Abstract
Chronic granulomatous disease (CGD) is a primary immunodeficiency caused by mutations of the phagocytic nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Autosomal recessive p47phox-deficient CGD (p47phox CGD) is the second most frequent form of the disease in western countries, and more than 94% of patients have a disease-causing dinucleotide deletion (ΔGT) in the neutrophil cytosolic factor 1 (NCF1) gene. The ΔGT mutation is most likely transferred onto the NCF1 from one of its two pseudogenes co-localized on the same chromosome. The presence of NCF1 pseudogenes in healthy individuals makes the genetic diagnostics of ΔGT p47phox CGD challenging, as it requires the distinction between ΔGT in NCF1 and in the two pseudogenes. We have developed a diagnostic tool for the identification of p47phox CGD based on PCR co-amplification of NCF1 and its pseudogenes, followed by band intensity quantification of restriction fragment length polymorphism products. The single-day, reliable p47phox CGD diagnostics allow for robust discrimination of homozygous ΔGT p47phox CGD patients from heterozygous carriers and healthy individuals, as well as for monitoring gene therapy efficacy.
Keywords: chronic granulomatous disease, CGD, NCF1, NCF1B, NCF1C, pseudogene, genetic diagnostics
Introduction
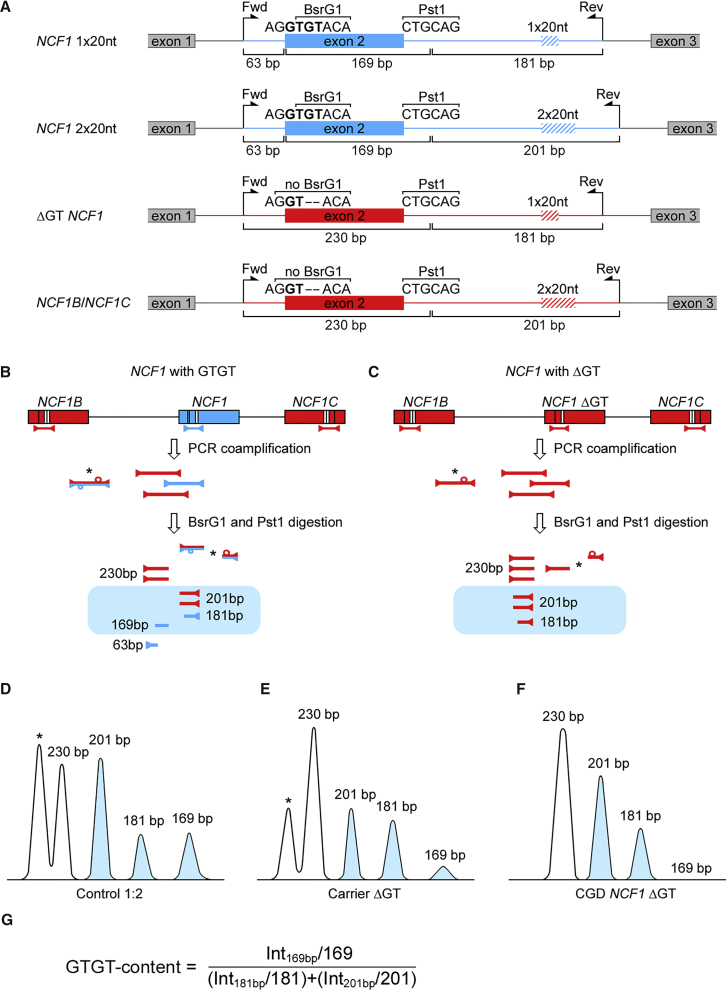
Chronic granulomatous disease (CGD) is a primary immunodeficiency of phagocytes, leading to recurrent severe bacterial and fungal infections due to impaired reactive oxygen species (ROS) production by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex.1 Disease-causing mutations are found in all NADPH oxidase subunits gp91phox, p47phox, p67phox, p22phox, and p40phox. In western countries, p47phox deficiency (p47phox CGD) is the second most frequent form of CGD. p47phox CGD is genetically exceptional, as 97% of patients share the same mutation, a dinucleotide deletion (ΔGT) within the GTGT sequence in exon 2 of the neutrophil cytosolic factor 1 (NCF1) gene.2 On chromosome 7, the NCF1 gene is accompanied by two pseudogenes (NCF1B and NCF1C) with 99.5% sequence homology to NCF1 (Figures 1A–1C).
