Escalating levels of antibiotic resistance in mycoplasmas, particularly macrolide resistance in Mycoplasma pneumoniae and M. genitalium, have narrowed our antibiotic arsenal. Further, mycoplasmas lack a cell wall and do not synthesize folic acid, rendering common antibiotics, such as beta-lactams, vancomycin, sulfonamides, and trimethoprim, of no value.
KEYWORDS: Mycoplasma genitalium, Mycoplasma pneumoniae, NH125 analogues, Ureaplasma, drug evaluation, nitroxoline, quinoline
ABSTRACT
Escalating levels of antibiotic resistance in mycoplasmas, particularly macrolide resistance in Mycoplasma pneumoniae and M. genitalium, have narrowed our antibiotic arsenal. Further, mycoplasmas lack a cell wall and do not synthesize folic acid, rendering common antibiotics, such as beta-lactams, vancomycin, sulfonamides, and trimethoprim, of no value. To address this shortage, we screened nitroxoline, triclosan, and a library of 20 novel, halogenated phenazine, quinoline, and NH125 analogues against Ureaplasma species and M. hominis clinical isolates from urine. We tested a subset of these compounds (n = 9) against four mycoplasma type strains (M. pneumoniae, M. genitalium, M. hominis, and Ureaplasma urealyticum) using a validated broth microdilution or agar dilution method. Among 72 Ureaplasma species clinical isolates, nitroxoline proved most effective (MIC90, 6.25 µM), followed by an N-arylated NH125 analogue (MIC90, 12.5 µM). NH125 and its analogue had significantly higher MICs against U. urealyticum isolates than against U. parvum isolates, whereas nitroxoline did not. Nitroxoline exhibited bactericidal activity against U. parvum isolates but bacteriostatic activity against the majority of U. urealyticum isolates. Among the type strains, the compounds had the greatest activity against M. pneumoniae and M. genitalium, with 8 (80%) and 5 (71.4%) isolates demonstrating MICs of ≤12.5 µM, respectively. Triclosan also exhibited lower MICs against M. pneumoniae and M. genitalium. Overall, we identified a promising range of quinoline, halogenated phenazine, and NH125 compounds that showed effectiveness against M. pneumoniae and M. genitalium and found that nitroxoline, approved for use outside the United States for the treatment of urinary tract infections, and an N-arylated NH125 analogue demonstrated low MICs against Ureaplasma species isolates.
INTRODUCTION
Annually in the United States, antibiotic-resistant organisms cause an estimated 2 million illnesses and 20,000 deaths (1). Reports of antibiotic resistance extend to include mycoplasmas, which are fastidious bacterial organisms that cause urogenital or respiratory tract infections in pediatric and adult populations (2–4). As mycoplasmas lack a cell wall and do not synthesize folic acid, they are refractory to many widely used antibiotics, such as beta-lactams, vancomycin, sulfonamides, and trimethoprim (2). Therefore, this restricts treatment options to drugs that interfere with DNA replication or protein synthesis (2). With the confluence of emerging antibiotic resistance in mycoplasmas and their innate resistance to many commonly prescribed pharmaceuticals, a great impetus exists to identify novel drug classes capable of treating infections caused by these pathogens.
Mycoplasma pneumoniae causes an estimated 2 million cases of pneumonia in the United States annually, and the highest number of cases cluster in school-aged children and adolescents (3). Current treatment options in the pediatric population center on macrolides, as quinolones are not recommended as first-line therapy (5) and tetracycline use is limited to patients older than age 8 years (6). In Asia, macrolide-resistant M. pneumoniae (MRMP) strains have been reported to be widespread. In Beijing, China, 90% of adult and pediatric M. pneumoniae respiratory isolates collected between 2008 and 2012 were macrolide resistant (7), and macrolide resistance was found in 50% to 93% of M. pneumoniae isolates from specimens collected across seven regions in Japan during the 2010 to 2012 epidemic (8). Conversely, within that same time period in the United States, the MRMP prevalence ranged from 10 to 13.2% (9, 10). High levels of MRMP in Japan have been attributed to the selective pressure created from elevated macrolide prescribing (11). As macrolide prescribing in the United States is on the rise and mirrors the proportion seen in some Japanese reports (12, 13), researchers emphasize the need to revisit existing drug classes, as well as identify novel ones, to treat emerging MRMP infections.
New treatment modalities are also desperately needed for M. genitalium, a sexually transmitted pathogen that is most known for causing nongonococcal urethritis (NGU) in men (14) and that is associated with cervicitis, pelvic inflammatory disease, preterm birth, and spontaneous abortion in women (4). Globally, extremely high levels of mutations conferring macrolide resistance in M. genitalium (MRMG) have been found in Greenland (100%), New Zealand (72% to 77%), and Australia (36% to 79.4%), whereas moderate to high levels were detected in Spain (35%), Japan (29% to 47%), Denmark (38% to 57%), Germany (52.6%), Norway (56.4%), Canada (47% to 58%), the United States (48 to 80%), and England (41% to 82%) (15–37). Lower levels of MRMG have been reported in Russia (4.6%), Belgium (6.5%), France (8.3% to 11.3%), Africa (9.8% to 14.6%), Estonia (10%), Sweden (17.6% to 18.1%), and the Netherlands (21%) (38–46). A few studies found indications that the rate of resistance may be rising over time, evidenced by a significant increase in azithromycin treatment failures (47) and an increase in mutations associated with macrolide or quinolone resistance compared to earlier periods (26, 27, 48). More worrisome is the finding that as azithromycin and quinolones serve as the first- and second-line anti-M. genitalium treatment regimens in the United States, respectively, the emergence of quinolone resistance-associated mutations (QRMs) and mutations conferring dual resistance to both macrolides and quinolones has further depleted treatment options (49). The levels of QRMs in Japanese M. genitalium specimens appear to be rising and occurred in 53% of samples collected from 2010 to 2017, with dual resistance being reported in 25% of M. genitalium isolates in specimens from separate populations of Japanese men with urethritis and female sex workers (26–28). Studies in Australasia have also reported concerning levels of QRMs among 23.3% of samples from New Zealand, 15.4% from Sydney, Australia, and 13.6% from Melbourne, Australia, wherein 12 (8.6%) samples in the last study had mutations conferring dual resistance (16, 19, 50). In two studies based in Birmingham, AL, QRMs and mutations conferring dual resistance were detected in nearly a third of specimens from a population of men who have sex with men and roughly 11% of African-American heterosexual partners (33, 34). In three separate Canadian studies, QRMs in M. genitalium were found in about 2% of isolates from specimens from women, 12% of isolates from specimens from men, and 20% of isolates from samples derived from a population comprised mostly of men (29–31). These multidrug-resistant organisms limit physicians to doxycycline, which exhibits low cure rates, ranging from 30% to 45%, or to imported drugs that require special permits (51–53). Europe and Australia have responded to rising resistance by developing resistance-guided prescribing recommendations (54, 55). Australia’s recently released guidelines now recommend pretreating M. genitalium infections with doxycycline, as it reduces the organism load and lacks a penchant for inducing resistance, followed by treating susceptible infections with either azithromycin or moxifloxacin (55).
In the United States, concerning levels of tetracycline resistance have been reported in Ureaplasma spp., a type of mycoplasma most recognized for its role in negative reproductive outcomes and infections in neonates (2, 56–59). Two separate studies detected tetracycline resistance in 33% and 34% of Ureaplasma species isolates (60, 61), and a separate report identified the tet(M) gene, which confers tetracycline resistance and which was present in 45% of clinical isolates sourced from distinct geographic areas (2). However, lower levels of tetracycline resistance (range, 0.4% to 1.4%) and quinolone resistance (range, 1.4% to 6%) have been described in the United States (62, 63). In contrast, levofloxacin resistance was detected in 57% and in 75% of isolates from a Japanese study and a Chinese study, respectively (64, 65). Besides these notable studies, Beeton and Spiller have provided a comprehensive review of recent studies evaluating antibiotic resistance among Ureaplasma species isolates, as well as discussions examining the shortcomings of different testing kits that may influence the comparability of results across studies (66).
