Carbapenem-resistant Klebsiella pneumoniae (CR-Kp) can cause biofilm-related bloodstream infections associated with significant morbidity and mortality worldwide. We investigated the bactericidal activities of colistin (CST), rifampin (RIF), meropenem (MEM), gentamicin (GEN), and tigecycline (TGC) alone and that of CST in combination with RIF, MEM, GEN, or TGC against CR-Kp mature biofilms.
KEYWORDS: Klebsiella pneumoniae, biofilms, colistin, synergistic activity
ABSTRACT
Carbapenem-resistant Klebsiella pneumoniae (CR-Kp) can cause biofilm-related bloodstream infections associated with significant morbidity and mortality worldwide. We investigated the bactericidal activities of colistin (CST), rifampin (RIF), meropenem (MEM), gentamicin (GEN), and tigecycline (TGC) alone and that of CST in combination with RIF, MEM, GEN, or TGC against CR-Kp mature biofilms. Twenty CR-Kp blood isolates were derived from an equal number of bloodstream infections in adult patients. Biofilm formation was assessed by staining with 0.4% crystal violet and measuring the optical density spectrophotometrically at 545 nm. Biofilm damage was measured as the percent reduction of metabolic activity by an XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt] assay. The MIC50 for biofilms was determined as the minimum concentration that caused ≥50% bacterial damage compared to that for untreated controls. Antibacterial drug interactions were analyzed by the Bliss independence model. Four of the 20 CR-Kp isolates were biofilm producers. Biofilm MIC50s of CST, RIF, MEM, GEN, and TGC for these isolates were 64, 8, >256, 128, and 8 mg/liter, respectively. Synergistic interactions were observed at 32 to 64 mg/liter of CST combined with 0.25 to 4 mg/liter of RIF, at 32 mg/liter of CST combined with 0.007 to 0.25 mg/liter of MEM, and at 16 to 32 mg/liter of CST combined with 16 to 64 mg/liter of TGC. The synergy was highest for CST plus RIF, with a mean ΔE ± standard error (SE) of 49.87% ± 9.22%, compared to 29.52% ± 4.97% for CST plus MEM (P < 0.001) and 32.44% ± 6.49% for CST plus TGC (P < 0.001). Indifferent results were exhibited by CST plus GEN. None of the combinations exhibited antagonism. These drug interaction findings, especially those for CST with RIF, may be of importance in the treatment of biofilm-related CR-Kp infections.
TEXT
The spread of antimicrobial resistance by carbapenemase-producing Enterobacteriaceae has taken global dimensions at an alarmingly fast pace, posing a major public health threat (1, 2). Infections caused by multidrug-resistant pathogens, including carbapenem-resistant Klebsiella pneumoniae (CR-Kp), are associated with high morbidity and mortality rates, and the available therapeutic options to treat patients infected with these organisms are extremely limited (3, 4).
The insufficiency of new chemical entities in the drug development pipeline has led to the revival of old antimicrobial agents, including colistin (CST), which is often the last-resort treatment for serious multidrug-resistant Gram-negative bacterial infections (5, 6). Colistin is a polypeptide antibiotic of the polymyxin family that causes rapid bacterial killing in a concentration-dependent manner (7, 8). It acts primarily on the Gram-negative bacterial cell wall, leading to rapid permeability changes in the cytoplasmic membrane and ultimately to cell death (9, 10). However, monotherapy with colistin is considered to have limited efficacy against infections caused by CR-Kp, contributing to the high crude mortality rates observed in patients infected with this pathogen (11–13). Clinical studies have concluded that colistin combination therapy for the treatment of multidrug- and extensively drug-resistant Gram-negative bacteria is associated with decreased failure rates and may present a survival benefit among critically ill patients (12–15). Systematic review and meta-analysis of the in vitro synergy of colistin and carbapenems against CR-Kp isolates support less resistance development with low antagonism rates (16).
The management of infections caused by K. pneumoniae is also highly challenging due to the ability of this pathogen to form biofilms on indwelling medical devices (17–19). Biofilms are microbial communities in which cells are embedded within a self-produced matrix of extracellular polymeric substance and constitute a significantly resistant phenotype (20, 21). Although significant synergy has been reported for the combination of colistin with carbapenems, rifampin (RIF), gentamicin (GEN), or tigecycline (TGC) against nonbiofilm infections caused by CR-Kp (15, 16, 22–28), there is a paucity of data regarding these combinations against biofilms caused by this pathogen. Motivated by the lack of information regarding this aspect, in this study, we aimed to investigate the antibiofilm activities of CST, RIF, meropenem (MEM), GEN, and TGC alone and that of CST in combination with RIF, MEM, GEN, or TGC against CR-Kp mature biofilms.
RESULTS
Biofilm formation and susceptibility of planktonic and biofilm cells to CST, RIF, MEM, GEN, and TGC.
