The spread of carbapenemase-producing Enterobacteriaceae (CPE), contributing to widespread carbapenem resistance, has become a global concern. However, the specific dissemination patterns of carbapenemase genes have not been intensively investigated in developing countries, including Myanmar, where NDM-type carbapenemases are spreading in clinical settings.
KEYWORDS: Enterobacteriaceae, Myanmar, blaNDM, carbapenemase, carbapenems, plasmid-mediated resistance
ABSTRACT
The spread of carbapenemase-producing Enterobacteriaceae (CPE), contributing to widespread carbapenem resistance, has become a global concern. However, the specific dissemination patterns of carbapenemase genes have not been intensively investigated in developing countries, including Myanmar, where NDM-type carbapenemases are spreading in clinical settings. In the present study, we phenotypically and genetically characterized 91 CPE isolates obtained from clinical (n = 77) and environmental (n = 14) samples in Yangon, Myanmar. We determined the dissemination of plasmids harboring genes encoding NDM-1 and its variants using whole-genome sequencing and plasmid analysis. IncFII plasmids harboring blaNDM-5 and IncX3 plasmids harboring blaNDM-4 or blaNDM-7 were the most prevalent plasmid types identified among the isolates. The IncFII plasmids were predominantly carried by clinical isolates of Escherichia coli, and their clonal expansion was observed within the same ward of a hospital. In contrast, the IncX3 plasmids were found in phylogenetically divergent isolates from clinical and environmental samples classified into nine species, suggesting widespread dissemination of plasmids via horizontal transfer. Half of the environmental isolates were found to possess IncX3 plasmids, and this type of plasmid was confirmed to transfer more effectively to recipient organisms at a relatively low temperature (25°C) compared to the IncFII plasmid. Moreover, various other plasmid types were identified harboring blaNDM-1, including IncFIB, IncFII, IncL/M, and IncA/C2, among clinical isolates of Klebsiella pneumoniae or Enterobacter cloacae complex. Overall, our results highlight three distinct patterns of the dissemination of blaNDM-harboring plasmids among CPE isolates in Myanmar, contributing to a better understanding of their molecular epidemiology and dissemination in a setting of endemicity.
INTRODUCTION
Carbapenemases are β-lactamases that hydrolyze almost all types of β-lactams, including carbapenems, which are the last line of defense against multidrug-resistant bacteria. Recent years have witnessed a rapid increase in the occurrence of Enterobacteriaceae species resistant to carbapenems; this resistance is mainly conferred by carbapenemase genes encoded by plasmids (1). Such carbapenemase-producing Enterobacteriaceae (CPE) are resistant to most of the commonly prescribed antibiotics, and infections by these pathogens are associated with poor prognosis, thereby raising serious concerns during treatment in clinical settings. Moreover, CPE have also been found in environmental samples such as sewage, tap water, and foodstuffs, posing a severe threat to public health (2–4).
The widespread dissemination of CPE may have been expedited by two mechanisms. The first is a clonal expansion of an organism carrying a carbapenemase-encoding plasmid, as represented by the spread of Klebsiella pneumoniae clonal complex 258 producing K. pneumoniae carbapenemase (KPC), mainly in the United States and some other countries (5, 6). The other potential mechanism is via the horizontal transfer of carbapenemase-encoding plasmids to naive Enterobacteriaceae present in the human and animal gut or even in the environment (7–9). Further, carbapenemase genes often readily transfer between replicons via a transposon-mediated mechanism, resulting in the emergence of novel carbapenemase-encoding plasmids or chromosomal carriage of the gene (10). Recently, whole-genome sequencing (WGS) has been exploited to conduct a high-resolution analysis of the dissemination of carbapenemase genes in clinical settings. However, to date, these analyses have mainly been conducted in the United States and European countries, and few studies have investigated the route of dissemination of carbapenemase genes in resource-poor settings (11–13), where CPE are often endemic (14). In South and Southeast Asian countries, the New Delhi metallo-β-lactamase (NDM) gene blaNDM is widely distributed, representing a main public health concern (6, 15). NDM-producing Enterobacteriaceae are also reported to be rapidly spreading in Balkan countries and China (14), and they have been frequently found in European countries and occasionally in the USA (6). Although blaNDM-1 and its variants have been found in various Enterobacteriaceae species harbored by different types of plasmids (16), there is a paucity of knowledge on the patterns of their dissemination. Thus, further investigation is needed to initiate effective control measures to prevent their further spread.
In a survey conducted in a tertiary-care hospital and two private hospitals in Yangon, Myanmar, Escherichia coli and K. pneumoniae isolates carrying blaNDM genes were isolated (17). E. coli isolates carrying the blaNDM genes were also found in another hospital in Yangon (18), suggesting that organisms carrying these genes are spreading in the region. We previously conducted WGS of eight carbapenem-resistant E. coli clinical isolates at a single tertiary-care hospital in Yangon; they were found to be phylogenetically distinct and to possess various types of blaNDM-harboring plasmids (19). To further understand the routes of dissemination and diversity of blaNDM-harboring bacteria, in the present study, we conducted a larger-scale WGS and plasmid analysis of 77 clinical and 14 environmental CPE isolates obtained in Yangon.
RESULTS
Characteristics of clinical and environmental CPE isolates.
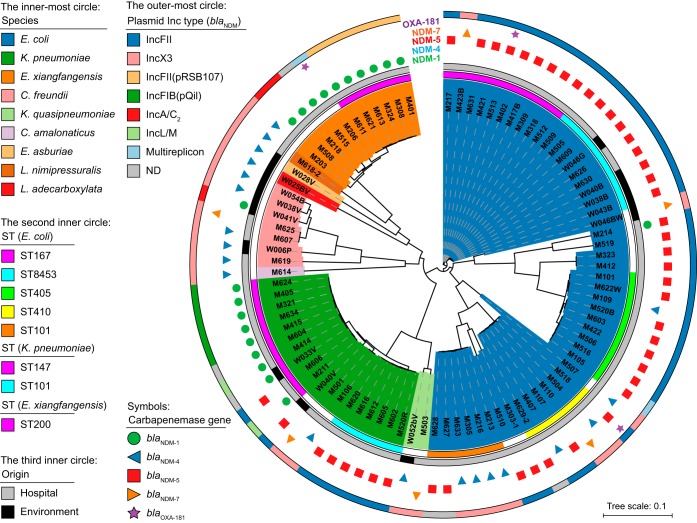
During a 21-month surveillance in a tertiary-care hospital in Yangon, a total of 2,262 Enterobacteriaceae were isolated from clinical specimens, 91 (4%) of which showed resistance to meropenem. We excluded isolates that originated from the same specimens and that were identified as same species and isolates without carbapenemase genes from further analyses. As a result, 77 isolates carrying carbapenemase genes, including 8 E. coli isolates that we previously reported (19), were selected for further analysis using Illumina sequencing. These included E. coli (n = 43), K. pneumoniae (n = 17), Klebsiella quasipneumoniae (n = 1), Citrobacter freundii (n = 3), Citrobacter amalonaticus (n = 1), and Enterobacter cloacae complex (n = 12) (Fig. 1; Tables S1 and S2). We identified four variants of blaNDM genes, namely, blaNDM-1 (n = 19), blaNDM-4 (n = 14), blaNDM-5 (n = 40), and blaNDM-7 (n = 5), among which blaNDM-5 was the most prevalent and was found in more than half of the total isolates (51.9%). Another type of carbapenemase gene, blaOXA-181, was found in three isolates (two E. coli isolates and one Enterobacter xiangfangensis isolate) that coexisted with blaNDM-1 or blaNDM-5.
