Treatment options for drug-resistant cytomegalovirus (CMV) are limited. Letermovir is a novel antiviral recently approved for CMV prophylaxis following hematopoietic cell transplantation, but its efficacy in other settings is unknown.
KEYWORDS: cytomegalovirus, ganciclovir-resistant, letermovir
ABSTRACT
Treatment options for drug-resistant cytomegalovirus (CMV) are limited. Letermovir is a novel antiviral recently approved for CMV prophylaxis following hematopoietic cell transplantation, but its efficacy in other settings is unknown. We recently used letermovir for salvage treatment in four solid organ transplant recipients with ganciclovir-resistant CMV retinitis. All patients improved clinically without known adverse drug events. However, three patients failed to maintain virologic suppression, including two patients who developed genotypically confirmed resistance to letermovir while on therapy.
INTRODUCTION
Ganciclovir-resistant cytomegalovirus (CMV) disease poses a substantial clinical problem within the transplant population. Mutations in either the viral kinase (UL97) or polymerase (UL54) can mediate resistance to ganciclovir and valganciclovir, which are first-line treatment agents. The incidence of ganciclovir resistance increases with cumulative drug exposure, ranging as high as 40% to 50% in patients receiving prolonged prophylaxis or repeated courses of treatment (1–3). Ganciclovir resistance is associated with worse clinical outcomes across a range of transplant types, including higher risk of recurrent CMV disease and increased mortality (4, 5). The FDA-approved alternative agents available to treat ganciclovir-resistant CMV are often poorly tolerated due to high rates of renal insufficiency (e.g., foscarnet and cidofovir) or neutropenia (e.g., high-dose ganciclovir and cidofovir). Brincidofovir and maribavir may also retain activity against ganciclovir-resistant CMV, but both drugs have failed phase 3 prophylaxis trials, and maribavir is limited by poor ocular and central nervous system (CNS) penetration (6–8). Letermovir is a novel agent which targets the viral terminase complex with a high specificity for CMV (9). Due to the unique mechanism of action, letermovir remains active against CMV carrying mutations in UL97 or UL54 (10). While it is currently approved for CMV prophylaxis in hematopoietic cell transplant recipients, experience with letermovir for treatment or secondary prophylaxis of CMV disease for other types of patients or scenarios is limited to case reports (11–14). This case series highlights our single-center experience with an off-label use of letermovir for the treatment of ganciclovir-resistant CMV disease in patients who either failed or were unable to tolerate traditional therapies for resistant CMV.
We analyzed clinical data from all adult patients at a tertiary care hospital in North Carolina who initiated letermovir for treatment of CMV disease between November 2017 and April 2018 (Table 1). Four patients received letermovir for treatment of ganciclovir-resistant CMV disease after failing therapy with ganciclovir-valganciclovir and developing nephrotoxicity from foscarnet. All patients had genotypically proven resistance to ganciclovir with a history of clinical failure on multiple traditional antiviral agents. Two of four patients in our cohort had CMV retinitis proven by CMV PCR from the aqueous obtained by an anterior chamber paracentesis (testing performed at University of Colorado Hospital, Denver, CO); two patients (A and C) were presumptively diagnosed with CMV retinitis based on CMV viremia with a fundoscopic examination showing retinitis. Plasma CMV viral loads at the time of letermovir initiation for the four-patient cohort ranged from 137 to 1,416 IU/ml. Induction letermovir doses were begun at 720 mg and in one case up-titrated to 960 mg daily due to a lack of effect. While efficacy at these higher doses has not been formally clinically assessed, these dose ranges were chosen in consultation with Merck pharmacists on the basis of tolerance and safety data from phase I studies (available in drug product insert). Three patients received concomitant CMV immune globulin, as well as intravitreal therapy with either foscarnet (2.4 mg/0.1 ml) or ganciclovir (4 mg/0.1 ml). Laboratory monitoring included serial hematologic, renal, and hepatic function testing. A genotypic assessment for resistance to letermovir at gene UL56 was performed for three patients (Viracor Eurofins, Lee’s Summit, MO).
TABLE 1.