Few studies that abide by validated methods have evaluated antibiotic resistance in M. hominis, a mycoplasma associated with pelvic inflammatory disease, pyelonephritis, and other reproductive sequelae (2). Studies in the United States and Germany have demonstrated increases in clindamycin and tetracycline resistance over time. In the U.S. study, clindamycin and tetracycline resistance levels increased from 2% and 7%, respectively, in the late 1970s to 10% and 27%, respectively, a decade later (67). Similarly, Krausse and Schubert measured tetracycline resistance in 2% of isolates from 1983, a rate which then increased to 17% from 1997 to 2004 (68). Clindamycin resistance also increased over the same time frame from 0% to 11% (68). In French studies, Degrange et al. found tetracycline resistance in nearly 19% of isolates in the early 2000s (69), while Meygret et al. found resistance in approximately 15% of isolates collected from 2010 to 2015 (90). A recent U.S. study that tested 10 M. hominis isolates from women with first-time urinary tract infection (UTI) found no resistance to tetracycline, clindamycin, or levofloxacin (62).
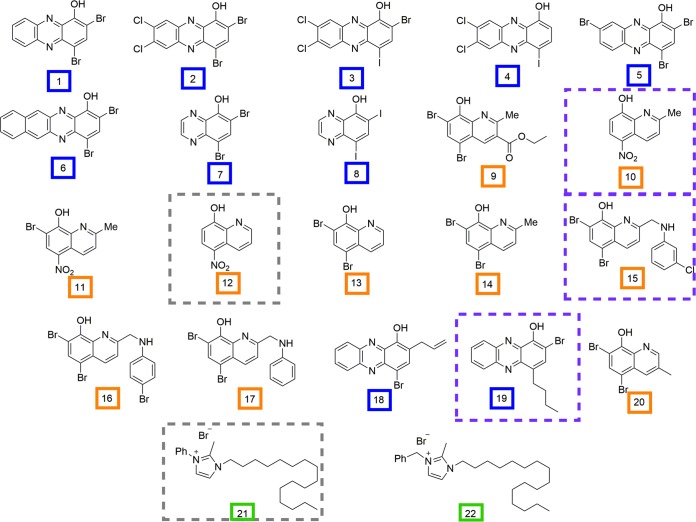
In order to expand our existing arsenal of antibiotics, we evaluated the MICs of 22 halogenated phenazine, quinoline, and NH125 analogues (Fig. 1) and triclosan against the following human mycoplasmas: M. genitalium, M. hominis, M. pneumoniae, and Ureaplasma spp. These analogue libraries were synthesized by substituting various halogen or methyl groups at critical positions along the base structure of a previously efficacious, representative compound for each class (70–72). This led to an expanded library of halogenated phenazine, quinoline, and NH125 analogues that had, in some cases, MICs comparable to or more efficacious than those of leading antimicrobials used to treat methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant S. epidermidis (MRSE), and vancomycin-resistant Enterococcus faecium (VRE) (70–72). Mechanistically, past investigations have accumulated evidence suggesting that the NH125 analogues exert their effects through bacterial membrane destruction (73), whereas the quinolines and halogenated phenazine compounds appear to work through a non-membrane-destroying, metal(II)-dependent mechanism (70, 74–76). As mycoplasmas lack a cell wall, which leaves an exposed cell membrane, we hypothesize that NH125 analogues will result in low MICs against human mycoplasmas.
FIG 1.
Compounds synthesized by the R. W. Huigens III lab. Halogenated phenazine, quinoline, and NH125 analogues have blue, orange, and green boxes around the compound numbers, respectively, designating each class. Compounds with a gray, dashed box represent those that demonstrated efficacious MICs against Ureaplasma species clinical isolates and M. genitalium and M. pneumoniae type strains. Those highlighted with a dashed purple box had efficacious MICs against both M. genitalium and M. pneumoniae, mycoplasmas with high levels of macrolide resistance in recent years. Me, methyl; Ph, phenyl.
RESULTS
Clinical isolate MICs.
The majority of compounds 1 to 22 had MICs of >12.5 µM against the screened, Ureaplasma species clinical isolates (Table 1), with the exception of compounds 12 (nitroxoline), 10 (a quinoline structurally similar to nitroxoline), 21 (an NH125 analogue), and 22 (NH125). Nitroxoline had the lowest MIC50 (3.13 µM) and MIC90 (6.25 µM) values against Ureaplasma spp. Interestingly, Ureaplasma species isolates that had MICs of ≤12.5 µM to compound 10 also had significantly lower MIC values to nitroxoline (P = 2.89 × 10−5). Compound 21 had the second lowest MIC50 and MIC90 values among the Ureaplasma species isolates. Ureaplasma species isolates with MICs of ≤12.5 µM to NH125 also had significantly lower MICs to compound 21 (P = 4.82 × 10−7). NH125 had MICs of ≤12.5 µM in 65.3% (n = 47) of isolates, while compound 10 had MICs of ≤12.5 µM in 50% (n = 17) of the screened isolates (n = 34). No significant differences existed when comparing MIC values for compound 10 and nitroxoline between Ureaplasma urealyticum and U. parvum isolates; however, compound 21 and its parent compound, NH125, had significantly lower (P < 0.0001) MICs against U. parvum isolates than against U. urealyticum isolates (Fig. 2). The levofloxacin- and tetracycline-resistant U. parvum isolates had MICs that fell in line with the MICs displayed by sensitive U. parvum isolates. The MICs of nitroxoline and compound 21 were 6.25 µM for both the levofloxacin- and tetracycline-resistant U. parvum isolates, which matches the MIC90 and MIC50 of these compounds, respectively. The MIC of compound 22 for both sets of isolates was 12.5 µM, which was the MIC50 for U. parvum isolates. In general, the MIC values of the compounds for U. urealyticum ATCC 33175 (Table 2) fell within the range of MICs observed for all clinical isolates, with the exception of compound 21 (MIC > 25 µM). Triclosan had MICs of >120 µM against the subset of Ureaplasma species and M. hominis clinical isolates and type strains tested (data not pictured). Initially, when screening nitroxoline against a subset of M. hominis isolates, we obtained MIC values of ≤12.5 µM against the type strain (6.25 µM) and two clinical isolates (12.5 µM). However, we could not reproduce these results and found that nitroxoline had a MIC of 50 µM against four clinical isolates and the type strain, while the remaining isolates had MICs of >50 µM.
TABLE 1.
Summary of MIC results for 72 Ureaplasma species clinical isolates for test compounds with MICs of ≤12.5 µM for >20% of the screened isolates
| Isolate and test compounda (no. of isolates) | MICb
|
No. (%) of resistant isolatesc | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| U. parvum (n = 59) | ||||
| Compound 10 (n = 26) | 12.5 to >12.5 | 12.5 | >12.5 | NA |
| Compound 12 (n = 59) | 1.56 to 6.25 | 3.13 | 6.25 | NA |
| Compound 21 (n = 59) | 0.39 to 12.5 | 6.25 | 12.5 | NA |
| Compound 22 (n = 59) | 6.25 to >12.5 | 12.5 | >12.5 | NA |
| Levofloxacin (n = 59)d | ≤0.25 to 4 | 0.5 | 1 | 1 (1.7) |
| Tetracycline (n = 59)d | ≤0.25 to 8 | ≤0.25 | 0.5 | 1 (1.7) |
| U. urealyticum (n = 13) | ||||
| Compound 10 (n = 8) | 12.5 to >12.5 | 12.5 | >12.5 | NA |
| Compound 12 (n = 13) | 1.56 to 6.25 | 3.13 | 6.25 | NA |
| Compound 21 (n = 13) | 3.13 to 12.5 | 12.5 | 12.5 | NA |
| Compound 22 (n = 13) | 12.5 to >12.5 | >12.5 | >12.5 | NA |
| Levofloxacin (n = 13)d | 0.5 to 1 | 1 | 1 | 0 |
| Tetracycline (n = 13)d | ≤0.25 to 1 | 1 | 1 | 0 |
Compound 12 is also known as nitroxoline, and compound 22 is known as NH125.