FIG 1.
Diversity of CPE isolates from Myanmar. The innermost colored regions show the species of the bacterial isolates. The next inner circle shows major MLST sequence types (STs) found more than two among the isolates. The third inner circle indicates the origin of each isolate. The next outer region indicates the carbapenemase gene carried by each isolate. The outermost circle shows the Inc replicon type of blaNDM-harboring plasmids carried by each CPE isolate. Isolate M214 carried an IncA/C2 plasmid harboring blaNDM-1 in addition to an IncFII plasmid harboring blaNDM-5. The genome sequence of E. coli K-12 MG1655 (GenBank accession number NC_000913.3) was used as a reference for the construction of the phylogenetic tree. ND, not determined; NA, not applicable.
Most of the clinical isolates were not susceptible to commonly used clinical antibiotics, such as levofloxacin (95.1%), minocycline (79.2%), and amikacin (76.6%) (Table S3), whereas the majority of the isolates were susceptible to colistin (97.4% [75/77]) and fosfomycin (63.6% [49/77]). The colistin resistance gene mcr was not detected in any of our Myanmar isolates.
A total of 54 sewage samples were collected from six locations adjacent to the hospital and were screened for the presence of isolates harboring carbapenemase genes. Of the 206 carbapenem-resistant isolates, there were 17 Enterobacteriaceae (8.3%), 14 of which carried the blaNDM variants blaNDM-1, blaNDM-4, blaNDM-5, and blaNDM-7 and 3 possessing none of the four major carbapenemase genes, namely, the blaNDM, blaKPC, blaIMP, and blaOXA-48-like genes. These environmental CPE isolates comprised eight species, including one isolate each of Enterobacter asburiae and Leclercia adecarboxylata that were found only in the environmental samples (Fig. 1; Table S1 and S2). The antimicrobial susceptibility profile of the environmental isolates was similar to that of the clinical isolates; however, there was a higher frequency of environmental isolates that were susceptible to aztreonam, aminoglycosides, quinolones, and chloramphenicol (Table S3). We also screened 54 drinking water samples and obtained three carbapenem-resistant Enterobacter cloacae isolates; however, the above-named four carbapenemase genes were not detected.
One of the other remarkable features of the Myanmar isolates was the prevalence of the extended-spectrum β-lactamase gene blaCTX-M-15, which was found in 70.3% (64/91) of the isolates that include five different species (E. coli, K. pneumoniae, K. quasipneumoniae, C. freundii, and E. xiangfangensis) isolated from clinical and environmental samples (Table S2).
Isolates carrying IncFII-type blaNDM-harboring plasmids.
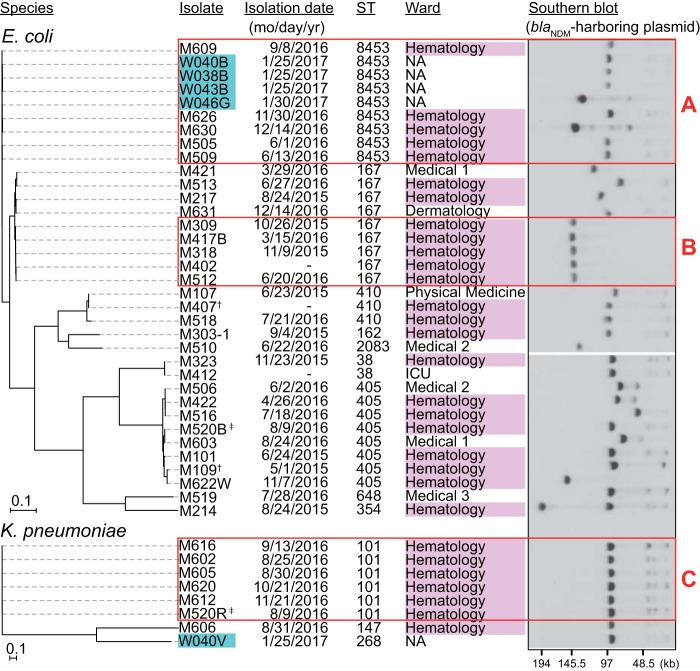
The Inc types of blaNDM-harboring plasmids carried by the isolates were determined by PlasmidFinder, BLAST, and Southern blot analysis. The IncFII-type plasmid was the most prevalent type of plasmid detected in our isolates, carried by E. coli and K. pneumoniae (Table S2). Almost all of the blaNDM-5 genes detected (41/45) were found on this plasmid type, which also carried the blaNDM-4 gene in two of the E. coli isolates. The IncFII plasmids were around 90 kb; however, several of the blaNDM-5-harboring plasmids showed different sizes, ranging from 50 to 150 kb (Fig. 2).
FIG 2.
Phylogenetic relationships among the CPE isolates with IncFII-type plasmids harboring blaNDM. All of the isolates, except for two blaNDM-4 carriers (†), harbored the blaNDM-5 gene on the IncFII-type plasmid. Environmental isolates are marked in cyan. blaNDM-harboring plasmids were detected by S1-PFGE-Southern blot analysis with a blaNDM probe. Red rectangles indicate closely related isolates. Cluster A, 7 to 20 SNPs, >99.9% ANI; cluster B, 6 to 22 SNPs, >99.9% ANI; cluster C, 8 to 28 SNPs, >99.9% ANI. SNPs between the isolates in cluster B and the other ST167 isolates were 833 to 1,051. Genome sequences of E. coli K-12 MG1655 and K. pneumoniae AATZP (GenBank accession number CP014755.1) were used as references for construction of the phylogenetic trees of respective species. Isolate M214 carried an IncA/C2 plasmid harboring blaNDM-1 (176 kb) in addition to an IncFII plasmid harboring blaNDM-5 (95 kb). ‡, M520B and M520R were isolated from the same specimen. -, unknown.
