Abstract
Fungal peritonitis is frequent on peritoneal dialysis, with rare cases by Exophiala dermatitidis. A 25-month-old female admitted to the pediatric ICU with acute renal failure was submitted to peritoneal dialysis. After 10 days patient presented fever. Peritoneal fluid culture showed yeast colonies molecularly identified as E. dermatitidis. Patient was treated with voriconazole and hemodialysis. The literature was reviewed. Disseminated infections are frequently fatal, but appropriate diagnose and therapeutic led to cure in this case.
Keywords: Phaeohyphomycosis, Fungal infection, Dialysis, Therapeutic management, Molecular diagnosis
1. Introduction
Phaeohyphomycosis covers a heterogeneous group of mycotic infections caused by dematiaceous fungi and characterized by the presence of melanized hyphal elements, yeast-like cells, or a combination of these in the host tissue. According to the extent and depth of invasion, the disease is classified as superficial, subcutaneous, systemic and cerebral [1]. Among the more important human pathogens are species of the genera of the order Chaetothyriales (black yeasts and relatives), such as Cladophialophora, Exophiala, Fonsecaea, Phialophora [1,2]. Some of the agents of phaeohyphomycosis are commonly encountered in nature as plant saprobes and nearly always infect immunocompromised patients, while members of the order Chaetothyriales mostly occupy hostile habitats and are able to cause infections both in immunocompromised and immunocompetent individuals [1]. Averaged over all melanized fungi, the number of cases has increased particularly in immunosuppressed patients [[1], [2], [3]].
A considerable number of cases have been published of cerebral phaeohyphomycosis in immunocompetent patients with no obvious risk factors identified [1]. Opportunistic fungus pathogens may lead to infection if additional predisposing factors are present [2]. As reported by Alabaz et al. [3], the risk of infection may also depend on the kind of contact, the fungal mass associated, and the patient's immune status. Malnutrition, genetic factors, and cystic fibrosis all have been established as risk factors for the respiratory tract colonization and dissemination. Although cerebral infection is the prevalent form of systemic phaeohyphomycosis, other localized deep forms of the disease such as arthritis and endocarditis have been reported. Disseminated infection is uncommon, but its incidence is increasing, particularly among immunocompromised individuals [1].
Cutaneous, subcutaneous, and corneal infections by members of Chaetothyriales occur worldwide, but are more common in tropical and subtropical climates. Traumatic cases generally occur in immunocompetent individuals. Another possible route of infection is through inhalation [1,2,4].
The genus Exophiala comprises a significant number of species potentially causing a variety of infections. The main species involved are Exophiala dermatitidis, E. xenobiotica and E. oligosperma [4]. Exophiala (Wangiella) dermatitidis presents high phenotypic plasticity, growing as yeast, with hyphae, or in meristematic cells [5]. Melanin in its cells walls and extracellular polysaccharides are important virulence factors of E. dermatitidis. The species is recognized as a dermatotropic and neurotropic agent, being the causative agent of cutaneous, subcutaneous and systemic infections [1].
Disseminated E. dermatitidis infections are almost always fatal [6]. Although fungal peritonitis is a frequent complication in cases of peritoneal dialysis [7], E. dermatitidis peritonitis is not common [[8], [9], [10]]. Given the risk of dissemination, careful management is required. This is the first report of E. dermatitidis peritonitis in a pediatric patient. The literature was reviewed with an overview of 110 clinical cases (Table S1).
2. Case
A 2-year-old female patient was admitted in our hospital with a 4-day history of diarrhea and vomiting. Her mother denied fever, mucus, blood or pus in the faeces. Due to significant reduction of diuresis, she was admitted to the pediatric ICU in oligoanuria. At initial physical examination the patient was in good general condition, pale, hydrated, active, reactive, irritated, with a blood pressure of 178/114 mm Hg and hypothermic (temperature of 35.5 °C).