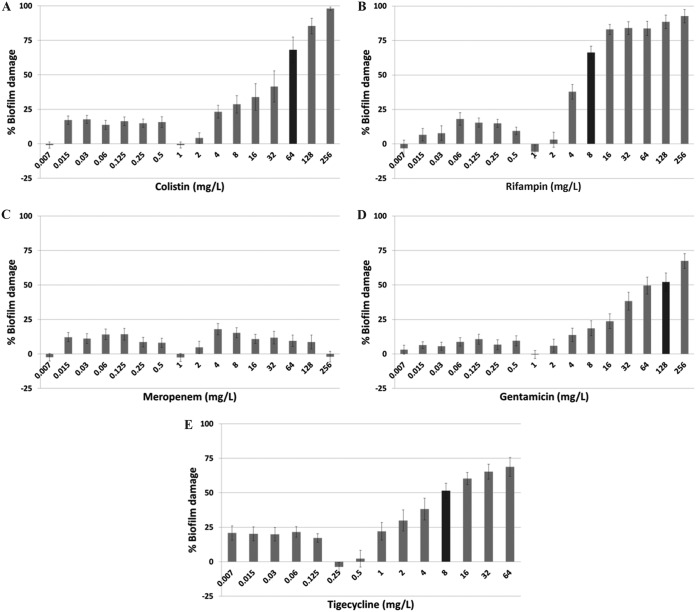
Biofilm formation assays performed on 20 CR-Kp isolates showed that 16 of 20 isolates were nonbiofilm producers (optical density [OD], ≤0.300), and 4 isolates were found to produce robust biofilms (OD range, 0.570 to 0.630). These 4 biofilm-producing strains were used for all subsequent experiments. Based on Clinical and Laboratory Standards Institute (CLSI) and European Committee of Antimicrobial Susceptibility Testing (EUCAST) susceptibility breakpoints, the planktonic cells of the 4 biofilm-forming isolates were resistant to MEM (MIC, 32 to 64 mg/liter) and TGC (MIC, 8 mg/liter) and sensitive to CST (MIC, 0.06 to 0.25 mg/liter). Three of the isolates were resistant to GEN (MIC, 16 to 32 mg/liter), whereas one isolate was GEN sensitive (MIC, 4 mg/liter). Since the MIC of RIF and the relevant susceptibility breakpoints for K. pneumoniae have not yet been defined by either CLSI or EUCAST methodology, the RIF MIC was not evaluated. When the four isolates were subsequently allowed to form mature biofilms, they showed highly resistant profiles for all antimicrobial agents. The biofilm MICs of CST, RIF, MEM, GEN, and TGC were 64, 8, >256, 128, and 8 mg/liter, respectively (Fig. 1A to E).
FIG 1.
Bacterial damage of biofilms of carbapenem-resistant K. pneumoniae bloodstream isolates caused by different concentrations of colistin (A), rifampin (B), meropenem (C), gentamicin (D), and tigecycline (E). The black column represents the MIC50 of each drug. Each concentration of each antibiotic was tested in pentaplicate per clinical isolate, and each drug-free control was tested in 16 replicates per experiment. All isolates were retested three times in independent experiments. The average values for these replicates were used in the data analysis to determine the mean ± standard error (SE) under each condition.
A paradoxical growth effect was observed with all antibiotics at concentrations ranging from 0.25 to 2 mg/liter against biofilms of the four CR-Kp isolates (Fig. 1). The maximum biofilm damage caused by CST and RIF was between 75% and 98% at concentrations ranging from 16 to 256 mg/liter (Fig. 1A and B). In contrast, GEN and TGC did not cause more than 75% biofilm damage across all tested concentrations (Fig. 1D and E), whereas MEM exhibited poor antibiofilm activity against CR-Kp biofilms, causing <25% damage throughout all concentrations (Fig. 1C).
Combinational interactions with colistin.
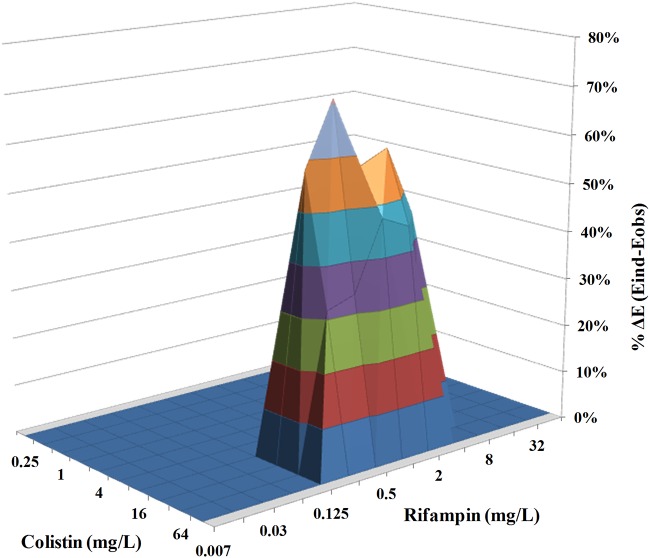
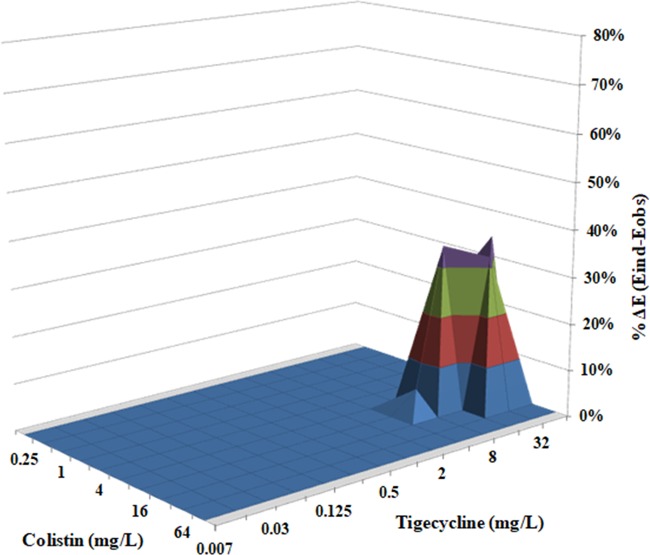
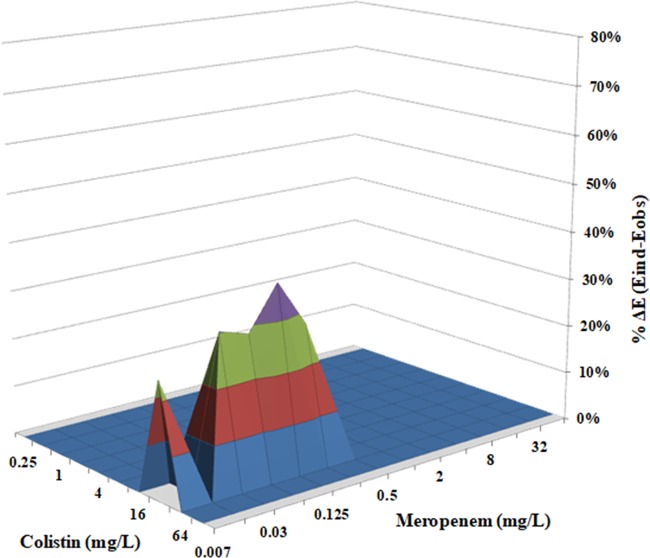