Figure 1.
PCR-RFLP Analysis of NCF1 Loci
(A) Co-amplified fragments of the NCF1 gene and pseudogenes. Positions of the GT-dinucleotide, 20-nt repeat, BsrG1 and Pst1 restriction sites, and primer-binding sites (forward and reverse) are shown. (B) In healthy individuals and (C) in patients with the ΔGT mutation in NCF1, the PCR co-amplification of NCF1 (correct, blue; mutated, red) and its pseudogenes (red) results in a mixture of PCR products with a defined stoichiometry. In the majority of individuals, co-amplified PCR products differ by 2-nt of the GT-dinucleotide locus, and in 20-nt of the intronic 20-nt repeat sequence. A significant fraction of the mixture comprises cross-hybridized PCR products derived from NCF1 and the pseudogenes (marked with an asterisk) (Figures S2–S6). BsrG1 and Pst1 restriction digestion leads to the appearance of up to seven different restriction fragments in healthy individuals (A) and up to five fragments in patients with ΔGT deletion in NCF1 (B) (Figure 2A). (D–F) Typical densitometry images of digested fragments in a polyacrylamide gel of (D) a healthy individual with NCF1 gene to pseudogene ratio 1:2 (Control 1:2), (E) a carrier of the ΔGT mutation with NCF1 gene to pseudogene ratio 1:5 (Carrier ΔGT), and (F) a ΔGT p47phox CGD patient (CGD NCF1 ΔGT). (G) Size-normalized band intensities of 169-, 181-, and 201-bp fragments (B–F, blue) are used for calculation of the GTGT content and for identification of ΔGT p47phox CGD patients and ΔGT mutation carriers.
The ΔGT mutation is also present in both pseudogenes, in healthy individuals, in CGD patients, and in carriers. NCF1 ΔGT results from unequal cross-over events between NCF1 and one of its pseudogenes during DNA replication or repair, leading to partial pseudogene sequence transfer, including ΔGT, onto the NCF1 gene.3, 4, 5 Genetic diagnostics of ΔGT p47phox CGD are challenging, as it requires the distinction between ΔGT in NCF1 and in the pseudogenes. Currently, diagnosis of ΔGT p47phox CGD relies on the gene-scan method,6 which is based on the comparison of fluorescence intensities of short co-amplified sequences of NCF1 and its pseudogenes, which differ in size by the 2-nt of the ΔGT mutation. Other diagnostic methods are allele-specific hybridization5 and determination of the ΔGT:GTGT ratio by the TaqMan copy number variation (CNV) assay.7
Results and Discussion
