Strategies are needed to improve time to optimal therapy in patients with bloodstream infections (BSI) due to resistant Gram-negative (GN) pathogens. Accelerate Pheno (ACC) can provide antimicrobial susceptibility results within 7 h of a positive culture and may more rapidly optimize therapy.
KEYWORDS: Accelerate Pheno, rapid diagnostic test, time to definitive therapy, time to effective therapy
ABSTRACT
Strategies are needed to improve time to optimal therapy in patients with bloodstream infections (BSI) due to resistant Gram-negative (GN) pathogens. Accelerate Pheno (ACC) can provide antimicrobial susceptibility results within 7 h of a positive culture and may more rapidly optimize therapy. The primary objective of this study was to evaluate the hypothetical impact of ACC on time to effective therapy (TTET) and time to definitive therapy (TTDT) among patients with BSI due to resistant GN pathogens. ACC was performed on resistant GN BSI isolates, and results were not available to clinicians in real time. A potential benefit of having ACC on TTET or TTDT was determined if modifications to antimicrobial regimens could have been made sooner with ACC. Comparisons on the impact of ACC in the presence or absence of testing by the Verigene Gram-negative blood culture test (Verigene GN-BC) were performed. Sixty-one patients with resistant GN BSI were evaluated. The median actual TTET and TTDT in the cohort were 25.9 h (interquartile range [IQR], 18.5, 42.1) and 47.6 h (IQR, 24.9, 79.6), respectively. Almost half of the patients had potential improvement in TTET and/or TTDT with ACC. In patients who would have had a benefit the median potential decreases in TTET and TTDT were 16.6 h (IQR, 5.5 to 30.6) and 29.8 h (IQR, 13.6 to 43), respectively. The largest potential improvements were seen in patients for whom Verigene results were not available. In conclusion, among patients with resistant GN BSI in a setting where other rapid diagnostic technologies are utilized, ACC results could have further improved TTET and TTDT.
INTRODUCTION
Nearly half of bloodstream infections (BSI) in the United States are caused by Gram-negative (GN) bacilli (1). The increasing incidence of BSI due to drug-resistant Gram-negative bacilli has complicated empirical therapy. In the United States, 17% of GN catheter-related BSI are caused by multidrug-resistant (MDR) pathogens (2). Furthermore, among hematopoietic stem cell transplant patients, 32.5% of GN BSI were due to MDR organisms (3). This is not without consequence, as the presence of MDR organisms in GN BSI is an independent predictor for inappropriate empirical therapy (4, 5), and analyses have demonstrated that delay in appropriate therapy in GN BSI is an independent predictor of both mortality and increased length of stay in survivors (6). Further complicating treatment strategies is that indiscriminant use of broad-spectrum therapies to cover MDR Gram-negative pathogens can enhance the development of antimicrobial resistance and/or lead to increased adverse events.
In order to minimize time to effective antibiotic treatment while limiting usage of broad-spectrum antibiotics, novel rapid diagnostic tests (RDTs) have been developed to decrease the time required to receive organism identification and in some cases identification of the presence of key resistance determinants compared with traditional culture methods. This information, when combined with real-time antimicrobial stewardship intervention, has the potential to decrease the time to effective therapy and improve outcomes. A recent meta-analysis demonstrated that when RDT methods were combined with antimicrobial stewardship interventions, the time to effective therapy was reduced and mortality rates were lower than with utilization of conventional testing methods (7).
The Verigene Gram-negative blood culture test (Verigene GN-BC) is an RDT which is based on a multiplex microarray and can detect Gram-negative organisms as well as genes for extended-spectrum beta-lactamases (CTX-M) and carbapenemases (including KPC, IMP, VIM, NDM, and OXA) (8). Recent studies have demonstrated that the main utility of Verigene has been for escalation when resistant determinants were detected, whereas the impact of this technology on de-escalation was modest (9).
The Accelerate Pheno system (ACC) (Accelerate Diagnostics, Tucson, AZ) is a novel RDT that is unique in that it provides both rapid identification of the organism (within 1.5 h of an organism growing in a blood culture) and antimicrobial susceptibility results (within 7 h). The system uses morphokinetic cellular analysis for phenotypic antimicrobial susceptibility testing and provides MICs and interpretations based on CLSI breakpoints (10). This technology has the potential to provide the most benefit in patients with MDR Gram-negative bacilli given that other methodologies will not provide definitive susceptibility data. Additionally, while a few resistance markers are detected with some molecular RDT platforms, such as Vergiene, they are limited to key beta-lactamase genes.
With the complexities seen in resistance of Gram-negative organisms, a more rapid and thorough assessment of resistance and susceptibility data should allow clinicians to improve the time to appropriate and optimal therapy and ultimately clinical outcomes. Currently data for assessment of the impact that ACC would have on time to implementation of more appropriate antimicrobial regimens in real-world settings are lacking.
The objective of this study was to determine the hypothetical impact of using ACC among patients who had BSI due to resistant Gram-negative pathogens compared to the current methodology used by the clinical microbiology laboratory at the Detroit Medical Center (DMC). In particular, this study aimed to assess the hypothetical impact of ACC on time to effective therapy (TTET) and time to definitive therapy (TTDT).
RESULTS
Seventy-six subjects with bacteremia due to resistant Gram-negative bacilli had ACC performed on their isolates. Fifteen subjects were excluded from the hypothetical analysis because of death or discharge prior to the time of culture positivity, leaving 61 eligible patients in the study.
