A major concern when using two-drug anti-HIV regimens is the risk of viral resistance. However, no techniques to evaluate the barrier to resistance of two-drug combinations in vitro have been reported.
KEYWORDS: barrier to resistance, dolutegravir, human immunodeficiency virus, two-drug regimen
ABSTRACT
A major concern when using two-drug anti-HIV regimens is the risk of viral resistance. However, no techniques to evaluate the barrier to resistance of two-drug combinations in vitro have been reported. We evaluated the emergence of drug-resistant mutants in a passage study with constant concentrations of two drugs simultaneously. The barrier to resistance of dolutegravir-containing two-drug combinations was higher than the other combinations evaluated in this study.
INTRODUCTION
Current antiretroviral regimens consist of one key drug and two nucleos(t)ide reverse transcriptase inhibitors [N(t)RTIs] (1). However, two-drug regimens with similar efficacy and durability to the standard 3-drug regimens would be preferable, as this approach would lessen problems, such as high cost, drug-drug interactions, and long-term side effects, and would reserve a class of drugs for a future treatment option (2). In a study of anti-retroviral therapy (ART)-naive patients, raltegravir (RAL)-ritonavir-boosted darunavir (DRV/r) was inferior to the standard 3-drug regimen in patients with low CD4 counts and high viral loads, and a high emergence of RAL-resistant mutants was seen (3). In another study, patients who switched to ritonavir-boosted atazanavir (ATV/r) and RAL had a higher rate of virologic rebound than those given ATV/r and tenofovir disoproxil fumarate-emtricitabine (TDF-FTC) (4). Therefore, it is important to evaluate the barrier to resistance of two-drug combinations in vitro to assess the occurrence of drug-resistant mutants and to support the clinical use of such combinations.
Currently, no methods have been established to evaluate the barrier to resistance of a two-drug combination. Usually, in vitro passage studies are used to isolate drug-resistant viruses but are conducted using only one drug. The in vitro two-drug combination evaluation with checkerboard method enables researchers to determine whether two drugs act synergistically, additively, or antagonistically, but it cannot provide information on viral resistance (5). In this study, we describe a quantitative method to compare the barrier to resistance of a two-drug combination with that of a single drug or other combinations in vitro. Here, we define the barrier to resistance based on the drug concentrations at which drug-resistant mutants emerge. This is a comparative ranking, and if a drug permits the emergence of resistant mutants up to a certain fold of its 50% effective concentration (EC50), but the comparison drug or combination of two drugs does not permit the emergence of drug-resistant mutants at the corresponding fold EC50 or combination EC50 (cEC50), then the former drug has a lower barrier to resistance than the comparison drug or combination.
Dolutegravir (DTG) was synthesized at Shionogi (Osaka, Japan) (6), and elvitegravir (EVG), lamivudine (3TC), and rilpivirine (RPV) were purchased from Sequoia Research Products (Pangbourne, UK). First, the EC50 and 90% effective concentration (EC90) of each drug were determined by a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay in MT-2 cells (7) infected with HIV NL-432 (8) as described previously (9). The EC50 and EC90 of each compound are shown in Table 1. It is well known that the EC50 of 3TC varies depending on the cell type (10). We selected MT-2 cells for this study because it is possible to isolate clinically relevant resistant viruses, although the EC50 of 3TC in MT-2 is relatively high (11).
TABLE 1.
EC50 and EC90 of drugs in the MT-2 MTT assay
| Drug | EC50 (nM)a | EC90 (nM)a |
|---|---|---|
| Dolutegravir | 2.1 ± 0.60 | 5.3 ± 1.3 |
| Elvitegravir | 1.4 ± 0.36 | 4.4 ± 1.3 |
| Rilpivirine | 1.2 ± 0.13 | 2.6 ± 0.49 |
| Lamivudine | 3310 ± 325 | 9055 ± 670 |
Data represent means and SDs that were calculated from data from three independent experiments.
