Fascioliasis is an infectious parasitic disease distributed globally and caused by the liver fluke Fasciola hepatica or F. gigantica. This neglected tropical disease affects both animals and humans, and it represents a latent public health problem due to the significant economic losses related to its effects on animal husbandry.
KEYWORDS: Fasciola hepatica, fasciocidal activity, in vitro screening, triclabendazole
ABSTRACT
Fascioliasis is an infectious parasitic disease distributed globally and caused by the liver fluke Fasciola hepatica or F. gigantica. This neglected tropical disease affects both animals and humans, and it represents a latent public health problem due to the significant economic losses related to its effects on animal husbandry. For decades, triclabendazole has been the unique anti-Fasciola drug that can effectively treat this disease. However, triclabendazole resistance in fascioliasis has more recently been reported around the world, and thus, the discovery of novel drugs is an urgent need. The aim of this study was to investigate the fasciocidal properties of 400 compounds contained in the Pathogen Box. The first stage of the screening was carried out by measuring the fasciocidal activity on metacercariae at a concentration of 33 μM each compound (the standard dose). Subsequently, the activities of the most active compounds (n = 33) at their 50% inhibitory concentration (IC50) values against metacercariae were assayed, and the results showed that 13 compounds had IC50s of ≤10 μM. The second stage queried the activities of these compounds at 33 μM against adult flukes, with seven of the compounds producing high mortality rates of >50%. Four hit compounds were selected on the basis of their predicted nontoxic properties, and the IC50 values obtained for adult worms were <10 μM; thus, these compounds represented the best fasciocidal compounds tested here. A cytotoxicity assay on four types of cell lines demonstrated that three compounds were nontoxic at their most active concentration. In conclusion, three hit compounds identified in this proof-of-concept study are potential candidates in the discovery of new fasciocidal drugs. Further studies are warranted.
INTRODUCTION
Fasciola hepatica is the etiological agent of fascioliasis, the most widespread trematodiasis that affects both humans and herbivorous mammals, such as sheep, cattle, goats, and other species (1, 2). In humans, fascioliasis can be acquired by the consumption of contaminated vegetables. Up to 17 million people in 51 countries are estimated to be infected with F. hepatica worldwide, and more than 91 million are at risk of infection by this parasite (3, 4). Among all the continents, the Andean region of South America is the most affected by Fasciola, where prevalence rates above 10% have been documented (5–8) and national treatment programs are being scaled up.
Triclabendazole (TCBZ) is the single most effective fasciocidal drug, with activity against both the infective larvae (metacercaria [MC]) and adult worms and efficacy that exceeds 90% in humans after a single oral dose (9, 10). Nonetheless, after decades of successful efficacy, TCBZ resistance has developed in both animals and humans (11). Cases of TCBZ-resistant Fasciola infection in both animals and humans have been reported in Australia, Europe, and Latin America (12–18). The development of TCBZ resistance represents an important public health concern throughout the world that mainly affects animal husbandry and leads to enormous economic losses (19). As a consequence, the discovery of novel drugs and vaccines effective against Fasciola is an urgent need for the global control of fascioliasis. Repurposing of praziquantel (PZQ) as an anti-Fasciola drug failed, whereas oxfebendazole was shown to be an effective drug in animals (20, 21). Currently, there is no other fasciocidal drug in clinical practice available for use in humans, and thus, TCBZ remains the unique treatment against this infectious disease.
Open-access drug discovery provides a substantial resource in research on those diseases that primarily affect people living in low-resource locations. The Medicines for Malaria Venture (MMV) foundation assembled a set of compounds, called Malaria Box, whose activities have been tested against various infectious agents, including Cryptosporidium parvum (22), Plasmodium falciparum (23, 24) Schistosoma mansoni (25, 26), Toxoplasma gondii (27), and mycobacteria (28, 29). Later, a new set of chemical entities was assembled and named the Pathogen Box collection. It contains 400 drug-like compounds that have shown inhibitory activity against various infectious diseases, such as hemonchosis, toxoplasmosis, tuberculosis, neosporosis, malaria, sleeping sickness, Chagas disease, leishmaniasis, and trypanosomiasis (30–36). The activities of the compounds in Pathogen Box against fungal diseases caused by Cryptococcus neoformans and Candida albicans have also been tested (37–39). The aim of this study was to identify the fasciocidal activity of 400 compounds contained in the Pathogen Box by in vitro testing.
RESULTS
In vitro activity of the Pathogen Box compounds against F. hepatica metacercariae.
