Abstract
Introduction
Preliminary studies have shown that treatment with plasma exchange (PE) plus therapeutic albumin replacement in patients with Alzheimer's disease (AD) induced mobilization of plasma and cerebrospinal fluid amyloid β protein, associated with an improvement in memory and language functions, as well as the stabilization of brain perfusion, which persisted after treatment discontinuation.
Methods
Alzheimer's Management By Albumin Replacement (AMBAR) is a multicenter, randomized, blinded and placebo-controlled, parallel-group, phase IIb/III trial enrolling patients with mild to moderate AD. The study evaluates PE with different replacement volumes of therapeutic albumin (5% and 20% Albutein®, Grifols), with or without intravenous immunoglobulin (Flebogamma® 5% DIF, Grifols). Patients are randomized to one of three active treatment groups or one control (sham PE) group (1:1:1:1). The intervention regime includes a first 6-week stage of intensive treatment, followed by a second 12-month stage of maintenance treatment. The change from the baseline to the end of treatment periods in the ADAS-Cog and ADCS-ADL scores are the coprimary efficacy variables. Secondary efficacy variables include change from the baseline in scores on cognitive, functional, behavioral, and overall progression tests; changes in plasma and cerebrospinal fluid levels of amyloid β and tau protein; and assessment of structural and functional changes in brain areas of interest. Safety and tolerability are assessed.
Results
The study has enrolled 496 patients from 41 centers (19 in Spain and 22 in the USA); 347 of these patients were randomized and underwent close to 5000 PEs, of which approximately 25% were sham PEs.
Discussion
We present an innovative approach for treating AD. The study has been designed to demonstrate clinical efficacy, defined as slow decline of the patient's cognition and brain function. The sample size has adequate power to detect differences between any of the active treatment groups and the control group, as well as between the three active treatment groups combined and the control group.
Keywords: Alzheimer's disease, Plasma exchange, Plasmapheresis, Clinical trial, Albumin, Albutein
Highlights
-
•
AMBAR approaches Alzheimer's disease with plasma exchange plus albumin replacement.
-
•
Clinical efficacy is to slow decline of the patient's cognition and brain function.
-
•
Sample size has power to detect differences between treatments and controls.
-
•
Interim results showed a safety profile similar to other plasma exchange indications.
1. Introduction
Alzheimer's disease (AD) is the most common cause of dementia in adults [1]. The presence of intracellular neurofibrillary tangles of phosphorylated tau protein deposits, as well as amyloid plaques formed from extracellular aggregates of amyloid β peptides (Aβ) are hallmarks of AD pathology [2], [3]. Although both neurofibrillary tangles and amyloid deposits are suspected to be responsible for cell death in the AD brain, the initial biological trigger of the pathology has not been fully elucidated.
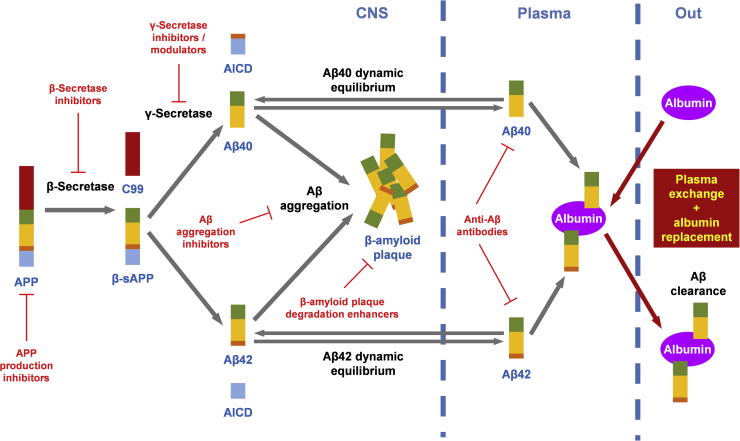
There are only symptomatic treatments approved for the treatment of AD, including cholinesterase inhibitors and N-methyl-d-aspartate receptor antagonists [4]. Therapies to prevent the accumulation of amyloid deposits or to reduce the existing plaque are currently being investigated for the treatment of AD, and several molecular targets of the amyloidogenic pathway are being or have been tested (see Fig. 1). Hence, interfering with factors that regulate the amyloid precursor protein production may affect intracellular levels of amyloid precursor protein and thus reducing the overall levels of Aβ [5], [6]. Similarly, inhibition or modulation of major players involved in the neurotoxic Aβ-generating, such as β-secretase and γ-secretase, appear to be key therapeutic targets against AD [7], [8]. Alternatively, downstream strategies targeting amyloid deposits in brain tissue may inhibit Aβ aggregation or disrupt the already formed plaque [9], [10]. Finally, there is the clearance of Aβ using both passive and active immunotherapies (direct use of anti-Aβ monoclonal antibody, and stimulation of the immune system through vaccination with Aβ peptide fragments, respectively) [11].
Fig. 1.
Amyloidogenic pathway and anti-Aβ therapeutic strategies. Abbreviation: Aβ, amyloid β.