The mean age of the cohort was 61 ± 18 years, 61% of the subjects were male, 76% were African-American, and 36% arrived from long-term care facilities (Table 1). The resistant Gram-negative bacilli assessed consisted of ESBL-producing Enterobacteriaceae (ESBL-E; n = 26 [42.5%]), carbapenem-resistant Enterobacteriaceae (CRE; n = 15 [25%]), Pseudomonas aeruginosa (n = 13 [21%]), and Acinetobacter spp. (n = 7 [11%]). Fifty-one (84%) patients acquired infection from health care facilities. The most common sources of bloodstream infection were the urinary tract (n = 19 [31%]), the respiratory tract (n = 16 [26%]), and vascular catheters (n = 12 [20%]). Other clinical characteristics are presented in Table 1. For 40 patients (65.6%), escalation of therapy was performed; de-escalation occurred in 15 patients (24.6%), including 10 subjects who were switched from a broader to a narrower agent (e.g., from meropenem to ertapenem for ESBL producers) and 5 subjects who were switched from combination therapy to monotherapy (e.g., two antipseudomonal agents to one), and no change in the antimicrobial regimen was made for 6 patients (9.8%).
TABLE 1.
Description of the cohort, demographics, comorbid conditions, and sources of infectiona
| Variable | Prevalence in the cohort (n = 61) |
|---|---|
| Age, yrs (mean ± SD) | 61 ± 18.4 |
| Sex (female) | 24 (39.3) |
| African-American race | 46 (75.6) |
| Residence in long-term care facility | 22 (36.1) |
| Comorbidities | |
| Lung disease | 25 (41.0) |
| Diabetes mellitus | 21 (34.4) |
| Chronic kidney disease | 19 (31.2) |
| Immunocompromise | 16 (26.2) |
| Congestive heart failure | 17 (27.9) |
| Cerebrovascular disease | 13 (21.7) |
| Coronary artery disease | 11 (18.0) |
| Dementia | 8 (13.1) |
| Malignancy | 6 (9.8) |
| Leukemia | 4 (6.6) |
| HSCT | 4 (6.6) |
| Lymphoma | 3 (4.9) |
| Charlson comorbidity index (median, IQR) | 5 (3, 7) |
| Median Pitt score when culture was collected (IQR) | 1 (0, 3) |
| Median SOFA score when culture was collected (IQR) | 4 (1, 6) |
| Epidemiologic categorization of infection | |
| Hospital acquired | 18 (29.5) |
| Health care facility acquired | 33 (54.1) |
| Community acquired | 10 (16.4) |
| Source of bloodstream infection | |
| Genitourinary | 19 (31.2) |
| Pulmonary | 16 (26.3) |
| Central venous catheter | 12 (19.7) |
| Intra-abdominal | 5 (8.2) |
| Skin and soft tissue | 3 (4.9) |
| Other | 2 (3.3) |
| Unknown | 4 (6.6) |
| Hospital location where blood culture was obtained | |
| General medicine | 33 (54.1) |
| Intensive care unit | 25 (41.0) |
| Emergency department | 3 (4.9) |
| Infectious diseases consult | 53 (86.9) |
| Pathogens | |
| E. coli, ESBL producing | 19 (31.1) |
| CRE | 15 (24.6) |
| P. aeruginosa | 13 (21.3) |
| K. pneumoniae, ESBL producing | 7 (11.4) |
| Acinetobacter spp. | 7 (11.4) |
Unless otherwise noted, data are presented as number (percent). HSCT, hematopoietic stem cell transplantation; ESBL, extended-spectrum beta-lactamase; CRE, carbapenem-resistant Enterobacteriaceae.
Impact of ACC on effective therapy and definitive therapy.
Sixty subjects (98%) received effective therapy for their BSI (Table 2). The median TTET was 25.9 h (interquartile range [IQR], 18.5, 42.1) and was longer among patients with CRE infection (60.2 h; IQR, 35.3, 78.6) than patients with ESBL (23.6 h; IQR, 18.5, 37.4) or nonfermenters (23.5; IQR, 9.3, 33.4) (P = 0.002 for CRE compared to other groups). Of subjects who received effective therapy, 24 patients (39%) would have had a potential benefit in TTET had ACC results been available in real time. Among patients who had a potential benefit in TTET, the potential median time improvement was 16.6 h (IQR, 5.5, 30.6).
TABLE 2.
Antimicrobial therapy of patients in the cohort: effective antimicrobial therapy and definitive antimicrobial therapy for the entire cohort and for each pathogen group
| Parameter | Valuea
for: |
|||
|---|---|---|---|---|
| Entire cohort (n = 61) | ESBL-E (n = 26) | CRE (n = 15) | Nonfermenters (n = 20) | |
| Median days of inpatient treatment (IQR) | 7.4 (5.2, 14.1) | 7.4 (4.4, 13.4) | 7.5 (5.2, 12.5) | 8.0 (5.8, 15.3) |
| Patients who received effective treatment | 60 (98.4) | 26 (100) | 14 (93.3) | 20 (100) |
| Patients with potential improved time to effective treatment | 24 (39.3) | 12 (46.2) | 8 (53.3) | 4 (20) |
| Time (h) from blood draw to effective treatment,b median (IQR) | 25.9 (18.5, 42.1) | 23.6 (18.5, 37.4) | 60.2 (35.3, 78.6) | 23.5 (9.3, 33.4) |
| Time (h) from positive culture to effective treatment,b median (IQR) | 6.7 (1.4, 25.5) | 6.4 (2.7, 20.4) | 45.6 (19.2, 50.9) | 2.6 (0, 6.7) |
| Potential improvement in hours from blood draw to effective treatment,c median (IQR) | 16.6 (5.5, 30.6) | 12.6 (3.8, 16.7) | 30.8 (19.3, 44.8) | 17.0 (2.6, 38.3) |
| Patients who received definitive treatment | 61 (100) | 26 (100) | 15 (100) | 20 (100) |
| Patients with potential improved time to definitive treatment | 28 (45.9) | 16 (61.5) | 8 (53.3) | 4 (20) |
| Time (h) from blood draw to definitive treatment,d median (IQR) | 47.6 (24.9, 79.6) | 33 (22.3, 51.2) | 73.3 (49.2, 86.7) | 49.0 (25.4, 108) |
| Time (h) from positive culture to definitive treatment,d median (IQR) | 28.1 (6.7, 62.8) | 14.2 (6.1, 30.4) | 50.4 (35.1, 69.3) | 30.1 (4.1, 92.1) |
| Potential improvement (h) from blood draw to definitive treatment,e median (IQR) | 29.8 (13.6, 43.0) | 16.7 (9.4, 33.9) | 37.8 (22.6, 48.9) | 35.9 (31.8, 42.4) |
Unless otherwise noted, data are presented as number (percent).