In the first stage of the study, the 400 compounds contained in the Pathogen Box were screened in vitro for activity against F. hepatica metacercariae. A total of 33 compounds showed mean mortality rates above 25% at 33 μM, but all these compounds were less active than TCBZ (mortality rate, 90%), as shown in Table 1. The fasciocidal activity of these 33 compounds was then assessed by determining the 50% inhibitory concentration (IC50) values (Table 1). As a result, 13 compounds showed potent inhibitory activities with IC50 values of between 0.31 μM and 8.23 μM and were then assayed for their activities against adult worms, despite their low r values (Table 1).
TABLE 1.
Summary of the chemical compounds that showed the best biological activity against metacercariae
| Compound plate codea | MMV identifierb | Molecular formulac | Mol wtc (g/mol) | Mean % mortality for MCd | SD for % mortality for MCd | IC50 (μM)e | rf | Activity against other infectious microorganism(s)g |
In silico toxicity featuresh
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute cytotoxicity (fathead minnow) (mg/liter) | Blood-brain barrier penetration (human) | Carcinogenicity (rodent) | Mutagenicity (S. enterica serovar Typhimurium) | Maximum recommended daily dose (human) (mg/kg bwi /day) | |||||||||
| TCBZ | NA | C14H9Cl3N2OS | 359.7 | 100j | 0j | 15k | NA | Schistosoma | 4.57 | Penetrating | Noncarcinogenic | Nonmutagenic | NA |
| PAA2 | MMV010764 | C14H16N4OS2 | 320.4 | 22 | 38.5 | 24.1 | −0.3 | Plasmodium | NA | NA | Noncarcinogenic | Nonmutagenic | NA |
| PAF4 | MMV676388 | C15H14N4O3S | 330.4 | 29 | 24.7 | 16.9 | 0.8 | Mycobacterium | 254.0 | Penetrating | Carcinogenic | Mutagenic | 2.44 |
| PAF5 | MMV202553 | C15H15N3O2 | 269.3 | 29 | 24.7 | 14.9 | 0.9 | Kinetoplastids | 7.58 | Penetrating | Noncarcinogenic | Mutagenic | 0.993 |
| PAG6 | MMV063404 | C19H24N3OCl | 345.9 | 54 | 7.2 | 5.3 | 1.0 | Mycobacterium | NA | Penetrating | Carcinogenic | Mutagenic | NA |
| PAH6 | MMV676539 | C20H16N2O3 | 332.4 | 17 | 28.9 | 24.7 | −1.0 | Mycobacterium | 25.9 | Penetrating | Carcinogenic | Mutagenic | 4.05 |
| PBD3 | MMV637953 | C51H40N6O23S6 | 1,435.3 | 25 | 9.9 | 21.8 | −0.6 | Trypanosoma and Onchocerca | NA | Penetrating | Noncarcinogenic | Nonmutagenic | NA |
| PBD7 | MMV019838 | C18H10N4OF6 | 412.3 | 26 | 11.6 | 12.4 | 0.0 | Plasmodium | NA | Penetrating | Noncarcinogenic | Mutagenic | NA |
| PBF4 | MMV003270 | C7H5N2OCl | 168.6 | 26 | 25.1 | 8.2 | −0.7 | Ancylostoma | 6.75 | Penetrating | Noncarcinogenic | Nonmutagenic | 15.5 |
| PBF6 | MMV688853 | C19H23N5O2 | 389.9 | 25 | 22.5 | 31.9 | −0.8 | Cryptosporidium | NA | Nonpenetrating | Noncarcinogenic | Mutagenic | NA |
| PBF11 | MMV085210 | C22H24N3O3ClS | 446.0 | 40 | 15.3 | 2.4 | 0.8 | Plasmodium | NA | Penetrating | Noncarcinogenic | Nonmutagenic | 1.64 |
| PBH10 | MMV676380 | C18H15N4O3Cl | 370.8 | 33 | 33.3 | 1.3 | 0.1 | Plasmodium | 132.0 | Penetrating | Noncarcinogenic | Nonmutagenic | 101.0 |
| PCA2 | MMV675997 | C24H29N4O2F | 424.5 | 22 | 38.4 | 18.1 | −0.2 | Kinetoplastids | NA | Penetrating | Noncarcinogenic | Mutagenic | 1.51 |
