Abstract
Urine samples provide a potential alternative to physician-taken or self-collected cervical samples for cervical screening. Screening by primary hrHPV testing requires additional risk assessment (so-called triage) of hrHPV-positive women. Molecular markers, such as DNA methylation, have proven most valuable for triage when applied to cervical specimens. This study was set out to compare hrHPV and DNA methylation results in paired urine and cervical scrapes, and to evaluate the feasibility of DNA methylation analysis in urine to detect cervical cancer. Urine samples (n = 41; native and sediment) and paired cervical scrapes (n = 38) from cervical cancer patients, and urine from 44 female controls, were tested for hrHPV and 6 methylation markers. Results on native urine and sediment were highly comparable. A strong agreement was found between hrHPV testing on urine and scrapes (kappa = 0.79). Also, methylation levels in urine were moderately to strongly correlated to those detected in scrapes (r = 0.508–0.717). All markers were significantly increased in urine from cervical cancer patients compared to controls and showed a good discriminatory power for cervical cancer (AUC = 0.744–0.887). Our results show a good agreement of urine-based molecular analysis with reference cervical samples, and suggest that urine-based DNA methylation testing may provide a promising strategy for cervical cancer detection.
Introduction
Cervical cancer is the fourth most common cancer in women worldwide, affecting 528,000 women and leading to 266,000 deaths each year1. Virtually all cervical cancers are caused by a persistent, high-risk HPV (hrHPV) infection. The slow progression from premalignant Cervical Intraepithelial Neoplasia (CIN) to a malignancy provides ample time for early detection and necessary action. Current cervical screening programs use cytology or primary hrHPV testing which focus on detection of abnormal cells and presence of hrHPV infection, respectively. Both approaches, however, have limitations. Cytology is limited by the subjectivity of the analysis and relatively limited and variable sensitivity to detect cervical (pre)malignancies, which ranges from 50–80%2. HrHPV screening cannot differentiate between a transient productive infection and a persistent transforming infection, which lowers the specificity of the test. While a combined approach, either co-testing or cytology triage of HPV positives, mitigates a number of these concerns, the subjective nature and low sensitivity of cytology testing remains a relevant problem. This indicates that efforts towards a more objective triage strategy are needed. Recent studies from others and us have shown that testing of hrHPV-positive women for hypermethylated genes offers an objective triage tool for the detection of CIN3 and cervical cancer3–7.
Besides the use of the most optimal screening test, the success of any screening program also depends on attendance. Many women experience obtaining cervical scrapes as an unpleasant and invasive procedure8,9. As a considerable percentage of cervical cancer is diagnosed in the population of screening non-attendees10, there is need for an approach that will reach these women. Although self-sampling of cervico-vaginal specimens increases screening attendance11, recent studies focusing on hrHPV detection in urine have established that urine sampling is preferred over cervical sample collection either by physician or by self-sampling9,12. The use of urine for screening has many additional benefits. It offers an easily manageable manner of sampling, with the potential of large-scale application13,14.
Given the need for triage testing in primary hrHPV-based screening, previous studies of us have focused on DNA methylation analysis in cervical scrapes and self-samples3,4. Furthermore, a number of studies have reported on the use of DNA methylation testing in urine for the detection of other cancer types, such as bladder (reviewed by15) and prostate cancer16,17. These studies, together with our encouraging data on DNA methylation analysis in cervical scrapes and self-samples, indicate that DNA methylation testing in urine could provide a promising noninvasive method for cervical cancer detection.
This study was set out to assess the feasibility of DNA methylation analysis in urine using 6 previously identified DNA methylation markers18,19. We collected urine and cervical scrapes from women with cervical cancer and first tested which urine component, sediment or unfractioned native urine, is most suitable for hrHPV detection and DNA methylation analysis. Secondly, we compared hrHPV and DNA methylation results in paired urine and cervical scrapes and evaluated the ability of urine-based DNA methylation testing for cervical cancer detection.
Methods
Sample collection and processing
Urine samples (n = 43) and paired cervical scrapes (n = 38) were collected from cervical cancer patients, aged 27 to 86 at the Department of Gynecology, the Netherlands Cancer Institute, Amsterdam The Netherlands between February 2016 and March 2017. Voided urine samples were provided by participating women prior to surgery and collected in containers. Urine samples were processed within 24 hours. Surgical pathology reports were verified to confirm presence of cervical cancer at time of sample collection. Tumor stage and age are indicated in Table 1. Informed written consent was obtained from all patients and research protocols were approved by the Medical Ethics Committee of the Netherlands Cancer Institute.
Table 1.