Unfortunately, clinical trials with small molecule pharmacotherapy and immunotherapies to reduce brain Aβ have not shown efficacy [12], [13], [14], [15], [16]. Persistent failure has led investigators to develop new therapeutic strategies for AD aimed at lowering Aβ accumulation in the brain by changing the transportation of Aβ through the blood-brain barrier. A therapeutic approach, which has recently been developed on the basis of performing plasma exchange (PE) with albumin replacement, can induce the shifting of the dynamic equilibrium existing between brain and plasma Aβ. This approach considers i) high levels of Aβ aggregate in the brain is associated with low levels of soluble Aβ in cerebrospinal fluid (CSF) in AD [17]; ii) albumin is the main transporter and the main extracellular antioxidant in the human body [18]; iii) around 90% of the circulating Aβ is bound to albumin [19]; and iv) therapeutic albumin has Aβ-binding capacity [20], [21]. The underlying hypothesis is that PE-mediated sequestration of albumin-bound Aβ in plasma would increase the transport of free Aβ from CSF to plasma (see Fig. 1) to restore the inherent balance between brain and blood levels of Aβ [22], [23], [24], [25], thereby decreasing brain Aβ burden. At the same time, PE would remove other toxic substances from patient plasma [26].
In preliminary pilot (EudraCT#: 2005-001616-45) [27] and phase II (EudraCT#: 2007-000414-36; ClinicalTrials.gov ID: NCT00742417) [28], [29] studies, mobilization of plasma and CSFAβ was found to be associated with an improvement in memory and language functions, as well as stabilization of brain perfusion. These observations, which persisted after treatment was discontinued, were assessed by neuroimaging and were observed in patients with AD who underwent PE with therapeutic albumin replacement (Albutein®, Grifols, Barcelona, Spain). On the basis of the results from those earlier studies, the Alzheimer's Management By Albumin Replacement (AMBAR) study (EudraCT#: 2011-001598-25; ClinicalTrials.gov ID: NCT01561053) was designed as a phase III trial in Europe (Spain) and a phase IIB trial in the United States to further evaluate the observed trends and occurrences by testing different replacement volumes of albumin, with or without intravenous immunoglobulin (IVIG) 5% (Flebogamma® DIF, Grifols) to correct a possible immunological deficit. The experimental phase of the AMBAR trial has been concluded according to schedule, giving way to data collection and evaluation and analysis of the results.
2. Methods
2.1. Global study design
The AMBAR study is a multicenter, randomized, blinded and placebo-controlled, parallel-group trial with a planned enrollment of at least 364 patients with mild to moderate AD from centers at 41 sites, 19 in Spain, and 22 in the United States. The Research Center and Memory Clinic Fundació ACE, Institut Català de Neurociències Aplicades, is the site of the coordinating investigator in Spain, and the University of Pittsburgh Medical Center is the site of the coordinating investigator in the United States.
The study includes four groups of patients: three PE treatment groups receiving different doses of albumin and IVIG and one control (placebo) group. Control patients are subjected to the same procedures as PE-treated patients, but with a simulated PE through a noninvasive procedure (sham) that mimics PE but without fluid replacement or albumin or IVIG administration. The patients, caregivers, and raters (investigators evaluating outcome measures: cognitive, functional, and behavioral changes, as well as neuroimaging evaluators and central laboratory analysis) are blinded as to the therapy received. A single code is used for the anonymization of patients.
2.2. Ethical aspects
The study strictly follows the ethical standards adopted by the XVIII World Medical Assembly Declaration of Helsinki (and subsequent revisions) as well as the European Union Standards of Good Clinical Practice relating to trials involving drug products [30]. Institutional review boards or ethics committees from the sites and the health authorities from both countries have approved the protocol, the informed consent form, and the patient information sheets.
The safety of the intervention is closely monitored. All serious and/or unexpected adverse reactions, as well as any additional information that may alter the study design or entail patient risk, are reported to the ethics committees and institutional review boards. In addition, an independent Data Safety Monitoring Board has been set up for this study.
2.3. Selection of study population
Participants in the AMBAR trial are men and women between 55-85 years of age at the time of signing the informed consent document. At the screening visit, patients must have a diagnosis of AD according to the National Institute of Neurological and Communication Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria [31], have a Mini-Mental State Examination (MMSE) [32] score from 18 to 26, and currently being treated with acetyl-cholinesterase-inhibitors and/or memantine with the previous 3 months at a stable dose. In addition, patients are free of cerebrovascular disease, evidenced by a brain computed axial tomography or magnetic resonance imaging (MRI) study obtained in the 12 months before screening. An MRI required during the screening period is used to rule out any finding that could affect patient safety such as microhemorrhages, infarction, hematoma, stroke, or brain tumors including meningioma. A stable caregiver is available and must attend the patient's study visits.
Exclusion criteria include any condition in which PE is contraindicated or not feasible (such as behavioral disorders, difficult venous access, or abnormal coagulation parameters) or replacement products cannot be administered (such as a history of frequent adverse reactions or thromboembolic complications associated with blood components, particularly hypersensitivity to albumin or allergies to any of the components of Albutein® or Flebogamma® DIF). Other exclusion criteria which may affect patient's safety include IgA deficiency, low hemoglobin (<10 g/dL), high plasma creatinine (>2 mg/dL), uncontrolled hypertension (systolic ≥160 mm Hg, diastolic ≥100 mm Hg, despite regular treatment during the last 3 months), liver disease (GPT >2.5 x ULN, or bilirubin >2 mg/dL), heart diseases, illness with less than 1 year of expected survival, drug or alcohol abuse, and pregnancy or nursing.
2.4. PE interventions
After the screening visit, the patients who meet the inclusion/exclusion criteria are randomized to one of the three PE treatment groups or the control (sham) group according to a [1:1:1:1] scheme. Randomization numbers are assigned sequentially across all sites participating in the study. Randomization numbers and their corresponding treatment allocations are assigned to patients per the randomization list by sequential block number and by sequential randomization numbers.