The E. coli isolates carrying IncFII-type plasmids (n = 35) were diverse, with nine different sequence types (STs) detected, including a novel ST (Fig. 2). The single nucleotide polymorphism (SNP)-based phylogenetic analysis identified two clusters consisting of highly related isolates, designated A and B. The isolates of cluster A differed from each other by 7 to 20 SNPs and were assigned to ST8453, a single locus variant of ST167. This group included five clinical isolates obtained from the hematology ward of the hospital and four environmental isolates, suggesting the spread of organisms with IncFII plasmids both inside and outside the hospital. Cluster B included five ST167 isolates that differed by 6 to 22 SNPs, which all harbored a relatively larger plasmid (137 kb) than the other IncFII-type plasmids found in this study (Fig. 2). All of the isolates included in cluster B were also obtained from the hematology ward, suggesting that clonal expansion of the organisms occurred in this ward of the hospital.
Of the eight K. pneumoniae isolates carrying IncFII-type plasmids, six were genotyped as ST101 (Fig. 2, cluster C) and were closely related (8 to 28 SNPs between isolates). Moreover, all of these isolates were also obtained from the hematology ward, suggesting their clonal spread. Notably, K. pneumoniae M520R and E. coli M520B were isolated from the same patient (Fig. 2, marked with a double dagger). This was the first detection of the IncFII plasmid in a K. pneumoniae isolate during the surveillance period; thus, it is likely that transfer of the IncFII plasmid occurred from M520B to M520R within a patient.
Genomic structure of the IncFII-type plasmid pM309-NDM5.
We determined the genomic structure of the blaNDM-5 gene-harboring plasmid pM309-NDM5, carried by a cluster B isolate (M309), using a long-read sequencer (Fig. S1A). The plasmid possessed IncFIA replication gene in addition to IncFII (FAB formula, F36:A4:B− or F36:A20:B−). Its multidrug resistance region appeared to consist of two parts. One harbored the blaNDM-5 gene and was entirely conserved in pM214_FII, a typical IncFII plasmid (F2:A-:B-) detected among our isolates (19) (Fig. S1B, region A). This genomic region was flanked by two intact IS26 sequences and coharbored other genes encoding β-lactamases and those conferring resistance to aminoglycoside, macrolide, sulfonamide, and trimethoprim. The other resistance region was bracketed by derivatives of Tn5403 and Tn2 and harbored blaCTX-M-15 and other resistance genes against tetracycline, aminoglycoside, and chloramphenicol (Fig. S1B, region B). Several plasmids, including E. coli plasmid pLZ135-CTX (GenBank accession number MF353155.1), were found to possess this resistance region by a database search. pLZ135-CTX shared a common type of plasmid backbone (F36:A4:B- or F36:A20:B-) with pM309-NDM5, and these plasmids were highly homologous (96% coverage and 99% nucleotide identity) except for the multidrug resistance region harboring blaNDM-5. In pM309-NDM5, the left and right sides of the resistance region containing blaNDM-5, including the two IS26 sequences, were bracketed by derivatives of Tn2 (ΔTn2) and ISSba14 (ΔISSba14), respectively. Of note, both of these gene configurations found at boundaries between the resistance region and the plasmid backbone, i.e., ΔTn2-IS26 and IS26-ΔISSba14, were also conserved in pLZ135-CTX.
Isolates carrying IncX3-type blaNDM-harboring plasmids.
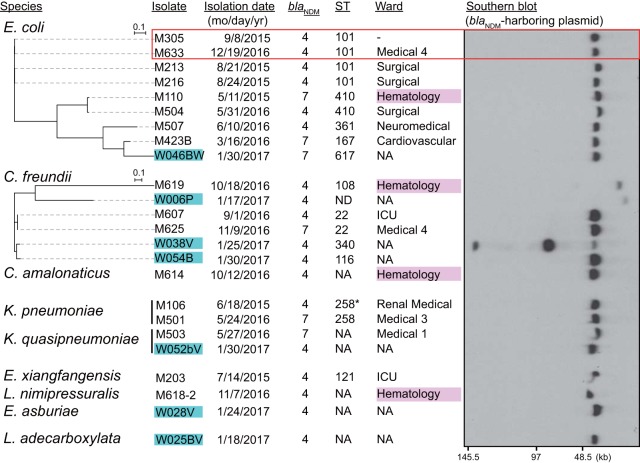
IncX3 was the second most prevalent plasmid type detected among blaNDM-harboring plasmids. The IncX3-type plasmids harbored blaNDM-4 or blaNDM-7 and were found in 24 isolates (26.4% of all isolates) consisting of 17 clinical and 7 environmental isolates, comprising nine different species. Unlike the IncFII plasmids, the clinical isolates harboring IncX3-type plasmids were obtained from 10 different wards of the hospital, and only two of them exhibited a close relationship, showing substantial phylogenetic diversity (Fig. 3). Half of the 14 environmental isolates examined possessed IncX3-type plasmids with a size of approximately 50 kb, except for those found in three C. freundii isolates that exhibited variable sizes. Thus, IncX3 plasmids have disseminated widely among Enterobacteriaceae species through horizontal transfer.
FIG 3.
Phylogenetic relationships among CPE isolates with IncX3-type plasmids harboring blaNDM. Environmental isolates are marked in cyan. The red rectangle indicates closely related isolates: 18 SNPs, >99.9% ANI. Genome sequences of E. coli K-12 MG1655 and C. freundii CFNIH1 (GenBank accession number CP007557.1) were used as references for construction of the phylogenetic trees of respective species. ST258* is a single locus variant of ST258.
Temperature-dependent transmissibility of IncFII- and IncX3-type blaNDM-harboring plasmids.
We assessed the efficiency of the conjugal transfer of the IncFII- and IncX3-type plasmids using the E. coli HST08 transformants. Although there was no significant difference in the transfer efficiency of the two plasmids, the optimal temperatures for conjugal transfer differed (Fig. 4). For the IncFII plasmids, the conjugal transfer was most efficient at 35°C, whereas the most appropriate temperature for transfer of the IncX3 plasmids was 25°C, which may explain their ability for broad dissemination in the environment. The means of the efficiency of transfer at 37°C for IncFII and IncX3 plasmids were 1.6 × 10−2 and 1.2 × 10−3, respectively.