| PCA6 | MMV688852 | C16H17N5ClF | 333.8 | 29 | 37.4 | 17.2 | −0.7 | Toxoplasma | NA | Penetrating | Noncarcinogenic | Mutagenic | NA |
| PCC2 | MMV688508 | C19H19N2O4F | 358.4 | 26 | 3.7 | 16.9 | −0.5 | Mycobacterium | NA | Penetrating | Noncarcinogenic | Mutagenic | NA |
| PCC5 | MMV687730 | C22H32N4O2 | 384.5 | 28 | 13.4 | 0.4 | −0.5 | Mycobacterium | NA | Penetrating | Carcinogenic | Nonmutagenic | NA |
| PCC6 | MMV687251 | C8H9N3O4S2 | 275.3 | 30 | 12.0 | 0.3 | −0.5 | Mycobacterium | NA | Penetrating | Noncarcinogenic | Nonmutagenic | 13.3 |
| PCC9 | MMV688361 | C21H19N5O | 357.4 | 32 | 11.5 | 17.2 | −0.7 | Kinetoplastids | NA | Penetrating | Carcinogenic | Mutagenic | NA |
| PCC10 | MMV689029 | C26H26N4O4S | 490.6 | 33 | 19.1 | 10.5 | 0.8 | Kinetoplastids | NA | Penetrating | Carcinogenic | Mutagenic | 11.9 |
| PCD11 | MMV1030799 | C20H18N4O | 330.4 | 28 | 11.7 | 1.5 | −0.3 | Plasmodium | 6.62 | Nonpenetrating | Carcinogenic | Mutagenic | NA |
| PCE5 | MMV687146 | C19H26N2O | 298.4 | 21 | 25.8 | 15.6 | 0.6 | Mycobacterium | NA | Penetrating | Noncarcinogenic | Mutagenic | NA |
| PCE6 | MMV687696 | C29H28N4O2ClF3 | 557.0 | 26 | 20.6 | 18.2 | −0.7 | Mycobacterium | NA | Nonpenetrating | Carcinogenic | Mutagenic | NA |
| PCE7 | MMV687170 | C17H13N4O2Cl | 340.8 | 34 | 25.3 | 13.1 | 0.0 | Mycobacterium | NA | Penetrating | Carcinogenic | Mutagenic | NA |
| PCE8 | MMV690102 | C22H23N7O2 | 417.5 | 38 | 15.6 | 2.1 | 0.7 | Kinetoplastids | NA | Penetrating | Noncarcinogenic | Mutagenic | 3.27 |
| PCE11 | MMV1029203 | C20H17N5OS | 375.5 | 33 | 29.7 | 7.1 | −0.4 | Plasmodium | 100.0 | Penetrating | Carcinogenic | Mutagenic | NA |
| PCF2 | MMV676053 | C18H16N3O3Cl | 357.8 | 38 | 12.5 | 1.9 | 0.6 | Cryptosporidium | 194.0 | Penetrating | Noncarcinogenic | Mutagenic | 0.991 |
| PCF3 | MMV688179 | C18H16N6OCl2 | 476.2 | 35 | 32.0 | 3.1 | −0.1 | Kinetoplastids | 4.62 | Penetrating | Carcinogenic | Mutagenic | 1.41 |
| PCF4 | MMV023969 | C24H24N4OS | 453.0 | 48 | 21.8 | 1.5 | 0.3 | Mycobacterium | NA | NA | Carcinogenic | Mutagenic | NA |
| PCF5 | MMV687138 | C19H17NO3S | 339.4 | 26 | 11.6 | 14.7 | −0.2 | Mycobacterium | 524.0 | Penetrating | Noncarcinogenic | Mutagenic | 89.7 |
| PCF11 | MMV688921 | C23H18N3O5Cl | 451.9 | 31 | 43.0 | 2.4 | −0.4 | Aedes aegypti- chikungunya virus | NA | Penetrating | Carcinogenic | Mutagenic | NA |
| PCG9 | MMV688891 | C18H11NO4BrF3 | 442.2 | 25 | 10.9 | 25.7 | −0.5 | Mycobacterium | NA | Penetrating | Carcinogenic | Mutagenic | 1.25 |
| PDH11 | MMV688980 | C16H18N3O2FS | 335.4 | 33 | 38.2 | 21.2 | 0.2 | Plasmodium | NA | Penetrating | Carcinogenic | Mutagenic | NA |
| PEC8 | MMV687765 | C25H26N6O | 463 | 28 | 25.5 | 20.6 | −0.8 | Mycobacterium | NA | Penetrating | Noncarcinogenic | Mutagenic | NA |
| PEG9 | MMV084864 | C17H12N6O | 316.3 | 40 | 18.7 | 17.3 | 0.8 | Plasmodium | 14.2 | Penetrating | Noncarcinogenic | Mutagenic | NA |
Coordinates used to identify the compounds in each plate. TCBZ, triclabendazole.