Next, we evaluated various drug combinations using a checkerboard method (5). We judged the interaction of each combination of drugs using the combination index (CI) and D values as described previously (5, 12). RPV-RPV was used as a control, as the effect is expected to be additive. The combinations that included 3TC showed very weak synergism. However, all of the combinations in this study displayed additive activity, as demonstrated by their D values (Table 2).
TABLE 2.
Interaction of two-drug combinationsa
| Drug combination | CI valueb | D values forc: |
||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Mean | ||
| DTG-RPV | 0.98 | −0.024 | 0.068 | 0.022 |
| DTG-3TC | 0.86 | −0.049 | −0.062 | −0.055 |
| EVG-RPV | 1.02 | 0.049 | 0.000 | 0.024 |
| EVG-3TC | 0.86 | −0.037 | 0.016 | −0.011 |
| RPV-3TC | 0.90 | −0.013 | −0.028 | −0.021 |
| RPV-RPV | 1.00 | 0.032 | −0.013 | 0.010 |
The interaction of each two-drug combination was determined using the CI values or average D values from two independent experiments. Overall, the interactions were additive.
CI values indicate synergistic (CI, <1), additive (CI, 1), and antagonistic (CI, >1) interactions.
D values indicate strong synergistic (−0.5 to −0.2), weak synergistic (−0.2 to −0.1), additive (−0.1 to 0.1), weak antagonistic (0.1 to 0.2), and strong antagonistic (0.2 to 0.5) interactions.
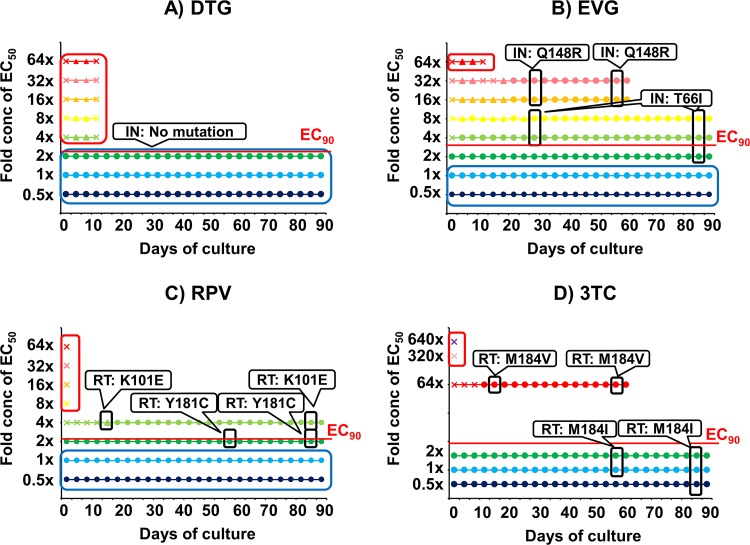
To determine the barrier to resistance of the drugs individually and in combination, we performed passage studies in MT-2 cells. The drug concentrations were kept consistent throughout the study, and the cells were passaged every 3 to 4 days. The cells were passaged with the addition of fresh MT-2 cells if a cytopathic effect (CPE) was observed. We then analyzed the HIV-1 proviral DNA sequence for mutations by PCR using a Taq kit (TaKaRa, Shiga, Japan) and specific primers (IN coding region, 5′-AACAAGTAGATAAATTAGTCAGT-3′ and 5′-TAGTGGGATGTGTACTTCTGAAC-3′; reverse transcriptase [RT] coding region, 5′-GCGGACATAAAGCTATAGGTACAG-3′, and 5′-CACTCCATGTACCGGTTCTTTTAG-3′). Sequencing was performed by the TaKaRa sequencing service. For comparison, passage studies of the four single drugs were conducted. The starting concentration of each drug was based on its EC50. For RPV, EVG, and DTG, the lowest concentration was half of their EC50s and then 2-fold increments up to 64-fold EC50. For 3TC, 0.5-, 1-, 2-, 64-, 320-, and 640-fold EC50 were used because our preliminary results suggested that a higher concentration was necessary for 3TC to inhibit HIV-1 replication completely (data not shown). Only DTG could stop HIV replication above its EC90, and no resistant virus emerged (Fig. 1A). As a single agent, DTG had the highest barrier to resistance, followed by RPV, EVG, and 3TC (Fig. 1B, C, and D).