FIG 4.
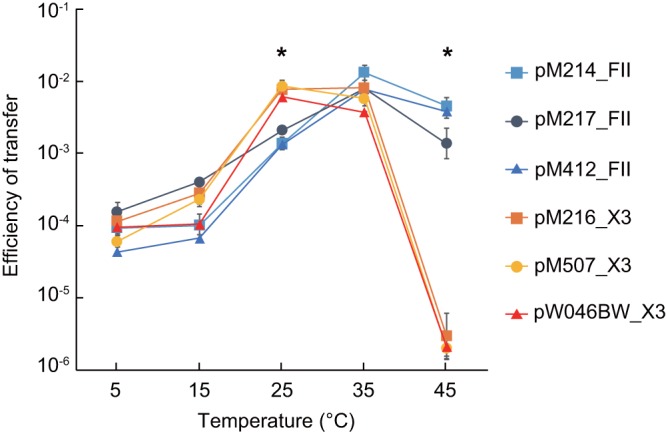
Comparison of the efficiencies of transfer of IncFII- and IncX3-type plasmids harboring blaNDM. The effect of incubation temperature on the efficiency of transfer of IncFII- or IncX3-type plasmids from their transformants to a recipient, E. coli ML4909, was assessed by a conjugation assay. E. coli HST08 transformed with IncFII plasmids harboring blaNDM-5 (pM214_FII, pM217_FII, or pM412_FII) and IncX3 plasmids harboring blaNDM-4 (pM216_X3 or pM507_X3) or blaNDM-7 (pW046BW) was used as the donor. Measurements were performed in triplicate (n = 3). *, P < 0.05 (t test).
Isolates carrying other plasmid types harboring bla NDM-1.
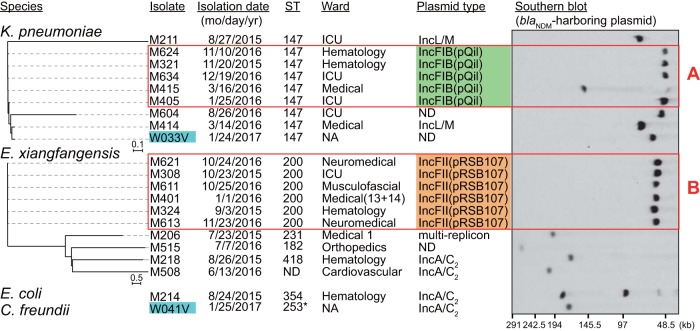
We further identified 21 isolates with a blaNDM-1-harboring plasmid, which were mainly dominated by K. pneumoniae (n = 9) and E. xiangfangensis (n = 10), with only 1 E. coli isolate detected in this group. We found five different replicon types among the plasmids harboring blaNDM-1: IncFIB(pQil), IncL/M, IncFII(pRSB107), IncA/C2, and a multireplicon-type plasmid harboring the IncFII(K), IncQ1, and IncR replication genes (Fig. S2A), thereby demonstrating the high diversity of blaNDM-1-harboring plasmids (Fig. 5).
FIG 5.
Phylogenetic relationship among CPE isolates carrying the blaNDM-1 gene. Environmental isolates are marked in cyan. Red rectangles denote closely related isolates. Cluster A, 20 to 72 SNPs, >99.9% ANI; cluster B, 8 to 30 SNPs, >99.8% ANI. Isolate M214 carried two blaNDM-harboring plasmids: the IncA/C2 plasmid harboring blaNDM-1 (176 kb) and the IncFII plasmid harboring blaNDM-5 (95 kb). Genome sequences of K. pneumoniae AATZP or E. xiangfangensis LMG27195 (GenBank accession number NZ_CP017183.1) were used as references for construction of the phylogenetic trees of the respective species. ST253* is a single locus variant of ST253.
All nine K. pneumoniae isolates carrying blaNDM-1 were typed as ST147. IncFIB(pQil)-type plasmids were found in five closely related clinical isolates with 20 to 72 SNPs between them, although the size of the plasmid was different in isolate M415 (Fig. 5, red rectangle A). Despite their sequence similarity, these isolates were obtained from three different wards of the hospital. The IncFIB(pQil)-type blaNDM-1-harboring plasmid carried by K. pneumoniae isolate M321, designated pM321-NDM1 (Fig. S2B), was almost identical (two nucleotide substitutions) to pNDM-1fa (20), harbored by the K. pneumoniae ST147 isolate AATZP, of Indian origin. Whole-genome comparison further confirmed that M321 and AATZP are closely related, with an average nucleotide identity (ANI) value of 99.95%. Two blaNDM-1-harboring plasmids carried by phylogenetically distant isolates (1,377 SNPs), M211 and M414, were determined as the IncL/M type. Assembled contig sequences of these two isolates were mapped with high confidence (93% coverage and 99% nucleotide identity) to a previously reported IncL/M plasmid carried by a clinical K. pneumoniae isolate of Omani origin, pNDM-OM (21), which was recently reclassified as IncM2 (22).
A cluster of closely related E. xiangfangensis isolates carrying blaNDM-1 (Fig. 5, red rectangle B) differing by 8 to 30 SNPs included isolates obtained from five different wards, suggesting dissemination from a common source. We conducted a more in-depth analysis of two of these plasmids, designated pM308-NDM1 and pM324-NDM1, identified in E. xiangfangensis isolates M308 and M324, respectively, using a long-read sequencer. Both plasmids were typed as IncFII(pRSB107) with PlasmidFinder, although the replicon sequence showed only 86.6% identity with the reference (GenBank accession number AJ851089). The sequences of pM308-NDM1 and pM324-NDM1 were highly conserved overall, except for the occurrence of a few insertions or deletions (Fig. S2C). Another four related isolates also possessed this type of plasmid with similar sizes; however, no homologous plasmids were found in the GenBank database.
IncFIB(pQil) was exclusively found in K. pneumoniae isolates, whereas the IncFII(pRSB107)-like plasmids were found only in E. xiangfangensis. Therefore, interspecies transmission of these plasmids did not occur among the Myanmar isolates. Indeed, these plasmids do not possess the genes necessary for plasmid transfer (Fig. S2B and C).
Plasmid harboring blaOXA-181.
We determined complete sequences of three blaOXA-181-harboring plasmids, which were all IncX3-type plasmids and were highly similar to each other (only 2 to 5 single nucleotide variations/51,479 bp) (Fig. S2D).