Identification codes assigned by the Medicines for Malaria Venture (MMV). NA, not applicable.
Molecular formulas and molecular weights were obtained from www.mmv.org. For TCBZ, these values were obtained from ChEMBL (https://www.ebi.ac.uk/chembl/).
Measured at 72 h after drug exposure for the metacercaria (MC) stage. Results are the mean and standard deviation for triplicate experiments at a concentration of 33 μM.
Compounds were serially diluted and tested in culture. Results are the means from triplicate experiments. Fasciolicidal activity values were determined with CompuSyn software.
r, correlation coefficient.
Activity shown against other agents causing infectious diseases, obtained from www.mmv.org.
Predictions were obtained using the lazar program (https://lazar.in-silico.de/predict). NA, not available.
bw, body weight.
Data represent the mean and standard deviation for 10 individual experiments performed in 5 plates.
Data were obtained from https://drugs.ncats.io.
In vitro activity of selected compounds against F. hepatica adult worms and in silico toxicology prediction.
The activities of the 13 selected compounds listed in Table 2 at 33 μM against adult worms were assayed. Seven compounds produced moderate or high mean mortality rates (>50%) (Table 2). These were MMV003270, MMV676380, MMV690102, MMV1029203, MMV063404, MMV1030799, and MMV688921. Six compounds showed low mortality rates (<50%), and for that reason, these were not considered in the next assays. Before we proceeded with the IC50 assay, the in silico safety profiles of the seven selected compounds were predicted by use of the lazar (lazy structure-activity relationship) program (Table 1). Whereas MMV003270 and MMV676380 were predicted noncarcinogenic and nontumorigenic compounds, MMV690102 was deemed noncarcinogenic and tumorigenic (Table 1). MMV1029203, MMV063404, MMV1030799, and MMV688921 were predicted to be carcinogenic and tumorigenic substances. Thus, the three compounds deemed noncarcinogenic as well as MMV1029203, a predicted carcinogenic substance that produced the highest mean mortality rate (78%), were tested in adult worms. These four compounds constituted our hit compounds.
TABLE 2.
Biological activity of the compounds screened on adult wormsa
| Compound plate codeb | MMV identifierc | Mean % mortality for adultsd | SD for % mortality for adultsd |
|---|---|---|---|
| TCBZ | NA | 100e | 0 |
| PAG6 | MMV063404 | 67 | 33.3 |
| PBF4 | MMV003270 | 67 | 0 |
| PBF11 | MMV085210 | 0 | 0 |
| PBH10 | MMV676380 | 78 | 19.2 |
| PCC5 | MMV687730 | 11 | 19.2 |
| PCC6 | MMV687251 | 33 | 33.3 |
| PCD11 | MMV1030799 | 67 | 33.3 |
| PCE8 | MMV690102 | 56 | 19.2 |
| PCE11 | MMV1029203 | 78 | 19.2 |
| PCF2 | MMV676053 | 0 | 0 |
| PCF3 | MMV688179 | 22 | 19.2 |
| PCF4 | MMV023969 | 33 | 33.3 |
| PCF11 | MMV688921 | 67 | 33.3 |
Compounds in italics were selected for evaluation of the IC50 for adult worms and for use in the cytotoxicity assay with cell lines. NA, not applicable.
Coordinates used to identify the compounds in each plate. TCBZ, triclabendazole.
Identification codes assigned by the Medicines for Malaria Venture (MMV).
Measured at 48 h after drug exposure for adult worms. Results are means and standard deviations from triplicate experiments at a concentration of 33 μM.
Mean and standard deviation from 6 individual experiments performed in 3 plates.
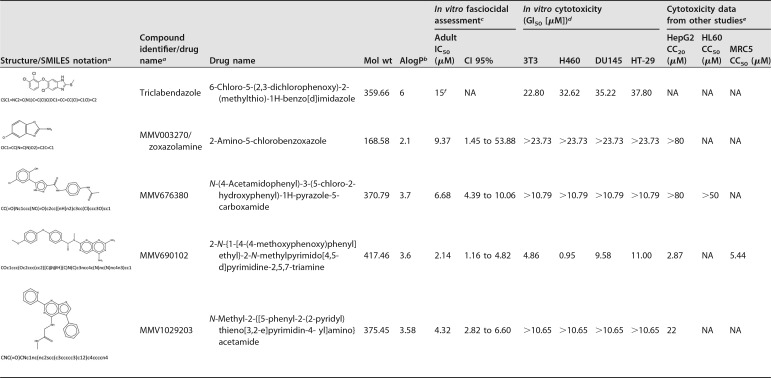
To determine which of the four hit compounds were the most potent at inhibiting the growth of F. hepatica adult worms, the IC50 values were determined. The hit compounds had IC50 values of <10 μM in adult worms (Table 3; see also Fig. S1 and Table S1 in the supplemental material). These four hit compounds were tested in the cytotoxicity study on cell cultures.