FIG 1.
In vitro passage studies of single drugs. HIV-1 WT NL-432 was propagated in MT-2 cells in the presence of DTG (A), EVG (B), RPV (C), or 3TC (D). The drug concentrations used were 0.5-fold (blue), 1-fold (light blue), 2-fold (green), 4-fold (yellowish green), 8-fold (yellow), 16-fold (orange), 32-fold (pink), 64-fold (red), 320-fold (lilac), or 640-fold (purple) EC50. A circle indicates that a cytopathic effect (CPE) was observed in more than 80% of the cells. A triangle means that CPE was observed in 30% to 80% of the cells. A cross means that CPE was observed in less than 30% cells. Mutations in the IN or RT coding regions of the virus are indicated in white rounded rectangles at the time points which they emerged. Red rounded rectangles indicate that no virus replicated. The blue rounded rectangles indicate that no mutations were observed in either the IN or RT coding regions despite HIV-1 replication. EC90s are shown in each figure as a red line. Results from one representative well are shown for each drug. The passages of 64-fold EC50 of 3TC (D) and both 16- and 32-fold EC50s of EVG (B) were stopped at day 60.
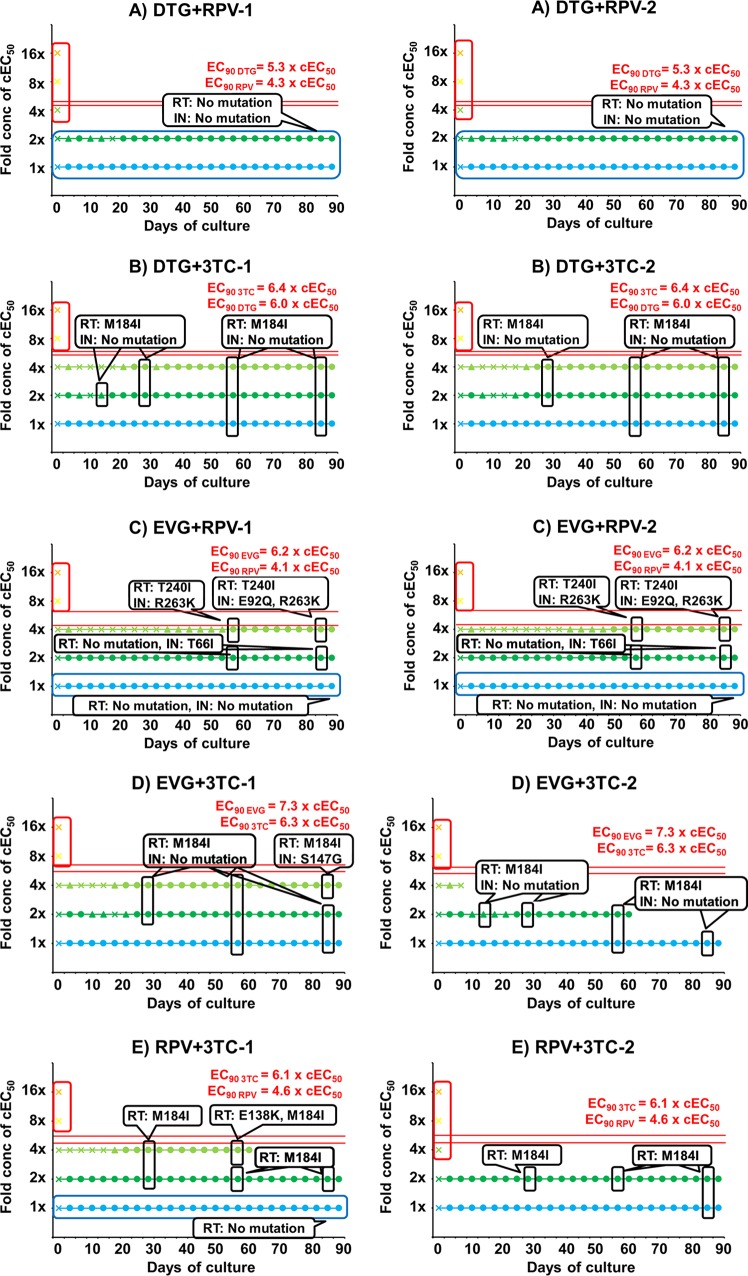
To specifically determine the barrier to resistance of the drugs in combination, we conducted passage studies with two-drug combinations based on their combination EC50 (cEC50). The cEC50 of drug n (Dn) was calculated with the EC50 and the fractional inhibitory concentration index (FICI) using the following formula: cEC50Dn = EC50Dn × FICI. The FICI was defined as the cross point of Y = x and on an approximate curve of an isobologram of the two-drug combination (12). The cEC50 of each drug was roughly equal to half of its EC50 (Table 3). These passage studies were done using the same methodology as that of the single-drug passages, with each combination repeated in two independent wells (Fig. 2). The starting drug concentrations were 1-, 2-, 4-, 8-, and 16-fold cEC50s of each combination (Table 3), and each drug was kept at the same fold cEC50 concentration throughout the passage experiment. In both wells containing RPV and DTG, wild-type HIV-1 could replicate at both 1- and 2-fold cEC50s and could not replicate at more than 2-fold cEC50s, which were less than their individual EC90s (Fig. 