DISCUSSION
We have identified phylogenetically divergent CPEs with different types of blaNDM-encoding plasmids in a tertiary-care hospital in Yangon, Myanmar, suggesting multiple independent introductions of these resistant organisms into the hospital, along with their clonal spread. Two types of blaNDM-harboring plasmids, IncFII and IncX3, were prevalent among the CPE isolates, along with various types of plasmids harboring blaNDM-1 detected at lower frequencies. These different types of plasmids showed distinct dissemination patterns, which appear to largely depend on the plasmid backbone and bacterial species harboring them.
IncFII plasmids spread among E. coli found in the fecal microbiota of humans and animals (23), which could explain the prevalence of E. coli carrying the IncFII-type plasmids among our CPE isolates. These plasmids were also found to be highly diverse in size, implying their high plasticity. We previously identified five IncFII plasmids with or without blaNDM from Myanmar E. coli isolates and determined their sequences, in which two to four copies of IS26 were found and traces of IS26-mediated insertion and mobilization of gene clusters were observed (19). In that study, the isolate M105 was lacking the IS26-bracketed antimicrobial resistance gene cluster containing blaNDM-5 in the IncFII plasmid; instead this cluster was found in a different plasmid backbone, resulting in a novel blaNDM-5-harboring plasmid (19). In this study, we further provide another example of the emergence of a novel plasmid harboring blaNDM-5. The plasmid pM309-NDM5 carrying FIA and FII replicons also possessed the IS26-bracketed multidrug resistance region containing blaNDM-5. Using a BLAST search, the plasmid backbone of pM309-NDM5 was found homologous to another plasmid with FIA and FII replicons, pLZ135-CTX. Although this plasmid lacked the resistance cluster harboring blaNDM-5, the intact IS26s and their neighboring sequences bracketing the resistance cluster were conserved in both plasmids. Thus, intermolecular homologous recombination could occur between a plasmid harboring the blaNDM-5 region, such as pM214_FII, and pLZ135-CTX like plasmid, resulting in the emergence of pM309-NDM5. Nevertheless, plasmids homologous to pLZ135-CTX have not yet been identified among the Myanmar isolates.
Most of the clinical isolates carrying the IncFII-type plasmids were obtained from the same hematology ward. Identification of three groups of closely related isolates carrying IncFII plasmids suggests the nosocomial spread of clonal lineages in this ward. These isolates were genotyped as E. coli ST167, its single locus variant ST8453, and K. pneumoniae ST101. The dose and frequency of the use of antimicrobials tend to be higher in the hematology ward than in other wards of the hospital since infections often become more severe in immunocompromised patients. This situation might allow these multidrug-resistant strains to spread in the ward. Of note, E. coli ST8453 isolates were also obtained from sewage samples, demonstrating the spread of clinically relevant organisms in the environment.
In contrast to the IncFII plasmids, we identified IncX3 plasmids harboring blaNDM-4 or blaNDM-7 in a wider variety of bacterial species of clinical and environmental origins, suggesting the dissemination of these plasmids via horizontal transfer. An IncX3 plasmid could be efficiently transferred in a conjugation assay at 25°C, whereas the optimum temperature was 35°C for the transfer of the IncFII plasmid. The IncX3 plasmids were prevalent in environmental isolates and were found in various Enterobacteriaceae isolates; therefore, conjugal transfer in environmental organisms might play a significant role in the dissemination of this plasmid. In this regard, the efficiency of transfer of IncA/C or nontypeable plasmids harboring blaNDM-1 from environmental Enterobacteriaceae isolates was reported to be better at 30°C than at 37°C (2). Efficient transfer of the IncX3 plasmid at a lower temperature could be one of the underlying mechanisms of its widespread dissemination. IncFII- and IncX3-type plasmids employ different types of type IV secretion machinery for plasmid transfer. IncFII-type plasmid possesses F-type conjugative pilus, while IncX3-type plasmid harbors P-type pilus homologous to Agrobacterium tumefaciens VirB/VirD4 (24). While F-type pili are typically long and flexible, P-type pili are thicker and more rigid; thus, the difference in physical properties of conjugative pilus might be related to temperature sensitivity of plasmid transfer.
The IncX3-type plasmid appears to be an efficient vector for carbapenemase genes, as it has been reported to carry the blaNDM, blaOXA-48-like, and blaKPC genes (25–27) and can disseminate the genes both inside (28) and outside (29, 30) of the clinical setting. IncX3 plasmids also harbor the hns gene, a homolog of which was reported to allow plasmids to be transferred to a bacterial host by minimizing their fitness cost (31). The hns gene is also involved in the temperature-dependent control of plasmid transfer (32), although the role of this gene, if any, in the temperature effects observed in the present study remains to be elucidated.
The blaNDM-1 gene was not found on either of the two most prevalent plasmids detected in our isolates, although it was previously found on IncFII (33, 34) and IncX3 (26) plasmids in other countries. It was found in other types of plasmids, such as IncFIB(pQil), IncFII(pRSB107)-like, IncL/M, and IncA/C2, indicating their independent acquisition of the gene rather than the dissemination of the gene with major transferable plasmids. It was noteworthy that phylogenetically closely related isolates were isolated from different wards. Five K. pneumoniae ST147 isolates carrying the IncFIB(pQil) plasmid were found in four different wards and six E. xiangfangensis ST200 isolates carrying the IncFII(pRSB107) plasmid were isolated from six different wards of a single hospital, suggesting their nosocomial spread from a common source and implying their persistent nature in the nosocomial environment. The intensive care unit could be the source of dissemination of these isolates, since two of the K. pneumoniae isolates and one of the E. xiangfangensis isolates were found in the ward.
We also characterized blaOXA-181-harboring plasmids for the first time in Myanmar isolates. The plasmid isolated from E. xiangfangensis M206, designated pM206-OXA181, was an IncX3-type plasmid, and its sequence was completely identical to those previously identified in a clinical E. coli isolate from China (25) and a porcine E. coli isolate from Italy (29), although epidemiological links of these isolates are unlikely.