We have developed a novel diagnostic tool for the identification of ΔGT p47phox CGD based on PCR co-amplification of NCF1 and its pseudogenes, followed by band intensity quantification of restriction fragment length polymorphism products (Figures 1B–1G). This 1-day method determines the NCF1 gene CNV by quantification of GTGT content in the NCF1 gene and pseudogene loci, and thus it detects the presence or absence of the ΔGT mutation within NCF1 gene and pseudogene alleles. It can be established in any molecular biology laboratory, and it allows for the robust discrimination of homozygous ΔGT p47phox CGD patients from heterozygous carriers and healthy individuals for rapid diagnostic purposes, as well as for the monitoring of NCF1 genome-editing-based gene therapy.8
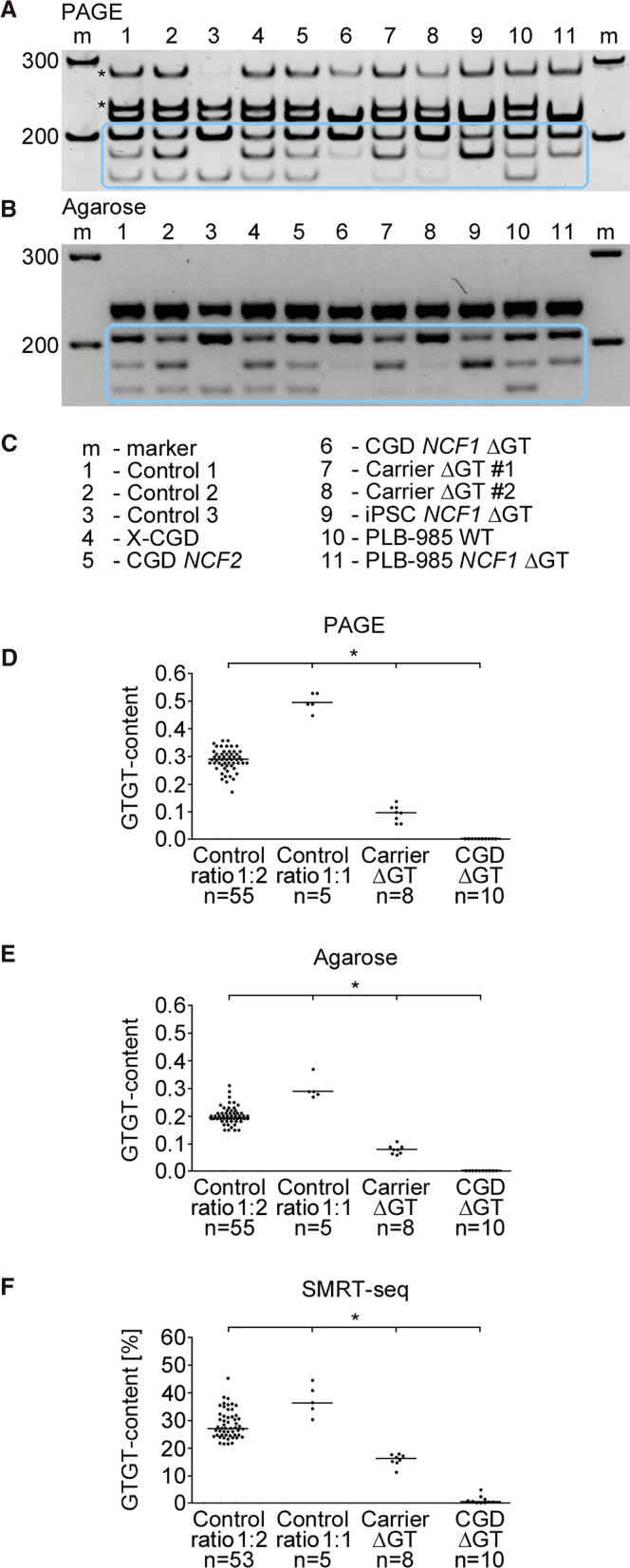
For quantification of the GTGT content, corresponding NCF1, NCF1B, and NCF1C sequences were co-amplified in one PCR reaction, digested, and visualized by PAGE or agarose electrophoresis (Figures 2A and 2B; detailed characterization of bands in polyacrylamide gel in Figures S2–S6). PCR-restriction fragment length polymorphism (RFLP) analysis was performed for 60 healthy individuals, 10 conventionally diagnosed ΔGT p47phox CGD patients, and 8 conventionally diagnosed ΔGT p47phox CGD carriers (see Table S1).
Figure 2.
GTGT Content Determination by PCR-RFLP
(A) PAGE and (B) agarose gel electrophoresis of BsrG1- and Pst1-digested PCR co-amplification products of the NCF1 loci (Figures 1B and 1C; full gel images available in Figure S7). The band of size 63-bp is not displayed in the gels. Bands of 201-, 181-, and 169-bp (blue box) were used to determine the GTGT content (Figure 1G). (C) List of samples presented in (A) and (B). Controls 1–3, GTGT sequence in both NCF1 gene alleles; Control 1, a single 20-nt intronic repeat (1 × 20 nt) in two and a double 20-nt repeat (2 × 20 nt) in four NCF1 alleles; Control 2, 1 × 20 nt in three of six NCF1 alleles whereas 2 × 20 nt in three remaining NCF1 alleles; Control 3, all six NCF1 alleles contain the 2 × 20 nt; X-CGD and CGD NCF2, gp91phox- and p67phox- deficient CGD, respectively; carrier ΔGT, heterozygous ΔGT mutation in NCF1; iPSC NCF1 ΔGT, induced pluripotent stem cell line with a homozygous ΔGT mutation in NCF1 (see Jiang et al.9); human acute myeloid leukemia cell line PLB-985 (wild-type); and a cellular model of ΔGT p47phox CGD, PLB-985 NCF1 ΔGT (see Wrona et al.8). (D and E) The GTGT content in polyacrylamide (D) and agarose gels (E). (F) The GTGT content determined by SMRT sequencing. Control ratio 1:2 (Figure S1A), the GTGT sequence in NCF1 and the ΔGT in NCF1B and NCF1C; Control ratio 1:1 (Figure S1B), the GTGT sequence in NCF1 and in one of four NCF1 pseudogene alleles. (D–F) Horizontal lines represent median values. *p < 0.01, n, number of samples.