Evaluated among patients who received effective therapy (n = 60).
Evaluated among patients who had potential benefit to reduce time to effective therapy by using Accelerate system’s results, compared to traditional culture results (n = 24).
Evaluated among patients who received definitive therapy (n = 61).
Evaluated among patients who had potential benefit to reduce time to definitive therapy by using Accelerate system’s results, compared to traditional culture results (n = 28).
All patients received definitive therapy for their BSI. The median TTDT was 47.6 h (IQR, 24.9, 79.6). Among patients who had CRE bloodstream infection, median TTDT was numerically higher (73.3 h; IQR, 49.2, 86.7) than for ESBL producers (33 h; IQR, 22.3, 51.2) and nonfermenters (49 h; IQR, 25.4, 108) (P = 0.13 for CRE compared to other groups). Twenty-eight patients (46%) could have received definitive therapy more rapidly had ACC results been available in real time. A potential benefit in TTDT was demonstrated in 8/15 (53%) patients with CRE, 16/26 (61.5%) patients with ESBL, and 4/20 (20%) patients with nonfermenters. The potential median decrease in TTDT among those who could have had a benefit if ACC was available was 29.8 h (IQR, 13.6, 43) and, while failing to reach statistical significance, was nearly a day greater among patients who had CRE (37.8 h; IQR, 22.6, 48.9) or nonfermenters (35.9 h; IQR, 31.8, 42.4) than among patients with ESBL infections (16.7 h; IQR, 9.4, 33.9) (P = 0.21 and P = 0.07, respectively) (Table 2).
Impact of Verigene GN-BC result availability on the impact of ACC.
Fifty-two (85.2%) patients had Verigene Gram-negative blood culture test (Verigene GN-BC) results available to help guide therapeutic decisions, while 9 (14.8%) did not. Among patients who had Verigene test results available, the median time from culture draw to Verigene test results was 19.5 h (IQR, 17.0, 24.3). The median times between Verigene results to effective therapy and to definitive therapy were 3.4 h (IQR, 0, 10.8) and 5.4 h (IQR, 2.1, 38.4), respectively. Table 3 summarizes the causative microbiology and potential impact on TTET and TTDT of ACC compared to the actual management of study patients in the presence or the absence of Verigene test results.
TABLE 3.
Description of effective antimicrobial therapy and definitive antimicrobial therapy in the presence and in the absence of Verigene results
| Parameter | Valuea
in the case that Verigene results were: |
P value | |
|---|---|---|---|
| Available (n = 52) | Absent (n = 9) | ||
| Pathogen group | <0.001 | ||
| ESBL-E | 26 (42.6) | 0 | |
| CRE | 8 (15.4) | 7 (46.7) | |
| Nonfermenters | 18 (32.8) | 2 (22.2) | |
| Management | |||
| Median days of inpatient treatment (IQR) | 7.4 (5.5, 14.1) | 7.8 (5, 13.4) | 0.87 |
| Patients who received effective treatment | 51 (98.1) | 9 (100) | 1 |
| Patients with potential improved time to effective treatment | 18 (34.6) | 6 (75) | 0.14 |
| Time (h) from blood draw to effective treatment,b median (IQR) | 23.6 (14.9, 37.4) | 57.8 (41.5, 78.6) | 0.002 |
| Time (h) from positive culture to effective treatment,b median (IQR) | 6.1 (0, 19.2) | 42.7 (28.5, 50.4) | <0.001 |
| Potential improvement (h) from blood draw to effective treatment,c median (IQR) | 12.6 (3.7, 21.7) | 30.8 (19.7, 49.1) | 0.01 |
| Patients who received definitive treatment | 52 (100) | 9 (100) | 1 |
| Patients with potential improved time to definitive treatment | 22 (42.3) | 6 (66.7) | 0.28 |
| Time from blood draw to definitive treatment,d median (IQR) | 40.2 (24.1, 77.7) | 78.6 (57.8, 86.7) | 0.04 |
| Time (h) from positive culture to definitive treatment,d median (IQR) | 22.3 (6.2, 62.4) | 50.4 (42.7, 129.3) | 0.05 |
| Potential improvement (h) from blood draw to definitive treatment,e median (IQR) | 26.7 (11.6, 39.3) | 42 (26.4, 49.1) | 0.07 |
Unless otherwise noted, data are presented as number (percent).
Evaluated among patients who received effective therapy in each group: n = 51 for the group with Verigene results available and n = 9 for the group for which Verigene results were absent.
Evaluated among patients who had potential benefit to reduce time to effective therapy by using Accelerate system’s results, compared to traditional culture results in each group: n = 18 for the group with Verigene results available and n = 6 for the group for which Verigene results were absent.
Evaluated among patients who received definitive therapy in each group, n = 52 for the group with Verigene results available and n = 9 for the group for which Verigene results were absent.
Evaluated among patients who had potential benefit to reduce time to definitive therapy by using Accelerate system’s results, compared to traditional culture results in each group, n = 22 for the group with Verigene results available and n = 6 for the group for which Verigene results were absent.