The parameters of Bliss independence drug interaction analysis for the interactions of CST with RIF, MEM, GEN, TGC, or GEN are summarized in Table 1. The range of concentrations used was chosen based on the MIC results obtained for each drug against CR-Kp biofilms (Fig. 1A to E). The interaction surface plots of CST combined with RIF, MEM, or TGC are shown in Fig. 2 and Fig. 4. In the static model that we used, CST at a concentration range of 32 to 64 mg/liter exhibited synergistic activity in the presence of RIF and MEM, with sub-MICs ranging from 0.25 to 4 mg/liter (Fig. 2) and from 0.007 to 0.25 mg/liter (Fig. 3), respectively. Finally, treatment of K. pneumoniae biofilms with CST at a concentration range from 16 to 32 mg/liter showed synergistic interactions with TGC over a concentration range from 16 to 64 mg/liter (Fig. 4).
TABLE 1.
In vitro interactions by Bliss independence analysis of colistin in combination with rifampin, meropenem, tigecycline, and gentamicin against biofilms of carbapenem-resistant K. pneumoniae isolatesa
| Antibacterial drug combination | Type of interaction | Concn (mg/liter) | Mean % ΔE value (range) | Mean % SE (range) |
|---|---|---|---|---|
| CST + RIF | Synergistic | COL, 32–64; RIF, 0.25–4 | 49.87 (31.63–71.13) | 9.22 (5.83–12.30) |
| CST + MEM | Synergistic | COL, 32; MEM, 0.007–0.25 | 29.52 (23.95–37.26) | 4.97 (4.04–5.54) |
| CST + TGC | Synergistic | COL, 16–32; TGC, 16–64 | 32.44 (28.80–34.56) | 6.49 (6.08–7.64) |
| CST + GEN | Indifferent | ND | ND | ND |
ND, not determined.
FIG 2.
In vitro interaction between colistin (0.25 to 128 mg/liter) and rifampin (0.007 to 64 mg/liter) against carbapenem-resistant K. pneumoniae biofilms. The x axis and y axis represent the concentrations of colistin and rifampin, and the z axis represents the percent ΔE (see the text). The zero plane (ΔE = 0) represents indifferent interactions, whereas volumes above (ΔE > 0) represent statistically significant synergistic interactions. The magnitude of the synergistic interactions is directly related to positive ΔE values. The different colors in the three-dimensional plots represent different percentile bands of synergy. The percent ΔE ± SE was 49.9% ± 9.2% for the colistin-rifampin combination (synergistic interaction).
FIG 4.
In vitro interaction between colistin (0.25 to 128 mg/liter) and tigecycline (0.007 to 64 mg/liter) against carbapenem-resistant K. pneumoniae biofilms. The x axis and y axis represent the concentrations of colistin and tigecycline, and the z axis represents the percent ΔE (for additional information, see the Fig. 2 legend). The percent ΔE ± SE was 32.4% ± 6.5% for the colistin-tigecycline combination (synergistic interaction).
FIG 3.