2A). Interestingly, mutations were not observed in either the IN coding region or in the RT coding region, even at the drug concentrations at which HIV-1 replicated. When RPV and DTG were compared to RPV or DTG alone, better resistance profiles were seen, especially for RPV (Fig. 2A versus Fig. 1A and C). In the wells containing 3TC and DTG (Fig. 2B), 3TC-resistant virus RT: M184I emerged on day 57 at 1-fold cEC50s, on day 14 or on day 28 at 2-fold cEC50s, and on day 28 at 4-fold cEC50s. No DTG-resistant viruses emerged during the 90-day monitoring period. HIV-1 could not replicate at more than 4-fold cEC50s. Even though 3TC-resistant mutants emerged in the 3TC-DTG combination, the 3TC concentration which permitted viral growth and resistance was decreased more than 32-fold in combination with DTG. This combination effect on 3TC was the highest among the four drugs. Similar combination effects on antiviral activity were seen in the 3TC-RPV, RPV-EVG, and 3TC-EVG combinations (Fig. 2C to E). However, viruses resistant to both drugs emerged in these combinations. RT: K101E (RPV-resistant) and IN: T66I or A (EVG-resistant) were found in the EVG-RPV wells. RT: M184I (3TC-resistant) and IN: S147G (EVG-resistant) emerged in the EVG-3TC wells. In the 3TC-RPV wells, RT: M184I and RT: E138K (RPV-resistant) emerged. These have all been previously reported to be drug-resistant mutations (13–17). Therefore, the barrier to resistance of DTG and 3TC was the second best of the drugs studied. Recently, the 3TC-DTG phase 3 clinical studies Gemini 1 and 2 showed that this combination was not inferior to the standard 3-drug regimen (18). The combination of DTG and RPV, which showed the greatest barrier to resistance, has been approved by the FDA as a two-drug maintenance regimen.
TABLE 3.
Combination EC50 of each drug in two-drug combination
| Drug combination | FICIa | cEC50 D1b (nM) | cEC50 D2c (nM) |
|---|---|---|---|
| DTG-RPV | 0.489 | 1.0 | 0.60 |
| DTG-3TC | 0.428 | 0.89 | 1416 |
| EVG-RPV | 0.509 | 0.71 | 0.63 |
| EVG-3TC | 0.431 | 0.60 | 1426 |
| RPV-3TC | 0.452 | 0.56 | 1496 |
| RPV-RPV | 0.4999 | 0.62 | 0.62 |
FICI, fractional inhibitory concentration index.
cEC50 D1, cEC50 of drug 1 in each combination of drug 1-drug 2.
cEC50 D2, cEC50 of drug 2 in each combination of drug 1-drug 2.