In the absence of Verigene test results, a nonsignificantly higher proportion of patients would have had a potential benefit to TTET had ACC results been available in real time than for those for whom a Verigene test result was available (75% versus 34.6%; P = 0.14). Additionally, among patients benefiting from ACC, the potential median decrease in TTET was greater in patients who did not have Verigene results available than in patients who did: 30.8 h (IQR, 19.7, 49.1), compared to 12.6 h (IQR, 3.7, 21.7) (P = 0.01).
Similarly to TTET, although numerically higher, there was no statistically significant difference in the proportion of patients who would have had a benefit in TTDT if ACC results had been available without Verigene test results compared to those with Verigene test results (66.7% versus 42.3%; P = 0.28). For those who would have had a benefit, the potential median decrease in TTDT was greater among patients who did not have Verigene results than among patients who did: 42 h (IQR, 26.4, 49.1) versus 26.7 h (IQR, 11.6, 39.3) (P = 0.07).
As different pathogens were observed in the group that had Verigene results and in the group without Verigene results (Table 3), a sensitivity analysis was performed to compare the impacts of ACC on TTET and TTDT for CRE only, as there were similar numbers in both groups (Table 4). In this subgroup analysis, results were similar to those for the entire cohort. Among 15 patients with CRE BSI, when Verigene results were absent (n = 7), compared to when it was present (n = 8), ACC would have potentially impacted TTET in a higher proportion of patients (85.7% versus 25%) and led to a 5-h difference decrease (30.8 versus 25.5 h) in TTET. Similarly, in the absence of Verigene results, a 16.5-h-greater potential decrease in TTDT was demonstrated (42 versus 25.5 h [Table 4]).
TABLE 4.
Description of effective antimicrobial therapy and definitive antimicrobial therapy among patients with CRE bloodstream infection, in the presence and in the absence of Verigene results
| Parameter | Valuea
for CRE in the case that Verigene results were: |
|
|---|---|---|
| Available (n = 8) | Absent (n = 7) | |
| Median days of inpatient treatment (IQR) | 7.5 (5.2, 12.5) | 7.4 (5.3, 14.3) |
| Patients who received effective treatment | 7 (87.5) | 7 (100) |
| Patients with potential improved time to effective treatment | 2 (25) | 6 (85.7) |
| Time (h) from blood draw to effective treatment,b median (IQR) | 35.3 (14.9, 72.3) | 73.3 (49.2, 86.7) |
| Time (h) from positive culture to effective treatment,b median (IQR) | 19.2 (7.4, 50.9) | 48.5 (35.1, 57.9) |
| Potential improvement (h) from blood draw to effective treatment,c median (IQR) | 25.5 (10.5, 40.4) | 30.8 (19.7, 49.1) |
| Patients who received definitive treatment | 8 (100) | 7 (100) |
| Patients with potential improved time to definitive treatment | 2 (50) | 6 (100) |
| Time (h) from blood draw to definitive treatment,d median (IQR) | 71.5 (28.4, 91.5) | 78.6 (57.8, 86.7) |
| Time (h) from positive culture to definitive treatment,d median (IQR) | 55.9 (13.6, 80.5) | 50.4 (42.7, 69.3) |
| Potential improvement (h) from blood draw to definitive treatment,e median (IQR) | 25.5 (10.5, 40.4) | 42 (26.4, 49.1) |
Unless otherwise noted, data are presented as number (percent).
Evaluated among patients who received effective therapy in each group: n = 7 for for the group with Verigene results available and n = 7 for the group for which Verigene results were absent.
Evaluated among patients who had potential benefit to reduce time to effective therapy by using Accelerate system’s results, compared to traditional culture results in each group: n = 2 for for the group with Verigene results available and n = 6 for the group for which Verigene results were absent.
Evaluated among patients who received definitive therapy in each group: n = 8 for for the group with Verigene results available and n = 7 for the group for which Verigene results were absent.
Evaluated among patients who had potential benefit to reduce time to definitive therapy by using Accelerate system’s results, compared to traditional culture results, in each group: n = 2 for for the group with Verigene results available and n = 6 for the group for which Verigene results were absent.
DISCUSSION
This study demonstrates the potential impact of ACC on TTET and TTDT among patients with BSI due to resistant Gram-negative bacilli in a setting where over 85% of patients had Verigene GN-BC results available and an antimicrobial stewardship program actively monitored and intervened on patients. Almost half of the patients would have had an improvement in TTET and/or TTDT had ACC results been available. The highest impact on TTET was observed among patients who had CRE, particularly when Verigene results were not available (Table 4). Among these patients, having ACC in place could have potentially reduced both the TTET and TTDT by greater than a day.
It is important to note that this impact was not limited to CRE. Thirty-nine percent of patients in the cohort would have had an improvement in TTET, with a median reduction in TTET of 17 h. Comparing these data to those from a similar analysis that was performed at our institution in all positive blood cultures clearly shows that the largest impact of ACC in improving TTET for Gram-negative bacilli will be in resistant pathogens. In the previous hypothetical analysis performed for all Gram-negative bloodstream infections, which largely consisted of susceptible Enterobacteriaceae, it was demonstrated that an improvement in TTET with ACC would have occurred in only 4/167 (2.4%) of BSI, with 3 of those patients having BSI with ESBL-producing Enterobacteriaceae (C. C. Cooper, O. Henig, K. S. Kaye, N. Hussain, Z. Hussain, K. Deed, S. Hossein, P. Lephart, U. Hayat, J. Patel, and J. M. Pogue, presented at IDweek, San Francisco, CA, 2017).