MICs are in micromolar for compounds 10, 12, 21, and 22 and micrograms per milliliter for levofloxacin and tetracycline.
Ureaplasma spp. determined by using CLSI interpretive guidelines. Ureaplasma species breakpoints were as follows: levofloxacin, ≥4 µg/ml; tetracycline, ≥2 µg/ml. NA, not applicable.
MIC values for levofloxacin and tetracycline were reported by Valentine-King and Brown (62).
FIG 2.
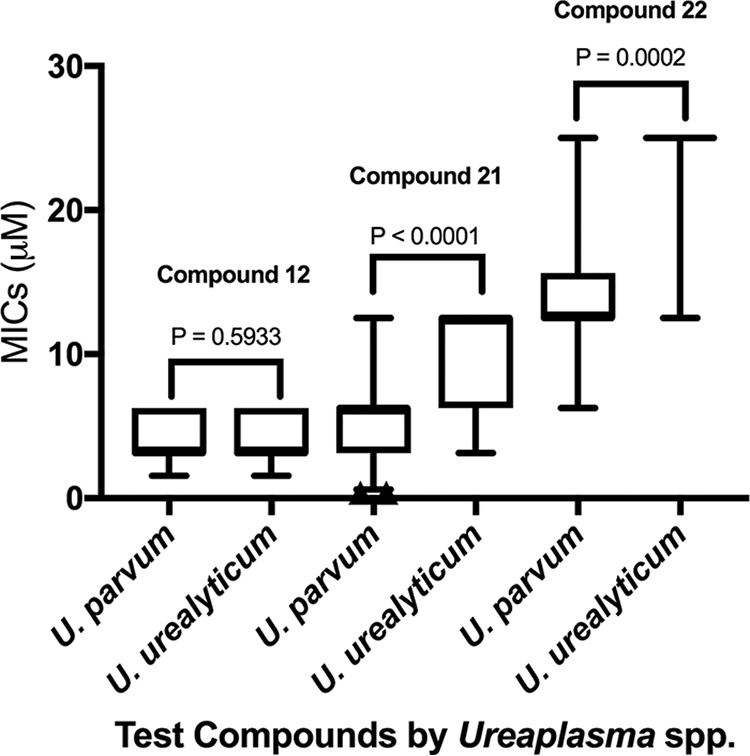
Box plots displaying MIC comparisons between Ureaplasma spp. for compounds 12, 21, and 22. To depict comparisons for antimicrobial compound 22, MICs of >12.5 µM were designated 25 µM for the purposes of this figure only. Whiskers depict 5th and 95th percentiles.
TABLE 2.
MIC results for a subset of the test compounds and triclosan against human mycoplasma type strains M. genitalium, M. hominis ATCC 23411, and U. urealyticum ATCC 33175e
| Organism | MIC (µM) for the following compounds: |
QC drug MIC (µg/ml) |
No. (%) of AMCs with MICs of ≤12.5 µM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 10 | 11 | 12 | 14 | 15 | 19 | 20 | 21 | 22 | Triclosan | Levofloxacin | Tetracycline | ||
| M. genitaliuma | NA | 12.5 | NA | 12.5 | >12.5 | 12.5 | 12.5 | >12.5 | 3.13 | NA | 120 | 1 | 5 (71.4) | |
| M. pneumoniaeb | >12.5 | 12.5 | >12.5 | 12.5 | 6.25 | 6.25 | 6.25 | 2.35c | 3.13 | 3.13 | 60 | 0.5 | 8 (80) | |
| U. urealyticumb | >12.5 | 12.5 | >12.5 | 3.13 | >25 | >25 | >25 | 25 | >25 | NA | >120 | 1 | 2 (22.2) | |
| M. hominisa | >12.5 | >12.5 | >12.5 | 50d | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 | >120 | 1 | (0) | |
| No. (%) of AMCs with MICs of ≤12.5 µM | 0 (0) | 3 (75) | 0 (0) | 3 (75) | 1 (25) | 2 (50) | 2 (50) | 1 (25) | 2 (50) | 1 (50) | ||||
Tested compounds against this organism in doubling dilutions ranging from 12.5 µM to 1.56 µM.
Tested compounds against this organism in doubling dilutions ranging from 25 µM to 3.13 µM, except for compounds 4 and 11, which were tested in doubling dilutions from 12.5 µM to 1.56 µM.
Midpoint value of the MIC range from two independent tests.
Tested in doubling dilutions ranging from 50 µM to 6.25 µM.
NA, not applicable (not tested); QC, quality control; AMC, antimicrobial compounds.
M. genitalium and M. pneumoniae type strain MICs.
The highest efficacy was observed against M. pneumoniae and M. genitalium type strains, with 8 (80%) and 5 (71%) compounds having MICs of ≤12.5 µM against each one, respectively (Table 2). In particular, nitroxoline and compounds 10, 15, 19, and 21 had efficacious MICs against both mycoplasmas, and compound 21 had the lowest MIC against both (MIC, 3.13 µM). Compound 20 had the lowest MIC against M. pneumoniae (2.35 µM), followed by compound 21 and NH125 (MICs, 3.13 µM) and then compounds 14, 15, and 19 (MICs, 6.25 µM). When comparing the MIC values of the same compounds between the two type strains, M. pneumoniae displayed lower average MICs (6.25 µM) than M. genitalium (12.5 µM). Both M. pneumoniae and M. genitalium appeared to display similar sensitivities to the drugs among the drug classes. Further, triclosan had MICs of ≤120 µM to both M. genitalium and M. pneumoniae, which varied by only 1 doubling dilution factor (Table 2).
MBC results.
We conducted minimum bactericidal concentration (MBC) assays using a subset of Ureaplasma species isolates and type strains and the compounds with efficacious MICs via broth microdilution. Nitroxoline demonstrated bactericidal effects against all U. parvum isolates tested (n = 10) but had mostly bacteriostatic effects against U. urealyticum (80%) isolates, one of which included the U. urealyticum ATCC 33175 type strain (Table 3). This difference in effect proved significantly different between species (P = 0.004). On the other hand, compound 21 (an N-arylated NH125 analogue) demonstrated bacteriostatic effects against all Ureaplasma spp. except for one U. parvum isolate (Table 3). Interestingly, this U. parvum isolate contained the tet(M) gene and displayed a tetracycline-resistant phenotype in a prior study (62).
TABLE 3.
