Abstract
Red cell distribution width (RDW) to platelet ratio (RPR) is a prognosticator in acute pancreatitis and myocardial infarction; however, the prognostic values of RDW and RPR in breast cancer have not been studied. This retrospective analysis of 299 breast cancer patients investigated the association between RDW and RPR and clinicopathological characteristics and prognosis, compared to platelet distribution width to platelet count ratio (PDW/P) which is a known independent prognostic factor in patients with breast cancer. We found a significant correlation between RPR, and age and HER2 status. An elevated RPR significantly correlated with age and HER2 status. After a median follow-up duration of 48 months, tumour size, nuclear grade, PDW/P, and RPR were recgnized to be significantly associated with lower disease-free survival rates (tumour size: p < 0.01; nuclear grade, PDW/P, and RPR: p < 0.05) in univariate analysis. Tumour size and RPR were significant prognostic factors for lower disease-free survival rates, with hazard ratios of 4.31 (95% confidence interval: 1.76–10.53) (p < 0.01)] and 2.79 [95% confidence interval: 1.01–87.69) (p < 0.05)], respectively, in a multivariate analysis using the Cox proportional hazards model. This is the first study showing that an elevated RPR could independently predict poor prognosis in patients with breast carcinoma. Thus, RPR could be a novel biomarker for prognostic estimation.
Introduction
Breast cancer is the most common malignant disease among women in Japan1. Despite the development of diagnostic and treatment modalities, breast cancer is one of the most frequent causes of cancer-related deaths2. For patients with breast cancer, prognostic estimation is crucial because it greatly impacts the selection of the most appropriate treatment. Several molecular diagnostic tests are applied to obtain reliable prognostic information in the United States and Europe, such as MammaPrint and Oncotype Dx. However, they are somewhat difficult to use in clinical situation in Japan, because the Japanese National Health Insurance could not follow their use owing to the high cost and regional unavailability of these kits3. Therefore, the liquid biopsy is used in the early detection of cancer; however, its clinical use is still limited due to its uncertain role and high cost4. Thus, there could be an urgent need to establish simple and low-cost prognostic biomarkers for breast cancer using routine haematological parameters of the complete blood count.
Red blood cell distribution width (RDW), an indicator of the variability in the sizes of circulating red blood cells, is routinely measured and automatically reported as part of the complete blood count, and has originally been used to differentiate the aetiology of anaemia for decades. Recently, RDW has gained substantial attention as an indicator of inflammation5, and a prognostic marker of cardiac and infectious disease6,7. Furthermore, accumulated evidence has also indicated that RDW could be a significant prognostic factor in esophageal8 and hepatocellular9 cancer. A novel index, RDW to platelet count ratio (RPR), has been shown to reflect the severity of inflammation and is used to predict fibrosis in chronic hepatitis10. Seretis et al. indicated that elevated RDW could be used as a supportive diagnostic tool to distinguish between benign and malignant breast tumours11. Seitanides et al. also revealed that RDW was significantly correlated with bone marrow metastatic spread in breast cancer patients12. However, to the best of our knowledge, no studies regarding the prognostic values of RDW and RPR in patients with breast cancer have been conducted. Recently, we demonstrated that platelet distribution width to platelet count ratio (PDW/P) was a significant prognostic factor in patients with breast cancer13. Hence, the purpose of the present study was to investigate the prognostic value of the RDW and RPR in breast cancer patients, compared with PDW/P.
Methods
Patients
This retrospective study comprised 299 patients with histologically confirmed breast cancer who underwent surgery at the Department of Thoracic and Breast Surgery, Oita University Faculty of Medicine between April 2006 and December 2017. As previously described in detail13, the exclusion criteria for our analysis included distant metastases at initial presentation, carcinoma in situ, bilateral breast carcinoma, and male breast carcinoma. Furthermore, we excluded patients with heart failure, on dialysis, and lacking the entire set of clinicopathological data in this study.
As previously described in detail13, adjuvant therapy was administered according to the recommendations of the St. Gallen panel14. A regular folow-up evaluation (3-month intervals during years 1–5 and at 6-month intervals during years 5–10 post-diagnosis) was performed. A radiological assessment (computed tomography and mammography), clinical examinations, and laboratory data analyses (carcinoembryonic antigen and carbohydrate antigen 15-3 levels) every 12 months during years 1–10 post-diagnosis were included as follow-up investigation.