In vitro interaction between colistin (0.25 to 128 mg/liter) and meropenem (0.007 to 64 mg/liter) against carbapenem-resistant K. pneumoniae biofilms. The x axis and y axis represent the concentrations of colistin and meropenem, and the z axis represents the percent ΔE (for additional information, see the Fig. 2 legend). The percent ΔE ± SE was 29.5% ± 5% for the colistin-meropenem combination (synergistic interaction).
The synergistic interaction was highest for the CST-RIF combination, as the mean ΔE ± standard error (SE) for the particular drug combination was 49.87% ± 9.22%, compared to a mean ΔE ±SE of 29.52% ± 4.97% for CST plus MEM (P < 0.001) and a mean ΔE ± SE of 32.44% ± 6.49% for CST plus TGC (P < 0.001). Indifferent results were observed for CST combined with GEN. None of the combinations exhibited antagonism (Table 1).
DISCUSSION
Biofilm-associated infections are very difficult to treat, since biofilms, through the activation of several defense mechanisms, exhibit decreased susceptibility to antimicrobial agents (19, 29). Controlling biofilm development and successfully treating mature biofilms still remain elusive, with very few new therapeutic options clinically available (30). In our study, the double-drug combinations with CST synergistically interacted to inhibit mature biofilms. Specifically, we found that CST in the presence of RIF exerts a greater synergistic effect against K. pneumoniae biofilms than in combinations with MEM or TGC, with both combinations exhibiting comparable magnitudes of synergy. Of note, while MEM exhibits a poor antibiofilm benefit when used alone, it shows considerable efficacy in combination with CST, at very low MEM concentrations. To the best of our knowledge, this is the first study to investigate the activity of these drug combinations against carbapenem-resistant K. pneumoniae biofilms.
Overall, the biofilms of the K. pneumoniae isolates examined in this study exhibited markedly decreased susceptibility to CST, RIF, MEM, GEN, and TGC compared to their corresponding planktonic forms. An in vitro biofilm study by Naparstek et al. investigating the susceptibility profile of extensively drug-resistant KPC-producing K. pneumoniae isolates to GEN alone showed that susceptibility to GEN in the planktonic state was retained in the biofilm state (31). However, those isolates were forming relatively low-mass biofilms (OD at 590 nm [OD590] range, 0.02 to 0.3) compared to our isolates (OD545 range, 0.570 to 0.630). Considering that the deposition of thick extracellular matrix perturbs antibiotic diffusion, causing decreased antibiofilm activity (32), the susceptibility profile observed in our study is well supported but noncomparable to the data in the study by Naparstek et al. due to the different biofilm-forming abilities of the isolates studied.
In our study, the biofilm MICs for RIF and TGC alone were 3 to >5 2-fold dilutions lower than the corresponding MICs for CST, MEM, and GEN. The efficacy of TGC in the reduction of biofilm cells, which was also exhibited in an in vitro catheter-related infection with K. pneumoniae (33), could be attributed to the probable interference of TGC with efflux pumps (34, 35). However, whether and to what extent TGC or any intracellularly acting antimicrobial agent may downregulate efflux pumps, increasing the antibiotic concentration inside biofilm cells and thus making antibiotics most effective, remain a hypothesis which needs to be further investigated. Despite the efficacy of TGC in reducing cell viability in biofilms, results of the in vitro catheter model study also demonstrated that the use of TGC alone may be associated with regrowth of K. pneumoniae (33). Although the biofilm forms of the isolates in our study showed a phenotype of resistance to all antimicrobial agents used alone, it is noteworthy that CST and RIF at high concentrations (>16 mg/liter) caused biofilm damage ranging from 75% to 98%, suggesting their potential use in antimicrobial lock therapy (ALT). In an in vitro catheter model study by Mataraci et al. (33), CST alone used as an ALT solution exhibited significant activity but did not eliminate K. pneumoniae colonization in biofilms. Regrowth following CST monotherapy has been demonstrated in several in vitro studies (23, 26, 27) and has been postulated to occur due to the selection of colistin-resistant subpopulations in the biofilm microenvironment, adaptive resistance, or the formation of persister cells (36).
Combination therapy has been shown to eliminate and possibly prevent the emergence of resistance (16). In the present study, CST plus RIF interacted in synergy to reduce biofilm cell viability at low RIF concentrations. The magnitude of the synergistic interaction was higher than those of the corresponding synergies observed with the other double combinations investigated. The observed interaction between the two antibiotics can be attributed to either the disruption of the outer bacterial membrane by CST, which allows RIF to reach sufficient intracellular concentrations and inhibit DNA transcription, or the mutual killing of resistant subpopulations by each drug. Both mechanistic and subpopulation synergy mechanisms, which are not mutually exclusive, have been proposed to explain the observed synergy of CST with other antimicrobial agents (37). Moreover, the ability of RIF to penetrate biofilm cells is supported by clinical combination studies using RIF to treat prosthetic material infections (38).
