana State University, LA) for providing M. leprae cells and stimulating discussions and Prof. Xiang-Yang Han (MD Anderson Cancer Center, TX, USA) for providing M. lepromatosis DNA. This study was supported by an R2STOP Research grant from Effect hope/ The Leprosy Mission Canada, The Order of Malta-Grants-for-Leprosy-Research (MALTALEP), the Q.M. Gastmann-Wichers Foundation, the Leprosy Research Initiative (LRI; ILEP#703.15.07) together with the Turing Foundation and the Principal’s Career Development PhD scholarship provided by the University of Edinburgh.
Author Contributions
M.T.C. and A.G. wrote the manuscript, M.T.C., T.W. and L.P. performed the experiments, K.A., J.C.R., W.R.F., H.M., T.P. and K.S. provided the samples, M.T.C. A.G., A.K.S. and K.S. edited the manuscript, A.G. and J.H.R. funding acquisition.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-39746-6.
References
- 1.World Health Organization. Global leprosy update, 2016: accelerating reduction of disease burden. 92: WHO; pp. 501–520 (2017). [PubMed]
- 2.Blok DJ, de Vlas SJ, Geluk A, Richardus JH. Minimum requirements and optimal testing strategies of a diagnostic test for leprosy as a tool towards zero transmission: A modeling study. PLOS Neglected Tropical Diseases. 2018;12:e0006529. doi: 10.1371/journal.pntd.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moet FJ, Meima A, Oskam L, Richardus JH. Risk factors for the development of clinical leprosy among contacts, and their relevance for targeted interventions. Leprosy review. 2004;75:310–326. [PubMed] [Google Scholar]
- 4.Liu H, et al. Discovery of six new susceptibility loci and analysis of pleiotropic effects in leprosy. Nature genetics. 2015;47:267–271. doi: 10.1038/ng.3212. [DOI] [PubMed] [Google Scholar]
- 5.Alter A, Grant A, Abel L, Alcais A, Schurr E. Leprosy as a genetic disease. Mammalian genome: official journal of the International Mammalian Genome Society. 2011;22:19–31. doi: 10.1007/s00335-010-9287-1. [DOI] [PubMed] [Google Scholar]
- 6.Hagge DA, et al. Opening a Can of Worms: Leprosy Reactions and Complicit Soil-Transmitted Helminths. EBioMedicine. 2017;23:119–124. doi: 10.1016/j.ebiom.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feenstra SG, Nahar Q, Pahan D, Oskam L, Richardus JH. Recent food shortage is associated with leprosy disease in Bangladesh: a case-control study. PLOS Neglected Tropical Diseases. 2011;5:e1029. doi: 10.1371/journal.pntd.0001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bratschi MW, Steinmann P, Wickenden A, Gillis TP. Current knowledge on Mycobacterium leprae transmission: a systematic literature review. Leprosy review. 2015;86:142–155. [PubMed] [Google Scholar]
- 9.Araujo S, Freitas LO, Goulart LR, Goulart IM. Molecular evidence for the aerial route of infection of Mycobacterium leprae and the role of asymptomatic carriers in the persistence of leprosy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;63:1412–1420. doi: 10.1093/cid/ciw570. [DOI] [PubMed] [Google Scholar]
- 10.Bonnar PE, et al. Leprosy in Nonimmigrant Canadian Man without Travel outside North America, 2014. Emerg Infect Dis. 2018;24:165–166. doi: 10.3201/eid2401.170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavania M, et al. Detection of Mycobacterium leprae DNA from soil samples by PCR targeting RLEP sequences. The Journal of communicable diseases. 2006;38:269–273. [PubMed] [Google Scholar]
- 12.Turankar RP, et al. Single nucleotide polymorphism-based molecular typing of M. leprae from multicase families of leprosy patients and their surroundings to understand the transmission of leprosy. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20:O142–149. doi: 10.1111/1469-0691.12365. [DOI] [PubMed] [Google Scholar]
- 13.Turankar RP, Lavania M, Singh M, Siva Sai KS, Jadhav RS. Dynamics of Mycobacterium leprae transmission in environmental context: deciphering the role of environment as a potential reservoir. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012;12:121–126. doi: 10.1016/j.meegid.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Turankar RP, et al. Presence of viable Mycobacterium leprae in environmental specimens around houses of leprosy patients. Indian journal of medical microbiology. 2016;34:315–321. doi: 10.4103/0255-0857.188322. [DOI] [PubMed] [Google Scholar]
- 15.Turankar RP, et al. Comparative evaluation of PCR amplification of RLEP, 16S rRNA, rpoT and Sod A gene targets for detection of M. leprae DNA from clinical and environmental samples. International journal of mycobacteriology. 2015;4:54–59. doi: 10.1016/j.ijmyco.2014.11.062. [DOI] [PubMed] [Google Scholar]