TABLE 3.
Hit compounds selected as new effective drugs for their fasciocidal activity against F. hepatica
aIdentification codes were assigned by MMV.
bA measurement of the octanol/water partition coefficient. Data were obtained from www.mmv.org.
cCompounds were serially diluted and tested in culture. The results are means from triplicate assays.
dCytotoxicity assays were performed on 3T3 cells and cells of the cancer cell lines H460, DU145, and HT-29. Results are the means from duplicate tests.
eObtained from www.mmv.org. HepG2, hepatocellular carcinoma; HL60, human promyelocytic leukemia cells; MRC5, fibroblasts derived from lung. CC20 and CC50, 20% and 50% cytotoxic concentrations, respectively; NA, not available.
fData were obtained from https://drugs.ncats.io.
In vitro cytotoxicity for cell lines.
The cytotoxicity of the four hit compounds for cell lines was evaluated in culture (Table 3). The 50% growth inhibitory concentration (GI50) values ranged from 0.95 and >23.73 μM across the four types of cell lines assayed (Table 3). MMV003270, MMV676380, MMV1029203, and TCBZ presented GI50 values above their IC50 values, meaning that these compounds are not toxic at their active concentrations. In one of the four cell lines, MMV690102 had a GI50 value below its IC50 value, thus suggesting that it may cause a level of toxicity in certain cell types when it is used at its active concentration (Table 3).
Computational recognition of targets.
As a result of a search of the ChEMBL database, a total of 27 targets were recognized for TCBZ, whereas MMV003270 was found to have 19 known targets, most of which were in humans (Table 4). MMV003270 and TCBZ have common human targets that comprise nuclear factor erythroid 2-related factor 2, microtubule-associated protein tau, and TAR DNA-binding protein 43. According to the data deposited in the ChEMBL database, MMV003270 targets a number of cytochrome P450 members of families 1, 2, and 3. MMV676380 and MMV023969 have identical cell targets that include the human glucose transporter and the hexose transporter of Plasmodium falciparum and Leishmania mexicana (Table 4). MMV1029203 and MMV676053 were also shown to have known targets, including human ferrochelatase and the IMP dehydrogenase of Cryptosporidium parvum, respectively. The remaining eight compounds had no known targets, according to the ChEMBL database (Table 4).
TABLE 4.
Potential targets of the 13 hits and TCBZ tested in adult worm assays
| Compound plate codea | MMV codeb | Target detailsc
|
||||
|---|---|---|---|---|---|---|
| No. of targets predicted | CHEMBL identifier | Preferred name | Organism | Protein target classification | ||
| PBH10 | MMV676380 | 3 | CHEMBL2535 | Glucose transporter | Homo sapiens | Transporter > electrochemical transporter > slc superfamily of solute carriers > slc02 family of hexose and sugar alcohol transporters |
| CHEMBL4697 | Hexose transporter 1 | Plasmodium falciparum | ||||
| CHEMBL3431938 | Glucose transporter | Leishmania mexicana | Transporter | |||
| PCE11 | MMV1029203 | 1 | CHEMBL3879831 | Ferrochelatase | Homo sapiens | Unclassified protein |
| PCF2 | MMV676053 | 1 | CHEMBL6145 | IMP dehydrogenase, probable | Cryptosporidium parvum | Enzyme |
| PCF4 | MMV023969 | 3 | CHEMBL2535 | Glucose transporter | Homo sapiens | Transporter > electrochemical transporter > slc superfamily of solute carriers > slc02 family of hexose and sugar alcohol transporters |
| CHEMBL4697 | Hexose transporter 1 | Plasmodium falciparum | ||||
| CHEMBL3431938 | Glucose transporter | Leishmania mexicana | Unclassified protein | |||
| PBF4 | MMV003270 | 19 | CHEMBL340 | Cytochrome P450 3A4 | Homo sapiens | Enzyme > cytochrome P450 > cytochrome P450 family 3 > cytochrome P450 family 3A > cytochrome P450 3A4 |
| CHEMBL289 | Cytochrome P450 2D6 | Homo sapiens | Enzyme > cytochrome P450 > cytochrome P450 family 2 > cytochrome P450 family 2D > cytochrome P450 2D6 | |||
| CHEMBL3397 | Cytochrome P450 2C9 | Homo sapiens | Enzyme > cytochrome P450 > cytochrome P450 family 2 > cytochrome P450 family 2C > cytochrome P450 2C9 | |||