MBC results for compounds efficacious against a subset of Ureaplasma species clinical isolates and representative type strains
| Compound, organism | No. of isolates for which MBC was: |
No. (%) of isolates for which treatment was: |
Range or avg (range) MBC (µM) | ||||
|---|---|---|---|---|---|---|---|
| 2× MIC | 4× MIC | >4× MIC | Bactericidal | Bacteriostatic | P value | ||
| Compound 12 | |||||||
| U. urealyticum (n = 5) | 1 | 0 | 4 | 1 (20) | 4 (80) | 0.004 | 6.25 to >12.5 |
| U. parvum (n = 10) | 7 | 3 | 10 (100) | 0 (0) | 12.5 (6.25 to 25) | ||
| Compound 21, Ureaplasma spp. (n = 9) | 1 | 0 | 8 | 1 (11.1) | 8 (88.9) | 12.5 to >12.5 | |
DISCUSSION
Widespread MRMP in Asia (7, 8) coupled with notable levels of MRMG globally (15–37) have depleted first-line treatment options against these pathogenic organisms. This is reflected in the newest sexually transmitted disease treatment guidelines for M. genitalium in Australia and Europe (54, 55). Additionally, concerning levels of tetracycline resistance in Ureaplasma species isolates in the United States have been reported (2, 60, 61). With rising levels of antibiotic resistance eliminating the drug classes available for treating mycoplasma infections, identifying new drug classes serves a critical need. To address this emerging necessity, we selected a chemically diverse library of drug analogues with different modes of action and antibacterial profiles for this study. We screened our library, and two agents were found to be active against a subset of urinary Ureaplasma species clinical isolates. We then tested a subset of our library against four human mycoplasma type strains and identified a number of compounds with low MICs against both M. genitalium and M. pneumoniae.
Among the Ureaplasma species clinical isolates, we found that nitroxoline and the N-arylated NH125 analogue (compound 21) demonstrated the highest efficacy. NH125 was the third most effective compound, followed by compound 10, an analogue of nitroxoline. Nitroxoline MICs did not differ by species; therefore, this would give clinicians higher confidence in prescribing nitroxoline to cover infections caused by either Ureaplasma species (MIC90, 6.25 µM, or 1.19 µg/ml). Importantly, nitroxoline was efficacious against the Ureaplasma spp. and the M. genitalium type strain (MIC, 12.5 µM, or 2.38 µg/ml). Therefore, nitroxoline could provide coverage against most urogenital Mollicutes. Nitroxoline also was effective against the tetracycline- and levofloxacin-resistant U. parvum isolates. A French study (77) that tested nitroxoline against 30 U. urealyticum isolates reported a MIC90 value of 0.35 mg/liter (1.84 µM) and a MIC90 of 3 mg/liter (15.78 µM) against M. hominis isolates, both of which are lower than what we observed using Clinical and Laboratory Standards Institute (CLSI) standards (78). As their study occurred prior to publication of CLSI standards for evaluating MICs in Mollicutes, they did not standardize the inoculum used, which ranged from 1 to 105 color-changing units (CCU)/ml for U. urealyticum and 101 to 106 CCU/ml for M. hominis (77). Thus, isolates tested with a CCU of 103 or less could have had higher MICs.
As mycoplasmas evolved in a reductionist manner from Gram-positive bacteria, we compared our results for Ureaplasma spp. to those of prior studies evaluating nitroxoline and compound 21 efficacies against MRSA and MRSE clinical isolates and type strains. Nitroxoline performed substantially better against Ureaplasma species isolates (MIC range, 1.56 to 6.25 µM; average MIC, 4.21 µM) than against its Gram-positive relatives (MIC range, 9.38 to 25 µM; average MIC, 16.16 µM) (79). However, compound 21 fared slightly better against MRSA and MRSE strains (MIC range, 1.17 to 3.13 µM; average MIC, 1.95 µM) than against Ureaplasma species isolates (MIC range, 0.39 to 12.5 µM; average MIC, 6.19 µM) (71).
A previous study found that nitroxoline exhibited bactericidal effects against U. urealyticum and M. hominis isolates; however, this was prior to the separation of Ureaplasma into two distinct species (77). When evaluating whether the compounds exerted bactericidal or bacteriostatic effects against Ureaplasma spp., we found that nitroxoline was significantly more likely to exert a bactericidal effect against U. parvum isolates than against U. urealyticum isolates. The mechanism behind the activity of nitroxoline in planktonic and sessile biofilm communities appears to involve metal ion chelation (75, 76); however, differences in metal requirements between Ureaplasma species have not been explored, based on the current literature. Therefore, the significance behind this difference is unknown. Unlike nitroxoline, compound 21 exhibited bacteriostatic effects against the majority of clinical Ureaplasma species isolates (89%), independent of species. As previous studies have indicated that N-arylated NH125 compounds target cell membranes and demonstrate hemolysis at low doses (71), we expected compound 21 to produce bactericidal effects against Ureaplasma spp. However, in a previous study, compound 21 required a dose of 7.84 µM to lyse 50% of red blood cells (71). Thus, perhaps a higher dose is required to obtain a bactericidal effect in Ureaplasma spp., since compound 21 had a MIC90 of 12.5 µM.
Until now, only one study has evaluated triclosan efficacy against mycoplasmas, reporting MICs ranging from 16 µg/ml (55 µM) to 64 µg/ml (221 µM) when tested against mycoplasmas that infect food and fiber animals (80). Here we found evidence that triclosan had comparable, if not lower, MICs against two human mycoplasmas: M. pneumoniae (60 µM) and M. genitalium (120 µM). However, triclosan showed no effect against both Ureaplasma species and M. hominis clinical isolates and type strains.
In light of the high levels of MRMP and MRMG in recent years (7, 8, 15–37), it is encouraging that we identified a number of promising antimicrobial agents and preexisting compounds that demonstrated efficacy against M. genitalium and M. pneumoniae. We identified eight compounds (compounds 10, 12, 14, 15, and 19 to 22) that were efficacious against M. pneumoniae and five (compounds 10, 12, 15, 19, and 21) that were effective against M. genitalium. When comparing the MICs of efficacious compounds between M. pneumoniae and M. genitalium, M. pneumoniae had lower MICs across the board. M. pneumoniae was tested via broth microdilution, and M. genitalium was tested via agar dilution. This could explain the lower MICs seen in M. pneumoniae, as previous research has shown that agar dilution can increase the MIC by a factor of 4-fold (81). In general, both M. pneumoniae and M. genitalium were largely susceptible to the same compounds, which seems reasonable, as both cluster together phylogenetically (82). Furthermore, nitroxoline demonstrated low MICs against Ureaplasma spp. and is a preexisting compound approved for the treatment of UTIs in Europe. As compounds 12, 14, 15, 21, and 22 (71, 79) eradicated MRSA and MRSE biofilms, these compounds may also have the potential to disrupt mycoplasma biofilms, which have been detected in M. pneumoniae and Ureaplasma spp. (83–85). All of the compounds represent new classes of antimicrobials separate from those currently approved for use in the United States and could increase the limited antimicrobial arsenal available for these pathogens.
MATERIALS AND METHODS
Study description.
The clinical isolates of Ureaplasma spp. and M. hominis tested originated from a study that prospectively followed college-age women presenting with first-time urinary tract infection (UTI) at a student health care center in Florida between 2001 and 2006 (86). Clinical isolates of Ureaplasma spp. and M. hominis were obtained from direct culture of urine collected at either the initial UTI presentation or any recurrent UTI episode(s). An antibiogram characterizing the MIC50 and MIC90 values of a panel of antibiotics against the Ureaplasma species and M. hominis clinical isolates was previously published (62). Two U. parvum samples had resistant phenotypes: one for resistance to tetracycline and one for resistance to levofloxacin (62). In the current study, we evaluated the efficacy of NH125, an N-arylated NH125 analogue, 10 phenazine analogues, 10 quinoline analogues, and triclosan against these urinary clinical isolates using a previously validated broth microdilution or agar dilution method (78). The chemical structures for the parent compounds and analogues are shown in Fig. 1. We screened a subset of clinical isolates against all compounds. Compounds with efficacious MICs (≤12.5 µM) against the majority of clinical isolates were chosen for full testing against all 13 U. urealyticum and 59 U. parvum isolates. Nine compounds also were tested against three human mycoplasma type strains, M. hominis ATCC 23114, M. pneumoniae ATCC 29342, and U. urealyticum ATCC 33175, and one clinical isolate, M. genitalium (a gift from J. Baseman, University of Texas Health Sciences Center, San Antonio, TX).