Clinicopathological characteristics
As previously described13, clinicopathological characteristics such as tumour size, nuclear grade, lymph node status, hormone receptor status, and human epidermal growth factor receptor 2 (HER2) status, were reviewed. Oestrogen receptor (ER) and progesterone receptor (PgR) statuses were evaluated via immunohistochemistry (IHC). Tumours with receptor expression scores above 0 were considered positive. HER2 status was assessed via IHC or fluorescence in situ hybridisation and was considered positive upon obtaining either an IHC score of 3 or at least a 2.2-fold stronger HER2 signal relative to the centromere enumerator probe 17 (CEP-17) signal in the tumour cells15.
Measurement of RDW indices
Blood samples were collected via peripheral venous puncture before the initiation of any treatment modality. RDW and platelet count were measured routinely using an automatic nephelometer (XN-9000; Sysmex Corporation, Kobe, Japan) according to the manufacturer’s instructions. The RPR was calculated by dividing the RDW by the platelet count (×104/μL). Both measurements were obtained from the same automated blood samples.
Statistical analysis
EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (the R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses. EZR is a modified version of the R Commander designed to include the statistical functions frequently used in biostatistics16. The student’s t-test was used for the comparison of variables between the two groups. The optimal cut-off values for PDW/P, RDW and RPR were determined by receiver operating characteristic (ROC) curve analysis by identifying the highest Youden index (sensitivity + specificity − 1).
The primary endpoint of the study was disease-free survival (DFS) defined as the interval between the dates of initial treatment and that of the first observation of disease relapse. Kaplan-Meier curve analysis and log-rank test were used to compare the survival of patients. Independent prognostic factors were identified via univariate analysis using a Cox proportional hazards model to identify any independent variables associated with DFS. Hazard ratios (HRs) estimated using Cox regression were reported as relative risks with their corresponding 95% confidence intervals (CIs). Multivariate Cox regression was performed for the parameters found to be significant in the univariate analysis. In this study, we opted to include factors with a p < 0.05 (instead of a p < 0.2) within the Cox regression model so as to be consistent and thus comparable with previous studies17,18. A p-value < 0.05 was considered statistically significant.
Data collection and ethics compliance
The institutional ethics review board (the clinical research board of Oita University, institutional ID: 1407) approved for this retrospective study and granted use of the opt-out consent method. All medical data from the participants were anonymised and compiled. Because the study plan and choice to freely refuse participation were announced through the bulletin at the Oita University Faculty of Medicine, patients were recognized to have consented to the study if they did not refuse participation.
All procedures used in this study were performed in accordance with the tenets of the Declaration of Helsinki (1975) and its later amendments.
Results
Patients’ characteristics
The baseline characteristics of the patients are outlined in Table 1. The median age was 64.2 (range: 31–92) years at the time of diagnosis. With regards to PDW/P, RDW, and RPR, patients were divided into two groups according to the optimised cut-off values determined by ROC analysis.
Table 1.
Baseline characteristics of the enrolled breast cancer patients.
| Characteristics | No. (%) |
|---|---|
| Age | |
| <50 | 39 (13) |
| ≥50 | 260 (87) |
| Oestrogen receptor | |
| Negative | 80 (27) |
| Positive | 219 (73) |
| Tumour size (mm) | |
| <20 | 201 (67) |
| ≥20 | 98 (33) |
| Nuclear grade | |
| 1 | 145 (48) |
| 2, 3 | 154 (52) |
| HER2 | |
| Negative | 248 (83) |
| Positive | 51 (17) |
| Progesterone receptor | |
| Negative | 125 (42) |
| Positive | 174 (48) |
| Lymph node involvement | |
| Negative | 229 (77) |
| Positive | 70 (23) |
| PDW/P | |
| <0.59 | 194 (65) |
| ≥0.59 | 105 (35) |
| RDW | |
| <13.7 | 216 (72) |
| ≥13.7 | 83 (28) |
| RPR | |
| <0.71 | 225 (75) |
| ≥0.71 | 74 (25) |
Abbreviations: No, number; HER2, human epidermal growth factor receptor 2; PDW/P, platelet distribution width to platelet count ratio; RDW, red cell distribution width; RPR, red cell distribution width to platelet count ratio.
ROC analysis showed that the optimal cut-off values for DFS were 0.59, 13.7 and 0.71 for the PDW/P, RDW and RPR, respectively (Table 2).
Table 2.