HrHPV test results in urine samples.
| No. | Cancer type | Stage | Age | Native urine | Urine sediment | Cervical scrape |
|---|---|---|---|---|---|---|
| 1^ | SCC | IIB | 52 | NA** | positive (other) | positive (other) |
| 2^ | SCC | IIB | 59 | NA** | positive (16) | NA*** |
| 3^ | SCC | IIB | 51 | positive (other) | positive (other) | positive (other)* |
| 4^ | AC | IBII | 43 | positive (16) | positive (16) | positive (16)* |
| 5 | SCC | IBI | 31 | positive (18) | positive (18) | positive (18) |
| 6 | SCC | IIB | 56 | positive (other) | positive (other) | positive (other) |
| 7 | AC | IBI | 52 | negative | negative | negative |
| 8^ | SCC | IIB | 82 | positive (16) | positive (16) | NA*** |
| 9 | SCC | IBI | 43 | positive (16) | positive (16) | positive (16) |
| 10 | SCC | IIB | 83 | positive (other) | positive (other) | positive (other) |
| 11 | SCC | IBI | 43 | positive (16) | positive (16) | positive (16) |
| 12 | ASC | IBI | 32 | positive (16, 18) | positive (16, 18) | positive (16, 18) |
| 13 | ASC | IBI | 34 | positive (18) | positive (18) | positive (18) |
| 14 | SCC | IIB | 36 | positive (18) | positive (18) | positive (18) |
| 15 | AC | IBI | 41 | positive (16) | positive (16) | positive (16) |
| 16 | SCC | IBII | 29 | positive (other) | positive (other) | positive (other) |
| 17 | SCC | IIAI | 75 | positive (16) | positive (16) | positive (16) |
| 18 | SCC | IIIB | 86 | positive (16) | positive (16) | positive (16) |
| 19 | SCC | IIB | 51 | positive (16) | positive (16) | positive (16) |
| 20 | SCC | IIB | 43 | positive (16) | positive (16) | positive (16) |
| 21 | SCC | IIB | 82 | positive (16) | positive (16) | positive (16) |
| 22 | SCC | IIB | 34 | positive (other) | positive (other) | positive (other) |
| 23 | SCC | IBI | 69 | negative | negative | positive (other) |
| 24 | SCC | IBI | 66 | positive (16) | positive (16) | positive (16) |
| 25 | SCC | IBI | 51 | negative | positive (16) | positive (16) |
| 26 | SCC | IIB | 76 | negative | positive (other) | positive (other) |
| 27 | SCC | IIB | 50 | positive (16) | positive (16) | positive (16) |
| 28 | AC | IBI | 46 | positive (16) | positive (16) | positive (16) |
| 29 | SCC | IIIB | 72 | positive (other) | positive (other) | positive (other) |
| 30^ | SCC | IVB | 35 | positive (16) | positive (16)* | positive (16, other) |
| 31 | SCC | IIB | 55 | positive (18) | negative | positive (18) |
| 32 | SCC | IVB | 80 | positive (16) | positive (16) | |
| 33 | SCC | IIAI | 55 | positive (16) | positive (16) | |
| 34 | SCC | IBI | 35 | negative | negative | |
| 35 | AC | IBII | 45 | positive (16) | positive (16) | |
| 36 | SCC | IIIB | 50 | positive (18) | positive (18) | |
| 37 | AC | IBI | 27 | positive (16, other) | positive (16, other) | |
| 38 | AC | IIAI | 82 | negative | negative | |
| 39 | SCC | IBI | 31 | positive (16) | positive (16) | |
| 40 | SCC | IIB | 46 | positive (other) | positive (other) | |
| 41 | AC | IBI | 62 | positive (18) | positive (18) | |
| 42 | SCC | IIB | 59 | negative | negative | |
| 43 | SCC | IBI | 47 | negative | positive (other) |
Native urine (n = 31), urine sediments (n = 43), and cervical scrapes (n = 43) from women with cervical cancer were included. Urine component analysis (sediment versus native urine) was performed on paired urine sediment and native urine. Excluded samples are indicated by ^; three samples were excluded based on low quality (indicated by*), for two patients there was insufficient amount of DNA from native urine (indicated by**), and two patients did not obtain a cervical scrape (indicated by***). This resulted in a total set of 28 samples for urine component analysis (upper part) and 38 samples for comparison between urine sediment and cervical scrapes (complete set). Abbreviations: SCC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous carcinoma; NA, not available; ND, not done.
Control samples (n = 47; all females aged 30 to 60) originated from women without known malignancy of which urine was collected for routine diagnostic purposes. These samples were supplied by the Department of Clinical Chemistry of the VU University Medical Center, Amsterdam, and were used anonymously in accordance with the code of conduct for responsible use20. Accordingly, no data was available on prior HPV and disease status.
Urine samples were processed within 24 hours. In order to evaluate different fractions of the urine, (1) 15 mL of urine was centrifuged at 800 × g for 10 minutes and the sediment (pellet) was stored at −20 °C and (2) 15 mL was stored as unfractioned native urine at −20 °C.
Cervical scrapes were collected with a Cervex-Brush (Rovers Medical Devices, Oss, The Netherlands) in Thinprep PreservCyt solution (Hologic, Bedwork, MA, US) and stored at 4 °C.