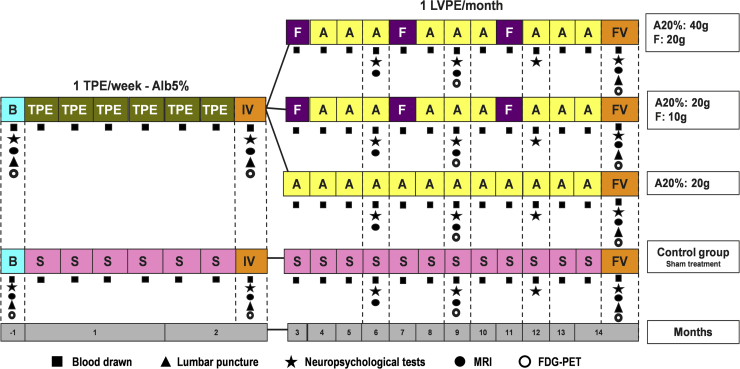
The intervention regime lasts 14 months, which includes a first 6-week stage of intensive treatment with one session of conventional therapeutic plasma exchange (TPE) per week, when all the patients assigned to any of the study groups (active treatment and control) follow the same treatment regime, followed by a second 12-month stage of maintenance treatment with one session of low-volume plasma exchange (LVPE) per month, as summarized in Fig. 2. Between these two treatment periods, there is an intermediate visit to assess patient's status and collect additional study-related data. At the corresponding PE sessions, sufficient EDTA-blood is collected for all the laboratory tests. Cerebrospinal fluid samples are collected before and after both treatment periods for all the laboratory tests. Aliquots of both plasma and CSF are stored at −70°C for future analysis. At the end of the maintenance period, the patient has the final visit which evaluates the same variables as the screening and intermediate visits. The window range for TPE (weeks 1 to 6) is ±1 day. For the intermediate visit (weeks 7 to 8), the window range is ±2 days. The window range for LVPE (weeks 9 to 53) is ±5 days. For the final visit (weeks 54-55), the window range is ±2 days.
Fig. 2.
AMBAR study interventions. Abbreviations: TPE, therapeutic plasma exchange; LVPE, low volume plasma exchange; F, Flebogamma® 5% DIF (intravenous immunoglobulin); A, Albutein® 5%-20% (albumin); S, sham treatment; B, baseline visit; IV, intermediate visit; FV, final visit.
Based on the previous experience from the pilot and phase II studies [27], [28], during TPE, 2500 to 3000 mL of the patient's plasma is being replaced with the same volume of Albutein® 5%, whereas during LVPE, only 650 mL to 880 mL of the patient's plasma is replaced with Albutein® 20%, 100 mL or 200 mL depending on the treatment arm randomization. There are three arms of LVPE: in one arm, 20 grams of Albutein® 20% are used for replacement in LVPE, whereas in the other two arms, visits for Albutein® 20% replacement (20 or 40 grams) are alternated with Flebogamma® DIF 5% (10 or 20 grams). A schematic chart of the interventions assigned to the four study groups is shown in Fig. 2.
Therapeutic plasma exchange is performed using a commercial continuous flow cell separator with either centrifugation- or filtration-based technology. Either a peripheral (e.g., radial/cubital vein) or central access (e.g., subclavian/jugular vein) is used based on the individual characteristics of the patient. Venous access implantation and maintenance is carried out according to the standard procedures used in each center and the correct placement of a central catheter is always confirmed by a chest X-ray. The volume of removed and replaced plasma depends on patient's characteristics (i.e., sex, height, weight and hematocrit) at approximately 35-45 mL/kg, corresponding to a volume of 2500-3000 mL.
Low-volume plasma exchange is carried out through a peripheral line by means of a prototype apheresis device based on the Auto-C™ device (Fenwal Inc, Lake Zurich, IL, USA) or the Aurora™ device (Fresenius Kabi, Bad Homburg, Germany), the newer version of Auto-C™. The removed plasma volume is similar to a plasma donation (650-880 mL) and depends on patient's weight. The volume of infused albumin is consistent with the treatment arm as described previously. Normal saline is used when there is a negative balance between the volume of removed plasma and infused albumin.
For the control group (sham TPE), the tip of a cut catheter is stitched to a colostomy adhesive patch (acting as a “second skin”) which is placed on the subclavicular or jugular region. Then, the patch with the catheter tip stitched is covered with gauze dressing and an adhesive film in a similar manner as in the active treatment groups. The cut catheter is similar to the catheters used in the active treatment groups. A conventional PE device is loaded with a saline solution colored with intravenous iron mimicking plasma and is working in a closed-circuit manner without any fluid interchange between the device and the subject. For the control group sham LVPE, an Auto-C- or Aurora-based prototype provides an apparently and realistic working status in which expired human blood from a local blood bank is circulated in a closed-circuit manner. Specific training on the sham procedures was provided to the investigators; several videos were recorded and were available at the participating sites during the entire study duration.
2.5. Outcome measures
The coprimary efficacy variables are i) the change from the baseline in the cognitive scores as measured with the ADAS-Cog scale [33]; and ii) the change from the baseline in the functional scores measured by the ADCS-ADL inventory [34]. In both cases, there are six measurements performed: one at the baseline (week −3, −2 or −1), one at end of intensive period (week 7-8), three during the maintenance period (months 6, 9, and 12), and one at end of the maintenance period (month 14). Fourteen months is the endpoint for the primary efficacy analysis. See Fig. 2 for details.