M. pneumoniae and M. genitalium were grown in standard laboratory medium preparations of SP4 broth and agar supplemented with glucose (pH 7.6 to 7.8); for M. hominis, we used SP4 supplemented with l-arginine (pH 7.2 to 7.4); for Ureaplasma spp., we used 10B broth and A8 agar with a pH range of 5.9 to 6.1.
Antimicrobial agents.
As an internal quality control, we included levofloxacin (U. urealyticum ATCC 33175 and M. pneumoniae ATCC 29342) and tetracycline or clindamycin (M. hominis ATCC 23114) to determine if the MICs fell within previously established ranges for these type strains (78). Although no quality control range exists for M. genitalium, we compared the MIC for levofloxacin against the values presented in previously published reports and used levofloxacin thereafter as an internal measure for quality control.
Levofloxacin powder was obtained from Sigma-Aldrich (St. Louis, MO, USA), clindamycin through Pfizer's Compound Transfer Program, and tetracycline through Cellgro (Herndon, VA, USA). When preparing stock solutions, we used established guidelines to adjust for drug purity (87) and used CLSI recommendations to dissolve and dilute the quality control drugs (88). The quality control drugs were tested in doubling dilutions that encompassed 1 dilution above and 1 dilution below the established quality control range. The 22 test compounds were provided at either 10 mM or 1 mM concentrations in dimethyl sulfoxide (DMSO) and were stored at room temperature with protection from light. Drugs were diluted in broth on the day of testing and tested within 6 months of receipt.
MIC determination.
We followed a previously validated broth microdilution or agar dilution method to evaluate the MICs, as previously described (62, 78). For the broth microdilution assay, we used sterile 96-well plates wherein each row contained an antimicrobial agent in doubling dilutions ranging from 12.5 µM to 0.2 µM for clinical isolates and from 25 µM to 3.13 µM for each type strain, in duplicate. Duplicate growth controls, drug controls, solvent controls, and medium controls were set up for each drug and organism tested. A 1:10 dilution of DMSO served as the solvent control. Plates were inoculated with 175 µl of organism at between 104 and 105 CFU/ml. The organisms had been preincubated in broth either for 1 h for the Ureaplasma spp. or for 2 h for all other mycoplasmas tested. The plates were sealed with sterile acetate sealers, with the exception of those with M. pneumoniae, which were sealed per the protocol of Waites et al. (78), and incubated at 37°C in ambient air until the growth control displayed a distinct color change. We read the MIC as the lowest concentration of drug that inhibited any color change.
For organisms that did not show a distinct color change and for compounds that altered the broth color, we used a validated agar dilution method to evaluate drug MICs. Briefly, the method consisted of incorporating 600 µl of antibiotic within 5.4 ml of molten agar by adding the appropriate volume of stock antibiotic to yield concentrations spanning from 25 to 3.13 µM for each drug. We created solvent and growth control plates by mixing 5.4 ml of molten agar with 600 µl of a 1:10 DMSO solution and with 600 µl of filter-sterilized, double-distilled water, respectively. Following a 2-h preincubation period, we added three separate 20-µl drops of organism at 103-, 104-, and 105-CFU/ml concentrations onto each agar plate. Using the organism dilution of between 104 and 105 CFU/ml, the MIC for each drug was read as the lowest antibiotic concentration that inhibited colony formation when the growth control plate exhibited colonies.
For compounds that had efficacious MICs against type strains, defined as a MIC of ≤12.5 µM, we performed a second, confirmatory MIC determination. For clinical isolates, we retested efficacious compounds against a subset of clinical isolates to confirm our results. We considered the MIC results valid only if the organism’s number of CFU ranged from 104 to 105 CFU/ml, which was confirmed on the day of testing.
MBC determination.
We evaluated the MBCs of efficacious compounds for a subset of Ureaplasma species clinical isolates using a previously published method (89). The MBC assay called for transferring 30-µl aliquots directly from the MIC microtiter plate at 1, 2, and 4 times the drug MIC into culture tubes with 2.97 ml of fresh broth immediately following MIC interpretation. For the positive and negative controls, we transferred 30 µl from the growth control and 30 µl from the medium control into separate tubes with 2.97 ml of fresh broth. Following inoculation, all tubes were incubated at 37°C with ambient air for 7 days. Compounds were considered bactericidal if the lowest concentration that did not show growth was within one to four times the predetermined MIC level following incubation. Although the guidelines of the journal Antimicrobial Agents and Chemotherapy call for the use of an inoculum of >5 × 105 CFU/ml for MBC assays with bacteria, the CLSI standardized assay for determining compound MICs in Ureaplasma spp. calls for the use of inoculum concentrations of between 104 and 105 CCU/ml or CFU/ml. Therefore, we conducted MBC assays using the inoculum range designated by the CLSI standardized assay.
Statistical considerations.
Statistical comparisons of MICs between U. parvum and U. urealyticum isolates were conducted using either a Wilcoxon-Mann-Whitney test for MIC data in interval form (compounds 12 and 21) or a chi-square test for MICs characterized as either greater than or less than 12.5 µM (compounds 10 and 22). We used the Fisher exact test to compare differences in MBC outcomes between Ureaplasma spp. Statistical calculations were conducted using RStudio (version 1.0.136) software. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
The clinical samples were obtained from a study funded by NIAID R01 AI45875 (MBB). Clindamycin was provided by Pfizer through their Compound Transfer Program. We acknowledge the National Institute of General Medical Sciences of the National Institutes of Health for providing support for this work (R35GM128621 to R.W.H.). M. A. Valentine-King, and K. Cisneros are supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida Clinical and Translational Science Awards TL1TR001428 and UL1TR001427.