FIG 2.
In vitro passage studies of two-drug combinations. HIV-1 WT NL-432 was propagated in MT-2 cells in the presence of DTG-RPV (A), DTG-3TC (B), EVG-RPV (C), EVG-3TC (D), or RPV-3TC (E). The drug concentrations are 1-fold (light blue), 2-fold (green), 4-fold (yellowish green), 8-fold (yellow), or 16-fold (orange) cEC50s of each combination. A circle indicates that cytopathic effect (CPE) was observed in more than 80% of the cells. A triangle means that CPE was observed in 30% to 80% of cells. A cross indicates that CPE was observed in less than 30% of the cells. Mutations in the IN and RT coding regions of the virus are indicated in white rounded rectangles at the time points which they emerged. Red rounded rectangles indicate that no virus replicated, and blue rounded rectangles indicate that no mutations were observed in the IN and RT coding regions despite HIV-1 replication. The EC90s of each drug are shown as red lines. Each passage was conducted in duplicate wells, and the results from both wells are shown as -1 and -2. The passages of 2-fold cEC50s of 3TC-EVG-2 and 4-fold cEC50s of 3TC-RPV-1 were stopped at day 60.
In all the combinations, viruses resistant to one or both drugs occurred at drug concentrations less than the EC90s of each drug, which allowed for some degree of viral replication (Fig. 2). However, for RPV and DTG, no viruses resistant to either drug emerged at 1- or 2-fold cEC50s. This concentration of 2-fold cEC50 of RPV plus 2-fold cEC50 of DTG is roughly equal to 2-fold EC50 of RPV alone or 2-fold EC50 of DTG alone. Similarly, no IN mutants emerged at 2-fold EC50 when DTG was passaged alone. However, the RT:Y181C mutant emerged at 2-fold EC50 (EC90) when RPV was used alone (Fig. 1C). DTG has a high genetic barrier; many of the single IN mutants which can stochastically emerge have similar susceptibility to DTG as the wild-type (WT) virus (9). On the other hand, viral fitness for IN single mutants usually decreases (19), and low selection pressure from DTG does not select for IN single mutants from WT virus (9). At the concentrations of 2-fold EC50 for RPV and DTG, viral growth was slow in the initial ∼20 days of passage (Fig. 2A). This low replication of virus may correlate with a low chance of the Y181C mutation emerging. Indeed, the emergence of resistance mutations was directly associated with viral growth (Fig. 1 and 2). Some synergistic effect may be seen when RPV and DTG are used in combination.
In our in vitro study, the barrier to resistance of 3TC and DTG was second to that of RPV and DTG. It was surprising that high concentrations of 3TC were necessary to prevent viral growth when used alone, but when 3TC was used in combination with DTG, EVG, or RPV, 8-fold cEC50 each was enough to prevent viral growth. 3TC and these drugs may have a positive synergistic effect that was not detected in the checkerboard method. Recently, it was reported that the combination of R263K in the IN coding region and M184I/V in the RT coding region decreases HIV-1 replicative capacity (20–22). This effect on viral fitness may be a reason for the higher barrier to resistance of the 3TC-DTG combination. When comparing 3TC resistance in vitro and in vivo, various factors, such as cell type, whether cells are resting or growing (10), and HIV subtype (23) should be considered.
In conclusion, our data suggest that two-drug combinations in vitro, especially with DTG, improve the barrier to resistance compared with each drug alone. The high genetic barrier to resistance of DTG likely contributes to this effect. These results may support future clinical use of the DTG-RPV and DTG-3TC combinations.
ACKNOWLEDGMENTS
We thank Akihiko Sato for critical reading of the manuscript.
Tomokazu Yoshinaga was involved in study design, analysis, interpretation of data, and writing the manuscript. Shigeru Miki was involved in study design, experiments, analysis, and writing the manuscript. Shinobu Kawauchi-Miki was involved in experiments and analysis. Takahiro Seki was involved in study design, interpretation of data, and writing the manuscript. Tamio Fujiwara was involved in data interpretation and writing the manuscript.