It is noteworthy that although biofilms were resistant to MEM alone, synergy in the double combination of CST plus MEM was observed at very low MEM concentrations (0.007 to 0.25 mg/liter). However, taking into consideration that the synergistic effect observed was at an increased CST concentration (32 mg/liter), such a high concentration in clinical settings is likely to raise toxicity issues. For this reason, the double combinations could be most efficacious in ALT for the treatment of catheter-related infections (39). Nevertheless, since human recommended dosing results in a dynamic peak-trough cycle, the effect of the pharmacokinetic profile of the combinations examined could be investigated in future experiments using a hollow-fiber model (40).
In the double combination of CST plus TGC, synergy was observed only at high concentrations of both drugs, while at low concentrations, the combination treatment exhibited indifferent results. A combinational study investigating the interaction of TGC with CST against planktonic NDM-1-producing Enterobacteriaceae found decreased bacterial killing at all concentrations tested, with some evidence of antagonism at lower concentrations. The authors of that study concluded that TGC, being bacteriostatic, may reduce the overall antibiotic benefit when the drug is combined with a bactericidal drug, such as CST (41).
All the antibiotics in this study exhibited a paradoxical growth effect at concentrations ranging from 0.25 to 2 mg/liter against K. pneumoniae biofilms. This is an in vitro phenomenon, described for the first time by Eagle and Musselman (42). They observed the activity of penicillin G against Gram-positive cocci to fall off as its concentration was increased. Recently, it was shown that increasing the dose intensity of polymyxin B amplifies the resistance of Acinetobacter baumannii (43).
One possible limitation of our study is the relatively small number of isolates studied, since only 4 of the 20 examined isolates could form a biofilm. However, these isolates were obtained in a time period of 2 years, and we assume that these are representative CR-Kp isolates. A future study with additional epidemiologically distinct biofilm-producing isolates is expected to validate our results. The present study elucidates the dose-dependent interactions of CST with RIF, MEM, GEN, or TGC, antimicrobial agents that are frequently used for the treatment of CR-Kp in everyday clinical practice (44). However, the effectiveness and the safety of these combinations in the treatment of biofilm-related infections have yet to be defined, as antimicrobial agents interact not only with each other but also with the host immune system (45). It is possible that in vivo models of tissues or device-related infections that are currently used could contribute to the knowledge of biofilm physiology in the host context (46).
In conclusion, rifampin and colistin alone significantly damage biofilms of carbapenem-resistant K. pneumoniae biofilms albeit at high concentrations. However, the double combination of colistin with rifampin or meropenem had a synergistic effect against K. pneumoniae biofilms at relatively low concentrations, although at high drug concentrations, synergy was also observed when colistin was combined with tigecycline. The dose-dependent synergistic interactions of colistin with rifampin, meropenem, and tigecycline against K. pneumoniae biofilms observed here may have important clinical implications for the treatment of biofilm-related infections caused by carbapenem-resistant K. pneumoniae and warrant further assessment in appropriate in vivo models.
MATERIALS AND METHODS
Clinical isolates and growth conditions.
Twenty CR-Kp blood isolates recovered from an equal number of adult patients with bloodstream infections hospitalized at the Hippokration General Hospital of Thessaloniki between 2012 and 2014 were studied. Species identification of these isolates was performed using a Vitek II system or an API ID32C kit (both from bioMérieux, Marcy l’Etoile, France) according to the manufacturer’s instructions. Stocks of these isolates were maintained in a solution containing 25% glycerol and 75% peptone at −80°C. All isolates were revived by subculture on Mueller-Hinton agar (Sigma-Aldrich, Darmstadt, Germany). During the day of the experiment, each isolate was grown for 2 h in Mueller-Hinton broth (Sigma-Aldrich) on an orbital shaker at 37°C under aerobic conditions. For biofilm formation, the bacterial concentration was measured with a turbidimeter (DEN 1; Grant Instruments Ltd., Cambridge, UK), and the cell density was adjusted to 5 × 106 CFU/ml in Mueller-Hinton broth. The isolates that could form robust biofilms were used in the subsequent experiments of antibiotic susceptibility assessment and of drug interactions.
Biofilm formation.
Biofilms were grown on the surface of flat-bottomed 96-well microtiter polystyrene plates. Specifically, 200 μl of the standardized K. pneumoniae suspension (5 × 106 CFU/ml) was incubated in the microtiter plates at 37°C for 48 h to form biofilms under static conditions. The medium was then aspirated, and nonadherent planktonic cells were removed by washing twice with sterile PBS (phosphate-buffered saline solution, pH 7.4; Sigma-Aldrich). Biofilm formation was assessed by staining the adherent bacterial cells with a 0.4% crystal violet solution for 15 min at room temperature. After two washes with PBS and the subsequent addition of 95% ethanol to each microtiter well, biofilm cell-associated dye was measured spectrophotometrically as the optical density (OD) at 545 nm using a microplate reader (ChroMate 4300; Awareness Technology, Inc., Palm City, FL). To distinguish biofilm formers from nonbiofilm formers, we followed the arbitrary classification described previously by Christensen et al. (47). We set as a cutoff value for biofilm formation the OD value defined as 3 standard deviations above the mean OD value of control wells that contained 0.4% crystal violet without the organism (negative control). All experiments were performed in triplicate on three separate days.
Antimicrobial agents.