Clinical features and outcomes for 4 patients with drug-resistant CMV treated with letermovir
| Feature | Information for patient: |
|||
|---|---|---|---|---|
| A (66-y-old male) | B (50-y-old male) | C (46-y-old male) | D (66-y-old male) | |
| CMV risk factor | Bilateral orthotopic lung transplant (CMV donor+/recipient−) | Bilateral orthotopic lung transplant (CMV donor+/recipient−) | Orthotopic heart transplant (CMV donor+/recipient−) | Orthotopic heart transplant (CMV donor+/recipient−) |
| Comorbidities | Sarcoidosis, chronic kidney disease | Interstitial lung disease, chronic kidney disease | ||
| Disease burden | CMV syndrome retinitis | CMV syndrome retinitis | CMV syndrome retinitis colitis | Retinitis |
| Plasma CMV DNA at start of letermovir | 342 IU/ml | 1,416 IU/ml | 745 IU/ml | <137 IU/ml |
| Prior CMV prophylaxis | Valganciclovir | Valganciclovir | Valganciclovir | Valganciclovir |
| Prior antiviral treatment | CMV IgG, ganciclovir, valganciclovir, maribavir, foscarnet | Ganciclovir, valganciclovir, maribavir, foscarnet | Ganciclovir, valganciclovir, foscarnet | CMV IgG, ganciclovir, valganciclovir, foscarnet |
| Known CMV mutations prior to letermovir initiation | M460V (UL97) | Q578H (UL54) | M460I (UL97), likely mixed population at N408K (UL54) | H520Q (UL97), C603W (UL97), T503I (UL54) |
| Letermovir dose (mg daily) | 720 | 960a | 720 | 720 |
| Concomitant therapies | CMV IgG, foscarnet (V)b | CMV IgG, foscarnet (V), ganciclovir (V) | n/a | CMV IgG, foscarnet (V) |
| Duration of follow-up (weeks) | 38 | 39 | 32 | 34 |
| Virologic suppression on letermovir | Unsuppressed | Unsuppressed | Unsuppressed | Suppressed |
| Mutations conferring letermovir resistance | Negative for UL56 mutations | C325F mutation detected in UL56 | C325Y mutation detected in UL56 | Letermovir resistance testing not performed |
| Management of rebound viremia and/or letermovir resistance | Letermovir stopped on day 138, transitioned to valganciclovir and CMV IgG; subsequently achieved virologic suppression | Letermovir stopped on day 110, transitioned to valganciclovir (given reversion of prior UL54 mutation); subsequently achieved virologic suppression | Letermovir stopped on day 102, transitioned to foscarnet; subsequently achieved virologic suppression | n/ac |
| Clinical outcome | Improved on retinal exam | Improved on retinal exam | Improved on retinal exam | Improved on retinal exam |
Patient B began at 720-mg dose letermovir, but the dose was up-titrated to 960 mg due to lack of response.
(V) indicates vitreal administration; all others systemic unless noted.
n/a, not applicable: patient D did not suffer any rebound viremia.
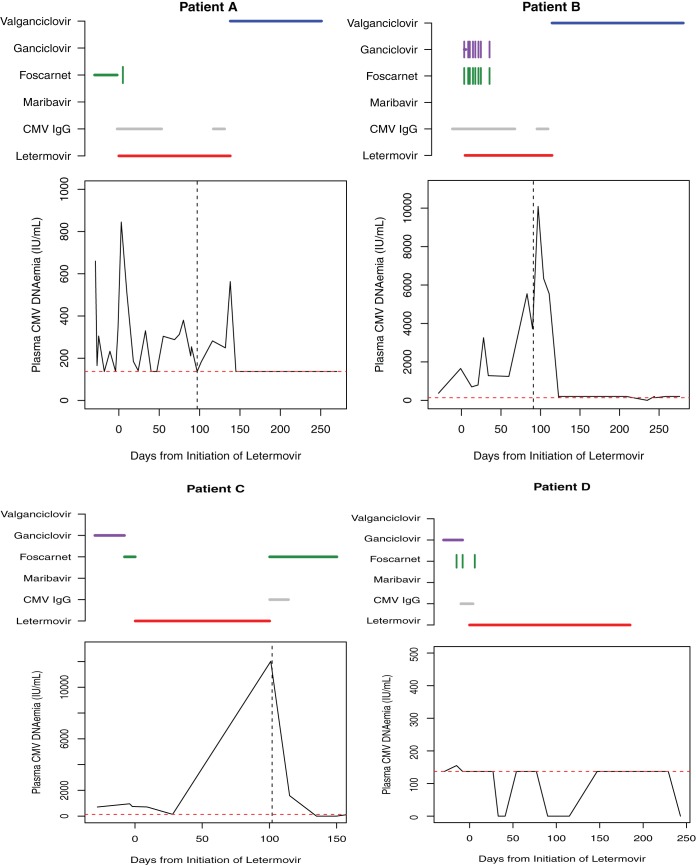
All four patients showed clinical and fundoscopic improvement with resolution of the retinitis. However, three patients failed to achieve sustained virologic suppression. Patient A exhibited prolonged, intermittent, low-level DNAemia, while patients B and C developed high-grade CMV DNAemia after more than a month of therapy with letermovir (Fig. 1). No patients developed adverse effects attributable to letermovir. As predicted by the inhibition of Cyp3A4 by letermovir, tacrolimus and warfarin required a downward dose adjustment and close therapeutic monitoring.
FIG 1.