| CHEMBL3622 | Cytochrome P450 2C19 | Homo sapiens | Enzyme > cytochrome P450 > cytochrome P450 family 2 > cytochrome P450 family 2C > cytochrome P450 2C19 | |||
| CHEMBL3356 | Cytochrome P450 1A2 | Homo sapiens | Enzyme > cytochrome P450 > cytochrome P450 family 1 > cytochrome P450 family 1A > cytochrome P450 1A2 | |||
| CHEMBL4040 | MAP kinase ERK2 | Homo sapiens | Enzyme > kinase > protein kinase > CMGC protein kinase group > CMGC protein kinase MAPK family > CMGC protein kinase ERK subfamily | |||
| CHEMBL2903 | Arachidonate 15-lipoxygenase | Homo sapiens | Enzyme | |||
| CHEMBL2756 | Monoamine oxidase B | Bos taurus | Enzyme | |||
| CHEMBL3254 | Monoamine oxidase A | Bos taurus | Enzyme | |||
| CHEMBL1075094 | Nuclear factor erythroid 2-related factor 2 | Homo sapiens | Unclassified protein | |||
| CHEMBL1293224 | Microtubule-associated protein tau | Homo sapiens | Unclassified protein | |||
| CHEMBL2362981 | TAR DNA-binding protein 43 | Homo sapiens | Unclassified protein | |||
| CHEMBL1293235 | Prelamin-A/C | Homo sapiens | Unclassified protein | |||
| CHEMBL1781865 | 78-kDa glucose-regulated protein | Homo sapiens | Unclassified protein | |||
| CHEMBL1977 | Vitamin D receptor | Homo sapiens | Transcription factor > nuclear receptor > nuclear hormone receptor subfamily 1 > nuclear hormone receptor subfamily 1 group I > nuclear hormone receptor subfamily 1 group I member 1 | |||
| CHEMBL1947 | Thyroid hormone receptor beta-1 | Homo sapiens | Transcription factor > nuclear receptor > nuclear hormone receptor subfamily 1 > nuclear hormone receptor subfamily 1 group A > nuclear hormone receptor subfamily 1 group A member 2 | |||
| CHEMBL1697668 | Solute carrier organic anion transporter family member 1B1 | Homo sapiens | Transporter > electrochemical transporter > slc superfamily of solute carriers > slc21/slco family of organic anion transporting polypeptides | |||
| CHEMBL1743121 | Solute carrier organic anion transporter family member 1B3 | Homo sapiens | ||||
| CHEMBL1741193 | Chromobox protein homolog 1 | Homo sapiens | Epigenetic regulator > reader > methyl-lysine/arginine binding protein > chromodomain | |||
| TCBZ | NA | 27 | CHEMBL1293278 | Geminin | Homo sapiens | Unclassified protein |
| CHEMBL1075094 | Nuclear factor erythroid 2-related factor 2 | Homo sapiens | Unclassified protein | |||
| CHEMBL1293224 | Microtubule-associated protein tau | Homo sapiens | Unclassified protein | |||
| CHEMBL1293258 | Mothers against decapentaplegic homolog 3 | Homo sapiens | Unclassified protein | |||
| CHEMBL2362981 | TAR DNA-binding protein 43 | Homo sapiens | Unclassified protein | |||
| CHEMBL2146310 | Aberrant vpr protein | Human immunodeficiency virus type 1 | Unclassified protein | |||
| CHEMBL2029198 | Rap guanine nucleotide exchange factor 4 | Homo sapiens | Unclassified protein | |||
| CHEMBL6152 | Alpha-synuclein | Homo sapiens | Unclassified protein | |||
| CHEMBL1293191 | Transcriptional regulator ERG | Homo sapiens | Unclassified protein | |||
| CHEMBL2007624 | Peripheral myelin protein 22 | Rattus norvegicus | Unclassified protein | |||
| CHEMBL1795086 | HSP90 | Plasmodium falciparum 3D7 | Unclassified protein | |||
| CHEMBL5567 | Luciferin 4-monooxygenase | Photinus pyralis | Enzyme | |||
| CHEMBL2007625 | Isocitrate dehydrogenase (NADP), cytoplasmic | Homo sapiens | Enzyme | |||
| CHEMBL3563 | Cruzipain | Trypanosoma cruzi | Enzyme > protease > cysteine protease > cysteine protease CA clan > cysteine protease C1A family | |||
| CHEMBL1293248 | 4′-Phosphopantetheinyl transferase FFP | Bacillus subtilis | Enzyme | |||
| CHEMBL1795087 | Ubiquitin carboxyl-terminal hydrolase 1 | Homo sapiens | Enzyme | |||
| CHEMBL1293234 | Putative fructose-1,6-bisphosphate aldolase | Giardia intestinalis | Enzyme | |||
| CHEMBL1293228 | Streptokinase A | Streptococcus pyogenes serotype M1 | Enzyme > kinase | |||
| CHEMBL2524 | Alpha-galactosidase A | Homo sapiens | Enzyme | |||
| CHEMBL1784 | Glucagon-like peptide 1 receptor | Homo sapiens | Membrane receptor > family B G protein-coupled receptor > peptide receptor (family B GPCR) > glucagon-like receptor > glucagon-like peptide receptor | |||