The co-amplification PCR products spanned the NCF1 gene and pseudogene GTGT locus within exon 2, as well as the surrounding intronic regions containing one or two repeats of a 20-nt sequence (1 × 20 nt or 2 × 20 nt; Figure 1A). Figure 1 displays possible DNA sequence variations of these loci, their configuration on chromosome 7, corresponding PCR co-amplification products, as well as PCR-RFLP results. CGD patients with homozygous ΔGT mutation in NCF1 can be identified by electrophoresis based on the absence of the 169-bp band (Figures 1C, 1F, and 2A, and 2B). The intensity of the 169-bp band was substantially weaker in heterozygous carriers. The absence of the 181-bp band (Figure 2, control 3) was observed in individuals who had two copies of the 20-nt repeat in intron 2 in all NCF1 gene and pseudogene alleles, a genotype that may be observed in healthy individuals. Calculation of the GTGT content (Figure 1G) allowed for the differentiation between NCF1 ΔGT mutation carriers and healthy individuals. Representative PCR-RFLP samples developed by PAGE and agarose electrophoresis (Figures 2A–2C) compare controls (healthy individuals with GTGT in two NCF1 alleles and different 20-nt intronic repeat numbers), X-CGD and autosomal recessive CGD NCF2 (gp91phox and p67phox deficiency, respectively), two NCF1 ΔGT carriers (Carrier ΔGT #1 and Carrier ΔGT #2), an induced pluripotent stem cell (iPSC) NCF1 ΔGT cell line,9 a human acute myeloid leukemia cell line PLB-985 (wild-type), and a PLB-985 NCF1 ΔGT cell line.8
The median GTGT content in 55 healthy individuals who carried two NCF1 gene alleles with the GTGT sequence and the ΔGT mutation in all four pseudogene alleles (Control ratio 1:2; Figure S1A) was 0.29 in the polyacrylamide gel and 0.20 in the agarose gel. With NCF1 allele PCR co-amplification, followed by single-molecule real-time (SMRT) sequencing (Table S1), we identified five healthy individuals with two functional NCF1 alleles with GTGT sequence plus one of four pseudogene alleles with GTGT sequence (Control ratio 1:1; Figure S1B). In these individuals, the determined GTGT content values were 0.49 and 0.29 in polyacrylamide and in agarose gel, respectively. The observed results in agarose gels were lower than theoretical values expected by the genetic background, which may be explained by a partial loss of signal intensity in the thick agarose gels. GTGT content values established for 8 ΔGT p47phox CGD carriers, in which one NCF1 allele carries the ΔGT mutation, was 0.10 in polyacrylamide gel (Figure 2D) and 0.08 in agarose gel (Figure 2E; data for individual samples and statistics in Table S1).
The results of the PCR-RFLP analysis were confirmed by SMRT sequencing: undigested pools of barcoded co-amplification PCR products (Figures 1A and 1B) were sequenced, and the frequencies of GTGT signature-containing reads were calculated (Figure 2F; Table S1). GTGT sequence was identified in 27% of reads from healthy individuals with two GTGT-carrying NCF1 alleles (Control ratio 1:2), in 37% of reads from healthy individuals with GTGT within two NCF1 alleles and one of four pseudogene alleles (Control ratio 1:1), and in 16% of reads from NCF1 ΔGT carriers. Percentage differences in the GTGT signature-containing reads were statistically significant, and they allowed for the discrimination of healthy individuals from NCF1 ΔGT carriers and CGD patients.