Receiver operating characteristic curve analyses of red cell distribution width and red cell distribution width to platelet count ratio in breast cancer patients.
| Characteristics | Optimal cut-off point | Specificity | Sensitivity | AUC (95%CI) |
|---|---|---|---|---|
| PDW/P | 0.59 | 0.67 | 0.58 | 0.59 (0.47–0.72) |
| RDW | 13.7 | 0.73 | 0.39 | 0.55 (0.44–0.67) |
| RPR | 0.71 | 0.77 | 0.42 | 0.52 (0.38–0.65) |
Abbreviations: AUC, area under the curve; PDW/P, platelet distribution width to platelet count ratio; RDW, red cell distribution width; RPR, red cell distribution width to platelet count ratio; CI, confidence interval.
The relationship between PDW/P, RDW & RPR and clinicopathological variables are shown in Table 3. The PDW/P was also found to be significantly correlated with age and HER2 status (p < 0.05).
Table 3.
Association between platelet distribution width to platelet count ratio, red cell distribution and red cell distribution width to platelet count ratio and clinicopathological characteristics in breast cancer patients.
| Variables | PDW/P | RDW | ||||
|---|---|---|---|---|---|---|
| Average | SD | p-value | Average | SD | p-value | |
| Age (years) | ||||||
| <50 | 0.49 | 0.15 | 0.018 | 13.51 | 1.46 | 0.36 |
| ≥50 | 0.57 | 0.21 | 13.32 | 1.21 | ||
| TS | ||||||
| <20 | 0.56 | 0.20 | 0.60 | 13.29 | 1.22 | 0.28 |
| ≥20 | 0.57 | 0.21 | 13.45 | 1.29 | ||
| NG | ||||||
| 1 | 0.57 | 0.20 | 0.37 | 13.34 | 1.31 | 0.98 |
| 2, 3 | 0.55 | 0.26 | 13.34 | 1.20 | ||
| LN | ||||||
| (−) | 0.56 | 0.19 | 0.70 | 13.28 | 1.18 | 0.09 |
| (+) | 0.57 | 0.26 | 13.56 | 1.43 | ||
| ER | ||||||
| (−) | 0.56 | 0.25 | 0.99 | 13.39 | 1.38 | 0.70 |
| (+) | 0.56 | 0.19 | 13.33 | 1.20 | ||
| PgR | ||||||
| (−) | 0.58 | 0.24 | 0.35 | 13.49 | 1.44 | 0.08 |
| (+) | 0.55 | 0.18 | 13.24 | 1.08 | ||
| HER2 | ||||||
| (−) | 0.58 | 0.21 | 0.018 | 13.40 | 1.32 | 0.10 |
| (+) | 0.50 | 0.18 | 13.08 | 0.76 | ||
| Variables | RPR | |||||
| Average | SD | p-value | ||||
| Age (years) | ||||||
| <50 | 0.54 | 0.11 | 0.0026 | |||
| ≥50 | 0.63 | 0.17 | ||||
| TS | ||||||
| <20 | 0.63 | 0.17 | 0.44 | |||
| ≥20 | 0.61 | 0.17 | ||||
| NG | ||||||
| 1 | 0.63 | 0.18 | 0.16 | |||
| 2, 3 | 0.61 | 0.16 | ||||
| LN | ||||||
| (−) | 0.62 | 0.17 | 0.46 | |||
| (+) | 0.61 | 0.16 | ||||
| ER | ||||||
| (−) | 0.60 | 0.17 | 0.29 | |||
| (+) | 0.63 | 0.17 | ||||
| PgR | ||||||
| (−) | 0.62 | 0.18 | 0.71 | |||
| (+) | 0.62 | 0.16 | ||||
| HER2 | ||||||
| (−) | 0.63 | 0.17 | 0.019 | |||
| (+) | 0.57 | 0.15 | ||||
Abbreviations: TS, tumour size; NG, nuclear grade; LN, lymph node involvement; ER, oestrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; SD, standard deviation; p, p-value; PDW/P, platelet distribution width to platelet count ratio; RDW, red cell distribution width; RPR, red cell distribution width to platelet count ratio.
Survival
After a median follow-up duration of 48 months, 26 patients (8.7%) had recurred. Univariate analysis revealed significant impacts of tumour size, nuclear grade, PDW/P, and RPR on DFS. Other variables were not found to be significantly correlated with DFS. On multivariate analysis, tumour size and RPR level were significantly correlated with poor prognosis for DFS, with HRs of 4.31 (95% CI: 1.76–10.53, p < 0.01) and 2.79 (95% CI: 1.01–7.69, p < 0.05), respectively (Table 4).