The antimicrobial agents used in this study were colistin sulfate (CST), rifampin (RIF), meropenem trihydrate (MEM), gentamicin sulfate (GEN), and tigecycline (TGC) (Sigma-Aldrich). Stock solutions of CST and GEN (10 mg/ml each) were prepared in sterile distilled water, whereas those of MEM (10 mg/ml), RIF (60 mg/ml), and TGC (20 mg/ml) were prepared in dimethyl sulfoxide. Except for TGC, which was used in experiments immediately after reconstitution, the remaining antibiotic stock solutions were maintained at −35°C for up to 1 month, according to the manufacturer’s instructions. An appropriate amount of each antibiotic was further diluted to 1,024 mg/liter in Mueller-Hinton broth and used to prepare a series of 2-fold dilutions ranging from 0.007 to 256 mg/liter.
Antibiotic susceptibility assessment.
Planktonic MICs were determined according to the method described in the CLSI M100 protocol (27th edition) (48). The MIC was determined as the lowest drug concentration at which a prominent decrease in turbidity was observed, corresponding to a ca. 80% reduction in visible growth. The MICs were recorded after incubation with each antimicrobial agent for 24 h. Except for CST and TGC, susceptibility classification was determined using CLSI breakpoints (48). Because CLSI has not yet issued interpretative criteria for CST and TGC regarding Enterobacteriaceae, the MIC breakpoints for these drugs were obtained from EUCAST (49). MICs were defined according to a previously described method for in vitro antibacterial susceptibility testing of biofilms, with minor modifications (50). Biofilms were grown on the surface of flat-bottomed 96-well microtiter plates at 37°C for 48 h. Mature biofilms were subsequently incubated with 2-fold dilutions of CST, RIF, MEM, and GEN at concentrations ranging from 0.007 to 256 mg/liter and TGC at concentrations ranging from 0.007 to 64 mg/liter for 24 h. Drug-free biofilms containing only Mueller-Hinton broth served as controls. Biofilm damage measured as the percent reduction of metabolic activity was assessed by 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)2H-tetrazolium-5-carboxanilide salt (XTT) (0.25 mg/ml; Sigma-Aldrich) and coenzyme Q0 (40 μg/ml; Sigma-Aldrich) assays spectrophotometrically at 450 nm with a reference wavelength of 690 nm. Antibiotic activity was calculated according to the formula percent biofilm damage = (1 − X/C) × 100, where X is the absorbance of experimental wells and C is the absorbance of control wells. The MIC for biofilms (MIC50) was determined as the minimum antibacterial drug concentration that caused ≥50% biofilm damage compared to untreated controls. Each concentration of CST, RIF, MEM, GEN, and TGC for every clinical isolate was tested in pentaplicate, and each untreated control was tested in 16 replicates per experiment. The average values for these replicates were used in the data analysis to determine the mean ± standard error (SE) under each condition. All the experiments were performed three times.
Combination antibiotic treatment against biofilms.
Using the two-dimensional (8-by-12) checkerboard microdilution method, mature biofilms of K. pneumoniae isolates were coincubated at 37°C for 24 h with serially 2-fold-diluted concentrations of CST ranging from 0.25 to 128 mg/liter; RIF, MEM, GEN, and TGC ranging from 0.007 to 64 mg/liter alone; or CST in combination with RIF, MEM, GEN, and TGC at the concentrations indicated above. Drug-free medium was used as a control. The range of drug concentrations investigated was based on the biofilm MIC50 determined for each antimicrobial agent. The combined effects of each of the antimicrobial agents with colistin were assessed by an XTT reduction assay as described above.
Analysis of drug interactions.
The in vitro interactions between colistin and each of the four antimicrobial agents were analyzed using the Bliss independence model (51). According to the Bliss model, the expected theoretical percentage of growth (Eind) (compared with an antimicrobial agent-free control) describing the effect of the combination of two antimicrobial agents was calculated using the equation Eind = EA × EB, where EA and EB are the experimental percentages of growth when each antimicrobial agent acts alone. For each combination of x mg/liter of antimicrobial agent A with y mg/liter of antimicrobial agent B, the experimental observed percentage of growth, Eobs, was subtracted from Eind. When ΔE (ΔE = Eind − Eobs) was positive and its 95% confidence interval (CI) did not include zero, statistically significant synergy was claimed for the specific combination, while when ΔE was negative with its 95% CI not including zero, significant antagonism was claimed. In any other case where the 95% CI of ΔE included zero, the conclusion was Bliss independence. ΔE was calculated for all the combinations of drug concentrations for each of the isolates and for each of the three different experiments. The mean and standard deviation of ΔE of all the combinations were calculated and are reported for the combinations, which are statistically significantly synergistic (ΔE ± 95% CI of >0) or antagonistic (ΔE ± 95% CI of <0). The ΔEs that were statistically different from zero were constructed as a three-dimensional plot, with peaks above and below the zero plane indicating synergistic and antagonistic interactions, respectively, and the zero plane indicating indifferent interactions (51, 52). Comparative analysis between synergistic interactions was performed by ordinary analysis of variance (ANOVA) with Dunnett’s posttest. A P value of <0.05 indicated statistical significance.