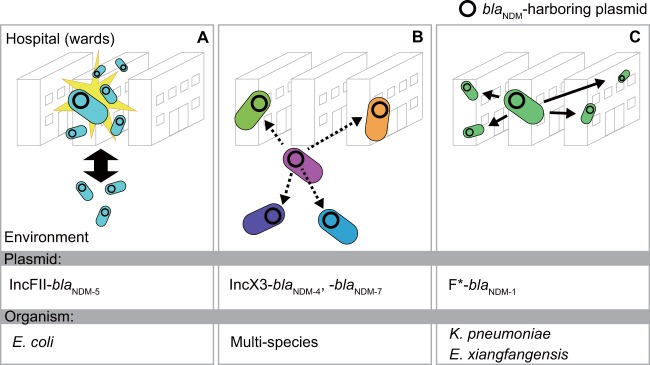
In conclusion, we have demonstrated the spread of diverse Enterobacteriaceae isolates harboring various blaNDM-harboring plasmids in a clinical setting and sewage from its adjacent area in Yangon, Myanmar, in which three patterns of dissemination of blaNDM-harboring plasmids were highlighted (Fig. 6). The IncFII- and IncX3-type plasmids are also spreading in other countries; thus, the implications of our results are not limited to Myanmar. In addition, we identified some novel plasmids, highlighting the vast pool of blaNDM-harboring plasmids in this Southeast Asian country. The presence of these various isolates in a tertiary-care hospital appears to result not only from the nosocomial transmission but also from multiple introductions into the hospital, implying their spread in the community. Further study is warranted to better understand the mechanism of spread of CPE outside clinical settings and to track their dissemination beyond Myanmar.
FIG 6.
Three different patterns of dissemination of blaNDM-harboring plasmid. (A) Clonal expansion of E. coli carrying IncFII-type plasmid harboring blaNDM was observed in the same hematology ward. Closely related isolates were also found in environmental samples. (B) Diverse clinical and environmental isolates possessed IncX3-type plasmids harboring blaNDM-4 or blaNDM-7, suggesting dissemination of the plasmids via horizontal plasmid transfer (dotted arrows). (C) Closely related K. pneumoniae or Enterobacter xiangfangensis carrying blaNDM-1-harboring plasmids were isolated in different wards in the hospital, suggesting clonal expansion among the different wards (arrows). *, the types of blaNDM-1-harboring plasmids are IncFIB(pQil) and IncFII(pRSB107) for K. pneumoniae and E. xiangfangensis, respectively.
MATERIALS AND METHODS
Bacterial isolates.
Enterobacteriaceae isolates were isolated from clinical specimens of patients at Yangon General Hospital, Yangon, Myanmar, from April 2015 to December 2016 as described previously (19). Ethical approval for the collection of patient specimens was obtained from the Ethics Committee of Osaka University Graduate School of Medicine and the Department of Medical Research, Myanmar, with a waiver of informed consent. All samples were anonymized before analysis. Environmental samples were collected at different locations within 500 m from the hospital in January 2017. Drinking water samples were collected from water storage container in individual households. Sewage samples were collected from a drainage canal collecting the flow of household effluents from nearby apartments. The drainage canal is not connected directly to the hospital drainage system. Bacteria were collected from water samples (15 ml) by centrifugation at 12,000 × g for 5 min and inoculated onto CHROMagar ECC (CHROMagar, Paris, France) supplemented with 0.25 μg/ml of meropenem and 70 μg/ml of ZnSO4 (35), to obtain carbapenem-resistant isolates. All the colonies with different morphologies and colors were stored and subjected to further analysis. Species identification was carried out using a matrix-assisted laser desorption ionization–time of flight mass spectrometry system (MALDI Sepsityper; Bruker Daltonics, Bremen, Germany) and an API 20E system (bioMérieux, Marcy l’Etoile, France). Drug susceptibility testing was performed by the broth microdiilution method using a MicroScan Walkaway Plus system with a Neg EN Combo1J panel (Beckman Coulter, Brea, CA) or an EIKEN dry plate (Eiken, Tokyo, Japan). Clinical breakpoints defined by the Clinical and Laboratory Standards Institute (M100-S22) (36) were used to interpret the results from the drug susceptibility tests. Carbapenemase genes (blaNDM, blaKPC, blaIMP, and blaOXA-48-like) in the bacterial isolates were detected using PCR-dipstick chromatography (37).
WGS and bioinformatics analysis.
Isolates were subjected to WGS using the HiSeq 3000 or MiSeq system (Illumina, San Diego, CA). Several isolates (M206, M308, M309, M321, and M324) were additionally analyzed using PacBio RSII (Pacific Biosciences, Menlo Park, CA) to obtain complete plasmid sequences, since the types of blaNDM-harboring plasmids carried by these isolates appeared novel in Myanmar. Genomic DNA was prepared using the DNeasy PowerSoil kit (Qiagen, Hilden, Germany). The genomic DNA library for Illumina sequencing was prepared using KAPA Frag (Kapa Biosystems, Woburn, MA) and TruSeq DNA Nano kit (Illumina). Sequence reads were de novo assembled using CLC Genomics Workbench 11.0.1 (CLC Bio, Aarhus, Denmark) and used for further analysis. Library preparation for PacBio RSII sequencing and de novo assembly of the obtained sequences were performed as described previously (19). Clonal relatedness of the isolates was assessed using CSI Phylogeny 1.4 (38), and the phylogenetic tree was drawn on iTOL (39). Multilocus sequence typing was conducted using MLST 1.8 (40) or PubMLST (https://pubmlst.org/). The ANI value, calculated on EZBioCloud (41), was used for identification of Enterobacter species and for assessing the degree of relatedness between isolates. Plasmid replicon typing, plasmid multilocus sequence typing, and identification of resistance genes were performed using PlasmidFinder 1.3 (42) pMLST 2.0 (42), and ResFinder 2.1 (43), respectively. Plasmids similar to those found in this study were identified by a National Center of Biotechnology Information BLAST search using whole-plasmid sequences or contigs containing blaNDM genes as queries. Assembled contigs from Illumina short reads were mapped to reference plasmids, and then the nucleotide identity and coverage were determined using BLAST on CLC Genomics Workbench. Plasmid sequences were annotated with MiGAP (https://www.migap.org/index.php/en), and the genomic structure was compared in EasyFig (44). Transposons and insertion sequences were determined using ISfinder (45).
Plasmid analysis.
The size and replicon types of blaNDM-harboring plasmids were determined by S1 nuclease pulsed-field gel electrophoresis (PFGE) followed by Southern hybridization. PFGE plugs prepared from the clinical or environmental isolates were treated with S1 nuclease (TaKaRa Bio, Shiga, Japan) and subjected to PFGE using the CHEF Mapper XA system (Bio-Rad, Hercules, CA). Separated DNA was transferred to a nylon membrane and probed with a digoxigenin-labeled DNA probe (Roche Diagnostics, Basel, Switzerland) specific to blaNDM and plasmid replicon IncFII, IncX3, IncFII(pRSB107), IncFIB(pQil), or IncL/M (42). Whole-plasmid sequences of blaOXA-181-harboring plasmids identified in isolates M513 and M518 were determined as follows. Contigs assembled from Illumina sequencing reads were mapped to the sequence of pM206_OXA181, a blaOXA-181-harboring plasmid identified in E. xiangfangensis M206. PCR primer pairs were designed to fill the intervals between mapped contigs, and the sequences of the PCR product were determined by Sanger sequencing.