Table 4.
Univariate and multivariate analyses of disease-free survival in breast cancer patients.
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Age (years) | ||||
| <50 | 1 | 0.08 | ||
| ≥50 | 0.46 (0.19–1.09) | |||
| ER | ||||
| Negative | 1 | 0.29 | ||
| Positive | 0.65 (0.29–1.45) | |||
| Tumour size | ||||
| <20 mm | 1 | 0.00057 | 1 | 0.0014 |
| ≥20 mm | 4.14 (1.84–9.30) | 4.31 (1.76–10.53) | ||
| NG | ||||
| 1 | 1 | 0.029 | 1 | 0.17 |
| 2, 3 | 2.62 (1.10–6.25) | 1.93 (0.76–4.93) | ||
| HER2 | ||||
| Negative | 1 | 0.71 | ||
| Positive | 0.79 (0.24–2.65) | |||
| PgR | ||||
| Negative | 1 | 0.86 | ||
| Positive | 0.94 (0.43–2.03) | |||
| LN | ||||
| Negative | 1 | 0.09 | ||
| Positive | 1.97 (0.89–4.34) | |||
| PDW/P | ||||
| <0.59 | 1 | 1 | 0.37 | |
| ≥0.59 | 2.23 (1.02–4.85) | 0.044 | 1.56 (0.59–4.13) | |
| RDW | ||||
| <13.7 | 1 | 0.26 | ||
| ≥13.7 | 1.58 (0.72–3.50) | |||
| RPR | ||||
| <0.71 | 1 | 1 | 0.048 | |
| ≥0.71 | 2.21 (1.10–4.81) | 0.046 | 2.79 (1.01–7.69) | |
Abbreviations: NG, nuclear grade; LN, lymph node involvement; ER, oestrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; PDW/P, platelet distribution width to platelet count ratio; RDW, red cell distribution width; RPR, red cell distribution width to platelet count ratio; CI, confidence interval.
The DFS rate in the elevated RPR group was significantly lower than in the low RPR group (5-year survival, 77.8% vs. 89.7%, respectively; p < 0.05) (Fig. 1).
Figure 1.
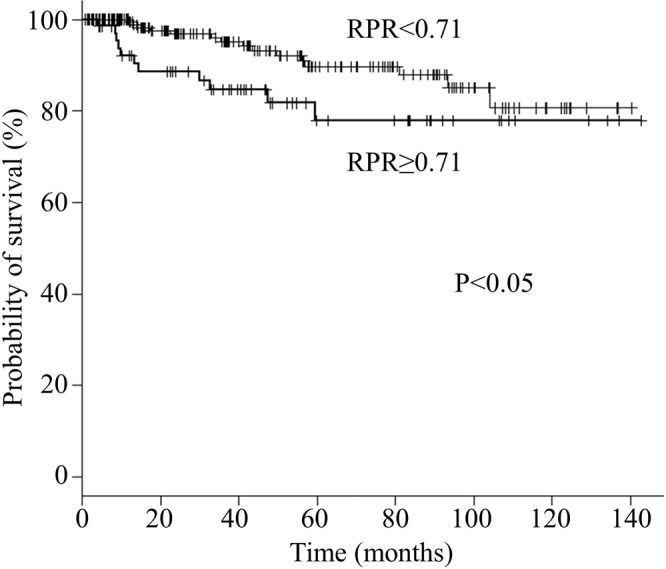
Kaplan-Meier analysis of disease-free survival stratified by the red cell distribution width to platelet count ratio in breast cancer patients.
Discussion
To the best of our knowledge, this is the first study to demonstrate that the elevated RPR could be an independent risk factor for prognosis in breast cancer patients and is more powerful than PDW/P as a prognostic factor.
RPR was recognised as a strong predictor of the severity of fibrosis and cirrhosis in patients with chronic hepatitis10 and a valuable prognostic marker of inflammation in acute pancreatitis and myocardial infarction19,20. These results showed that RPR was regarded as an indicator of systemic inflammatory response. We have already demonstrated elevated levels of inflammatory markers, such as C-reactive protein and platelet to lymphocyte ratio to be related to poorer survival among breast cancer patients21,22. Therefore, it was biologically feasible that RPR was a reliable prognostic indicator in breast cancer, although there have been no reports regarding the value of RPR in the prognosis of malignant disease.