REFERENCES
- 1.Gueler A, Moser A, Calmy A, Günthard HF, Bernasconi E, Furrer H, Fux CA, Battegay M, Cavassini M, Vernazza P, Zwahlen M, Egger M, Swiss HIV Cohort Study, Swiss National Cohort. 2017. Life expectancy in HIV-positive persons in Switzerland: matched comparison with general population. AIDS 31:427–436. doi: 10.1097/QAD.0000000000001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soriano V, Fernandez-Montero JV, Benitez-Gutierrez L, Mendoza C, Arias A, Barreiro P, Peña JM, Labarga P. 2017. Dual antiretroviral therapy for HIV infection. Expert Opin Drug Saf 16:923–932. doi: 10.1080/14740338.2017.1343300. [DOI] [PubMed] [Google Scholar]
- 3.Lambert-Niclot S, George EC, Pozniak A, White E, Schwimmer C, Jessen H, Johnson M, Dunn D, Perno CF, Clotet B, Plettenberg A, Blaxhult A, Palmisano L, Wittkop L, Calvez V, Marcelin AG, Raffi F, NEAT 001/ANRS 143 Study Group. 2016. Antiretroviral resistance at virological failure in the NEAT 001/ANRS 143 trial: raltegravir plus darunavir/ritonavir or tenofovir/emtricitabine plus darunavir/ritonavir as first-line ART. J Antimicrob Chemother 71:1056–1062. doi: 10.1093/jac/dkv427. [DOI] [PubMed] [Google Scholar]
- 4.van Lunzen J, Pozniak A, Gatell JM, Antinori A, Klauck I, Serrano O, Baakili A, Osiyemi O, Sevinsky H, Girard PM. 2016. Brief report: switch to ritonavir-boosted atazanavir plus raltegravir in virologically suppressed patients With HIV-1 infection: a randomized pilot study. J Acquir Immune Defic Syndr 71:538–543. doi: 10.1097/QAI.0000000000000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selleseth DW, Talarico CL, Miller T, Lutz MW, Biron KK, Harvey RJ. 2003. Interactions of 1263W94 with other antiviral agents in inhibition of human cytomegalovirus replication. Antimicrob Agents Chemother 47:1468–1471. doi: 10.1128/AAC.47.4.1468-1471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johns BA, Kawasuji T, Weatherhead JG, Taishi T, Temelkoff DP, Yoshida H, Akiyama T, Taoda Y, Murai H, Kiyama R, Fuji M, Tanimoto N, Jeffrey J, Foster SA, Yoshinaga T, Seki T, Kobayashi M, Sato A, Johnson MN, Garvey EP, Fujiwara T. 2013. Carbamoyl pyridone HIV-1 integrase inhibitors 3. A diastereomeric approach to chiral nonracemic tricyclic ring systems and the discovery of dolutegravir (S/GSK1349572) and (S/GSK1265744). J Med Chem 56:5901–5916. doi: 10.1021/jm400645w. [DOI] [PubMed] [Google Scholar]
- 7.Harada S, Koyanagi Y, Yamamoto N. 1985. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 8.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, Foster SA, Hazen RJ, Miki S, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. 2011. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 55:813–821. doi: 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aquaro S, Perno CF, Balestra E, Balzarini J, Cenci A, Francesconi M, Panti S, Serra F, Villani N, Caliò R. 1997. Inhibition of replication of HIV in primary monocyte/macrophages by different antiviral drugs and comparative efficacy in lymphocytes. J Leukoc Biol 62:138–143. doi: 10.1002/jlb.62.1.138. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Nakahara K, Seki T, Miki S, Kawauchi S, Suyama A, Wakasa-Morimoto C, Kodama M, Endoh T, Oosugi E, Matsushita Y, Murai H, Fujishita T, Yoshinaga T, Garvey E, Foster S, Underwood M, Johns B, Sato A, Fujiwara T. 2008. Selection of diverse and clinically relevant integrase inhibitor-resistant human immunodeficiency virus type 1 mutants. Antiviral Res 80:213–222. doi: 10.1016/j.antiviral.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Chou TC, Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 13.Schuurman R, Nijhuis M, van Leeuwen R, Schipper P, de Jong D, Collis P, Danner SA, Mulder J, Loveday C, Christopherson C, Kwok S, Sninsky J, Boucher CAB. 1995. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC). J Infect Dis 171:1411–1419. doi: 10.1093/infdis/171.6.1411. [DOI] [PubMed] [Google Scholar]