Laboratory tests showed anemia (Hb 4.5 g/dL), kidney failure and high levels of serum potassium (urea 357 mg/dL, creatinine 9.8 mg/dL, K 7.0 mmol/L). Accordingly, patient was diagnosed with Acute Renal Failure due to Hemolytic Uremic Syndrome. The patient needed red blood cell transfusion, bicarbonate replacement, blood pressure control and renal replacement therapy. Peritoneal dialysis was initiated, being performed for 16 days due to anuria. On the tenth day, patient developed abdominal pain and fever (day 0). During screening, the analysis of peritoneal fluid showed: 437 cells/mm3, 389 leukocytes/mm3 (29% neutrophils and 59% lymphocytes), pH 7.68, glucose 243 mg/dL, protein 1.4 g/dL and LDH 215 U/L.
Samples of peritoneal fluid were sent to the Laboratory of Clinical Analysis for culture of microorganisms. Direct microscopic examination revealed yeast cells. Treatment was started with fluconazole intravenous, 6 mg/kg/day (day +4). Hemodialysis was started replacing the peritoneal dialysis. The sample was inoculated into flasks with BACTEC Plus Aerobic/FBD culture medium (Becton-Dickinson, Sparks, U.S.A.) and incubated in BACTEC FX equipment (Becton-Dickinson). After six days of incubation growth of microorganisms was observed. An aliquot was seeded on blood agar and colonies developed with smooth and waxy appearance, olive green in colour (Fig. 1A).
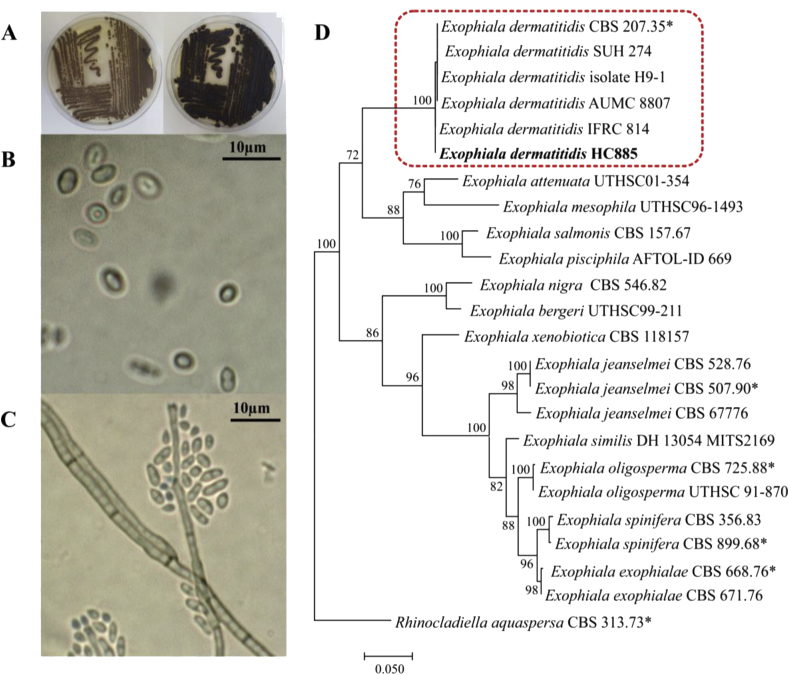
Fig. 1.
Identification of Exophiala dermatitidis strain HC885 isolated from peritoneal fluid. A: Macromorphology showing the greyish to olive colonies; B and C: Micromorphology, presence of yeasts cells and dark septate hyphae with conidia around phialide openings; D: Phylogenetic tree based on confidently aligned rDNA Internal Transcribed Spacer (ITS) sequences constructed with Maximum likelihood implemented in MEGA v.7. Bootstrap values > 80% from 100 resampled datasets are shown with the branches.
The catheter content sample was inoculated on Sabouraud, Mycosel and BHI media, and incubated at 37 °C. Fungal growth was observed on all three media. Initially, the colonies presented mucoid growth, with moist and yeast-like appearance. Subsequently, colonies developed greyish to olive mycelium, and the colony reverse became dark grey (Fig. 1A). Microscopically, yeasts were present (Fig. 1B), in addition to dark, septate hyphae with few annellated zones with circular heads of globose to ellipsoidal conidia (Fig. 1C). The morphology was consistent with descriptions of the species [5], but molecular methods were used to head the definitive diagnosis (Fig. 1D).