Mycoplasma genitalium was a gift from J. Baseman, University of Texas Health Sciences Center, San Antonio, TX.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 2.Waites KB, Katz B, Schelonka RL. 2005. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev 18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. 2017. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev 30:747–809. doi: 10.1128/CMR.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lis R, Rowhani-Rahbar A, Manhart LE. 2015. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 61:418–426. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 5.Bradley JS, Jackson MA, Committee on Infectious Diseases, American Academy of Pediatrics. 2011. The use of systemic and topical fluoroquinolones Pediatrics 128:e1034–e1045. doi: 10.1542/peds.2011-1496. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee A, O’Keefe C. 2015. Pediatric mycoplasma infections medication. Medscape, New York, NY: https://emedicine.medscape.com/article/966785-medication. [Google Scholar]
- 7.Zhao F, Liu G, Wu J, Cao B, Tao X, He L, Meng F, Zhu L, Lv M, Yin Y, Zhang J. 2013. Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother 57:1521–1523. doi: 10.1128/AAC.02060-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai Y, Miyashita N, Kubo M, Akaike H, Kato A, Nishizawa Y, Saito A, Kondo E, Teranishi H, Wakabayashi T, Ogita S, Tanaka T, Kawasaki K, Nakano T, Terada K, Ouchi K. 2013. Nationwide surveillance of macrolide-resistant Mycoplasma pneumoniae infection in pediatric patients. Antimicrob Agents Chemother 57:4046–4049. doi: 10.1128/AAC.00663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz MH, Benitez AJ, Winchell JM. 2015. Investigations of Mycoplasma pneumoniae infections in the United States: trends in molecular typing and macrolide resistance from 2006 to 2013. J Clin Microbiol 53:124–130. doi: 10.1128/JCM.02597-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, Lee S, Selvarangan R, Qin X, Tang YW, Stiles J, Hong T, Todd K, Ratliff AE, Crabb DM, Xiao L, Atkinson TP, Waites KB. 2015. Macrolide-resistant Mycoplasma pneumoniae, United States. Emerg Infect Dis 21:1470–1472. doi: 10.3201/eid2108.150273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, Chiba N, Iwata S, Ubukata K. 2012. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis 55:1642–1649. doi: 10.1093/cid/cis784. [DOI] [PubMed] [Google Scholar]
- 12.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Taylor TH. 2014. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrob Agents Chemother 58:2763–2766. doi: 10.1128/AAC.02239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida S, Takeuchi M, Kawakami K. 2017. Prescription of antibiotics to pre-school children from 2005 to 2014 in Japan: a retrospective claims database study. J Public Health (Oxf) 40:397–403. doi: 10.1093/pubmed/fdx045. [DOI] [PubMed] [Google Scholar]
- 14.Taylor-Robinson D, Jensen JS. 2011. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gesink DC, Mulvad G, Montgomery-Andersen R, Poppel U, Montgomery-Andersen S, Binzer A, Vernich L, Frosst G, Stenz F, Rink E, Rosing Olsen O, Koch A, Skov Jensen J. 2012. Mycoplasma genitalium presence, resistance and epidemiology in Greenland. Int J Circumpolar Health 71:18203. doi: 10.3402/ijch.v71i0.18203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson T, Coughlan E, Werno A. 2017. Mycoplasma genitalium macrolide and fluoroquinolone resistance detection and clinical implications in a selected cohort in New Zealand. J Clin Microbiol 55:3242–3248. doi: 10.1128/JCM.01087-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu I, Roberts SA, Bower JE, Henderson G, Reid M. 2017. High macrolide resistance in Mycoplasma genitalium strains causing infection in Auckland, New Zealand. J Clin Microbiol 55:2280–2282. doi: 10.1128/JCM.00370-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bissessor M, Tabrizi SN, Twin J, Abdo H, Fairley CK, Chen MY, Vodstrcil LA, Jensen JS, Hocking JS, Garland SM, Bradshaw CS. 2015. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 60:1228–1236. doi: 10.1093/cid/ciu1162. [DOI] [PubMed] [Google Scholar]
- 19.Tagg KA, Jeoffreys NJ, Couldwell DL, Donald JA, Gilbert GL. 2013. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol 51:2245–2249. doi: 10.1128/JCM.00495-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trembizki E, Buckley C, Bletchly C, Nimmo GR, Whiley DM. 2017. High levels of macrolide-resistant Mycoplasma genitalium in Queensland, Australia. J Med Microbiol 66:1451–1453. doi: 10.1099/jmm.0.000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couldwell DL, Jalocon D, Power M, Jeoffreys NJ, Chen SC, Lewis DA. 2018. Mycoplasma genitalium: high prevalence of resistance to macrolides and frequent anorectal infection in men who have sex with men in western Sydney. Sex Transm Infect 94:406–410. doi: 10.1136/sextrans-2017-053480. [DOI] [PubMed] [Google Scholar]
- 22.Barbera MJ, Fernandez-Huerta M, Jensen JS, Caballero E, Andreu A. 2017. Mycoplasma genitalium macrolide and fluoroquinolone resistance: prevalence and risk factors among a 2013-2014 cohort of patients in Barcelona, Spain. Sex Transm Dis 44:457–462. doi: 10.1097/OLQ.0000000000000631. [DOI] [PubMed] [Google Scholar]
- 23.Salado-Rasmussen K, Jensen JS. 2014. Mycoplasma genitalium testing pattern and macrolide resistance: a Danish nationwide retrospective survey. Clin Infect Dis 59:24–30. doi: 10.1093/cid/ciu217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unemo M, Salado-Rasmussen K, Hansen M, Olsen AO, Falk M, Golparian D, Aasterod M, Ringlander J, Nilsson CS, Sundqvist M, Schonning K, Moi H, Westh H, Jensen JS. 2018. Clinical and analytical evaluation of the new Aptima Mycoplasma genitalium assay, with data on M. genitalium prevalence and antimicrobial resistance in M. genitalium in Denmark, Norway and Sweden in 2016. Clin Microbiol Infect 24:533–539. doi: 10.1016/j.cmi.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Dumke R, Thurmer A, Jacobs E. 2016. Emergence of Mycoplasma genitalium strains showing mutations associated with macrolide and fluoroquinolone resistance in the region Dresden, Germany. Diagn Microbiol Infect Dis 86:221–223. doi: 10.1016/j.diagmicrobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi M, Ito S, Yasuda M, Tsuchiya T, Hatazaki K, Takanashi M, Ezaki T, Deguchi T. 2014. Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan. J Antimicrob Chemother 69:2376–2382. doi: 10.1093/jac/dku164. [DOI] [PubMed] [Google Scholar]
- 27.Hamasuna R, Le PT, Kutsuna S, Furubayashi K, Matsumoto M, Ohmagari N, Fujimoto N, Matsumoto T, Jensen JS. 2018. Mutations in ParC and GyrA of moxifloxacin-resistant and susceptible Mycoplasma genitalium strains. PLoS One 13:e0198355. doi: 10.1371/journal.pone.0198355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deguchi T, Yasuda M, Horie K, Seike K, Kikuchi M, Mizutani K, Tsuchiya T, Yokoi S, Nakano M, Hoshina S. 2015. Drug resistance-associated mutations in Mycoplasma genitalium in female sex workers, Japan. Emerg Infect Dis 21:1062–1064. doi: 10.3201/eid2106.142013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chernesky MA, Jang D, Martin I, Hoang LMN, Naidu P, Levett PN, Wylie J, Rebbapragada A, Ratnam S, Smieja M, Weinbaum B, Getman D. 2017. Mycoplasma genitalium antibiotic resistance-mediating mutations in Canadian women with or without Chlamydia trachomatis infection. Sex Transm Dis 44:433–435. doi: 10.1097/OLQ.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 30.Gratrix J, Plitt S, Turnbull L, Smyczek P, Brandley J, Scarrott R, Naidu P, Parker P, Blore B, Bull A, Shokoples S, Bertholet L, Martin I, Chernesky M, Read R, Singh A. 2017. Prevalence and antibiotic resistance of Mycoplasma genitalium among STI clinic attendees in western Canada: a cross-sectional analysis. BMJ Open 7:e016300. doi: 10.1136/bmjopen-2017-016300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gesink D, Racey CS, Seah C, Zittermann S, Mitterni L, Juzkiw J, Jamieson H, Greer J, Singh S, Jensen JS, Allen V. 2016. Mycoplasma genitalium in Toronto, Ont: estimates of prevalence and macrolide resistance. Can Fam Physician 62:e96–e101. [PMC free article] [PubMed] [Google Scholar]