Furthermore, when including the 5 patients in this cohort who could have received more rapid de-escalation, there was an opportunity for an improvement of TTDT in 46% of patients in the cohort. This percentage of patients that could have improvement in TTDT is similar to findings in a study previously reported by our group which included all BSI. In this study, which included BSI due to Gram-positive cocci, Gram-negative bacilli, and Candida spp., 31% of patients in the cohort had opportunities for improvements in TTDT (Cooper et al., presented at IDweek). The potential decreases in TTDT were similar between the current and previous analyses as well (30 h and 25 h, respectively). The primary difference was that in the current study, which consisted only of resistant organisms, 23/28 (82%) of the more rapid initiations in definitive therapy would have been driven by escalation to appropriate therapy, whereas in the other analysis which included primarily susceptible pathogens, 48/55 (87%) would have occurred due to more rapid de-escalation of therapy. These findings highlight the impact that can be expected when ACC results are available for subjects with resistant as opposed to susceptible pathogens.
The results from this study were complicated by changing standard methodology in the clinical microbiology laboratory over the study period. In 2015, the Verigene test was introduced, which led to more rapid organism identification and identification of key resistance determinants by 2 days, which significantly influenced stewardship pharmacists’ ability to positively impact TTET and TTDT. While the introduction of the Verigene test during the study period complicated the study, it also allowed for an interesting analysis of the hypothetical impact of ACC in the setting of having Verigene results available versus not having these results available. Although small numbers limit the ability to make statistical inferences, a clear demarcation of the hypothetical impact of ACC in the presence or absence of this technology was demonstrated. In patients who would have had benefit from ACC, there was an 18-h difference in impact on TTET and a 15-h difference in impact on TTDT as a function of whether or not Verigene results were available. In the absence of Verigene results, ACC was associated with the potential for an impressive 31-h improvement in TTET in patients who were not on appropriate therapy. The importance of this cannot be overstated given the known association between delays in implementation of effective therapy and poor outcomes in bacteremic patients (11–13). Importantly, the magnitude of the potential impact of ACC on TTET and TTDT in the presence or absence of Verigene results held true when the analysis was limited to CRE only, the only pathogens present in appreciable numbers in each group, strengthening the validity of the association.
The main difference between ACC and the Verigene test is that the former provides actual susceptibility results and the latter provides resistance markers which represent most, but not all, of the clinically relevant beta-lactam resistance mechanisms for Enterobacteriaceae. Additionally, while the Verigene test detects most carbapenemases leading to carbapenem resistance in Acinetobacter baumannii, none of the major mechanisms of beta-lactam resistance in P. aeruginosa are detected. As our analysis demonstrates, the potential benefit of ACC for CRE is limited in the setting of Verigene results, as the major mechanism of carbapenem resistance will be identified by Verigene. However, in institutions where other mechanisms of carbapenem resistance predominate (such as ESBL/AmpC plus porin deficiencies), the value of ACC over Verigene will become more pronounced. In fact, in this setting the partial information obtained from Verigene (i.e., CTX-M positivity) might lead to inappropriate assumption of carbapenem activity. Furthermore, the presence or absence of beta-lactamase genes does not inform the activity of antibiotics other than beta-lactams.
Additionally, the potential value of ACC over Vergiene for nonfermenter organisms was not demonstrated in our analysis, but it is partially related to our definition of “resistant” isolates. We included all P. aeruginosa and A. baumannii isolates, and the isolates included ended up being susceptible to empirical therapy (this is reflected by the short median time between the time of positive culture to receipt of effective therapy, less than 3 h). Unfortunately, this study was underpowered to show the impact of ACC among multidrug resistant nonfermenters, where ACC would be expected to have an additional benefit over that of molecular rapid diagnostic methods.
However, the above-described considerations need to be taken into account by stewardship programs when deciding between the use of a multiplex PCR platform such as Verigene and a rapid susceptibility testing platform such as Accelerate. Institutions with more “susceptible” antibiograms for nonfermenters and/or Enterobacteriaceae resistance driven by the common genes detected likely do not warrant the extra cost that is associated with a rapid susceptibility platform. However, even though algorithms for escalation and de-escalation have been developed based on presence/absence of these key resistance genes (14, 15), the comparative ability for stewardship personnel to make interventions based on known (accelerate) or presumed (Verigene) susceptibilities remains unknown.
There are limitations to this study. First, the sample size was small. Only 61 patients were included in the analysis, and not all potentially resistant organisms were isolated. Second, the study was not an interventional study, and the analyses were based on the assumption that had susceptibilities been available earlier, a response to these results with regard to changes in antimicrobial therapy would have been made earlier. Additionally, the hypothetical time of response to results and modification of therapy to ACC results was assumed to be the same as the time of response to automated susceptibility test (AST) results in cases where actual therapy was modified after AST results were known. However, this time of response was unknown in cases where actual therapy was modified prior to actual AST results becoming available to the providers. In such cases, 2 h was added to ACC final results in order to account for human factors (i.e., intervention by the stewardship team, entering of orders, delivery from the pharmacy, and administration by the nursing staff) that would have impacted the benefit of ACC. Importantly, any efforts to fully realize the benefits of ACC would mandate real-time stewardship intervention based on test results.
In this study, the accuracy of ACC was not compared to that of traditional cultures, and categorical agreement was not evaluated. This evaluation has been published elsewhere (16). In order to account for ACC performance, when organism identify or susceptibility results were not provided by Accelerate due to technical error, they were considered as having provided no potential benefit of ACC, as this is what would have occurred in the real-world setting. Finally, this study was not designed to evaluate costs. If costs were to be evaluated, patients who died before the culture turned positive would have been included in the analysis, because the test would have been run as the microbiology laboratory would not have been notified to the patient’s death.
In conclusion, this study has demonstrated the additional benefit of a unique RDT that rapidly provides susceptibility results. Among patients who had BSI due to resistant GN bacilli, having ACC results in real time would potentially improve the time to receipt of effective antimicrobial therapy and reduce unnecessary broad-spectrum exposure, even in settings where RDTs and antimicrobial stewardship are already in place.
MATERIALS AND METHODS
Study design and study population.