Secondary efficacy variables are i) changes from the baseline in scores on cognitive, functional, behavioral, and overall progression tests, measured with the MMSE [32], a neuropsychological battery (NPS) [35], [36], [37], [38], neuropsychiatric inventory (NPI) [39], Clinical Dementia Rating Sum of Boxes (CDR-Sb) [40], Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change (ADCS-CGIC) [41], Cornell Scale for Depression in Dementia (CSDD) [42], Columbia-Suicide Severity Rating Scale [43], Quality of Life-Alzheimer's Disease (QoL-AD) [44], and Resource Utilization in Dementia (RUD-Lite®); ii) changes in plasma levels of Aβ1-40 and Aβ1-42 before and after each PE; iii) changes in CSF levels of Aβ1-40 and Aβ1-42 between the finalization and beginning of each of the two treatment periods; iv) changes in CSF levels of T-tau and P-tau throughout the study; v) assessment of structural changes in hippocampal volume, posterior cingulate volume, and other areas of interest as shown by MRI (six measurements as in the primary efficacy variables); and vi) assessment of functional changes in the brain as detected by positron emission tomography with 18F-fludeoxyglucose (4 measurements, months 6 and 12 are omitted).
2.6. Specific methodology for cognition/behavior assessment
The ADAS-Cog [33] is an instrument specifically designed to evaluate the severity of the fundamental alterations in cognitive and behavioral function that are characteristic of patients with AD.
Functional ability is assessed by means of the ADCS-ADL test [34], which offers detailed descriptions of each activity and requests the informer to describe the actions or behaviors observed.
Mini-Mental State Examination [32] is widely used to assess cognitive alterations and is the most commonly used brief screening test.
The specific NPS battery consists of processing speed tests such as the Symbol Digit Modalities Test [38], developed principally for examining visual attention and tracking, concentration, and psychomotor speed; language/attention tests such as the Semantic Verbal Fluency [35] allowing voluntary access to a certain vocabulary assessing reduction in verbal spontaneity and fluency difficulties; the Neuropsychological Assessment Battery Naming Test [36], designed to highlight deficits in visual confrontation naming skills and to identify aphasia; and the Rey Auditory Verbal Learning Test [37] consisting of words presented in the same order that the subject listens to and must remember and repeat.
Neuropsychiatric disorders assessment includes NPI, to evaluate the most frequent neuropsychiatric manifestations of dementia and also to determine their frequency and intensity; CSDD to evaluate the signs and symptoms of major depression in patients with dementia; CDR-Sb, a clinical test validated in patients with AD that assesses six domains: memory, orientation, judgment and problem solving, social and occupational activities, domestic activities and hobbies, and personal care; and ADCS-CGIC, an instrument for the reliable assessment of global change from the baseline in a clinical trial.
Other tests include the Columbia-Suicide Severity Rating Scale to quantify the severity of suicidal ideation and behavior to estimate the rate of suicidality; the QoL-AD designed specifically to obtain a rating of the quality of life from both the patient and the caregiver; and the RUD-Lite to assess the amount of health-related resource use among patients.
Importantly, the evaluators/raters of the tests in the trial have no access to any information allowing them to identify patient assignment to treatment. Blinding of the evaluators to patient treatment is confirmed when evaluators sign a document to that effect.
The established order of tests at each visit is as follows: with the patient, the first evaluator rates MMSE (when stated by the protocol), ADAS-Cog, NPS battery, QoL-AD, and Columbia-Suicide Severity Rating Scale. Next, the global impairment and clinical impression scales (CDR-Sb and ADCS-CGIC) are administered by the second evaluator. With the caregiver, the second evaluator (while the first evaluator is administering the aforementioned battery with the patient) administers the global impairment and clinical impression scale (CDR-Sb, ADCS-CGIC). The first evaluator, once he/she has finished the NPS battery with the patient, interviews the caregiver to evaluate the patient functionality and behavior: NPI, ADCS-ADL, CSDD, QoL-AD, and RUD-Lite.
2.7. Safety considerations
Therapeutic plasma exchange is regularly used worldwide following a well-known evidence-based approach [45], and TPE appears to be a safe procedure in geriatric patients when performed by experienced practitioners [46]. Both the previous pilot and phase II studies have shown that PE is feasible in elder patients with AD. Plasma exchange may induce adverse reactions, mostly expected and therefore preventable and controllable (e.g., hypocalcemia, hypotension) [47], [48].
Considering the special vulnerability of the patients studied, to minimize the risks, vital signs and laboratory test parameters are monitored more frequently than in a typical clinical setting. In addition, patients are required to remain in the center before and after the PE procedure for longer periods of time than usual, and the caregiver is present except during the actual PE intervention to maintain its blinded character. On rare occasions, agitated or anxious patients can be accompanied by the caregiver during the procedure to ease patient's anxiety. However, the blinding protocol is maintained for both the patient and the caregiver.
The primary criterion of safety assessment is the percentage of TPE and LVPE procedures (including the infusion of albumin and IVIG) associated with at least one adverse event that may be related to the study procedure (adverse reaction). In addition, vital signs (blood pressure, heart rate, respiration rate, and body temperature) and laboratory test parameters (blood cell counts, platelet count, prothrombin time [Quick], activated partial thromboplastin time [aPTT], fibrinogen, total proteins, and calcium) are recorded before, during, and after each PE session.
The adverse events are coded according to the adverse events classification of the World Health Organization (MeDRA version 17.1) and are described by a synonym (Lowest Level Term) and the affected organ/system, the intensity, causality, and seriousness.