ACKNOWLEDGMENTS
This work was funded by IKY Fellowships of Excellence for Postgraduate Studies in Greece-Siemens Program in the framework of the Hellenic Republic-Siemens Settlement Agreement and by the European Social Fund (ESF) and the Greek State (5393) 2014, within the framework of the action Aristeia II of the operational program Education and Lifelong Learning (action’s beneficiary, General Secretariat for Research and Technology).
We thank Theodoros Karampatakis for providing stocks of CR-Kp blood isolates and Ioanna Stamouli for technical assistance.
E.R. has received research grant support from Pfizer and Gilead; has served as consultant to Astellas, Gilead, Pfizer, and Merck; and has been in the speakers’ bureaus of Merck, Aventis, Astellas, and Pfizer. The remaining authors have no relevant disclosures.
REFERENCES
- 1.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordmann P. 2014. Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect 44:51–56. doi: 10.1016/j.medmal.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Kohler PP, Volling C, Green K, Uleryk EM, Shah PS, McGeer A. 2017. Carbapenem resistance, initial antibiotic therapy, and mortality in Klebsiella pneumoniae bacteremia: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 38:1319–1328. doi: 10.1017/ice.2017.197. [DOI] [PubMed] [Google Scholar]
- 4.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. 2014. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 5.Falagas ME, Kasiakou SK. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Turnidge J, Milne R, Nation RL, Coulthard K. 2001. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother 45:781–785. doi: 10.1128/AAC.45.3.781-785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen RJ, Li J, Nation RL, Spelman D. 2007. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J Antimicrob Chemother 59:473–477. doi: 10.1093/jac/dkl512. [DOI] [PubMed] [Google Scholar]
- 9.Newton BA. 1956. The properties and mode of action of the polymyxins. Bacteriol Rev 20:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schindler M, Osborn MJ. 1979. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry 18:4425–4430. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch EB, Tam VH. 2010. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother 65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 12.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrosillo N, Giannella M, Lewis R, Viale P. 2013. Treatment of carbapenem-resistant Klebsiella pneumoniae: the state of the art. Expert Rev Anti Infect Ther 11:159–177. doi: 10.1586/eri.12.162. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenhard JR, Nation RL, Tsuji BT. 2016. Synergistic combinations of polymyxins. Int J Antimicrob Agents 48:607–613. doi: 10.1016/j.ijantimicag.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. 2013. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piperaki ET, Syrogiannopoulos GA, Tzouvelekis LS, Daikos GL. 2017. Klebsiella pneumoniae: virulence, biofilm and antimicrobial resistance. Pediatr Infect Dis J 36:1002–1005. doi: 10.1097/INF.0000000000001675. [DOI] [PubMed] [Google Scholar]
- 18.Donlan RM. 2001. Biofilms and device-associated infections. Emerg Infect Dis 7:277–281. doi: 10.3201/eid0702.700277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vuotto C, Longo F, Balice MP, Donelli G, Varaldo PE. 2014. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens 3:743–758. doi: 10.3390/pathogens3030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 21.de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock REW. 2013. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16:580–589. doi: 10.1016/j.mib.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Tascini C, Tagliaferri E, Giani T, Leonildi A, Flammini S, Casini B, Lewis R, Ferranti S, Rossolini GM, Menichetti F. 2013. Synergistic activity of colistin plus rifampin against colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 57:3990–3993. doi: 10.1128/AAC.00179-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pournaras S, Vrioni G, Neou E, Dendrinos J, Dimitroulia E, Poulou A, Tsakris A. 2011. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int J Antimicrob Agents 37:244–247. doi: 10.1016/j.ijantimicag.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Nastro M, Rodriguez CH, Monge R, Zintgraff J, Neira L, Rebollo M, Vay C, Famiglietti A. 2014. Activity of the colistin-rifampicin combination against colistin-resistant, carbapenemase-producing Gram-negative bacteria. J Chemother 26:211–216. doi: 10.1179/1973947813Y.0000000136. [DOI] [PubMed] [Google Scholar]
- 25.Gaibani P, Lombardo D, Lewis RE, Mercuri M, Bonora S, Landini MP, Ambretti S. 2014. In vitro activity and post-antibiotic effects of colistin in combination with other antimicrobials against colistin-resistant KPC-producing Klebsiella pneumoniae bloodstream isolates. J Antimicrob Chemother 69:1856–1865. doi: 10.1093/jac/dku065. [DOI] [PubMed] [Google Scholar]
- 26.Lagerback P, Khine WW, Giske CG, Tangden T. 2016. Evaluation of antibacterial activities of colistin, rifampicin and meropenem combinations against NDM-1-producing Klebsiella pneumoniae in 24 h in vitro time-kill experiments. J Antimicrob Chemother 71:2321–2325. doi: 10.1093/jac/dkw213. [DOI] [PubMed] [Google Scholar]