DNA isolation and bisulfite modification
DNA from urine sediment (15 mL original volume) was isolated using the DNA mini and blood mini kit (Qiagen, Hilden, Germany). DNA from native urine was isolated using the Quick DNA urine kit (Zymo Research, Irvine, CA, US). Following isolation, DNA was eluted in 50 µL elution buffer. DNA from cervical scrapes was isolated using the Nucleo-Spin 96 Tissue kit (Macherey-Nagel, Germany) and Microlab Star robotic system (Hamilton, Germany). All procedures were performed according to the recommendations of the manufacturer.
DNA concentrations were quantified using a NanoDrop 1000 (ThermoFisher Scientific, Waltham, MA, US). Isolated DNA was subjected to bisulfite treatment using the EZ DNA Methylation Kit (Zymo Research, Irvine, CA, US), according to manufacturer’s instructions.
HrHPV testing
DNA was subjected to hrHPV testing using the HPV-Risk assay (Self-screen B.V., Amsterdam, The Netherlands), a multiplex real-time PCR-based assay for the clinical detection of 15 (probably) hrHPV types, with partial genotyping for HPV16 and HPV1821.
Quantitative methylation specific PCR (qMSP) analysis
Two multiplex qMSPs for each 3 targets (FAM19A4, PHACTR3, PRDM1419 and GHSR, SST, ZIC118) and ACTB was performed using 50 ng of bisulfite-converted DNA as described before22 on an ABI-7500 real-time PCR-system (Applied Biosystems, Waltham, MA, US). ACTB was used as a reference gene for quantification and as a quality control. The methylation values of the targets were normalized to ACTB using the comparative Cq method (2−ΔCq × 100) to obtain Cq ratios. Samples with an ACTB Cq value > 32 were considered to be of insufficient quality and excluded from further analysis. Based on this criterion, 3 native urines (2 controls, 1 cervical cancer), 4 urine sediments (3 controls, 1 cervical cancer), and 2 scrapes from cervical cancer patients were excluded.
Data analysis
Agreement of hrHPV test results between urine sediment and native urine or cervical scrapes was assessed using Cohen’s kappa statistics23. HrHPV agreement was defined as slight (kappa ≤ 0.20), weak (kappa = 0.21–0.40), moderate (kappa = 0.41–0.60), strong (kappa = 0.61–0.80), near-perfect (kappa = 0.81–0.99), and perfect (kappa = 1.000).
The Spearman correlation coefficient r was calculated based on log2-transformed Cq ratios to assess correlation for each DNA methylation marker between urine sediment and native urine or cervical scrapes.
Differences in DNA methylation levels between patient and control groups were determined using the non-parametric Mann-Whitney U-test. P values < 0.05 were considered statistically significant.
Logistic regression analysis was performed to assess the prediction ability of the 6 DNA methylation markers for detection of cervical cancer. First, the Cq ratio of each marker was log2-transformed. Next, univariable logistic regression model was fitted to observe the performance of each marker. The performance of the models was assessed by leave-one-out cross validation (LOOCV), then visualized by the receiver operating characteristics (ROC) curve and quantified by area under the curve (AUC).
All statistical analyses were performed in R open source software, using the pROC package for logistic regression analysis24.
Statement
All experiments and methods were performed in accordance with relevant guidelines and regulations.
Results
Comparison of hrHPV and methylation analysis in urine sediment versus native urine
To evaluate which urine component (sediment or native urine) is best suited for hrHPV detection and DNA methylation analysis we analyzed paired urine sediments and native urine from 28 women with cervical cancer. HrHPV was detected in 25 (89%) urine sediments and 24 (86%) native urine samples from cervical cancer patients (Table 1; upper part), with the majority of the hrHPV-positive women being HPV16/18 positive. We found a strong agreement in the detection of hrHPV, overall and at the genotype level, between urine sediments and native urine with a kappa statistic of 0.79 (95% confidence interval (CI) 0.58–1.00).
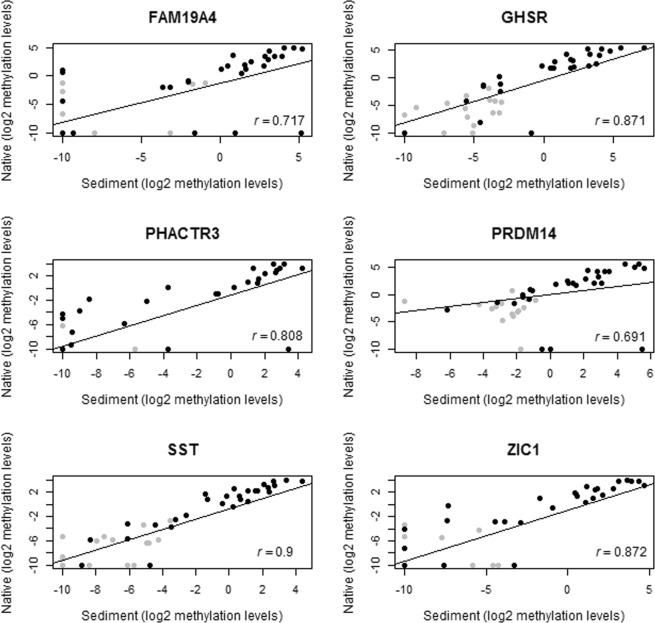
In addition, a strong correlation was observed between urine sediments and native urine for all 6 DNA methylation markers (FAM19A4, GHSR, PHACTR3, PRDM14, SST, and ZIC1), with correlation coefficient varying from 0.691 (PRDM14) to 0.9 (SST) (Fig. 1).