CMV Plasma DNAemia response kinetics on letermovir treatment. Horizontal lines above each graph represent the time period on systemic therapy with each agent. Vertical tick marks indicate intravitreal doses. The horizontal dashed line indicates the threshold for quantitative detection of CMV plasma DNA by the assays used in this series (137 IU/ml). The vertical dashed line indicates timing of letermovir drug resistance testing.
The finding of three patients (A, B, and C) with sustained or recurrent plasma CMV DNAemia raised a concern for the emergence of resistance on letermovir therapy. A genotypic assessment demonstrated UL56 mutations within the same codon for patients B (C325F) and C (C325Y). Resistance testing did not reveal in vitro resistance for patient A; however, a mutation conferring resistance could have occurred at a UL56 site that was not sequenced or a different terminase complex component (e.g., UL51 or UL89) (15). Patient A was transitioned back to valganciclovir plus CMV IgG and demonstrated virologic suppression. For patient B, a reemergence of virus with a wild-type UL-54 permitted the resumption of valganciclovir with subsequent virologic suppression. Patient C had previously developed renal injury on foscarnet but was successfully rechallenged with this agent and achieved virologic suppression.
This case series highlights several promising features of letermovir that led us to use it as an off-label salvage therapy for refractory CMV infection. First, as predicted by its unique mechanism of action, letermovir should retain in vitro activity in patients with phenotypic and/or genotypic resistance to ganciclovir. Second, it was well-tolerated, both in our experience at doses up to 960 mg daily and in the previously published trials within the hematopoietic cell transplantation (HCT) population. Finally, it appeared at least initially effective as a component of variable, real-world treatment for CMV retinitis. All four of our patients had CMV retinal disease, and while three of four patients also received intravitreal injections of foscarnet during initial treatment with letermovir, none experienced any recurrent retinitis or vision loss while on letermovir for ongoing suppression.
These cases also emphasize the important concern for emergence of resistance while receiving letermovir. Three patients failed to achieve sustained virologic suppression despite demonstrable clinical improvement in retinitis. Genotyping confirmed treatment-emergent UL56 mutations in two patients, while a third patient had clinical evidence of resistance. Serial viral passage under letermovir selective pressure has been associated with a relatively rapid selection of UL56 mutations, particularly within codons 231 to 369 (16, 17). The possibility exists that the observed cases of letermovir resistance resulted from a selection of resistant subpopulations of CMV rather than as a consequence of a low barrier to resistance. However, regardless of the mechanism, the high rate of clinically significant resistance in our cohort has important implications. In particular, the use of letermovir to treat active CMV infection requires caution and close clinical monitoring, particularly in the setting of persistent viremia. Fortunately, in each instance of confirmed resistance in this cohort, it was possible to transition to an alternative agent, namely, in one case due to the reversion of a prior UL54 mutation and in the second due to tolerance of foscarnet upon rechallenge.
In addition to potential resistance development, letermovir inhibits Cyp3A4, leading to many potentially significant drug interactions. We made preemptive dose adjustments for statins, warfarin, and tacrolimus without noting adverse clinical effects. Serial tacrolimus drug levels and close international normalized ratio (INR) monitoring (for patients receiving warfarin) were required.
In our patients, letermovir was well tolerated and was associated with the resolution of CMV retinitis. There was no recurrence of retinitis during the follow-up period after cessation of intravitreal therapy. However, three patients developed recurrent or persistent DNAemia while receiving letermovir, including two patients with confirmed treatment-emergent UL56 resistance. Although letermovir may prove to be a useful treatment for some patients with CMV infection who have either failed prior therapies or are unable to tolerate traditional antiviral agents, providers will need to remain vigilant for treatment failure and emergence of resistance.
ACKNOWLEDGMENTS
N.T. was supported in part by an Antibacterial Resistance Leadership Group fellowship (National Institute of Allergy and Infectious Diseases, grant UM1AI104681). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors report no relevant financial disclosures. A.W.B., S.A., E.K.M., J.H.S., A.S., and C.R.W. are subinvestigators for a multicenter institutional review board (IRB)-approved study of maribavir for refractory CMV. This study does not include letermovir as a study drug or investigator-given therapy, and none of the authors receive study-related compensation.