2.8. Statistics
A sample size of 312 subjects (78 in each of the 4 groups) would make it possible to detect, with nearly 92% power for the first of the coprimary efficacy variables (the change from the baseline of the ADAS-Cog scores), a difference in the mean of 3 points between any of the treatment groups and the control group, assuming the common standard deviation (SD) to be 5.55 (according to the data obtained in the phase II study) [28], with a level of significance of 5%. The sample size of 312 subjects provides over 98% power for the second of the coprimary endpoint variables (the changes from the baseline of the ADCS-ADL inventory scores) to detect a difference in the mean of 6.69 points between any of the treatment groups and the control group, assuming the common SD to be 10.0 (according to the data obtained in the phase II study) [28], with a level of significance of 5%. The study has sufficient power for these coprimary endpoints of at least 90% (0.92*0.98 = 0.90). The calculation makes the conservative assumption that these endpoints are independent. Because these endpoints may be positively correlated, 90% should serve as a lower bound on power. On the other hand, because all patients from the three active treatment groups share exactly the same plasmapheresis component (plasma removal), the difference between the three active treatment groups combined and the control group will be assessed. Therefore, the study intends to include 312 patients for evaluation, which implies 364 to 496 patients enrolled assuming a global dropout rate of 15% to 30%, respectively.
The changes from the baseline of the ADAS-Cog scores and the ADCS-ADL inventory will be analyzed over time using a mixed model for repeated measures (MMRM) approach. This model will include fixed-effects factors for month (2, 6, 9, 12, and 14 months), treatment group, and the month-by-treatment interaction, with adjustments for age, disease severity (MMSE ≤ 21vs. ≥22) and the baseline ADAS-Cog score or ADCS-ADL inventory.
Efficacy will be determined by the change in the total ADAS-Cog score and in the ADCS-ADL inventory score from the baseline to 14 months. Because the endpoints are coprimary, both should be statistically significant for confirming efficacy. To account for the three dose group comparisons with the control group, and to maintain the overall significance level of 0.05, adjustment for α will be made for multiple dose groups according to the Hochberg procedure [49]. This procedure has been demonstrated to control the overall type I error at 0.05. The coprimary endpoints ADAS-Cog and ADCS-ADL will also be analyzed by AD severity (MMSE score: 18-21 and MMSE score: 22-26). The same primary analysis will be performed as for the entire population, with the exception that the MMRM will not be adjusted for AD severity. All other efficacy endpoints with the baseline and postbaseline data will analyze change from the baseline to that specific time point using analysis of covariance with treatment group as a fixed effect, and the corresponding baseline value, age, and AD severity as a covariate. Expanded use of MMRM into secondary analyses may be considered if the results for the MMRM and analysis of covariance methodologies for the primary analysis are qualitatively different.
2.9. Study progress
Recruitment started in April 2012. A preliminary, interim, descriptive analysis was performed as of June 2015 on the data available for 186 patients whose data were presented together (randomization codes not broken) [50]. As of December 16, 2016 (date of last patient recruited), 496 patients had been invited to participate in the AMBAR study; 347 of the patients were randomized and underwent close to 5000 PEs, of which approximately 25% were sham PEs. The last patient visit took place in March 2018.
3. Discussion
The AMBAR study is designed to examine whether PE with infusion of human albumin combined with IVIG can prevent or delay progression of the cognitive and functional impairment in patients with mild to moderate AD. ADAS-Cog and ADCS-ADL scores are the coprimary endpoints to determine clinical efficacy. Additional cognitive measures are included to better understand previous study results where a persistent beneficial effect in memory and language functions was detected [27], [28], [29].
Plasma exchange is a technique that separates plasma from blood cells, before the latter are returned to the bloodstream. During therapeutic apheresis, plasma may be removed to eliminate pathogenic elements [51] being replaced with an equivalent volume of healthy plasma, or of colloid or crystalloid solutions. Because approximately 90% of plasma Aβ is bound to circulating albumin [19] which favors its degradation by the liver [52], therapeutic apheresis using albumin as a replacement fluid is especially interesting as a potential therapy for AD.
The AMBAR approach is considered relevant on the basis of the clinical results obtained in the phase II trial (randomized, placebo-controlled), as well as because PE removes not only albumin and Aβ but many other active plasma components. Consequently, the possibility that one or more unidentified agents could play a role in the observed effects of PE on the patients with AD cannot be ruled out [53]. Given the consistent failure of anti-Aβ strategies to date, a multiple-path hypothesis for AD pathogenesis is getting attention. On the other hand, even within the amyloid hypothesis, a potential explanation of the Aβ-related failures is that a relevant Aβ species is insufficiently affected, which is not the case of PE because it affects all Aβ species [54].
In the phase II study [28], the primary objective was to determine whether PE treatment with albumin replacement was able to modify the concentration of Aβ in CSF and, secondarily, in plasma of patients with AD with a similar profile to that of the AMBAR study (MMSE score from 18 to 26). In the phase II study, this modification was indeed observed for plasma Aβ1-42 levels which were lower in the PE-treated group after each treatment period (P < .05), and for CSF Aβ1-42 levels (marginal P = .072 after the last PE compared with the baseline). However, assessment of the effects of PE on cognitive, functional, and behavioral outcomes were secondary objectives, and the study was not powered to detect statistically significant differences in the cognition tests applied. Even so, PE-treated patients exhibited better scores than untreated controls in measures of global cognition (borderline significances of P = .081 with the MMSE and P = .094 with the ADAS-cog) and particularly in language functions (P < .05 with the Boston Naming Test and Semantic Verbal Fluency) which persisted after PE was discontinued. Moreover, neuroimaging studies confirmed that PE-treated patients had less hypoperfusion than controls in frontal, temporal, and parietal areas, and perfusion stabilization in Brodmann area BA38-R during the PE treatment period (P < .05); BA38 is assumed to play a significant role in language [55].