- 32.Getman D, Jiang A, O'Donnell M, Cohen S. 2016. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 54:2278–2283. doi: 10.1128/JCM.01053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dionne-Odom J, Geisler WM, Aaron KJ, Waites KB, Westfall AO, Van Der Pol B, Xiao L. 2018. High prevalence of multidrug-resistant Mycoplasma genitalium in human immunodeficiency virus-infected men who have sex with men in Alabama. Clin Infect Dis 66:796–798. doi: 10.1093/cid/cix853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L, Waites KB, Van Der Pol B, Aaron KJ, Hook EW, Geisler WM. 2019. Mycoplasma genitalium infections with macrolide and fluoroquinolone resistance-associated mutations in heterosexual African American couples in Alabama. Sex Transm Dis 46:18–24. doi: 10.1097/OLQ.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allan-Blitz LT, Mokany E, Campeau S, Wee R, Shannon C, Klausner JD. 2018. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 45:632–635. doi: 10.1097/OLQ.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. 2014. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 58:631–637. doi: 10.1093/cid/cit752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitt R, Fifer H, Woodford N, Alexander S. 2018. Detection of markers predictive of macrolide and fluoroquinolone resistance in Mycoplasma genitalium from patients attending sexual health services in England. Sex Transm Infect 94:9–13. doi: 10.1136/sextrans-2017-053164. [DOI] [PubMed] [Google Scholar]
- 38.Shipitsyna E, Rumyantseva T, Golparian D, Khayrullina G, Lagos AC, Edelstein I, Joers K, Jensen JS, Savicheva A, Rudneva N, Sukhanova L, Kozlov R, Guschin A, Unemo M. 2017. Prevalence of macrolide and fluoroquinolone resistance-mediating mutations in Mycoplasma genitalium in five cities in Russia and Estonia. PLoS One 12:e0175763. doi: 10.1371/journal.pone.0175763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coorevits L, Traen A, Binge L, Descheemaeker P, Boelens J, Reynders M, Padalko E. 2018. Macrolide resistance in Mycoplasma genitalium from female sex workers in Belgium. J Glob Antimicrob Resist 12:149–152. doi: 10.1016/j.jgar.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Chrisment D, Charron A, Cazanave C, Pereyre S, Bebear C. 2012. Detection of macrolide resistance in Mycoplasma genitalium in France. J Antimicrob Chemother 67:2598–2601. doi: 10.1093/jac/dks263. [DOI] [PubMed] [Google Scholar]
- 41.Le Roy C, Pereyre S, Hénin N, Bébéar C. 2017. French prospective clinical evaluation of the Aptima Mycoplasma genitalium CE-IVD assay and macrolide resistance detection using three distinct assays. J Clin Microbiol 55:3194–3200. doi: 10.1128/JCM.00579-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hay B, Dubbink JH, Ouburg S, Le Roy C, Pereyre S, van der Eem L, Morré SA, Bébéar C, Peters RPH. 2015. Prevalence and macrolide resistance of Mycoplasma genitalium in South African women. Sex Transm Dis 42:140–142. doi: 10.1097/OLQ.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 43.Balkus JE, Manhart LE, Jensen JS, Anzala O, Kimani J, Schwebke J, Shafi J, Rivers C, Kabare E, McClelland RS. 2018. Mycoplasma genitalium infection in Kenyan and US women. Sex Transm Dis 45:514–521. doi: 10.1097/OLQ.0000000000000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hadad R, Golparian D, Lagos AC, Ljungberg J, Nilsson P, Jensen JS, Fredlund H, Unemo M. 2018. Macrolide and fluoroquinolone resistance in Mycoplasma genitalium in two Swedish counties, 2011–2015. APMIS 126:123–127. doi: 10.1111/apm.12792. [DOI] [PubMed] [Google Scholar]
- 45.Bjornelius E, Magnusson C, Jensen JS. 2017. Mycoplasma genitalium macrolide resistance in Stockholm, Sweden. Sex Transm Infect 93:167–168. doi: 10.1136/sextrans-2016-052688. [DOI] [PubMed] [Google Scholar]
- 46.Braam JF, Slotboom B, Van Marm S, Severs TT, Van Maarseveen NM, Van Zwet T, Boel ECH, Berkhout H, Hagen F, Van De Bovenkamp JHB, Van Dommelen L, Kusters JG. 2017. High prevalence of the A2058T macrolide resistance-associated mutation in Mycoplasma genitalium strains from the Netherlands. J Antimicrob Chemother 72:1529–1530. doi: 10.1093/jac/dkw584. [DOI] [PubMed] [Google Scholar]
- 47.Twin J, Jensen JS, Bradshaw CS, Garland SM, Fairley CK, Min LY, Tabrizi SN. 2012. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS One 7:e35593. doi: 10.1371/journal.pone.0035593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anagrius C, Lore B, Jensen JS. 2013. Treatment of Mycoplasma genitalium. Observations from a Swedish STD clinic. PLoS One 8:e61481. doi: 10.1371/journal.pone.0061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Workowski KA, Bolan GA, Prevention CDCa. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommend Rep 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 50.Murray GL, Bradshaw CS, Bissessor M, Danielewski J, Garland SM, Jensen JS, Fairley CK, Tabrizi SN. 2017. Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium. Emerg Infect Dis 23:809–812. doi: 10.3201/eid2305.161745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwebke JR, Rompalo A, Taylor S, Seña AC, Martin DH, Lopez LM, Lensing S, Lee JY. 2011. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens—a randomized clinical trial. Clin Infect Dis 52:163–170. doi: 10.1093/cid/ciq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mena LA, Mroczkowski TF, Nsuami M, Martin DH. 2009. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis 48:1649–1654. doi: 10.1086/599033. [DOI] [PubMed] [Google Scholar]
- 53.Manhart LE, Gillespie CW, Lowens MS, Khosropour CM, Colombara DV, Golden MR, Hakhu NR, Thomas KK, Hughes JP, Jensen NL, Totten PA. 2013. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin Infect Dis 56:934–942. doi: 10.1093/cid/cis1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen JS, Cusini M, Gomberg M, Moi H. 2016. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol 30:1650–1656. doi: 10.1111/jdv.13849. [DOI] [PubMed] [Google Scholar]
- 55.Australian Sexual Health Alliance. 2018. Australian STI management guidelines for use in primary care: Mycoplasma genitalium. http://www.sti.guidelines.org.au/sexually-transmissible-infections/mycoplasma-genitalium#management.
- 56.Kallapur SG, Kramer BW, Jobe AH. 2013. Ureaplasma and BPD. Semin Perinatol 37:94–101. doi: 10.1053/j.semperi.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClure EM, Goldenberg RL. 2009. Infection and stillbirth. Semin Fetal Neonatal Med 14:182–189. doi: 10.1016/j.siny.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, Yoon BH. 2010. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 203:e1–e8. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viscardi RM. 2014. Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed 99:F87–F92. doi: 10.1136/archdischild-2012-303351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao L, Crabb DM, Duffy LB, Paralanov V, Glass JI, Waites KB. 2012. Chromosomal mutations responsible for fluoroquinolone resistance in Ureaplasma species in the United States. Antimicrob Agents Chemother 56:2780–2783. doi: 10.1128/AAC.06342-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao L, Crabb DM, Duffy LB, Paralanov V, Glass JI, Hamilos DL, Waites KB. 2011. Mutations in ribosomal proteins and ribosomal RNA confer macrolide resistance in human Ureaplasma spp. Int J Antimicrob Agents 37:377–379. doi: 10.1016/j.ijantimicag.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 62.Valentine-King MA, Brown MB. 2017. Antibacterial resistance in Ureaplasma species and Mycoplasma hominis isolates from urine cultures in college-aged females. Antimicrob Agents Chemother 61:e01104-17. doi: 10.1128/AAC.01104-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez J, Karau MJ, Cunningham SA, Greenwood-Quaintance KE, Patel R. 2016. Antimicrobial susceptibility and clonality of clinical Ureaplasma isolates in the United States. Antimicrob Agents Chemother 60:4793–4798. doi: 10.1128/AAC.00671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawai Y, Nakura Y, Wakimoto T, Nomiyama M, Tokuda T, Takayanagi T, Shiraishi J, Wasada K, Kitajima H, Fujita T, Nakayama M, Mitsuda N, Nakanishi I, Takeuchi M, Yanagihara I. 2015. In vitro activity of five quinolones and analysis of the quinolone resistance-determining regions of gyrA, gyrB, parC, and parE in Ureaplasma parvum and Ureaplasma urealyticum clinical isolates from perinatal patients in Japan. Antimicrob Agents Chemother 59:2358–2364. doi: 10.1128/AAC.04262-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song J, Qiao Y, Kong Y, Ruan Z, Huang J, Song T, Zhang J, Xie X. 2015. Frequent topoisomerase IV mutations associated with fluoroquinolone resistance in Ureaplasma species. J Med Microbiol 64:1315–1320. doi: 10.1099/jmm.0.000153. [DOI] [PubMed] [Google Scholar]