In conclusion, we propose a package of complementary methods to be used for single-day reliable p47phox CGD diagnostics, based on PCR-RFLP, giving comparable results to SMRT sequencing. Furthermore, the PCR-RFLP diagnostic method represents an attractive alternative to the existing methods used in CGD diagnostics in terms of appliance requirements and costs per tested sample (Table 1). In addition to diagnostics, both methods can be also effectively applied for the assessment of correction of the ΔGT mutation upon genome-editing-based gene therapy.
Table 1.
Comparison of Methods Used for ΔGT p47phox CGD Diagnostics
| Method | Time (Days) | Primer Labeling | Equipment | Cost |
|---|---|---|---|---|
| PCR-RFLP (new method described in this article) | 1* | no* | PAGE or agarose electrophoresis system* | * |
| Gene scan | 1* | yes (fluorescently labeled)** | DNA sequencer*** | *** |
| Allele-specific hybridization | 2** | yes (32P oligonucleotides)** | autoradiography equipment* | ** |
| TaqMan CNV | 1* | yes (fluorescently and MGB-labeled probe)*** | qPCR instrument*** | *** |
Asterisks indicate time and resource requirements (*lowest, ***highest). MGB, minor groove binder.
Materials and Methods
DNA Isolation and PCR Amplification
Sample processing was covered by ethical vote KEK ZH 2015/0135, BASEC-Nr. PB_2016-02202. Genomic DNA from healthy individuals, diagnosed ΔGT p47phox CGD patients, and their family members was isolated using DNeasy Blood & Tissue Kit (QIAGEN, Hombrechtikon, Switzerland). The 411- to 433-bp fragments of NCF1, NCF1B, and NCF1C genes were PCR co-amplified using published PCR primers6 (Microsynth, Balgach, Switzerland). Phusion High-Fidelity DNA Polymerase and deoxyribonucleotides (dNTPs) were from Thermo Fisher Scientific (Reinach, Switzerland). PCR reaction included GC 10× buffer, dNTPs (200 μM each), primers (240 nM each), 0.04 U/μL Phusion High-Fidelity DNA Polymerase, and 2.5 ng/μL DNA. Initial 3-min denaturation (95°C) was followed by 36–40 cycles of denaturation (95°C, 30 s), annealing (65°C, 30 s), and elongation (72°C, 15 s) and final elongation (72°C, 3 min).
Determination of the GTGT Content by RFLP
The PCR co-amplification products of NCF1, NCF1B, and NCF1C were digested with BsrG1 and Pst1 (New England Biolabs, Frankfurt am Main, Germany) (37°C, 180 min), followed by enzyme inactivation (80°C, 20 min) (Figures 1A–1C). The digestion fragments were developed in a 7.5% polyacrylamide (ratio 29:1) gel or a 5% (w/v) agarose gel stained with GelRed Nucleic Acid Gel Stain (Biotum, Fremont, CA, USA) and visualized using Gel Logic 100 Imaging System (Kodak, Eysins, Switzerland). Band intensities were quantified with ImageJ software.10 For determination of the GTGT content (Figure 1G), RFLP band intensities of 169-, 181-, and 201-bp BsrG1/Pst1 digestion products (Figures 1D–1F, 2A, and 2B) were size normalized by dividing band intensities by their length (number of base pairs). The size-normalized band intensity of the 169-bp band was divided by the sum of normalized band intensities of the 181- and 201-bp bands (Figure 1G).
SMRT Sequencing
PCR co-amplification products of the NCF1 gene and its pseudogenes were produced using individually barcoded Fwd1 primers, utilizing PCR conditions described above. PCR products were purified using the QIAquick Gel Purification Kit (QIAGEN). 20 ng gel-purified barcoded PCR products of individual subjects were pooled and analyzed with SMRT sequencing11 by Functional Genomics Center Zurich, ETH/University of Zurich, Switzerland, as described.8
Statistical Analysis
The Kruskal-Wallis tests with post hoc Dunn’s multiple comparison tests were performed using IBM SPSS Statistics version 23.0 (IBM, Armonk, NY, USA).