Taking into account the previous experience with PE and the results of the pilot and phase II studies [27], [28], we expect AMBAR to show the expected results in the primary and secondary outcomes. We believe that the sample size has the adequate power to detect statistical differences between any of the active treatment groups and the control group, as well as between the three active treatment groups combined and the control group. Changes in the concentration of Aβ in plasma, neuroimaging, and CSF measures are secondary objectives and they will help in better understanding the outcome of the study.
The approach presented in this report is highly innovative to the treatment of AD. Specifically, the AMBAR trial integrates different therapeutic components using different doses and procedures. In the AMBAR trial, albumin is infused in combination with IVIG (Flebogamma® DIF). Although it is known that IVIG contains antibodies against Aβ [56], [57] and preliminary studies have confirmed that Flebogamma® DIF is able to bind to Aβ [58], results of a randomized, placebo-controlled trial showed that IVIG did not have any effect on cognition or function in patients with AD [59]. In the AMBAR trial, IVIG is used to correct a possible immunological deficit caused by plasma depletion. In fact, this combination approach of PE with albumin plus IVIG replacement has already been used for selected neurologic diseases [60], [61]. The AMBAR trial evaluates the maintenance period including combination of monthly LVPE (similar to regular plasma donation plasmaphereses) with IVIG every 4 months. The maintenance therapy follows the load treatment during the intensive period, with six TPE in six weeks. This design was derived from previous studies. The two different types of apheresis would play a fundamental role in the new therapeutic strategies.
To summarize, the AMBAR study is designed to confirm previous findings in patients with AD treated with PE plus albumin replacement. The different types of apheresis combination approaches would play a key role in defining newly designed therapeutic strategies in a new landscape for the treatment of AD.
Research in context.
-
1.
Systematic review: So far, clinical trials testing small molecule pharmacotherapy and immunotherapies aimed to reduce brain amyloid β have failed to improve clinical symptoms of Alzheimer's disease.
-
2.
Interpretation: The AMBAR (Alzheimer's Management By Albumin Replacement) trial addresses plasma exchange with therapeutic albumin replacement as a new approach directly applicable to clinical practice for Alzheimer's disease treatment.
-
3.
Future directions: Confirmation of previous findings on cognitive and functional improvement in patients with Alzheimer's disease treated with plasma exchange plus albumin replacement will open a promising new landscape in designing therapeutic strategies for this devastating disease.
Acknowledgments
The authors thank the patients for their indispensable contribution. Jordi Bozzo PhD CMPP (Grifols) is acknowledged for medical writing and editorial assistance in the preparation of this manuscript.
The AMBAR study is sponsored by Grifols, a manufacturer of therapeutic human serum albumin and intravenous immune globulin.
Footnotes
Conflict of interest: MB has been a consultant for Araclon, Avid, Bayer, Elan, Grifols, Janssen/Pfizer, Lilly, Neuroptix, Nutricia, Roche, Sanofi, and Servier; and received fees for lectures and funds for research from Araclon, Esteve, Grifols, Janssen, Novartis, Nutricia, Piramal, Pfizer-Wyett, Roche, and Servier. OL has been a consultant Grifols and Lundbeck. ZMS has been on the board of directors or an advisory committee for AABB, National Marrow Donor Program, Fenwal/Fresenius Kabi and Grifols, and has received research grants from Therakos. LN, MT, CG, and AP are employees of Grifols.
References
- 1.Ferri C.P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blennow K., de Leon M.J., Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 3.Ittner L.M., Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 4.Parsons C.G., Danysz W., Dekundy A., Pulte I. Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer's disease. Neurotox Res. 2013;24:358–369. doi: 10.1007/s12640-013-9398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winblad B., Giacobini E., Frolich L., Friedhoff L.T., Bruinsma G., Becker R.E. Phenserine efficacy in Alzheimer's disease. J Alzheimers Dis. 2010;22:1201–1208. doi: 10.3233/JAD-2010-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Y., Ryder J., Ni B. Inhibition of Abeta production and APP maturation by a specific PKA inhibitor. FEBS Lett. 2003;546:407–410. doi: 10.1016/s0014-5793(03)00645-8. [DOI] [PubMed] [Google Scholar]
- 7.Islam M.A., Pillay T.S. Beta-secretase inhibitors for Alzheimer's disease: identification using pharmacoinformatics. J Biomol Struct Dyn. 2018:1–20. doi: 10.1080/07391102.2018.1430619. [DOI] [PubMed] [Google Scholar]
- 8.Kumar D., Ganeshpurkar A., Kumar D., Modi G., Gupta S.K., Singh S.K. Secretase inhibitors for the treatment of Alzheimer's disease: Long road ahead. Eur J Med Chem. 2018;148:436–452. doi: 10.1016/j.ejmech.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q., Yu X., Li L., Zheng J. Inhibition of amyloid-beta aggregation in Alzheimer's disease. Curr Pharm Des. 2014;20:1223–1243. doi: 10.2174/13816128113199990068. [DOI] [PubMed] [Google Scholar]