Figure 1.
Correlation between paired urine sediment and native urine from 28 women with cervical cancer (black) and 15 controls (gray). Log2 methylation levels of FAM19A4, GHSR, PHACTR3, PRDM14, SST and ZIC1 were used. Spearman correlation coefficient (r) values are shown.
Given similar findings and based on practical considerations, such as ease of storage and costs of DNA isolation, we decided to continue with urine sediments.
hrHPV and DNA methylation detection in paired urine sediments and cervical scrapes
We investigated how hrHPV and DNA methylation test results in urine sediment correlated to cervical scrapes for which we had 38 paired samples available. In the paired samples, hrHPV infection was detected in 31 (82%) urine sediments and 34 (89%) cervical scrapes (Table 1; complete set), resulting in a near-perfect agreement overall and at the genotype level (with a kappa value of 0.85 (95% confidence interval (CI) 0.64–1.00).
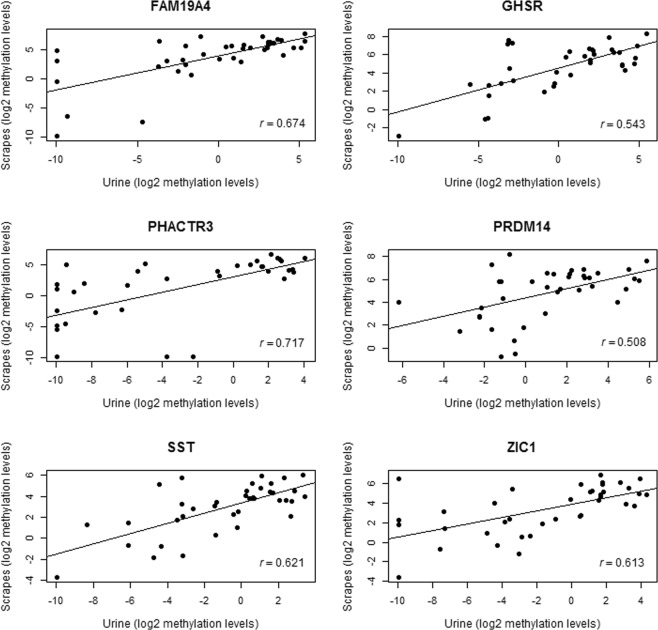
For the DNA methylation markers we obtained a moderate to strong correlation of DNA methylation levels between urine sediments and cervical scrapes (correlation coefficient varying from 0.508 (PRDM14) to 0.717 (PHACTR3); Fig. 2). Notably, the DNA methylation levels in cervical scrapes were considerably higher than those in urine (data not shown).
Figure 2.
Correlation between paired urine sediment and cervical scrapes from 38 women with cervical cancer. Log2 methylation levels of FAM19A4, GHSR, PHACTR3, PRDM14, SST and ZIC1 were used. Spearman correlation coefficient (r) values are shown.
Clinical performance of DNA methylation markers in urine sediments
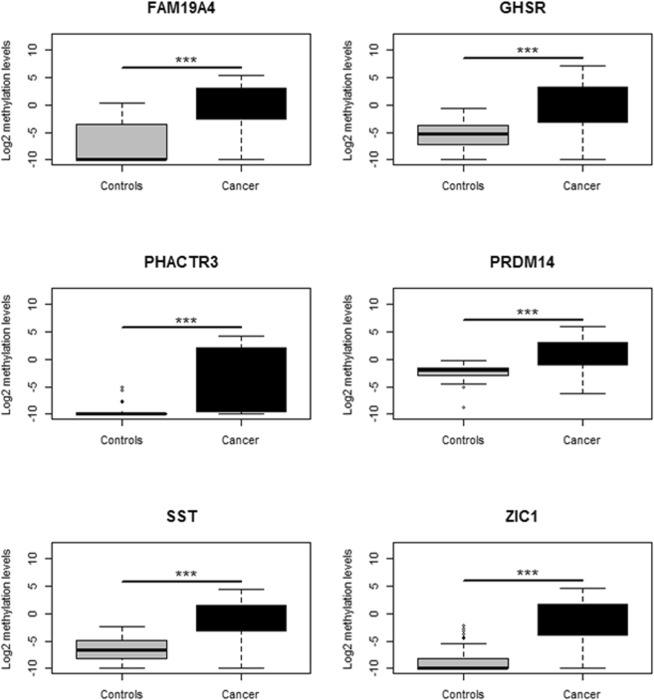
Subsequently, we assessed whether the 6 DNA methylation markers can discriminate between urine sediments from women with and without cervical cancer. Comparison of urine sediments from cervical cancer patients to controls revealed a significant increase in DNA methylation levels for all DNA methylation markers in cervical cancer patients (P < 0.001; Fig. 3).