REFERENCES
- 1.Young PG, Rubin J, Angarone M, Flaherty J, Penugonda S, Stosor V, Ison MG. 2016. Ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients: a single-center retrospective cohort study. Transpl Infect Dis 18:390–395. doi: 10.1111/tid.12537. [DOI] [PubMed] [Google Scholar]
- 2.Reddy AJ, Zaas AK, Hanson KE, Palmer SM. 2007. A single-center experience with ganciclovir-resistant cytomegalovirus in lung transplant recipients: treatment and outcome. J Heart Lung Transplant 26:1286–1292. doi: 10.1016/j.healun.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Cunha-Bang Cd, Kirkby N, Sønderholm M, Sørensen SS, Sengeløv H, Iversen M, Rasmussen A, Gustafsson F, Frederiksen CM, Kjaer J, Lepri AC, Lundgren JD. 2013. The time course of development and impact from viral resistance against ganciclovir in cytomegalovirus infection. Am J Transplant 13:458–466. doi: 10.1111/ajt.12042. [DOI] [PubMed] [Google Scholar]
- 4.Avery RK, Arav-Boger R, Marr KA, Kraus E, Shoham S, Lees L, Trollinger B, Shah P, Ambinder R, Neofytos D, Ostrander D, Forman M, Valsamakis A. 2016. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation 100:e74–e80. doi: 10.1097/TP.0000000000001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minces LR, Nguyen MH, Mitsani D, Shields RK, Kwak EJ, Silveira FP, Abdel-Massih R, Pilewski JM, Crespo MM, Bermudez C, Bhama JK, Toyoda Y, Clancy CJ. 2014. Ganciclovir-resistant cytomegalovirus infections among lung transplant recipients are associated with poor outcomes despite treatment with foscarnet-containing regimens. Antimicrob Agents Chemother 58:128–135. doi: 10.1128/AAC.00561-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koszalka GW, Johnson NW, Good SS, Boyd L, Chamberlain SC, Townsend LB, Drach JC, Biron KK. 2002. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother 46:2373–2380. doi: 10.1128/AAC.46.8.2373-2380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, Brundage TM, Robertson AT, Godkin S, Mommeja-Marin H, Boeckh M. 2013. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med 369:1227–1236. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 8.Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young JA, Rodriguez T, Maertens J, Schmitt M, Einsele H, Ferrant A, Lipton JH, Villano SA, Chen H, Boeckh M. 2011. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 11:284–292. doi: 10.1016/S1473-3099(11)70024-X. [DOI] [PubMed] [Google Scholar]
- 9.Marschall M, Stamminger T, Urban A, Wildum S, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. 2012. In vitro evaluation of the activities of the novel anticytomegalovirus compound AIC246 (letermovir) against herpesviruses and other human pathogenic viruses. Antimicrob Agents Chemother 56:1135–1137. doi: 10.1128/AAC.05908-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. 2011. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J Virol 85:10884–10893. doi: 10.1128/JVI.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemaly RF, Ullmann AJ, Stoelben S, Richard MP, Bornhäuser M, Groth C, Einsele H, Silverman M, Mullane KM, Brown J, Nowak H, Kölling K, Stobernack HP, Lischka P, Zimmermann H, Rübsamen-Schaeff H, Champlin RE, Ehninger G. 2014. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med 370:1781–1789. doi: 10.1056/NEJMoa1309533. [DOI] [PubMed] [Google Scholar]
- 12.Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, Haider S, Ullmann AJ, Katayama Y, Brown J, Mullane KM, Boeckh M, Blumberg EA, Einsele H, Snydman DR, Kanda Y, DiNubile MJ, Teal VL, Wan H, Murata Y, Kartsonis NA, Leavitt RY, Badshah C. 2017. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 377:2433–2444. doi: 10.1056/NEJMoa1706640. [DOI] [PubMed] [Google Scholar]
- 13.Kaul DR, Stoelben S, Cober E, Ojo T, Sandusky E, Lischka P, Zimmermann H, Rubsamen-Schaeff H. 2011. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am J Transplant 11:1079–1084. doi: 10.1111/j.1600-6143.2011.03530.x. [DOI] [PubMed] [Google Scholar]
- 14.Chong PP, Teiber D, Prokesch BC, Arasaratnam RJ, Peltz M, Drazner MH, Garg S. 2018. Letermovir successfully used for secondary prophylaxis in a heart transplant recipient with ganciclovir-resistant cytomegalovirus syndrome (UL97 mutation). Transplant Infect Dis 20:e12965. doi: 10.1111/tid.12965. [DOI] [PubMed] [Google Scholar]
- 15.Chou S. 2017. A third component of the human cytomegalovirus terminase complex is involved in letermovir resistance. Antiviral Res 148:1–4. doi: 10.1016/j.antiviral.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou S. 2015. Rapid in vitro evolution of human cytomegalovirus UL56 mutations that confer letermovir resistance. Antimicrob Agents Chemother 59:6588–6593. doi: 10.1128/AAC.01623-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou S, Satterwhite LE, Ercolani RJ. 2018. A new locus of drug resistance in the human cytomegalovirus UL56 Gene revealed by in vitro exposure to letermovir and ganciclovir. Antimicrob Agents Chemother 62:e00922-18. doi: 10.1128/aac.00922-18. [DOI] [PMC free article] [PubMed] [Google Scholar]