Author Contributions
D.W. conducted experiments. D.W., U.S., and J.R. designed experiments, analyzed data, made figures, and wrote the manuscript.
Conflicts of Interest
The described diagnostic tool has been submitted for intellectual property (IP) filing.
Acknowledgments
This study was supported by the CGD Society (grant CGDS16/01) and Hochspezialisierte Medizin Schwerpunkt Immunologie (HSM-2-Immunologie). D.W. received a research grant from the University of Zurich (Forschungskredit, grant FK-17-041). J.R. is supported by the Uniscientia Foundation and the Clinical Research Priority Program ImmuGene of the University of Zurich. We thank the Functional Genomics Center Zurich for performing SMRT sequencing and data analysis.
Footnotes
Supplemental Information includes seven figures and two tables and can be found with this article online at https://doi.org/10.1016/j.omtm.2019.02.001.
Supplemental Information
References
- 1.Segal B.H., Leto T.L., Gallin J.I., Malech H.L., Holland S.M. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Noack D., Rae J., Cross A.R., Ellis B.A., Newburger P.E., Curnutte J.T., Heyworth P.G. Autosomal recessive chronic granulomatous disease caused by defects in NCF-1, the gene encoding the phagocyte p47-phox: mutations not arising in the NCF-1 pseudogenes. Blood. 2001;97:305–311. doi: 10.1182/blood.v97.1.305. [DOI] [PubMed] [Google Scholar]
- 3.Hayrapetyan A., Dencher P.C.D., van Leeuwen K., de Boer M., Roos D. Different unequal cross-over events between NCF1 and its pseudogenes in autosomal p47(phox)-deficient chronic granulomatous disease. Biochim. Biophys. Acta. 2013;1832:1662–1672. doi: 10.1016/j.bbadis.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Roesler J., Curnutte J.T., Rae J., Barrett D., Patino P., Chanock S.J., Goerlach A. Recombination events between the p47-phox gene and its highly homologous pseudogenes are the main cause of autosomal recessive chronic granulomatous disease. Blood. 2000;95:2150–2156. [PubMed] [Google Scholar]
- 5.Vázquez N., Lehrnbecher T., Chen R., Christensen B.L., Gallin J.I., Malech H., Holland S., Zhu S., Chanock S.J. Mutational analysis of patients with p47-phox-deficient chronic granulomatous disease: The significance of recombination events between the p47-phox gene (NCF1) and its highly homologous pseudogenes. Exp. Hematol. 2001;29:234–243. doi: 10.1016/s0301-472x(00)00646-9. [DOI] [PubMed] [Google Scholar]
- 6.Dekker J., de Boer M., Roos D. Gene-scan method for the recognition of carriers and patients with p47(phox)-deficient autosomal recessive chronic granulomatous disease. Exp. Hematol. 2001;29:1319–1325. doi: 10.1016/s0301-472x(01)00731-7. [DOI] [PubMed] [Google Scholar]
- 7.Heyworth P.G., Noack D., Cross A.R. Identification of a novel NCF-1 (p47-phox) pseudogene not containing the signature GT deletion: significance for A47 degrees chronic granulomatous disease carrier detection. Blood. 2002;100:1845–1851. doi: 10.1182/blood-2002-03-0861. [DOI] [PubMed] [Google Scholar]
- 8.Wrona D., Siler U., Reichenbach J. CRISPR/Cas9-generated p47phox-deficient cell line for Chronic Granulomatous Disease gene therapy vector development. Sci. Rep. 2017;7:44187. doi: 10.1038/srep44187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y., Cowley S.A., Siler U., Melguizo D., Tilgner K., Browne C., Dewilton A., Przyborski S., Saretzki G., James W.S. Derivation and functional analysis of patient-specific induced pluripotent stem cells as an in vitro model of chronic granulomatous disease. Stem Cells. 2012;30:599–611. doi: 10.1002/stem.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levene M.J., Korlach J., Turner S.W., Foquet M., Craighead H.G., Webb W.W. Zero-mode waveguides for single-molecule analysis at high concentrations. Science. 2003;299:682–686. doi: 10.1126/science.1079700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.