Figure 3.
Methylation levels of FAM19A4, GHSR, PHACTR3, PRDM14, SST and ZIC1 in urine sediments from 42 cervical cancer patients (black) and 42 controls (gray). Methylation levels were determined by multiplex qMSP and represented by the log2-transformed Cq ratios.
Next, we performed univariable logistic regression analysis and LOOCV to assess the performance of each DNA methylation marker in urine sediments. We found that all 6 DNA methylation markers had a high discriminatory power for cervical cancer detection with an AUC varying from 0.744 (PHACTR3) to 0.887 (SST) (Fig. 4). At the threshold corresponding to 80% specificity in controls, all 6 DNA methylation markers revealed a high sensitivity varying from 76% (FAM19A4 and PHACTR3) to 83% (PRDM14 and ZIC1) for cervical cancer detection in urine sediments. As shown in Table 2 all but one urine sample of cervical cancer patients tested positive for at least one out of 6 DNA methylation markers.
Figure 4.
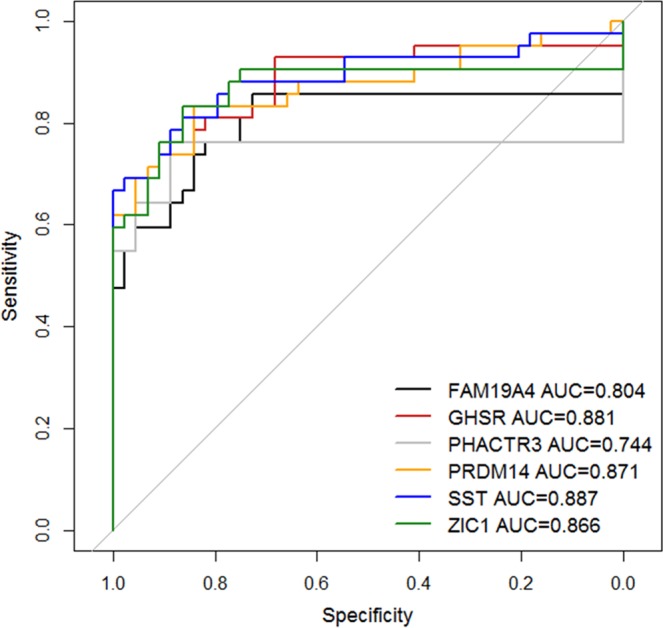
Receiver operating characteristics (ROC) curve and AUC of FAM19A4 (black), GHSR (red), PHACTR3 (gray), PRDM14 (orange), SST (blue) and ZIC1 (green) in urine sediments from 42 cervical cancer patients and 44 controls.
Table 2.
Overview of logistic regression results in urine sediments from cervical cancer patients.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FAM19A4 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| GHSR | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| PHACTR3 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| PRDM14 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| SST | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| ZIC1 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
Results from the 6 DNA methylation markers (FAM19A4, GHSR, PHACTR3, PRDM14, SST, and ZIC1) are defined as methylation-positive (indicated by X) and methylation-negative (white boxes) at the threshold corresponding to 80% specificity in controls. Numbers indicate cervical cancer patients and correspond to Table 1.
Discussion
Urine collection has been proposed as a promising alternative for cervical cancer screening when combined with hrHPV testing12. This study shows that DNA methylation marker testing is feasible on urine and enables the detection of cervical cancer. Comparison of hrHPV testing in urine sediments, native urines and cervical scrapes yielded a strong to near-perfect agreement (sediments versus native: kappa = 0.79; 95% CI 0.58–1.00, sediments versus scrapes: kappa = 0.85; 95% CI 0.64–1.00). Similarly, analysis of 6 DNA methylation markers (FAM19A4, GHSR, PHACTR3, PRDM14, SST and ZIC1)18,19 showed a strong correlation between DNA methylation levels detected in urine sediment versus native urine and versus cervical scrapes. All markers revealed a high discriminatory power for cervical cancer detection in urine sediments. Logistic regression analysis yielded AUCs ranging from 0.744 (PHACTR3) to 0.887 (ZIC1). This indicates the potential of hrHPV DNA and DNA methylation testing in urine for the detection of cervical cancer.