- 27.Tangden T, Hickman RA, Forsberg P, Lagerback P, Giske CG, Cars O. 2014. Evaluation of double- and triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time-kill experiments. Antimicrob Agents Chemother 58:1757–1762. doi: 10.1128/AAC.00741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clancy CJ, Hao B, Shields RK, Chen L, Perlin DS, Kreiswirth BN, Nguyen MH. 2014. Doripenem, gentamicin, and colistin, alone and in combinations, against gentamicin-susceptible, KPC-producing Klebsiella pneumoniae strains with various ompK36 genotypes. Antimicrob Agents Chemother 58:3521–3525. doi: 10.1128/AAC.01949-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aslam S. 2008. Effect of antibacterials on biofilms. Am J Infect Control 36:S175.e9–S175.e11. doi: 10.1016/j.ajic.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. 2017. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15:740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naparstek L, Carmeli Y, Navon-Venezia S, Banin E. 2014. Biofilm formation and susceptibility to gentamicin and colistin of extremely drug-resistant KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother 69:1027–1034. doi: 10.1093/jac/dkt487. [DOI] [PubMed] [Google Scholar]
- 32.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 33.Mataraci Kara E, Ozbek Celik B. 2018. Investigation of the effects of various antibiotics against Klebsiella pneumoniae biofilms on in vitro catheter model. J Chemother 30:82–88. doi: 10.1080/1120009X.2017.1390633. [DOI] [PubMed] [Google Scholar]
- 34.Kvist M, Hancock V, Klemm P. 2008. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol 74:7376–7382. doi: 10.1128/AEM.01310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Cao J, Zhou C, Liu H, Zhang X, Zhou T. 2017. Biofilm formation restrained by subinhibitory concentrations of tigecyclin in Acinetobacter baumannii is associated with downregulation of efflux pumps. Chemotherapy 62:128–133. doi: 10.1159/000450537. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez L, Breidenstein EB, Hancock RE. 2011. Creeping baselines and adaptive resistance to antibiotics. Drug Resist Updat 14:1–21. doi: 10.1016/j.drup.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Bergen PJ, Forrest A, Bulitta JB, Tsuji BT, Sidjabat HE, Paterson DL, Li J, Nation RL. 2011. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother 55:5134–5142. doi: 10.1128/AAC.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forrest GN, Tamura K. 2010. Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev 23:14–34. doi: 10.1128/CMR.00034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vassallo M, Dunais B, Roger PM. 2015. Antimicrobial lock therapy in central-line associated bloodstream infections: a systematic review. Infection 43:389–398. doi: 10.1007/s15010-015-0738-1. [DOI] [PubMed] [Google Scholar]
- 40.Drusano GL. 2017. Pre-clinical in vitro infection models. Curr Opin Pharmacol 36:100–106. doi: 10.1016/j.coph.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Albur M, Noel A, Bowker K, MacGowan A. 2012. Bactericidal activity of multiple combinations of tigecycline and colistin against NDM-1-producing Enterobacteriaceae. Antimicrob Agents Chemother 56:3441–3443. doi: 10.1128/AAC.05682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eagle H, Musselman AD. 1948. The rate of bactericidal action of penicillin in vitro as a function of its concentration, and its paradoxically reduced activity at high concentrations against certain organisms. J Exp Med 88:99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuji BT, Landersdorfer CB, Lenhard JR, Cheah SE, Thamlikitkul V, Rao GG, Holden PN, Forrest A, Bulitta JB, Nation RL, Li J. 2016. Paradoxical effect of polymyxin B: high drug exposure amplifies resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 60:3913–3920. doi: 10.1128/AAC.02831-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawkey PM, Warren RE, Livermore DM, McNulty CAM, Enoch DA, Otter JA, Wilson APR. 2018. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother 73:iii2–iii78. doi: 10.1093/jac/dky027. [DOI] [PubMed] [Google Scholar]
- 45.Labro MT. 2012. Immunomodulatory effects of antimicrobial agents. Part I: antibacterial and antiviral agents. Expert Rev Anti Infect Ther 10:319–340. doi: 10.1586/eri.12.11. [DOI] [PubMed] [Google Scholar]
- 46.Lebeaux D, Chauhan A, Rendueles O, Beloin C. 2013. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2:288–356. doi: 10.3390/pathogens2020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing, 27th ed, M100-S27 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 49.European Committee on Antimicrobial Susceptibility Testing. 2018. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.0, 2018. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf.
- 50.Chatzimoschou A, Simitsopoulou M, Antachopoulos C, Walsh TJ, Roilides E. 2015. Antipseudomonal agents exhibit differential pharmacodynamic interactions with human polymorphonuclear leukocytes against established biofilms of Pseudomonas aeruginosa. Antimicrob Agents Chemother 59:2198–2205. doi: 10.1128/AAC.04934-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meletiadis J, Verweij PE, TeDorsthorst DT, Meis JF, Mouton JW. 2005. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med Mycol 43:133–152. [DOI] [PubMed] [Google Scholar]
- 52.Katragkou A, McCarthy M, Alexander EL, Antachopoulos C, Meletiadis J, Jabra-Rizk MA, Petraitis V, Roilides E, Walsh TJ. 2015. In vitro interactions between farnesol and fluconazole, amphotericin B or micafungin against Candida albicans biofilms. J Antimicrob Chemother 70:470–478. doi: 10.1093/jac/dku374. [DOI] [PMC free article] [PubMed] [Google Scholar]