| CHEMBL1793 | Parathyroid hormone receptor | Homo sapiens | Membrane receptor > family B G protein-coupled receptor > peptide receptor (family B GPCR) > parathyroid hormone receptor > parathyroid hormone receptor | |||
| CHEMBL5162 | Neuropeptide S receptor | Homo sapiens | Membrane receptor > family A G protein-coupled receptor > peptide receptor (family A GPCR) > short peptide receptor (family A GPCR) > neuropeptide receptor | |||
| CHEMBL1293231 | Nuclear receptor ROR-gamma | Mus musculus | Transcription factor > nuclear receptor > nuclear hormone receptor subfamily 1 > nuclear hormone receptor subfamily 1 group F > nuclear hormone receptor subfamily 1 group F member 3 | |||
| CHEMBL1871 | Androgen receptor | Homo sapiens | Transcription factor > nuclear receptor > nuclear hormone receptor subfamily 3 > nuclear hormone receptor subfamily 3 group C > nuclear hormone receptor subfamily 3 group C member 4 | |||
| CHEMBL3880 | Heat shock protein HSP90-alpha | Homo sapiens | Other cytosolic protein | |||
| CHEMBL6032 | Histone-lysine N-methyltransferase, H3 lysine-9 specific 3 | Homo sapiens | Epigenetic regulator > writer > protein methyltransferase | |||
| CHEMBL4377 | Guanine nucleotide-binding protein G(s), subunit alpha | Homo sapiens | Other membrane protein | |||
Coordinates used to identify the compounds in each plate. For the following hits, no target was identified: MMV063404, MMV687730, MMV687251, MMV1030799, MMV690102, MMV085210, MMV688179, and MMV688921.
Identification codes assigned by MMV except for TCBZ.
Obtained by consulting ChEMBL. Abbreviations: MAP, mitogen-activated protein; MAPK, mitogen-activated protein kinase; ERK2, extracellular signal-regulated kinase 2; ERK extracellular signal-regulated kinase; slc, solute carrier; CMGC, cyclin-dependent kinase; MAPK, mitogen-activated protein kinase; GSK, glycogen synthase kinase; CLK, and CDC-like kinase; GPCR, G protein-coupled receptors; NA, not available.
DISCUSSION
In the present study, the Pathogen Box was queried to identify compounds with in vitro activity against both metacercariae and adult worms of Fasciola (Fig. 1). We found 13 compounds with potent inhibitory activity against metacercariae (IC50 < 10 μM), meaning that 3% of the substances within the Pathogen Box are effective against the infective form of F. hepatica. Two out of the 13 compounds (MMV687730 and MMV687251) had the most potent activity against metacercariae, with IC50 values being below 1 μM, but showed mild effects on adult worms (Tables 1 and 2). Since we were interested in identifying hit compounds that were active against the larval and adult stages, these two compounds were not further studied (Table 2). When assayed on adult worms, seven promising compounds showed mortality rates above 50% (Table 2). As a criterion for hit prioritization during the screening on adult worms, we prepared a list of hit compounds that mostly excluded the predicted carcinogenic/tumorigenic compounds (Table 3). Thus, three (MMV676380, MMV003270, and MMV690102) of the seven most promising candidates were included in the list of hit compounds since they were predicted noncarcinogenic agents (Table 1). One additional compound (MMV1029203) that was predicted to be a carcinogenic compound was also included due to its very strong effect on adult worms. According to our results, the four hit compounds were potent molecules that inhibited both the MC and adult stages (Table 3). The cytotoxicity assay revealed that three hit compounds (MMV676380, MMV003270, and MMV1029203) were nontoxic agents when assayed at their most active concentrations on cell lines (Table 3). In contrast, MMV690102 may cause cell cytotoxicity at its most active concentration, meaning that it is not a primary candidate for drug development (Table 3). Our results are consistent with those of previous cytotoxicity assays on HepG2, HL60, and MRC5 cells, as shown in Table 3 (data provided by the MMV as part of the supporting information for the open-access Malaria Box).