The near-perfect agreement (kappa = 0.88) of hrHPV detection in paired urine samples and cervical scrapes is in concordance with earlier research, in which agreement of hrHPV detection in urine and cervical scrapes varied from 70% to 96%25–33. These studies also indicated the need for optimization and standardization on urine sampling methods, storage conditions, and DNA testing. Of note, in our study 8 out of 42 urine sediments from cervical cancer patients were hrHPV-negative, while for 4 patients the paired cervical scrape was hrHPV-positive. Since higher hrHPV detection rates in cervical samples as compared to urine are commonly observed34, this likely explains discrepancies in hrHPV-positivity between urine sediments and cervical scrapes. Testing of the corresponding tissue specimens for the 4 patients that were hrHPV-negative in both urine and cervical samples confirmed a hrHPV-negative test result in three cases (data not shown). Interestingly, all 8 hrHPV-negative urine samples tested methylation-positive for at least 1 DNA methylation marker.
In order to optimize methods, we evaluated detection of hrHPV and DNA methylation markers in urine sediments and native urines and found no differences. The decision to proceed with urine sediments instead of native urine samples was based on practical considerations, such as ease of storage and costs of DNA isolation.
We did not standardize timing of urine collection (first void, midstream or random), as opposed to previous studies35,36. Pathak and colleagues (2014) demonstrated that the sensitivity of the hrHPV test could increase when first void urine samples are used35. However, results must be interpreted with caution due to heterogeneity in testing methods and results of individual studies. A different definition on “first void” is used in individual studies, as some defined it as the initial stream (correct definition) while others as the first urine sample of the day.
To the best of our knowledge, only three studies compared hrHPV detection in different urine samples in the same population of women. Senkomago and colleagues (2016) found in 27 hrHPV-positive women, no differences in hrHPV detection for first-void, initial stream, and mid-stream urine for sediments37. However, Vorsters (2014) found in a pilot study of 10 hrHPV-positive women higher hrHPV DNA detection in first void than mid-stream urine38. Leeman and colleagues (2017) reported that physician-taken smears, brush-based self-sampling, morning first-void urine and first-void urine from later during the day showed similar high sensitivity for detection of CIN2+ in their referral population, measured with two different hrHPV tests39.
Similar to our hrHPV results, we also showed a strong correlation between DNA methylation levels detected in urine sediment versus native urine (r = 0.691 to 0.9) and in urine sediment versus cervical scrapes (r = 0.508 to 0.717).
Two previous studies report on DNA methylation analysis in urine for the detection of cervical cancer, but did not compare urine fractions and included other DNA methylation markers40,41. Feng and colleagues (2007) found a moderate to high agreement for detection of DNA methylation in urine and cervical scrapes, varying from 49% (DAPK1) and 59% (RARB) to 80% (TWIST1; CDH13)40, which is consistent with our findings. At least one of the four genes was positive for hypermethylation in 62% of urine from cervical cancer cases, compared with 97% positive for at least one of the six markers in our study. Moreover, in our study, only one out of 42 urine samples from cervical cancer patients was methylation-negative for all six markers.
At the individual gene level all six markers had a high discriminatory power for cervical cancer detection in urine sediments (AUC varying from 0.744 (PHACTR3) to 0.887 (SST). When it can be confirmed that these DNA methylation markers allow for the detection of clinically relevant precancerous lesions as well, urine-based DNA methylation testing comes into reach for cervical screening. In fact, recent data showed that analysis of a 4-gene methylation classifier (ZNF516, FKBP6, INTS1 and HPV16-L1) in urine resulted in an AUC of 0.86 when comparing women with CIN1 or no cervical neoplasia (NILM) to CIN2+ lesions41.
The advantages of cervical screening using urine over conventional cervical scrapes and self-samples relate to the fact that use of urine is expected to largely increase screening attendance9,12,35,42. Furthermore, the use of urine for primary hrHPV-based screening offers an easily manageable manner of sampling13.
A study limitation includes the fact that the control urine samples were obtained from women for which we lack knowledge on prior HPV and disease status. Besides the inclusion of better defined controls, e.g. from a regular screening population, further studies including women with cervical precancerous lesions are needed to determine the potential of urine-based cervical screening. Moreover, further standardization of sample collection and optimization of hrHPV testing and DNA methylation marker selection is warranted.
In conclusion, our study demonstrates a good agreement of both urine-based HPV DNA and urine-based DNA methylation analysis with reference cervical samples, and shows the feasibility of DNA methylation testing in urine for the detection of cervical cancer.
Acknowledgements
This work was in part supported by an unrestricted grant of HANARTH Foundation. The source of funding did not have any influence on the design of the study, collection, analysis and interpretation of the data and in writing the manuscript.
Author Contributions
R.S. and N.v.T. were involved in the conceptualization of the study. A.v.S., N.v.T., D.H., M.B., M.v.G., W.V., H.S. and W.R. were involved in sample and data collection. B.S., A.v.S. R.S., N.v.T., M.B., M.v.R. and D.H., were involved in data interpretation. B.S. performed the statistical analysis. B.S., R.S., N.v.T. and M.v.R. drafted the manuscript. All authors critically reviewed the manuscript and approved the final version.