The fungal isolated was identified by Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) using Vitek MS (bioMerieux, Durham, NC) platform. In consonance with the manufacturer's instructions, the direct colony technique was used for applying the isolate on the target slide. After application of the isolate, 0.5 μL of formic acid was added and maintained at room temperature until completely dried. A volume of 1 μL of CHCA matrix was added to the spot. Analysis was performed in duplicate. Spectra were analysed using VITEK MS Myla software IVD database version 2.0. Both spots were identified Exophiala dermatitidis with confidence value of 99.9.
DNA extraction was performed using glass beads (Sigma G9143) based on the protocol described previously [11]. The rDNA Internal Transcribed Spacer (ITS) was amplified and sequenced using primers ITS1 and ITS4. Sequences were analysed on an ABI Prism 3700 DNA Sequencer (Perkin-Elmer, Norwalk, Foster City, U.S.A.). For phylogenetic analysis, the alignment of obtained sequences was performed according to Gomes et al. [11] using the online MAFFT interface. A tree was constructed with 100 bootstrap replicates using Maximum Likelihood Implemented in Mega v.7 software applying the best evolutionary model to this dataset (Fig. 1 D). The clinical strain clustered with 100 bootstrap supports in the E. dermatitidis clade with 99.8% of similarity to the type strain CBS 207.35 (accession number NR 121268).
Once the etiological agent was identified, the therapy was reviewed, replacing fluconazole by voriconazole IV, 4 mg/kg/dose 12/12hs (day +10). The removed catheter as well as other samples of peritoneal fluid were analysed confirming the presence of E. dermatitidis. Additional investigations with cranial tomography, chest X-ray, abdominal ultrasound, echocardiogram and fundoscopy exam were with normal limits. Following 14 days of treatment with voriconazole, with requirement of three hemodialysis sessions, the patient showed positive outcome in renal function, but blood pressure controls showed hypertension. She was discharged after 37 days of hospitalization with prescription of antihypertensives (amlodipine and propranolol), and ambulatory follow-up.
Clinical cases published of E. dermatitidis infection reported in literature since 1977 (date of species description by de Hoog and Hermanides-Nijhof) to 2018 were revised. The cases were searched in PubMed using search terms (i) Exophiala dermatitidis, (ii) Exophiala dermatitidis infection, (iii) Wangiella dermatitidis and (iv) Exophiala. In addition, we also reviewed cases reported in clinical reports reviews; results are presented as supplementary material (Table S1).
3. Discussion
Several extensive reviews on Phaeohyphomycosis caused by black fungi have been published during the last decades. Gostincar et al. [12] showed that members of the order Chaetothyriales are exceptional among black fungi by their polyextremotolerance determining their opportunistic potential. The order contains species that show relatively frequent opportunism in healthy patients; particularly members of the genera Exophiala, Cladophialophora, Phialophora, and Ramichloridium are commonly involved with E. dermatitidis as one of the prevalent opportunists [[1], [2], [3]].
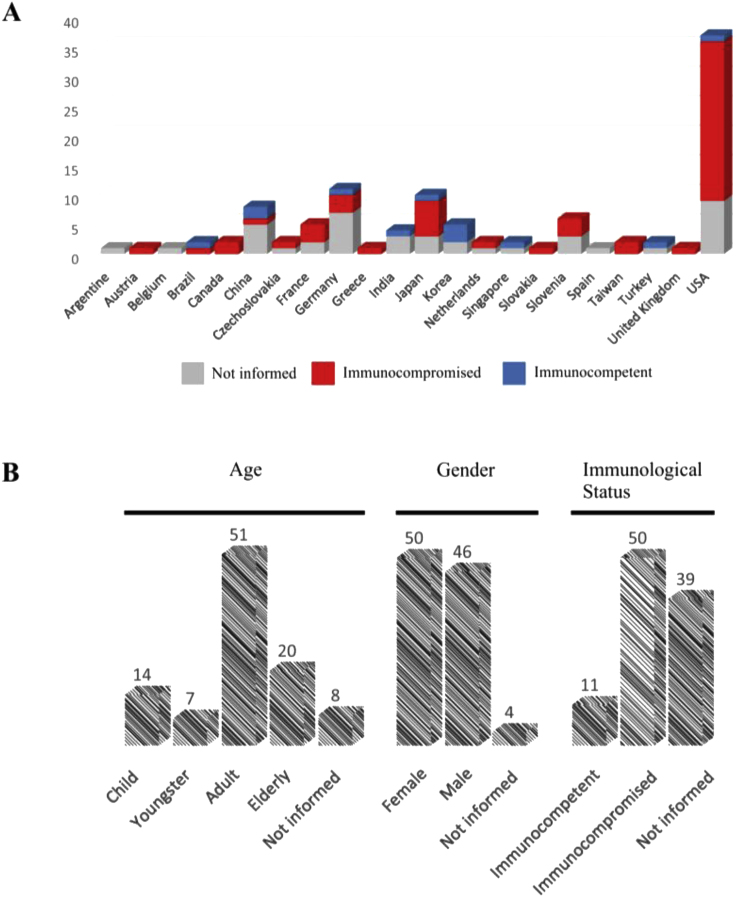