- 66.Beeton ML, Spiller OB. 2017. Antibiotic resistance among Ureaplasma species isolates: cause for concern? J Antimicrob Chemother 72:330–337. doi: 10.1093/jac/dkw425. [DOI] [PubMed] [Google Scholar]
- 67.Cummings MC, McCormack WM. 1990. Increase in resistance of Mycoplasma hominis to tetracyclines. Antimicrob Agents Chemother 34:2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krausse R, Schubert S. 2010. In-vitro activities of tetracyclines, macrolides, fluoroquinolones and clindamycin against Mycoplasma hominis and Ureaplasma ssp. isolated in Germany over 20 years. Clin Microbiol Infect 16:1649–1655. doi: 10.1111/j.1469-0691.2009.03155.x. [DOI] [PubMed] [Google Scholar]
- 69.Degrange S, Renaudin H, Charron A, Bebear C, Bebear CM. 2008. Tetracycline resistance in Ureaplasma spp. and Mycoplasma hominis: prevalence in Bordeaux, France, from 1999 to 2002 and description of two tet(M)-positive isolates of M. hominis susceptible to tetracyclines. Antimicrob Agents Chemother 52:742–744. doi: 10.1128/AAC.00960-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Basak A, Abouelhassan Y, Huigens RW. 2015. Halogenated quinolines discovered through reductive amination with potent eradication activities against MRSA, MRSE and VRE biofilms. Org Biomol Chem 13:10290–10294. doi: 10.1039/c5ob01883h. [DOI] [PubMed] [Google Scholar]
- 71.Abouelhassan Y, Basak A, Yousaf H, Huigens RW III.. 2017. Identification of N-arylated NH125 analogues as rapid eradicating agents against MRSA persister cells and potent biofilm killers of Gram-positive pathogens. Chembiochem 18:352–357. doi: 10.1002/cbic.201600622. [DOI] [PubMed] [Google Scholar]
- 72.Garrison AT, Abouelhassan Y, Yang H, Yousaf HH, Nguyen TJ, Huigens RW III.. 2016. Microwave-enhanced Friedländer synthesis for the rapid assembly of halogenated quinolines with antibacterial and biofilm eradication activities against drug resistant and tolerant bacteria. Medchemcomm 8:720–724. doi: 10.1039/c6md00381h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Basak A, Abouelhassan Y, Zuo R, Yousaf H, Ding Y, Huigens RW. 2017. Antimicrobial peptide-inspired NH125 analogues: bacterial and fungal biofilm-eradicating agents and rapid killers of MRSA persisters. Org Biomol Chem 15:5503–5512. doi: 10.1039/c7ob01028a. [DOI] [PubMed] [Google Scholar]
- 74.Garrison AT, Abouelhassan Y, Norwood VMT, Kallifidas D, Bai F, Nguyen MT, Rolfe M, Burch GM, Jin S, Luesch H, Huigens RW III.. 2016. Structure-activity relationships of a diverse class of halogenated phenazines that targets persistent, antibiotic-tolerant bacterial biofilms and Mycobacterium tuberculosis. J Med Chem 59:3808–3825. doi: 10.1021/acs.jmedchem.5b02004. [DOI] [PubMed] [Google Scholar]
- 75.Sobke A, Klinger M, Hermann B, Sachse S, Nietzsche S, Makarewicz O, Keller PM, Pfister W, Straube E. 2012. The urinary antibiotic 5-nitro-8-hydroxyquinoline (nitroxoline) reduces the formation and induces the dispersal of Pseudomonas aeruginosa biofilms by chelation of iron and zinc. Antimicrob Agents Chemother 56:6021–6025. doi: 10.1128/AAC.01484-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pelletier C, Prognon P, Bourlioux P. 1995. Roles of divalent cations and pH in mechanism of action of nitroxoline against Escherichia coli strains. Antimicrob Agents Chemother 39:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonissol C, Pua K, Stoiljkovic B. 1986. In vitro activity of nitroxoline on urogenital mycoplasmas. Pathol Biol (Paris) 34:1001–1005. (In French.) [PubMed] [Google Scholar]
- 78.Waites KB, Duffy LB, Bebear CM, Matlow A, Talkington DF, Kenny GE, Totten PA, Bade DJ, Zheng X, Davidson MK, Shortridge VD, Watts JL, Brown SD. 2012. Standardized methods and quality control limits for agar and broth microdilution susceptibility testing of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum. J Clin Microbiol 50:3542–3547. doi: 10.1128/JCM.01439-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abouelhassan Y, Yang Q, Yousaf H, Nguyen MT, Rolfe M, Schultz GS, Huigens RW. 2017. Nitroxoline: a broad-spectrum biofilm-eradicating agent against pathogenic bacteria. Int J Antimicrob Agents 49:247–251. doi: 10.1016/j.ijantimicag.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 80.Li L, Shen W, Zhang K, Tang X, Guo N, Shen F, Xing M, Liua L, Yuan P, Shi Q, Liang J, Yu L. 2012. In-vitro antimycoplasmal activity of triclosan in combination with fluoroquinolones against five Mycoplasma species. Iran J Pharm Res 11:1111–1119. [PMC free article] [PubMed] [Google Scholar]
- 81.Waites KB, Figarola TA, Schmid T, Crabb DM, Duffy LB, Simecka JW. 1991. Comparison of agar versus broth dilution techniques for determining antibiotic susceptibilities of Ureaplasma urealyticum. Diagn Microbiol Infect Dis 14:265–271. [DOI] [PubMed] [Google Scholar]
- 82.Brown DR. 2010. Phylum XVI. Tenericutes Murray 1984a, 356VP (effective publication: Murray 1984b, 33.), p 567–723. In Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB (ed), Bergey’s manual of systematic bacteriology, vol 4 The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes. Springer New York, New York, NY. [Google Scholar]
- 83.Simmons WL, Daubenspeck JM, Osborne JD, Balish MF, Waites KB, Dybvig K. 2013. Type 1 and type 2 strains of Mycoplasma pneumoniae form different biofilms. Microbiology 159:737–747. doi: 10.1099/mic.0.064782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pandelidis K, McCarthy A, Chesko KL, Viscardi RM. 2013. Role of biofilm formation in Ureaplasma antibiotic susceptibility and development of bronchopulmonary dysplasia in preterm neonates. Pediatr Infect Dis J 32:394–398. doi: 10.1097/INF.0b013e3182791ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia-Castillo M, Morosini MI, Galvez M, Baquero F, del Campo R, Meseguer MA. 2008. Differences in biofilm development and antibiotic susceptibility among clinical Ureaplasma urealyticum and Ureaplasma parvum isolates. J Antimicrob Chemother 62:1027–1030. doi: 10.1093/jac/dkn337. [DOI] [PubMed] [Google Scholar]
- 86.Vincent CR, Thomas TL, Reyes L, White CL, Canales BK, Brown MB. 2013. Symptoms and risk factors associated with first urinary tract infection in college age women: a prospective cohort study. J Urol 189:904–910. doi: 10.1016/j.juro.2012.09.087. [DOI] [PubMed] [Google Scholar]
- 87.Hannan PC. 2000. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary Mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet Res 31:373–395. doi: 10.1051/vetres:2000100. [DOI] [PubMed] [Google Scholar]
- 88.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. CLSI document M100-S22, vol 32 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 89.Waites KB, Crabb DM, Bing X, Duffy LB. 2003. In vitro susceptibilities to and bactericidal activities of garenoxacin (BMS-284756) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother 47:161–165. doi: 10.1128/AAC.47.1.161-165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meygret A, Le Roy C, Renaudin H, Bébéar C, Pereyre S. 2018. Tetracycline and fluoroquinolone resistance in clinical Ureaplasma spp. and Mycoplasma hominis isolates in France between 2010 and 2015. J Antimicrob Chemother 73:2696–2703. doi: 10.1093/jac/dky238. [DOI] [PubMed] [Google Scholar]