Data Availability
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
D. Heideman and R. Steenbergen have a minority stake in Self-screen B.V., a spin-off company of VU University Medical Center Amsterdam, which owns patents related to this work. D. Heideman has been on the speaker’s bureau of Qiagen, and serves occasionally on the scientific advisory board of Pfizer and Bristol-Meyer Squibb. All other authors declare that they have no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nienke E. van Trommel and Renske D. M. Steenbergen jointly supervised this work.
References
- 1.Ferlay J, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int. J. Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 3.De Strooper LMA, et al. Methylation analysis of the FAM19A4 gene in cervical scrapes is highly efficient in detecting cervical carcinomas and advanced CIN2/3 lesions. Cancer Prev. Res. 2014;7:1251–1257. doi: 10.1158/1940-6207.CAPR-14-0237. [DOI] [PubMed] [Google Scholar]
- 4.Verhoef VMJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): A randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315–322. doi: 10.1016/S1470-2045(14)70019-1. [DOI] [PubMed] [Google Scholar]
- 5.Luttmer R, et al. FAM19A4 methylation analysis in self-samples compared with cervical scrapes for detecting cervical (pre)cancer in HPV-positive women. Br. J. Cancer. 2016;115:579–587. doi: 10.1038/bjc.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brentnall AR, et al. HPV33 DNA methylation measurement improves cervical pre-cancer risk estimation of an HPV16, HPV18, HPV31 and \textit{EPB41L3} methylation classifier. Cancer Biomarkers. 2015;15:669–675. doi: 10.3233/CBM-150507. [DOI] [PubMed] [Google Scholar]
- 7.Yin A, et al. JAM3 methylation status as a biomarker for diagnosis of preneoplastic and neoplastic lesions of the cervix. Oncotarget. 2015;6:44373–87. doi: 10.18632/oncotarget.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzuba IG, et al. The Acceptability of Self-Collected Samples for HPV Testing vs. the Pap Test as Alternatives in Cervical CancerScreening. J. Womens. Health Gend. Based. Med. 2002;11:265–275. doi: 10.1089/152460902753668466. [DOI] [PubMed] [Google Scholar]
- 9.Sellors JW, et al. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ. 2000;163:513–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Rozemeijer K, et al. Offering Self-Sampling to Non-Attendees of Organized Primary HPV Screening: When Do Harms Outweigh the Benefits? Cancer Epidemiol. Biomarkers Prev. 2015;24:773–82. doi: 10.1158/1055-9965.EPI-14-0998. [DOI] [PubMed] [Google Scholar]
- 11.Gök M, et al. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ. 2010;340:c1040. doi: 10.1136/bmj.c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vorsters A, et al. Detection of human papillomavirus DNA in urine. A review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:627–640. doi: 10.1007/s10096-011-1358-z. [DOI] [PubMed] [Google Scholar]
- 13.Fontenot HB. Urine-Based HPV Testing as a Method to Screen for CervicalCancer. Nurs. Womens. Health. 2015;19:59–65. doi: 10.1111/1751-486X.12176. [DOI] [PubMed] [Google Scholar]
- 14.Dijkstra MG, et al. Cervical cancer screening: on the way to a shift from cytology to full molecular screening. Ann. Oncol. 2014;25:927–935. doi: 10.1093/annonc/mdt538. [DOI] [PubMed] [Google Scholar]
- 15.Bosschieter J, et al. The diagnostic accuracy of methylation markers in urine for the detection of bladder cancer: a systematic review. Epigenomics. 2018;10:673–687. doi: 10.2217/epi-2017-0156. [DOI] [PubMed] [Google Scholar]
- 16.Moreira-Barbosa C, et al. Comparing diagnostic and prognostic performance of two-gene promoter methylation panels in tissue biopsies and urines of prostate cancer patients. Clin. Epigenetics. 2018;10:132. doi: 10.1186/s13148-018-0564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brikun I, et al. A panel of DNA methylation markers for the detection of prostate cancer from FV and DRE urineDNA. Clin. Epigenetics. 2018;10:91. doi: 10.1186/s13148-018-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verlaat W, et al. Genome-wide DNA Methylation Profiling Reveals Methylation Markers Associated with 3q Gain for Detection of Cervical Precancer and Cancer. Clin. Cancer Res. 2017;23:3813–3822. doi: 10.1158/1078-0432.CCR-16-2641. [DOI] [PubMed] [Google Scholar]
- 19.Steenbergen RD, et al. Methylation-specific digital karyotyping of HPV16E6E7-expressing human keratinocytes identifies novel methylation events in cervical carcinogenesis. J. Pathol. 2013;231:53–62. doi: 10.1002/path.4210. [DOI] [PubMed] [Google Scholar]
- 20.Human Tissue and Medical Research: Code of conduct for responsible use (2011).