Exophiala dermatitidis has been found affecting different organs, causing superficial and cutaneous, but particularly subcutaneous tissue and disseminated infections, in the latter case with secondary cutaneous eruptions (Table S1). Sites of infection commonly are lymph nodes, lungs and internal organs. Phaeohyphomycosis associated with E. dermatitidis has been reported around the world, especially in Europe and the USA, with most cases occurring in immunocompromised patients (Fig. 2A). As shown in Fig. 2B, the majority of these patients are adults (51%) with an equivalent number of cases registered in males and females. Invasive infections in immunocompetent human hosts are rare (11%). Disseminated infections are extremely rare and probably all associated with inherited immunological diseases such as CARD9 innate immune deficiency [2]. Other predisposing factors for invasive disease include peritoneal dialysis, steroid use, human immunodeficiency virus infection, cancer, bronchiectasis, and diabetes mellitus, demonstrating that numerous changes in patient's condition have been correlated with disease progression [13].
Fig. 2.
Exophiala dermatitidis human infections. (A): Geographic distribution of reported cases with regional immunological status; (B): percentage of cases related to age-groups, genre and immunological status.
In our overview of 110 cases (Table S1), E. dermatitidis infections were reported from the respiratory tract, pancreas, blood, ocular globe, central nervous system, intestines, heart and bone. We came across three reported cases of peritonitis caused by E. dermatitidis, all in adult patients. Our patient represents the first case reported in a child. Exophiala dermatitidis infections are very rare in pediatric (14%) and adolescent patients (7%). Hong et al. [14] reported a case of liver cirrhosis caused by E. dermatitidis in a previously healthy child, while a pulmonary infection in a child after allogenic stem cell transplantation was reported by Tanuskova et al. [13]. Recently, a case of phaeohyphomycosis breast infection in a teenage girl was reported [15]. In the present case, the patient developed a peritonitis infection despite the fact that the child was immunocompetent judging from cytological findings and medical history. The agent was not recovered from blood, and although we isolated the fungus only from aspiration fluid, we did not find evidence of dissemination. Paracentesis was crucial for culture and diagnosis of the fungal infection. Molecular identification assisted in correct diagnosis and treatment. In the present case, peritonitis remained after dialysis, indicating that the fungus infection can be associated with dialysis procedure.
Fungemia due to E. dermatitidis have been reported as being catheter-associated [16]. In general, the environmental source of infection is obscure, particularly when the patient is without either skin or mucous membrane lesions as visible portals of entry (Table S1). The fungus is likely to be inhaled with aerosols in bathing facilities [17] or might be ingested with the food, entering the digestive tract and possibly invading tissues via this route [18]. De Hoog et al. [19], based on a wide screening faecal samples (n = 2300) of humans with and without underlying disease, revealed intestinal presence of E. dermatitidis at a frequency of 5.2%. Although E. dermatitidis is not a common environmental saprobe, the fungus can be found in artificial, humid, low-nutrient habitats [2,18].
In our case there are evidences of infection related to peritoneum dialysis. The association of fungal infection to this procedure have been shown repeatedly in the literature on peritonitis in adult [7,[20], [21], [22]] and children [23], mostly caused by Candida species. Fungi commonly are involved in biofilm formation on indwelling and prosthetic materials, interfering with eradication the infection [22]. Therefore, after final diagnosis the catheter as likely source of infection was removed.