Transformation and conjugation.
Transformants with blaNDM-harboring plasmids were obtained by electroporation using the E. coli strain HST08 (TaKaRa Bio) as a recipient, as previously described (19). Bacterial conjugation was performed using the transformants as donors and E. coli ML4909 (46) as a recipient. Mating was conducted on nitrocellulose membranes on a Luria-Bertani agar plate by incubation at 5, 15, 25, 35, or 45°C for 2 h. Transconjugants were selected on a brain heart infusion agar plate supplemented with 0.25 µg/ml of meropenem and 100 µg/ml of rifampin. The conjugation frequency was calculated as the number of CFU of the transconjugants/number of CFU of the donor and transconjugants.
Accession number(s).
The sequence data and details of the sequenced samples, including the date and location of collection and source of specimen, were submitted to the DDBJ/GenBank/ENA database under BioProject number PRJDB5126.
Supplementary Material
ACKNOWLEDGMENTS
We thank Norihisa Yamamoto, Yoshihiro Fujiya, and Geoffrey Kumwenda for their helpful comments and discussion. We appreciate Noriyasu Iwase and Satomi Tanaka for their technical assistance on WGS and Akiko Ueda, Yumi Sasaki, and Kazuhiro Maeda for their technical assistance on species identification and antimicrobial susceptibility testing.
This work was supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from Ministry of Education, Culture, Sport, Science and Technology in Japan and the Japan Agency for Medical Research and Development (AMED) under grant number JP18fm0108003. This work was also supported by a JSPS Grant-in-Aid for Research Activity start-up grant (number 16H06946) to Y.S.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01924-18.
REFERENCES
- 1.Temkin E, Adler A, Lerner A, Carmeli Y. 2014. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci 1323:22–42. doi: 10.1111/nyas.12537. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 3.Zurfluh K, Poirel L, Nordmann P, Klumpp J, Stephan R. 2015. First detection of Klebsiella variicola producing OXA-181 carbapenemase in fresh vegetable imported from Asia to Switzerland. Antimicrob Resist Infect Control 4:38. doi: 10.1186/s13756-015-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janecko N, Martz SL, Avery BP, Daignault D, Desruisseau A, Boyd D, Irwin RJ, Mulvey MR, Reid-Smith RJ. 2016. Carbapenem-resistant enterobacter spp. in retail seafood imported from southeast Asia to Canada. Emerg Infect Dis 22:1675–1677. doi: 10.3201/eid2209.160305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitout JD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Duin D, Doi Y. 2017. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AK, Carroll J, Scheld WM, Hazen KC, Sifri CD. 2011. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. mBio 2:e00204-11. doi: 10.1128/mBio.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidjabat HE, Silveira FP, Potoski BA, Abu-Elmagd KM, Adams-Haduch JM, Paterson DL, Doi Y. 2009. Interspecies spread of Klebsiella pneumoniae carbapenemase gene in a single patient. Clin Infect Dis 49:1736–1738. doi: 10.1086/648077. [DOI] [PubMed] [Google Scholar]
- 9.Conlan S, Thomas PJ, Deming C, Park M, Lau AF, Dekker JP, Snitkin ES, Clark TA, Luong K, Song Y, Tsai Y-C, Boitano M, Dayal J, Brooks SY, Schmidt B, Young AC, Thomas JW, Bouffard GG, Blakesley RW, Mullikin JC, NISC Comparative Sequencing Program, Korlach J, Henderson DK, Frank KM, Palmore TN, Segre JA. 2014. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci Transl Med 6:254ra126. doi: 10.1126/scitranslmed.3009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheppard AE, Stoesser N, Wilson DJ, Sebra R, Kasarskis A, Anson LW, Giess A, Pankhurst LJ, Vaughan A, Grim CJ, Cox HL, Yeh AJ, Modernising Medical Microbiology (MMM) Informatics Group, Sifri CD, Walker AS, Peto TE, Crook DW, Mathers AJ. 2016. Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob Agents Chemother 60:3767–3778. doi: 10.1128/AAC.00464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoesser N, Giess A, Batty EM, Sheppard AE, Walker AS, Wilson DJ, Didelot X, Bashir A, Sebra R, Kasarskis A, Sthapit B, Shakya M, Kelly D, Pollard AJ, Peto TEA, Crook DW, Donnelly P, Thorson S, Amatya P, Joshi S. 2014. Genome sequencing of an extended series of NDM-producing Klebsiella pneumoniae isolates from neonatal infections in a Nepali hospital characterizes the extent of community- versus hospital-associated transmission in an endemic setting. Antimicrob Agents Chemother 58:7347–7357. doi: 10.1128/AAC.03900-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoesser N, Sheppard AE, Shakya M, Sthapit B, Thorson S, Giess A, Kelly D, Pollard AJ, Peto TE, Walker AS, Crook DW. 2015. Dynamics of MDR Enterobacter cloacae outbreaks in a neonatal unit in Nepal: insights using wider sampling frames and next-generation sequencing. J Antimicrob Chemother 70:1008–1015. doi: 10.1093/jac/dku521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas LJ, Weinstock GM, De La Cadena E, Diaz L, Rios R, Hanson BM, Brown JS, Vats P, Phillips DS, Nguyen H, Hujer KM, Correa A, Adams MD, Perez F, Sodergren E, Narechania A, Planet PJ, Villegas MV, Bonomo RA, Arias CA. 2017. An analysis of the epidemic of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: convergence of two evolutionary mechanisms creates the “perfect storm.” J Infect Dis 217:82–92. doi: 10.1093/infdis/jix524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu LY, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. 2017. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in south and southeast Asia. Clin Microbiol Rev 30:1–22. doi: 10.1128/CMR.00042-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wailan AM, Paterson DL. 2014. The spread and acquisition of NDM-1: a multifactorial problem. Expert Rev Anti Infect Ther 12:91–115. doi: 10.1586/14787210.2014.856756. [DOI] [PubMed] [Google Scholar]
- 17.Tin OM, Rachel FH, Khwar NZ, Wah WH, Kyu KW, John AC, David RM, James EU. 2017. ESBL- and carbapenemase-producing Enterobacteriaceae in patients with bacteremia, Yangon, Myanmar, 2014. Emerg Infect Dis 23:857–859. doi: 10.3201/eid2305.161100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aung MS, San N, Maw WW, San T, Urushibara N, Kawaguchiya M, Sumi A, Kobayashi N. 2018. Prevalence of extended-spectrum beta-lactamase and carbapenemase genes in clinical isolates of Escherichia coli in Myanmar: dominance of blaNDM-5 and emergence of blaOXA-181. Microb Drug Resist 24:1333–1344. doi: 10.1089/mdr.2017.0387. [DOI] [PubMed] [Google Scholar]