Although the specific mechanism underlying the poor prognosis of breast cancer patients with elevated RPR remains uncertain, it may be partially attributed to the inflammatory response and malnutrition. As tumour size increased, an extensive inflammatory reaction might be triggered and lead to an increase in the levels of circulating cytokines such as interleukin-6, tumour necrosis factor-α, and hepcidin23,24. These cytokines might suppress erythrocyte maturation and accelerate the entry of newer, larger reticulocytes into the peripheral circulation, thereby causing elevated RDW25. With regard to the association between RDW and nutrition status, malnutrition caused by deficiency of iron, folate and vitamin B12 owing to loss of appetite due to cancer could affect haematopoiesis, and thus amplify the heterogeneity of red blood cells, leading to an increase in RDW26. On the other hand, platelets are known to be associated with tumour growth and metastasis due to the release of various growth factors such as platelet-derived growth factor, vascular endothelial growth factor, and platelet factor 427. However, the reason why the imbalances between RDW and platelet count could be a significant prognostic factor remains uncertain. Additional investigations are required to clarify the exact mechanisms bridging RPR and the survival of breast cancer patients.
The AUC of RPR was 0.52 which may be considered a small value. The AUC can be thought of as an indicator of overall ‘accuracy.’ A major practical fault of the AUC as an index of diagnostic performance is that it summarises the entire ROC curve, including regions that are frequently irrelevant to practical applications28. A large part of the area arose from the right side of the AUC where the high false positive fraction is of minimal clinical relevance29. Comparison of the AUCs between different screening or diagnostic tests may be meaningless30.
Some limitations of this study should be taken into account when interpreting the results. First, the analyses were performed on a small sample size, with a short-term follow-up period and single-centre. Secondly, there may inevitably be the potential for bias and inaccuracy in data collection as in most retrospectively studies. Third, the variability of the Youden index also depends on the clinical situation, because it could not differentiate between differences in sensitivity and specificity30. The cut-off point must, therefore, be judged in the context of the diagnostic situation to which the test is applied31. Furthermore, relevant laboratory data that can influence RPR levels, such as iron and vitamin B12 deficiency, were not collected and evaluated in this study.
Our study is the first to indicate that elevated preoperative RPR levels are indicative of unfavourable prognosis in patients with breast cancer. RPR, a cost-effective and easily calculated index almost universally available using two most common haematological parameters, can improve risk evaluation. However, our results are not conclusive and should not be considered for clinical practice unless further validation and feasibility studies have been completed.
Author Contributions
H.T., Y.T. and T.O. participated in manuscript preparation, data analysis and editing. H.T., M.A. and T.H. participated in data collection and data analysis. H.T. and M.M. participated in study design, data analysis, and manuscript preparation. H.T. and K.S. participated in study design and manuscript revision. All authors reviewed the manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hortobagyi GN, et al. The global breast cancer burden: variation in epidemiology and survival. Clin. Breast Cancer. 2005;6:391–401. doi: 10.3816/CBC.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 2.Qiu D, Katanoda K, Marugame T, Sobue T. A joinpoint regression analysis of long-term trends in cancer mortality in Japan (1958–2004) Int. J. Cancer. 2009;124:443–448. doi: 10.1002/ijc.23911. [DOI] [PubMed] [Google Scholar]
- 3.Drukker CA, et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int. J. Cancer. 2013;133:929–936. doi: 10.1002/ijc.28082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuniyoshi RK, et al. Gene profiling and circulating tumor cells as biomarker to prognostic of patients with locoregional breast cancer. Tumour Biol. 2015;36:8075–8083. doi: 10.1007/s13277-015-3529-5. [DOI] [PubMed] [Google Scholar]
- 5.Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin. Chem. Lab. Med. 2014;52:e197–e199. doi: 10.1515/cclm-2014-0353. [DOI] [PubMed] [Google Scholar]
- 6.Chen PC, et al. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am. J. Epidemiol. 2010;171:214–220. doi: 10.1093/aje/kwp360. [DOI] [PubMed] [Google Scholar]