The study was a retrospective cohort analysis of patients admitted to the DMC. The DMC is a tertiary-care health system located throughout metropolitan Detroit, MI, and consists of 6 acute-care hospitals with >2,000 inpatient beds. The institutional review boards of Wayne State University and the DMC approved the study.
This was a convenience cohort to explore the hypothetical impact of ACC in patients with BSI due to MDR Gram-negative bacilli which were identified by traditional methods that were used by the microbiology laboratory (see “Laboratory procedures” below) and was a subset of an unrelated analysis assessing the accuracy of ACC compared to that of traditional AST. Patients were eligible for inclusion in this study if they had a BSI due to extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae (CRE), Pseudomonas aeruginosa, or Acinetobacter baumannii between October 2013 and March 2017, and their isolates were either identified in real time during the period of the accuracy study or stored in the DMC Microbiology Laboratory and subsequently able to be tested by ACC. For the purposes of this study, an ESBL producer was defined through the algorithm utilized by the BD Phoenix automated susceptibility testing panel and was based on resistance to third-generation cephalosporins with reductions in MICs demonstrated by the addition of clavulanic acid. CRE were defined as isolates resistant to meropenem and/or imipenem. While the molecular epidemiology of CRE was not explicitly assessed as part of the study, the isolates that were evaluated by Verigene (described in detail below) all were positive for Klebsiella pneumoniae carbapenemase production.
Patients with resistant isolates analyzed by ACC were excluded from the hypothetical analysis if they died or were discharged prior to the blood culture becoming positive.
Laboratory procedures.
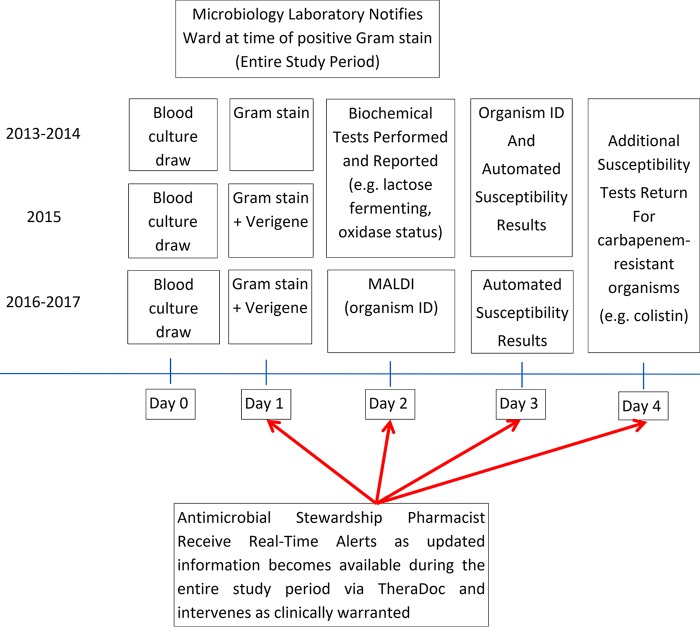
In this study, the hypothetical impact of ACC on TTET and TTDT was compared to how the patient was actually managed by treating providers (i.e., actual TTET and TTDT) in the setting of traditional organism identification and antimicrobial susceptibility testing procedures utilized over the study period at the DMC as described in Fig. 1.
FIG 1.
Detroit Medical Center laboratory process.
Although antimicrobial stewardship processes (described below) remained consistent at the DMC over the length of the study, this was not the case for the microbiological tests performed. During the entire study period, the DMC clinical microbiology lab utilized the BD Bactec automated blood culture system (BD Diagnostic Systems, Franklin Lakes, NJ) for the growth and detection of microorganisms present in blood culture samples. Once the culture turned positive, Gram staining was performed and the microbiology laboratory personnel called the nursing manager on the patient care ward to inform them of the positive result. The procedure for the identification of the causative pathogens changed over the study period and is detailed in Fig. 1. From 2013 through 2014, after the culture turned positive with a sufficient growth of the organism, identification of the organisms and automated susceptibility testing (AST) were performed by the BD Phoenix automated microbiology system (BD Diagnostic Systems) and were reported when results were complete. Other biological tests (lactose fermentation and oxidase status) were performed once sufficient growth of the organism occurred, usually 1 day after culture positivity. In 2015, Verigene Gram-negative blood culture (GN-BC) nucleic acid tests (Luminex Corporation, Austin, TX) were introduced in addition to the aforementioned process to identify Gram-negative microorganisms and to determine the presence of selected resistance genes within hours of culture positivity. In 2016, after bacterial colonies grew, matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry was used for bacterial identification, while the BD Phoenix was still used for AST.
Clinical and Laboratory Standards Institute (CLSI) guidelines were followed to determine antibiotic susceptibility test results (10). Throughout the entire study period, other than initial notification to the patient care ward at the time of culture positivity, no further active updates to clinicians were made via direct notifications and results were only updated in the electronic medical record. However, antimicrobial stewardship pharmacists, through the use of real-time automated email alerts, continually monitored positive blood culture results and intervened as warranted during normal business hours. As ACC was run either as part of a validation study or on stored isolates, the results from the ACC were not available to treating clinicians and did not influence the care of patients. However, all other microbiology procedures that are described above were performed in real time.
Data collection.
Data pertaining to demographics and comorbid conditions included in Charlson’s comorbidity index (17) were extracted from the electronic medical record. In addition, vital signs and laboratory results were obtained on the day of blood culture positivity to assess severity of illness via Pitt bacteremia score and SOFA score (18, 19). Clinical and infection courses were obtained from the care provider’s notes as well as infectious diseases consults, when available. Acquisition of infection was classified as hospital acquired, health care associated, and community acquired as previously described (20).
Antimicrobial treatment during the admission was captured and included dates and times that each antimicrobial agent was administered and discontinued. Additionally, blood culture information collected included dates and times of culture collection, first positive culture result, and final susceptibility results. When Verigene results were available, dates and times of Verigene results were captured. In order to assess the hypothetical impact of ACC on TTET and TTDT, the amount of time it took for ACC to provide organism identification and AST results was added to the time of the first positive culture result.