- 14.Wainberg MA, Salomon H, Gu Z, Montaner JSG, Cooley TP, McCaffrey RP, Ruedy J, Hirst HM, Cammack N, Cameron J, Nicholson W. 1995. Development of HIV-1 resistance to (-) 2′- deoxy-3′-thiacytidine in patients with AIDS or advanced AIDS-related complex. AIDS 9:351–357. [PubMed] [Google Scholar]
- 15.Tisdale M, Kemp SD, Parry NR, Larder BA. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 39-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci U S A 90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azijn H, Tirry I, Vingerhoets J, de Béthune MP, Kraus G, Boven K, Jochmans D, Van Craenenbroeck E, Picchio G, Rimsky LT. 2010. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother 54:718–727. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimura K, Kodama E, Sakagami Y, Matsuzaki Y, Watanabe W, Yamataka K, Watanabe Y, Ohata Y, Doi S, Sato M, Kano M, Ikeda S, Matsuoka M. 2008. Broad antiretroviral activity and resistance profile of the novel human immunodeficiency virus integrase inhibitor elvitegravir (JTK-303/GS-9137). J Virol 82:764–774. doi: 10.1128/JVI.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahn P, Madero JS, Arribas JR, Antinori A, Ortiz R, Clarke AE, Hung CC, Rockstroh JK, Girard PM, Sievers J, Man C, Currie A, Underwood M, Tenorio AR, Pappa K, Wynne B, Fettiplace A, Gartland M, Aboud M, Smith K, GEMINI Study Team. 2018. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet doi: 10.1016/S0140-6736(18)32462-0. [DOI] [PubMed] [Google Scholar]
- 19.Nakahara K, Wakasa-Morimoto C, Kobayashi M, Miki S, Noshi T, Seki T, Kanamori-Koyama M, Kawauchi S, Suyama A, Fujishita T, Yoshinaga T, Garvey EP, Johns BA, Foster SA, Underwood MR, Sato A, Fujiwara T. 2009. Secondary mutations in viruses resistant to HIV-1 integrase inhibitors that restore viral infectivity and replication kinetics. Antiviral Res 81:141–146. doi: 10.1016/j.antiviral.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Singhroy DN, Wainberg MA, Mesplède T. 2015. Combination of the R263K and M184I/V resistance substitutions against dolutegravir and lamivudine decreases HIV replicative capacity. Antimicrob Agents Chemother 59:2882–2885. doi: 10.1128/AAC.05181-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham HT, Mesplède T, Wainberg MA. 2016. Effect on HIV-1 viral replication capacity of DTG-resistance mutations in NRTI/NNRTI resistant viruses. Retrovirology 13:31. doi: 10.1186/s12977-016-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira M, Ibanescu RI, Pham HT, Brenner B, Mesplède T, Wainberg MA. 2016. The M184I/V and K65R nucleoside resistance mutations in HIV-1 prevent the emergence of resistance mutations against dolutegravir. AIDS 30:2267–2273. doi: 10.1097/QAD.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 23.Quashie PK, Oliviera M, Veres T, Osman N, Han YS, Hassounah S, Lie Y, Huang W, Mesplède T, Wainberg MA. 2015. Differential effects of the G118R, H51Y, and E138K resistance substitutions in different subtypes of HIV integrase. J Virol 89:3163–3175. doi: 10.1128/JVI.03353-14. [DOI] [PMC free article] [PubMed] [Google Scholar]