- 16.Lavania M, et al. Detection of viable Mycobacterium leprae in soil samples: insights into possible sources of transmission of leprosy. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2008;8:627–631. doi: 10.1016/j.meegid.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Holanda MV, et al. Presence of Mycobacterium leprae genotype 4 in environmental waters in Northeast Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2017;50:216–222. doi: 10.1590/0037-8682-0424-2016. [DOI] [PubMed] [Google Scholar]
- 18.Arraes M, et al. Natural environmental water sources in endemic regions of northeastern Brazil are potential reservoirs of viable Mycobacterium leprae. Memorias do Instituto Oswaldo Cruz. 2017;112:805–811. doi: 10.1590/0074-02760170117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desikan KV. & Sreevatsa. Extended studies on the viability of Mycobacterium leprae outside the human body. Leprosy review. 1995;66:287–295. doi: 10.5935/0305-7518.19950032. [DOI] [PubMed] [Google Scholar]
- 20.Mohanty PS, et al. Viability of Mycobacterium leprae in the environment and its role in leprosy dissemination. Indian journal of dermatology, venereology and leprology. 2016;82:23–27. doi: 10.4103/0378-6323.168935. [DOI] [PubMed] [Google Scholar]
- 21.Wheat WH, et al. Long-term survival and virulence of Mycobacterium leprae in amoebal cysts. PLoS Neglected Tropical Diseases. 2014;8:e3405. doi: 10.1371/journal.pntd.0003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avanzi C, et al. Red squirrels in the British Isles are infected with leprosy bacilli. Science (New York, N.Y.) 2016;354:744–747. doi: 10.1126/science.aah3783. [DOI] [PubMed] [Google Scholar]
- 23.Simpson V, et al. Leprosy in red squirrels on the Isle of Wight and Brownsea Island. The Veterinary record. 2015;177:206–207. doi: 10.1136/vr.h4491. [DOI] [PubMed] [Google Scholar]
- 24.Truman RW, et al. Probable zoonotic leprosy in the Southern United States. The New England journal of medicine. 2011;364:1626–1633. doi: 10.1056/NEJMoa1010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honap TP, et al. Mycobacterium leprae genomes from naturally infected nonhuman primates. PLOS Neglected Tropical Diseases. 2018;12:e0006190. doi: 10.1371/journal.pntd.0006190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donoghue HD, Holton J, Spigelman M. PCR primers that can detect low levels of Mycobacterium leprae DNA. Journal of medical microbiology. 2001;50:177–182. doi: 10.1099/0022-1317-50-2-177. [DOI] [PubMed] [Google Scholar]
- 27.Vera-Cabrera L, et al. Mycobacterium lepromatosis infections in Nuevo León, Mexico. Journal of clinical microbiology. 2015;53:1945–1946. doi: 10.1128/JCM.03667-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monot M, et al. On the origin of leprosy. Science (New York, N.Y.) 2005;308:1040–1042. doi: 10.1126/science/1109759. [DOI] [PubMed] [Google Scholar]
- 29.Tsai YL, Olson BH. Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Applied and Environmental Microbiology. 1992;58:2292–2295. doi: 10.1128/aem.58.7.2292-2295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson RJ, Blackwell B. Purification and characterization of a common soil component which inhibits the polymerase chain reaction. Canadian journal of microbiology. 2000;46:633–642. doi: 10.1139/w00-043. [DOI] [PubMed] [Google Scholar]
- 31.da Silva MBP, et al. Evidence of zoonotic leprosy in Pará, Brazilian Amazon, and increased anti-PGL-I titer in individuals who consume armadillos in their diet. PLOS Neglected Tropical Diseases. 2018;12:e0006532. doi: 10.1371/journal.pntd.0006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh P, et al. Insight into the evolution and origin of leprosy bacilli from the genome sequence of Mycobacterium lepromatosis. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:4459–4464. doi: 10.1073/pnas.1421504112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole ST, Supply P, Honoré N. Repetitive sequences in Mycobacterium leprae and their impact on genome plasticity. Leprosy review. 2001;72:449–461. doi: 10.5935/0305-7518.20010053. [DOI] [PubMed] [Google Scholar]
- 34.Braet, S. et al. The repetitive element RLEP is a highly specific target for detection of Mycobacterium leprae. Journal of clinical microbiology56 (2018). [DOI] [PMC free article] [PubMed]
- 35.Monot M, et al. Comparative genomic and phylogeographic analysis of Mycobacterium leprae. Nature genetics. 2009;41:1282–1289. doi: 10.1038/ng.477. [DOI] [PubMed] [Google Scholar]
- 36.Benjak A, et al. Phylogenomics and antimicrobial resistance of the leprosy bacillus Mycobacterium leprae. Nature communications. 2018;9:352. doi: 10.1038/s41467-017-02576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