Definitions pertaining to antimicrobial therapy.
An effective antibiotic was defined as an antibiotic with in vitro activity against the identified infecting pathogen. A definitive antibiotic regimen was defined as the final antibiotic regimen selected by the treating team after susceptibility information was made available. It is worth noting that definitive therapy was not always the narrowest-spectrum therapeutic option available or an in vitro active therapy. Escalation of therapy was defined as an antibiotic regimen that was changed from a narrower to a broader spectrum of activity. De-escalation of therapy was defined as an antibiotic regimen that was changed from a broader to a narrower spectrum of activity.
Cases were reviewed and were adjudicated by 3 investigators (O.H., J.M.P., and K.S.K.) to determine whether effective and definitive therapy had been provided to patients and if effective and/or definitive therapy could have been provided to the patient more rapidly if results from ACC had been available in real time. It was determined that there would have been a potential benefit of having had the ACC results if, once final antimicrobial susceptibility results became available per standard clinical microbiology processes, the treating clinicians transitioned therapy from an ineffective to an effective antibiotic or if the treating clinician transitioned from an initial to a definitive antibiotic regimen. In any case where ACC did not provide identification of the organisms and/or susceptibilities (owing to technical failure), it was considered that there would have been no benefit of ACC for this case.
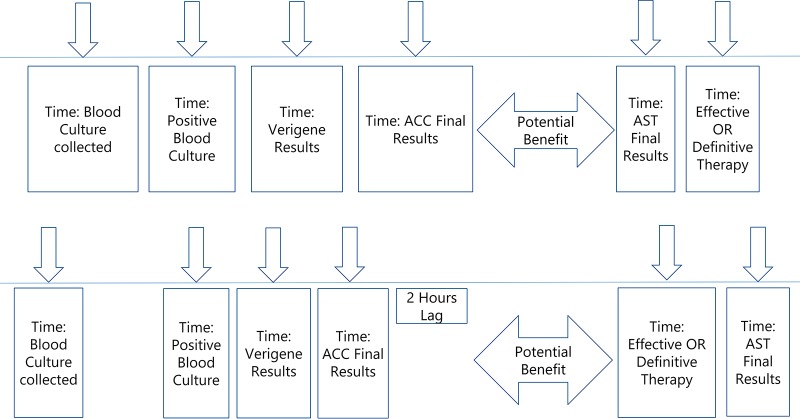
For each case the TTET and TTDT were calculated. Time to effective therapy was defined as the difference (in hours) between the time that the blood culture was drawn and the time when an effective antibiotic with in vitro activity was first administered. Time to definitive therapy was defined as the time from the blood culture being drawn to the time that definitive therapy was provided. Time to ACC-AST was calculated as the time of the actual positive culture plus 6.75 h, which was the average time for ACC to provide susceptibility results. In addition, time to Verigene results was calculated as the difference (in hours) between the time that blood cultures were drawn and the time Verigene results were available, and times between Verigene results and effective therapy or definitive therapy were calculated and presented. For each case where it was determined that there would have been a potential benefit to having had the ACC results available in real time, potential decreases in TTET and TTDT were calculated. In the first scenario, if actual effective or definitive therapy modifications were made after final traditional AST results, the potential decrease in TTET or TTDT was calculated by determining the differences in times when final results became available by traditional AST results and when the ACC results would have been made available with the assumption that the time from final traditional AST to therapy modification would be the same as the time from ACC to therapy modification (Fig. 2, top). In the second scenario, if patients were transitioned to effective or definitive therapy prior to final AST results becoming available, but after the results of ACC would hypothetically have become available, the potential decreases in TTET or TTDT were calculated as the difference between the time of the actual intervention (therapy) and the time ACC results would have been available plus 2 h. The 2 h was added to reflect an estimated amount of time from when the ACC results would have been made available to when interventions would have been made and therapy been modified and administered (Fig. 2, bottom). This potential decrease in time to implementation of effective or definitive therapy was evaluated for the entire cohort and analyzed separately for ESBL-producing Enterobacteriaceae, CRE, and nonfermenters (P. aeruginosa and A. baumannii). A comparison was also performed between patients who had Verigene results available and patients who did not.
FIG 2.
Potential time benefit for effective or definitive therapy. (Top) In scenarios where therapy modifications occurred after traditional AST, returned potential decreases in TTET and TTDT were calculated as the differences in times when traditional final AST results became available and when the ACC results would have been made available. (Bottom) In scenarios where therapy modifications occurred and patients were transitioned to effective or definitive therapy prior to final AST results becoming available, but after the results of ACC would hypothetically have been available, the potential decreases in TTET or TTDT were calculated as the difference between the time of the actual intervention and the time ACC results would have been available. In addition, 2 h was added to the time of ACC results to reflect the time required to address and intervene on ACC results.
Data analysis.
A description of the cohort was presented using means and standard deviations (SD) as well as medians with interquartile ranges (IQRs) where appropriate. Duration of antibiotic treatment-associated variables (i.e., total duration of treatment, TTET, TTDT, and potential decrease in TTET or TTDT) were presented using medians with IQR. Patients who had Verigene results were compared to patients who had no Verigene results using Fisher’s exact test or chi-square test for categorical variables and Wilcoxon rank sum test for continuous variables. Similar analyses were performed to compare patients who had CRE to patients who had other pathogens.