- 21.Hesselink AT, et al. Clinical Validation of the HPV-Risk Assay, a Novel Real-Time PCR Assay for Detection of High-Risk Human Papillomavirus DNA by Targeting the E7 Region. J. Clin. Microbiol. 2014;52:890–896. doi: 10.1128/JCM.03195-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snellenberg S, et al. Development of a multiplex methylation-specific PCR as candidate triage test for women with an HPV-positive cervical scrape. BMC Cancer. 2012;12:551. doi: 10.1186/1471-2407-12-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol. Bull. 1968;70:213–20. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 24.Robin X, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, et al. Human papillomavirus DNA in urine samples of women with or without cervical cancer and their male partners compared with simultaneously collected cervical/penile smear or biopsy specimens. J. Clin. Virol. 2006;37:190–194. doi: 10.1016/j.jcv.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Payan C, et al. Human Papillomavirus Quantification in Urine and Cervical Samples by Using the Mx4000 and LightCycler General Real-Time PCR Systems. J. Clin. Microbiol. 2007;45:897–901. doi: 10.1128/JCM.02022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanzi E, et al. High performance of a new PCR-based urine assay for HPV-DNA detection and genotyping. J. Med. Virol. 2013;85:91–98. doi: 10.1002/jmv.23434. [DOI] [PubMed] [Google Scholar]
- 28.Brinkman JA, et al. Detection of human papillomavirus DNA in urine specimens from human immunodeficiency virus-positive women. J. Clin. Microbiol. 2002;40:3155–61. doi: 10.1128/JCM.40.9.3155-3161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendez K, et al. Urine-based human papillomavirus DNA testing as a screening tool for cervical cancer in high-risk women. Int. J. Gynecol. Obstet. 2014;124:151–155. doi: 10.1016/j.ijgo.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson DL, et al. Concordance of human papillomavirus in the cervix and urine among inner city adolescents. Pediatr. Infect. Dis. J. 2000;19:722–8. doi: 10.1097/00006454-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Stanczuk GA, et al. Detection of human papillomavirus in urine and cervical swabs from patients with invasive cervical cancer. J. Med. Virol. 2003;71:110–114. doi: 10.1002/jmv.10456. [DOI] [PubMed] [Google Scholar]
- 32.Cuschieri K, et al. Urine testing as a surveillance tool to monitor the impact of HPV immunization programs. J. Med. Virol. 2011;83:1983–1987. doi: 10.1002/jmv.22183. [DOI] [PubMed] [Google Scholar]
- 33.Alameda F, et al. Human Papillomavirus Detection in Urine Samples. J. Low. Genit. Tract Dis. 2007;11:5–7. doi: 10.1097/01.lgt.0000230204.65742.e4. [DOI] [PubMed] [Google Scholar]
- 34.Sehgal A, Gupta S, Parashari A, Sodhani P, Singh V. Urine HPV-DNA detection for cervical cancer screening: Prospects and prejudices. J. Obstet. Gynaecol. (Lahore). 2009;29:583–589. doi: 10.1080/01443610903061736. [DOI] [PubMed] [Google Scholar]
- 35.Pathak N, Dodds J, Zamora J, Khan K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ. 2014;349:g5264. doi: 10.1136/bmj.g5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson DJ, Calderaro AC, Roberts KA. Variation in Nuclear DNA Concentrations During Urination. J. Forensic Sci. 2007;52:110–113. doi: 10.1111/j.1556-4029.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- 37.Senkomago V, et al. Comparison of urine specimen collection times and testing fractions for the detection of high-risk human papillomavirus and high-grade cervical precancer. J. Clin. Virol. 2016;74:26–31. doi: 10.1016/j.jcv.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Vorsters A, et al. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:2005–2014. doi: 10.1007/s10096-014-2147-2. [DOI] [PubMed] [Google Scholar]
- 39.Leeman A, et al. HPV testing in first-void urine provides sensitivity for CIN2+ detection comparable with a smear taken by a clinician or a brush-based self-sample: cross-sectional data from a triage population. BJOG An Int. J. Obstet. Gynaecol. 2017;124:1356–1363. doi: 10.1111/1471-0528.14682. [DOI] [PubMed] [Google Scholar]
- 40.Feng Q, et al. Promoter Hypermethylation of Tumor Suppressor Genes in Urine from Patients with CervicalNeoplasia. Cancer Epidemiol. Biomarkers Prev. 2007;16:1178–1184. doi: 10.1158/1055-9965.EPI-06-0694. [DOI] [PubMed] [Google Scholar]
- 41.Guerrero-Preston R, et al. Molecular Triage of Premalignant Lesions in Liquid-Based Cervical Cytology and Circulating Cell-Free DNA from Urine, Using a Panel of Methylated Human Papilloma Virus and Host Genes. Cancer Prev. Res. 2016;9:915–924. doi: 10.1158/1940-6207.CAPR-16-0138. [DOI] [PubMed] [Google Scholar]
- 42.Kitchener HC, Owens GL. Urine testing for HPV. BMJ. 2014;349:g5542. doi: 10.1136/bmj.g5542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.