- 7.Bojakowski K, et al. A high red blood cell distribution width predicts failure of arteriovenous fistula. PLoS ONE. 2012;7:e36482. doi: 10.1371/journal.pone.0036482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, G. P., Huang, Y., Yang, X. & Feng, J. F. A nomogram to Predict Prognostic Value of Red Cell Distribution Width in Patients with Esophageal Cancer. Mediators Inflamm. 854670 (2015). [DOI] [PMC free article] [PubMed]
- 9.Smirne C, et al. Evaluation of the red cell distribution width as a biomarker of early mortality in hepatocellular carcinoma. Dig. Liver Dis. 2015;47:488–494. doi: 10.1016/j.dld.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Taefi A, Huang CC, Kolli K, Ebrahimi S, Patel M. Red cell distribution width to platelet ratio, a useful indicator of liver fibrosis in chronic hepatitis patients. Hepatol. Int. 2015;9:454–460. doi: 10.1007/s12072-015-9638-9. [DOI] [PubMed] [Google Scholar]
- 11.Seretis C, Seretis F, Lagoudianakis E, Gemenetzis G, Salemis NS. Is red cell distribution width a novel biomarker of breast cancer activity? Data from a pilot study. J. Clin. Med. Res. 2013;5:121–126. doi: 10.4021/jocmr1214w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seitanides B, Giakoumakis G, Tsakona C. Increased red cell volume distribution width in patients with bone marrow metastases. J. Clin. Pathol. 1988;41:1246. doi: 10.1136/jcp.41.11.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi H, et al. The prognostic impact of the platelet distribution width-to-platelet count ratio in patients with breast cancer. PLoS ONE. 2017;12:e0189166. doi: 10.1371/journal.pone.0189166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldhirsch A, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J. Breast Cancer. 2013;16:55–59. doi: 10.4048/jbc.2013.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JY, et al. Biological significance and prognostic relevance of peripheral blood neutrophil-to-lymphocyte ratio in soft tissue sarcoma. Sci Rep. 2018;8:11959. doi: 10.1038/s41598-018-30442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui L, Fan P, Qiu C, Hong Y. Single institution analysis of incidence and risk factors for post-mastectomy pain syndrome. Sci Rep. 2018;8:11494. doi: 10.1038/s41598-018-29946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cetinkaya E, Şenol K, Saylam B, Tez M. Red cell distribution width to platelet ratio: New and promising prognostic marker in acute pancreatitis. World J. Gastroenterology. 2014;20:14450–14454. doi: 10.3748/wjg.v20.i39.14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celik T, et al. Predictive value of admission red cell distribution width-platelet ratio for no-reflow phenomenon in acute ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiol. J. 2016;23:84–92. doi: 10.5603/CJ.a2015.0070. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi H, et al. The prognostic significance of the preoperative platelet-lymphocyte ratio in Japanese patients with localized breast cancer. Adv. Breast Cancer Res. 2016;5:49–57. doi: 10.4236/abcr.2016.52005. [DOI] [Google Scholar]
- 22.Takeuchi H, et al. Comparison of the prognostic values of preoperative inflammation-based parameters in patients with breast cancer. PLoS ONE. 2017;12:e0177137. doi: 10.1371/journal.pone.0177137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Gonzalo-Calvo D, et al. Interleukin 6, soluble tumor necrosis factor receptor I and blood cell distribution width as biological markers of functional dependence in an elderly population: a translational approach. Cytokine. 2012;58:193–198. doi: 10.1016/j.cyto.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes CJ, et al. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J. Am. Coll. Cardiol. 2011;58:300–309. doi: 10.1016/j.jacc.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 25.Chen B, Ye B, Zhang J, Ying L, Chen Y. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS ONE. 2013;8:e68780. doi: 10.1371/journal.pone.0068780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goyal H, Gupta S, Singla U. Level of red cell distribution width is affected by various factors. Clin. Chem. Lab. Med. 2016;54:e387. doi: 10.1515/cclm-2016-0195. [DOI] [PubMed] [Google Scholar]
- 27.Peterson JE, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis. 2012;15:265–273. doi: 10.1007/s10456-012-9259-z. [DOI] [PubMed] [Google Scholar]
- 28.Ma H, Bandos AI, Rockette HE, Gur D. On use of partial area under the ROC curve for evaluation of diagnostic performance. Stat. Med. 2013;32:3449–3458. doi: 10.1002/sim.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J. Intern. Med. 2013;4:627–635. [PMC free article] [PubMed] [Google Scholar]
- 30.Wald NJ, Bestwick JP. Is the area under an ROC curve a valid measure of the performance of a screening or diagnostic test? J. Med. Screen. 2014;21:51–56. doi: 10.1177/0969141313517497. [DOI] [PubMed] [Google Scholar]
- 31.Carter JV, Pan J, Rai SN, Galandiuk S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery. 2016;159:1638–1645. doi: 10.1016/j.surg.2015.12.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