REFERENCES
- 1.Anderson DJ, Moehring RW, Sloane R, Schmader KE, Weber DJ, Fowler VG Jr, Smathers E, Sexton DJ. 2014. Bloodstream infections in community hospitals in the 21st century: a multicenter cohort study. PLoS One 9:e91713. doi: 10.1371/journal.pone.0091713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner LM, Fridkin SK, Aponte-Torres Z, Avery L, Coffin N, Dudeck MA, Edwards JR, Jernigan JA, Konnor R, Soe MM, Peterson K, Clifford McDonald L. 2016. Vital signs: preventing antibiotic-resistant infections in hospitals—United States, 2014. Am J Transplant 16:2224–2230. doi: 10.1111/ajt.13893. [DOI] [PubMed] [Google Scholar]
- 3.Averbuch D, Tridello G, Hoek J, Mikulska M, Akan H, Yaňez San Segundo L, Pabst T, Özçelik T, Klyasova G, Donnini I, Wu D, Gülbas Z, Zuckerman T, Botelho de Sousa A, Beguin Y, Xhaard A, Bachy E, Ljungman P, de la Camara R, Rascon J, Ruiz Camps I, Vitek A, Patriarca F, Cudillo L, Vrhovac R, Shaw PJ, Wolfs T, O’Brien T, Avni B, Silling G, Al Sabty F, Graphakos S, Sankelo M, Sengeloev H, Pillai S, Matthes S, Melanthiou F, Iacobelli S, Styczynski J, Engelhard D, Cesaro S. 2017. Antimicrobial resistance in Gram-negative rods causing bacteremia in hematopoietic stem cell transplant recipients: intercontinental prospective study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin Infect Dis 65:1819–1828. doi: 10.1093/cid/cix646. [DOI] [PubMed] [Google Scholar]
- 4.Carrara E, Pfeffer I, Zusman O, Leibovici L, Paul M. 2018. Determinants of inappropriate empirical antibiotic treatment: systematic review and meta-analysis. Int J Antimicrob Agents 51:548–553. doi: 10.1016/j.ijantimicag.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Marchaim D, Gottesman T, Schwartz O, Korem M, Maor Y, Rahav G, Karplus R, Lazarovitch T, Braun E, Sprecher H, Lachish T, Wiener-Well Y, Alon D, Chowers M, Ciobotaro P, Bardenstein R, Paz A, Potasman I, Giladi M, Schechner V, Schwaber MJ, Klarfeld-Lidji S, Carmeli Y. 2010. National multicenter study of predictors and outcomes of bacteremia upon hospital admission caused by Enterobacteriaceae producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother 54:5099–5104. doi: 10.1128/AAC.00565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battle SE, Bookstaver PB, Justo JA, Kohn J, Albrecht H, Al-Hasan MN. 2017. Association between inappropriate empirical antimicrobial therapy and hospital length of stay in Gram-negative bloodstream infections: stratification by prognosis. J Antimicrob Chemother 72:299–304. doi: 10.1093/jac/dkw402. [DOI] [PubMed] [Google Scholar]
- 7.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. 2017. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 64:15–23. doi: 10.1093/cid/ciw649. [DOI] [PubMed] [Google Scholar]
- 8.Luminex Corporation. 2018. https://www.luminexcorp.com/gram-negative-blood-culture/. Accessed 15 October 2018.
- 9.Rivard KR, Athans V, Lam SW, Gordon SM, Procop GW, Richter SS, Neuner E. 2017. Impact of antimicrobial stewardship and rapid microarray testing on patients with Gram-negative bacteremia. Eur J Clin Microbiol Infect Dis 36:1879–1887. doi: 10.1007/s10096-017-3008-6. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing. 26th informational supplement; CLSI document M100S Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Morata L, Cobos-Trigueros N, Martínez JA, Soriano A, Almela M, Marco F, Sterzik H, Núñez R, Hernández C, Mensa J. 2012. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 56:4833–4837. doi: 10.1128/AAC.00750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 54:4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andria N, Henig O, Kotler O, Domchenko A, Oren I, Zuckerman T, Ofran Y, Fraser D, Paul M. 2015. Mortality burden related to infection with carbapenem-resistant Gram-negative bacteria among haematological cancer patients: a retrospective cohort study. J Antimicrob Chemother 70:3146–3153. doi: 10.1093/jac/dkv218. [DOI] [PubMed] [Google Scholar]
- 14.Rodel J, Karrasch M, Edel B, Stoll S, Bohnert J, Loffler B, Saupe A, Pfister W. 2016. Antibiotic treatment algorithm development based on a microarray nucleic acid assay for rapid bacterial identification and resistance determination from positive blood cultures. Diagn Microbiol Infect Dis 84:252–257. doi: 10.1016/j.diagmicrobio.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Pogue JLP, Kaye K, Painter T, Burger T, Taylor M, Cooper C, Salimnia H. 2017. Evaluation of the Accelerate PhenoTM system in a clinical setting: comparison of identification and antibiotic susceptibility test results of 224 prospective blood cultures to standard laboratory methods at Detroit Medical Center. Abstr 27th Eur Congr Clin Microbiol Infect Dis, Vienna, Austria, April 2017. [Google Scholar]
- 16.Charnot-Katsikas A, Tesic V, Love N, Hill B, Bethel C, Boonlayangoor S, Beavis KG. 2018. Use of the Accelerate Pheno system for identification and antimicrobial susceptibility testing of pathogens in positive blood cultures and impact on time to results and workflow. J Clin Microbiol 56:e01166-17. doi: 10.1128/JCM.01166-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Rhee JY, Kwon KT, Ki HK, Shin SY, Jung DS, Chung DR, Ha BC, Peck KR, Song JH. 2009. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock 31:146–150. doi: 10.1097/SHK.0b013e318182f98f. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent J-L, Wiersinga WJ, Zimmerman JL, Dellinger RP. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso T, Almeida M, Friedman ND, Aragao I, Costa-Pereira A, Sarmento AE, Azevedo L. 2014. Classification of healthcare-associated infection: a systematic review 10 years after the first proposal. BMC Med 12:40. doi: 10.1186/1741-7015-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]