There is no standard approach for treatment of phaeohyphomycosis. Most of guidelines are based on uncontrolled experiments, clinical experience, opinion of expertise and descriptive case studies. Moreover, most of all studies suggest that Exophiala species, in general, are susceptible to the azole class of agents, specially, posaconazole, voriconazole and itraconazole (Table S1). The patient was treated with voriconazol based on the antifungal susceptibility studies of Exophiala in vitro, which demonstrated variable activity of posaconazole, itraconazole, voriconazole and amphotericin B [4]. Although invasive E. dermatitidis infections are potentially fatal, this case was cured due to precise etiologic agent identification and adjustment of therapeutic management.
Conflict of interest
The authors have no conflicts of interest to declare and confirm that each one has made substantial contributions to conception the manuscript, acquisition and analysis of data.
Acknowledgements
We thank the team of the pediatric service ambulatory and the team from Central Laboratory of Paraná State-LACEM and Molecular Microbiology Laboratory-LabMicro/UFPR for their cooperation and assistance. The work of R.R. Gomes and M.F. Voidaleski was supported by a fellowship from the Brazilian Federal Agency for Support and Evaluation of Graduate: Education Coordination for the Improvement of Higher Education Personnel-CAPES (www.capes.gov.br). V.A. Vicente received a fellowship from the National Counsel of Technological and Scientific Development (http://cnpq.br/), Brasilia, Brazil.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mmcr.2019.02.001.
Contributor Information
Renata R. Sakiyama, Email: re_sakiyama@hotmail.com.
Vânia A. Vicente, Email: vaniava63@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Homa M., Manikandan P., Saravanan V., Revathi R., Anita R., Narendran V. Exophiala dermatitidis endophthalmitis: case report and literature review. Mycopathologia. 2018;183:603–609. doi: 10.1007/s11046-017-0235-4. [DOI] [PubMed] [Google Scholar]
- 2.Babič M.N., Zupančič J., Gunde-Cimerman N., de Hoog G.S., Zalar P. Ecology of the human opportunistic black yeast Exophiala dermatitidis indicates preference for human-made habitats. Mycopathologia. 2018;183:201–212. doi: 10.1007/s11046-017-0134-8. [DOI] [PubMed] [Google Scholar]
- 3.Alabaz D., Kibar F., Arikan S., Sancak B., Celik U., Aksaray N. Systemic phaeohyphomycosis due to Exophiala (Wangiella) in an immunocompetent child. Med. Mycol. 2009;47:653–657. doi: 10.1080/13693780802715815. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhary A., Meis J.F., Guarro J., de Hoog G.S., Kathuria S., Arendrup M.C. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin. Microbiol. Infect. 2014;20:47–75. doi: 10.1111/1469-0691.12515. [DOI] [PubMed] [Google Scholar]
- 5.de Hoog G.S., Guarro J., Gené J., Figueras M.J. 2 ed. Universitat Rovira i Virgilli; Utrecht: 2000. Atlas of Clinical Fungi. [Google Scholar]
- 6.Kenney R.T., Kwon-Chung K.J., Waytes A.T., Melnick D.A., Pass H.I., Merino M.J. Successful treatment of systemic Exophiala dermatitidis infection in a patient with chronic granulomatous disease. Clin. Infect. Dis. 1992;14:235–242. doi: 10.1093/clinids/14.1.235. [DOI] [PubMed] [Google Scholar]
- 7.García-Agudo R., García-Martos P. Clinical and microbiological aspects of fungal peritonitis in peritoneal dialysis. Nefrologia. 2009;29:506–517. doi: 10.3265/Nefrologia.2009.29.6.5650.en.full. [DOI] [PubMed] [Google Scholar]
- 8.Lye W.C. Peritonitis due to Wangiella dermatitidis in a patient on CAPD. Perit. Dial. Int. 1993;13:319–320. [PubMed] [Google Scholar]
- 9.Vlassopoulos D., Kouppari G., Arvanitis D., Papaefstathiou K., Dounavis A., Velegraki A. Wangiella dermatitidis peritonitis in a CAPD patient. Perit. Dial. Int. 2001;39:2261–2266. [PubMed] [Google Scholar]