- 19.Sugawara Y, Akeda Y, Sakamoto N, Takeuchi D, Motooka D, Nakamura S, Hagiya H, Yamamoto N, Nishi I, Yoshida H, Okada K, Zin KN, Aye MM, Tomono K, Hamada S. 2017. Genetic characterization of blaNDM-harboring plasmids in carbapenem-resistant Escherichia coli from Myanmar. PLoS One 12:e0184720. doi: 10.1371/journal.pone.0184720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlan S, Lau AF, NISC Comparative Sequencing Program, Palmore TN, Frank KM, Segre JA. 2016. Complete genome sequence of a Klebsiella pneumoniae strain carrying blaNDM-1 on a multidrug resistance plasmid. Genome Announc 4:e00664-16. doi: 10.1128/genomeA.00664-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnin RA, Nordmann P, Carattoli A, Poirel L. 2013. Comparative genomics of IncL/M-type plasmids: evolution by acquisition of resistance genes and insertion sequences. Antimicrob Agents Chemother 57:674–676. doi: 10.1128/AAC.01086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carattoli A, Seiffert SN, Schwendener S, Perreten V, Endimiani A. 2015. Differentiation of IncL and IncM plasmids associated with the spread of clinically relevant antimicrobial resistance. PLoS One 10:e0123063. doi: 10.1371/journal.pone.0123063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christie PJ. 2016. The mosaic type IV secretion systems. EcoSal Plus 7:ESP-0020-2015. doi: 10.1128/ecosalplus.ESP-0020-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Feng Y, Wu W, Xie Y, Wang X, Zhang X, Chen X, Zong Z. 2015. First report of OXA-181-producing Escherichia coli in China and characterization of the isolate using whole-genome sequencing. Antimicrob Agents Chemother 59:5022–5025. doi: 10.1128/AAC.00442-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho PL, Li Z, Lo WU, Cheung YY, Lin CH, Sham PC, Cheng VC, Ng TK, Que TL, Chow KH. 2012. Identification and characterization of a novel incompatibility group X3 plasmid carrying blaNDM-1 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. Emerg Microbes Infect 1:e39. doi: 10.1038/emi.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho PL, Cheung YY, Lo WU, Li Z, Chow KH, Lin CH, Chan JFW, Cheng VCC. 2013. Molecular characterization of an atypical IncX3 plasmid pKPC-NY79 carrying blaKPC-2 in a Klebsiella pneumoniae. Curr Microbiol 67:493–498. doi: 10.1007/s00284-013-0398-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Liu L, Zhou H, Chan EW, Li J, Fang Y, Li Y, Liao K, Chen S. 2017. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine 19:98–106. doi: 10.1016/j.ebiom.2017.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulss S, Semmler T, Prenger-Berninghoff E, Bauerfeind R, Ewers C. 2017. First report of an Escherichia coli strain from swine carrying an OXA-181 carbapenemase and the colistin resistance determinant MCR-1. Int J Antimicrob Agents 50:232–236. doi: 10.1016/j.ijantimicag.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Ho PL, Wang Y, Liu MCJ, Lai ELY, Law PYT, Cao H, Chow KH. 2018. IncX3 epidemic plasmid carrying blaNDM-5 in Escherichia coli from swine in multiple geographic areas in China. Antimicrob Agents Chemother 62:e02295-17. doi: 10.1128/AAC.02295-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle M, Fookes M, Ivens A, Mangan MW, Wain J, Dorman CJ. 2007. An H-NS-like stealth protein aids horizontal DNA transmission in bacteria. Science 315:251–252. doi: 10.1126/science.1137550. [DOI] [PubMed] [Google Scholar]
- 32.Forns N, Banos RC, Balsalobre C, Juarez A, Madrid C. 2005. Temperature-dependent conjugative transfer of R27: role of chromosome- and plasmid-encoded Hha and H-NS proteins. J Bacteriol 187:3950–3959. doi: 10.1128/JB.187.12.3950-3959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonnin RA, Poirel L, Carattoli A, Nordmann P. 2012. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS One 7:e34752. doi: 10.1371/journal.pone.0034752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiett J, Baraniak A, Izdebski R, Sitkiewicz I, Żabicka D, Meler A, Filczak K, Hryniewicz W, Gniadkowski M. 2014. The first NDM metallo-β-lactamase-producing Enterobacteriaceae isolate in Poland: evolution of IncFII-type plasmids carrying the blaNDM-1 gene. Antimicrob Agents Chemother 58:1203–1207. doi: 10.1128/AAC.01197-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto N, Kawahara R, Akeda Y, Shanmugakani RK, Yoshida H, Hagiya H, Hara N, Nishi I, Yukawa S, Asada R, Sasaki Y, Maeda K, Sakamoto N, Hamada S, Tomono K. 2017. Development of selective medium for IMP-type carbapenemase-producing Enterobacteriaceae in stool specimens. BMC Infect Dis 17:229. doi: 10.1186/s12879-017-2312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. CLSI document M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 37.Shanmugakani RK, Akeda Y, Yamamoto N, Sakamoto N, Hagiya H, Yoshida H, Takeuchi D, Sugawara Y, Kodera T, Kawase M, Laolerd W, Chaihongsa N, Santanirand P, Ishii Y, Hamada S, Tomono K. 2017. PCR-dipstick chromatography for differential detection of carbapenemase genes directly in stool specimens. Antimicrob Agents Chemother 61:e00067-17. doi: 10.1128/AAC.00067-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaas RS, Leekitcharoenphon P, Aarestrup FM, Lund O. 2014. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS One 9:e104984. doi: 10.1371/journal.pone.0104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Letunic I, Bork P. 2016. Interactive Tree Of Life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. 2017. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 42.Carattoli A, Zankari E, Garciá-Fernández A, Larsen MV, Lund O, Villa L, Aarestrup FM, Hasman H. 2014. In silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma L, Ishii Y, Ishiguro M, Matsuzawa H, Yamaguchi K. 1998. Cloning and sequencing of the gene encoding Toho-2, a class A β-lactamase preferentially inhibited by tazobactam. Antimicrob Agents Chemother 42:1181–1186. doi: 10.1128/AAC.42.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.