- 10.Yamin G., Ono K., Inayathullah M., Teplow D.B. Amyloid beta-protein assembly as a therapeutic target of Alzheimer's disease. Curr Pharm Des. 2008;14:3231–3246. doi: 10.2174/138161208786404137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo J.J., Li J.Y., Yang Z., Liu Z., Feng J.S. Efficacy and safety of anti-amyloid-beta immunotherapy for Alzheimer's disease: a systematic review and network meta-analysis. Ann Clin Transl Neurol. 2017;4:931–942. doi: 10.1002/acn3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 13.Salloway S., Sperling R., Brashear H.R. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer's disease. N Engl J Med. 2014;370:1460. doi: 10.1056/NEJMc1402193. [DOI] [PubMed] [Google Scholar]
- 14.Moussa C.E. Beta-secretase inhibitors in phase I and phase II clinical trials for Alzheimer's disease. Expert Opin Investig Drugs. 2017;26:1131–1136. doi: 10.1080/13543784.2017.1369527. [DOI] [PubMed] [Google Scholar]
- 15.Villemagne V.L., Rowe C.C., Barnham K.J., Cherny R., Woodward M., Bozinosvski S. A randomized, exploratory molecular imaging study targeting amyloid beta with a novel 8-OH quinoline in Alzheimer's disease: The PBT2-204 IMAGINE study. Alzheimers Dement. 2017;3:622–635. doi: 10.1016/j.trci.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketter N., Liu E., Di J., Honig L.S., Lu M., Novak G. A Randomized, Double-Blind, Phase 2 Study of the Effects of the Vaccine Vanutide Cridificar with QS-21 Adjuvant on Immunogenicity, Safety and Amyloid Imaging in Patients with Mild to Moderate Alzheimer's Disease. J Prev Alzheimers Dis. 2016;3:192–201. doi: 10.14283/jpad.2016.118. [DOI] [PubMed] [Google Scholar]
- 17.Grimmer T., Riemenschneider M., Forstl H., Henriksen G., Klunk W.E., Mathis C.A. Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65:927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo G., Clerici M., Giustarini D., Rossi R., Milzani A., Dalle-Donne I. Redox albuminomics: oxidized albumin in human diseases. Antioxid Redox Signal. 2012;17:1515–1527. doi: 10.1089/ars.2012.4702. [DOI] [PubMed] [Google Scholar]
- 19.Biere A.L., Ostaszewski B., Stimson E.R., Hyman B.T., Maggio J.E., Selkoe D.J. Amyloid β-Peptide Is Transported on Lipoproteins and Albumin in Human Plasma. J Biol Chem. 1996;271:32916–32922. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- 20.Costa M., Ortiz A.M., Jorquera J.I. Therapeutic albumin binding to remove amyloid-beta. J Alzheimers Dis. 2012;29:159–170. doi: 10.3233/JAD-2012-111139. [DOI] [PubMed] [Google Scholar]
- 21.Milojevic J., Costa M., Ortiz A.M., Jorquera J.I., Melacini G. In Vitro Amyloid-beta Binding and Inhibition of Amyloid-beta Self-Association by Therapeutic Albumin. J Alzheimers Dis. 2014;38:753–765. doi: 10.3233/JAD-131169. [DOI] [PubMed] [Google Scholar]
- 22.Deane R., Wu Z., Zlokovic B.V. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004;35:2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 23.DeMattos R.B., Bales K.R., Parsadanian M., O'Dell M.A., Foss E.M., Paul S.M. Plaque-associated disruption of CSF and plasma amyloid-beta (Abeta) equilibrium in a mouse model of Alzheimer's disease. J Neurochem. 2002;81:229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 24.Roberts K.F., Elbert D.L., Kasten T.P., Patterson B.W., Sigurdson W.C., Connors R.E. Amyloid-beta efflux from the central nervous system into the plasma. Ann Neurol. 2014;76:837–844. doi: 10.1002/ana.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marques M.A., Kulstad J.J., Savard C.E., Green P.S., Lee S.P., Craft S. Peripheral amyloid-beta levels regulate amyloid-beta clearance from the central nervous system. J Alzheimers Dis. 2009;16:325–329. doi: 10.3233/JAD-2009-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russi G., Marson P. Urgent plasma exchange: how, where and when. Blood Transfus. 2011;9:356–361. doi: 10.2450/2011.0093-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boada M., Ortiz P., Anaya F., Hernandez I., Munoz J., Nunez L. Amyloid-targeted therapeutics in Alzheimer's disease: use of human albumin in plasma exchange as a novel approach for Abeta mobilization. Drug News Perspect. 2009;22:325–339. doi: 10.1358/dnp.2009.22.6.1395256. [DOI] [PubMed] [Google Scholar]
- 28.Boada M., Anaya F., Ortiz P., Olazaran J., Shua-Haim J.R., Obisesan T.O. Efficacy and Safety of Plasma Exchange with 5% Albumin to Modify Cerebrospinal Fluid and Plasma Amyloid-beta Concentrations and Cognition Outcomes in Alzheimer's Disease Patients: A Multicenter, Randomized, Controlled Clinical Trial. J Alzheimers Dis. 2017;56:129–143. doi: 10.3233/JAD-160565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuberas-Borrós G., Roca I., Boada M., Tárraga L., Hernández I., Buendia M. Longitudinal neuroimaging analysis in mild-moderate Alzheimer's disease patients treated with plasma exchange with 5% human albumin. J Alzheimers Dis. 2018;61:321–332. doi: 10.3233/JAD-170693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Note for guidance. Good Clinical Practice. 1996. CPMP/ICH/135/95. [Google Scholar]