- 10.Greig J., Harkness M., Taylor P., Hashmi C., Liang S., Kwan J. Peritonitis due to the dematiaceous mold Exophiala dermatitidis complicating continuous ambulatory peritoneal dialysis. Clin. Microbiol. Infect. 2003;9:713–715. doi: 10.1046/j.1469-0691.2003.00569.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomes R.R., Vicente V.A., de Azevedo C.M.P.S., Salgado C.G., da Silva M.B., Queiroz-Telles F. Molecular epidemiology of agents of human chromoblastomycosis in Brazil with the description of two novel species. PLoS Neglected Trop. Dis. 2016;11 doi: 10.1371/journal.pntd.0005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gostincar C., Muggia L., Grube M. Polyextremotolerant black fungi: oligotrophism, adaptive potential, and a link to lichen symbioses. Front. Microbiol. 2012;3:e390. doi: 10.3389/fmicb.2012.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanuskova D., Horakova J., Buzassyova D., Poczova M., Bodova I., Svec P. A case of Exophiala dermatitidis infection in a child after allogeneic stem cell transplantation: case report and literature review of paediatric cases. JMM Case Rep. 2017;4 doi: 10.1099/jmmcr.0.005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong K.H., Kim J.W., Jang S.J., Yu E., Kim E.C. Liver cirrhosis caused by Exophiala dermatitidis. J. Med. Microbiol. 2009;58:674–677. doi: 10.1099/jmm.0.002188-0. [DOI] [PubMed] [Google Scholar]
- 15.Gupta J., Singh M., Yadav S., Khurana N., Jain S.L., Chawla R. Phaeohyphomycosis breast masquerading as fibroadenoma in a young teenage girl. Diagn. Cytopathol. 2017:1–4. doi: 10.1002/dc.23755. [DOI] [PubMed] [Google Scholar]
- 16.Nachman S., Alpan O., Malowitz R., Spitzer E.D. Catheter-associated fungemia due to Wangiella (Exophiala) dermatitidis. J. Clin. Microbiol. 1996;34:1011–1013. doi: 10.1128/jcm.34.4.1011-1013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lian X., de Hoog G.S. Indoor wet cells harbour melanized agents of cutaneous infection. Med. Mycol. 2010;48:622–628. doi: 10.3109/13693780903405774. [DOI] [PubMed] [Google Scholar]
- 18.Sudhadham M., Gerrits van den Ende A.H.G., Sihanonth P., Sivichai S., Chaiyarat R., Menken S.B. Elucidation of distribution patterns and possible infection routes of the neurotropic black yeast Exophiala dermatitidis using AFLP. Fungal Biol. 2011;115:1051–1065. doi: 10.1016/j.funbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 19.de Hoog G.S., Matos T., Sudhadham M., Luijsterburg K.F., Haase G. Intestinal prevalence of the neurotropic black yeast Exophiala (Wangiella) dermatitidis in healthy and impaired individuals. Mycoses. 2005;48:142–145. doi: 10.1111/j.1439-0507.2004.01083.x. [DOI] [PubMed] [Google Scholar]
- 20.Auricchio S., Giovenzana M.E., Pozzi M., Galassi A., Santorelli G., Dozio B., Scanziani R. Fungal peritonitis in peritoneal dialysis: a 34-year single centre evaluation. Clin. Kidney J. 2018;11:874–880. doi: 10.1093/ckj/sfy045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacobino J., Montelli A.C., Barretti P., Nascimento A.B., Caramori J.T., Barbosa L., Bagagli E. Fungal peritonitis in patients undergoing peritoneal dialysis (PD) in Brazil: molecular identification, biofilm production and antifungal susceptibility of the agents. Med. Mycol. 2016;54:725–732. doi: 10.1093/mmy/myw. [DOI] [PubMed] [Google Scholar]
- 22.Kumar K.V., Mallikarjuna H.M., Gokulnath, Jayanthi S. Fungal peritonitis in continuous ambulatory peritoneal dialysis: the impact of antifungal prophylaxis on patient and technique outcomes. Indian J. Nephrol. 2014;24:297–301. doi: 10.4103/0971-4065.133005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warady B.A., Bashir M., Donaldson L.A. Fungal peritonitis in children receiving peritoneal dialysis: a report of the NAPRTCS. Kidney Int. 2000;58:384–389. doi: 10.1046/j.1523-1755.2000.00176.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.