- 31.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 32.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 34.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M. An Inventory to Assess Activities of Daily Living for Clinical Trials in Alzheimerʼs Disease. Alzheimer Dis Assoc Disord. 1997;11:33–39. [PubMed] [Google Scholar]
- 35.Benton A., Hamsher K. ASA Associates; Iowa City: 1983. Multilingual aphasia examination. [Google Scholar]
- 36.Kaplan E., Goodglass H., Weintraub S. Lea & Febiger; Philadelphia: 1983. The Boston naming test. [Google Scholar]
- 37.Rey A. Presses Universitaires de France; Paris: 1964. L'examen clinique en psychologie. [Google Scholar]
- 38.Smith A. Western Psychological Services; Los Angeles: 1973. Symbol Digits Modality Test. [Google Scholar]
- 39.Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 40.Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 41.Schneider L.S., Olin J.T., Doody R.S., Clark C.M., Morris J.C., Reisberg B. Validity and Reliability of the Alzheimerʼs Disease Cooperative Study-Clinical Global Impression of Change. Alzheimer Dis Assoc Disord. 1997;11:22–32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 42.Alexopoulos G.S., Abrams R.C., Young R.C., Shamoian C.A. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 43.Mundt J.C., Greist J.H., Jefferson J.W., Federico M., Mann J.J., Posner K. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. 2013;74:887–893. doi: 10.4088/JCP.13m08398. [DOI] [PubMed] [Google Scholar]
- 44.Merchant C., Hope K.W. The Quality of Life in Alzheimer's Disease Scale: direct assessment of people with cognitive impairment. J Clin Nurs. 2004;13:105–110. doi: 10.1111/j.1365-2702.2004.01050.x. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz J., Padmanabhan A., Aqui N., Balogun R.A., Connelly-Smith L., Delaney M. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher. 2016;31:149–162. doi: 10.1002/jca.21470. [DOI] [PubMed] [Google Scholar]
- 46.Ataca P., Marasuna O.A., Ayyildiz E., Bay M., Ilhan O. Therapeutic plasmapheresis in geriatric patients: favorable results. Transfus Apher Sci. 2014;51:64–67. doi: 10.1016/j.transci.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Szczeklik W., Wawrzycka K., Wludarczyk A., Sega A., Nowak I., Seczynska B. Complications in patients treated with plasmapheresis in the intensive care unit. Anaesthesiology Intensive Ther. 2013;45:7–13. doi: 10.5603/AIT.2013.0002. [DOI] [PubMed] [Google Scholar]
- 48.Vucic S., Davies L. Safety of plasmapheresis in the treatment of neurological disease. Aust New Zealand J Med. 1998;28:301–305. doi: 10.1111/j.1445-5994.1998.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 49.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1998;75:800–802. [Google Scholar]
- 50.Boada M., Lopez O.L., Rodriguez-Gomez O., Núñez L., Torres M., Afonso N. Changes in cognitive status of Alzheimer's disease patients treated with plasma exchange and replacement with human albumin plus immunoglobulin: Interim global results of the AMBAR trial Alzheimers. Dement. 2016;12:P419–P420. [Google Scholar]
- 51.Meca-Lallana J.E., Rodriguez-Hilario H., Martinez-Vidal S., Saura-Lujan I., Carreton-Ballester A., Escribano-Soriano J.B. Plasmapheresis: its use in multiple sclerosis and other demyelinating processes of the central nervous system. An observation study. Rev Neurol. 2003;37:917–926. [PubMed] [Google Scholar]
- 52.Bohrmann B., Tjernberg L., Kuner P., Poli S., Levet-Trafit B., Naslund J. Endogenous proteins controlling amyloid beta-peptide polymerization. Possible implications for beta-amyloid formation in the central nervous system and in peripheral tissues. J Biol Chem. 1999;274:15990–15995. doi: 10.1074/jbc.274.23.15990. [DOI] [PubMed] [Google Scholar]
- 53.Villeda S.A., Plambeck K.E., Middeldorp J., Castellano J.M., Mosher K.I., Luo J. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pascual B., Masdeu J.C., Hollenbeck M., Makris N., Insausti R., Ding S.L. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb Cortex. 2015;25:680–702. doi: 10.1093/cercor/bht260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Relkin N.R., Szabo P., Adamiak B., Burgut T., Monthe C., Lent R.W. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30:1728–1736. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 57.DeMattos R.B., Bales K.R., Cummins D.J., Dodart J.C., Paul S.M., Holtzman D.M. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boada M., Nuñez L., Lafuente A., Hernández I., Buendía M., Tárraga L. Peripheral amyloid-beta mobilization with the intravenous immunoglobulin Flebogamma DIF® in Alzheimer's disease patients. Alzheimers Dement. 2013;7:S456–S457. [Google Scholar]
- 59.Relkin N.R., Thomas R.G., Rissman R.A., Brewer J.B., Rafii M.S., van Dyck C.H. A phase 3 trial of IV immunoglobulin for Alzheimer disease. Neurology. 2017;88:1768–1775. doi: 10.1212/WNL.0000000000003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lehmann H.C., Hartung H.-P. Plasma exchange and intravenous immunoglobulins: Mechanism of action in immune-mediated neuropathies. J Neuroimmunology. 2011;231:61–69. doi: 10.1016/j.jneuroim.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Ludwig K.P., Thiesset H.F., Gayowski T.J., Schwartz J.J. Plasmapheresis and Intravenous Immune Globulin Improve Neurologic Outcome of Central Pontine Myelinolysis Occurring Post Orthotopic Liver Transplant. Ann Pharmacother. 2011;45:e10. doi: 10.1345/aph.1P371. [DOI] [PubMed] [Google Scholar]