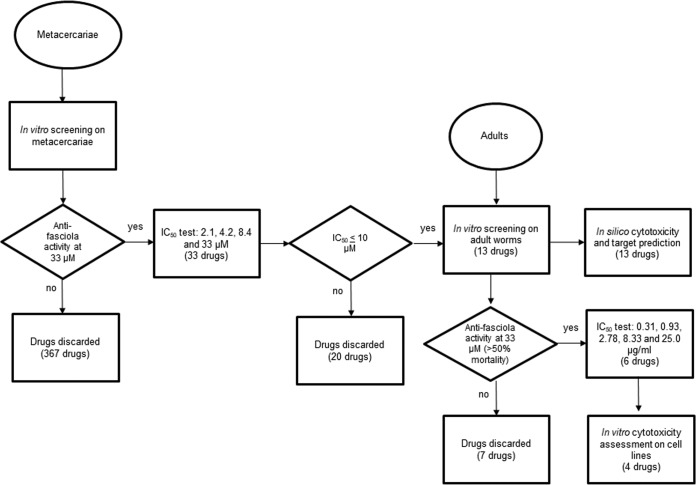
FIG 1.
Flow chart of the study.
Repurposing of hits with activity against F. hepatica obtained using analysis of the compounds in the Pathogen Box is highly relevant since TCBZ is the only existing drug effective against Fasciola, but resistance to this agent is known (40–42). Previous works tried to repurpose albendazole, nitroxinil, and closantel as candidate fasciocidal drugs, but treatment failed (43, 44). In the present study, 4 out of 400 compounds contained in the Pathogen Box showed potent inhibitory activity against the infective form of F. hepatica as well as against its adult form (Table 3). Such a finding represents a relevant contribution to the identification of dual drug candidates that are able to act against the initial stages of the infective larvae (metacercaria) and adult forms of liver flukes, similar to TCBZ. Additionally, 13 other compounds showed biological activity at <20 μM against metacercaria (Table 1). Since MC represents the initial infective form of the parasites, it should be primarily controlled through potent compounds such as those identified here (Table 1). Future exploration of the activities of the compounds in the Pathogen Box against newly juvenile metacercaria is desirable, given that some compounds may not have penetrated the cyst wall of the larvae. By testing the activities of the compounds on juvenile worms, some additional molecules that are active against adult worms might be recognized.
The four hit compounds identified in this study have previously been characterized to have activity against Plasmodium falciparum, Ancylostoma ceylanicum, Trypanosoma cruzi, and Leishmania donovani (data provided by the MMV as part of the supporting information for the open-access Malaria Box). Therefore, a common mechanism of action or target among the hit compounds across such pathogens is plausible. For instance, MMV676380 has previously been shown to have a lethal effect on P. falciparum and here was found to be an inhibitory compound with potent activity against F. hepatica (36, 45). Known targets of MMV676380 are the glucose and hexose transporters, suggesting that such a mechanism may be affected in both parasites in the presence of such a compound (Table 4). On the other hand, MMV003270 (zoxazolamine), which is also active against A. ceylanicum, was found to have 19 targets, including 3 human proteins that are also targeted by TCBZ (Table 4). Two of these proteins are transcription regulators (nuclear factor erythroid 2-related factor 2 and TAR DNA-binding protein 43) whose disruption may affect gene expression. Such a finding is in accordance with a hypothetical mechanism of action of TCBZ that involves a direct effect of the drug on protein synthesis (11, 46). Similarly, the microtubule-associated protein tau is a known target both of TCBZ and of MMV003270. TCBZ is a benzimidazole derivative that disrupts the assembly of microtubules in helminths by binding to tubulin molecules (47). Our results suggest that MMV003270 also affects the microtubule formation mechanism. Common targets of TCBZ and MMV003270 may be partially explained by the similar scaffold structures. MMV1029203, one of the four hit compounds, targets a human ferrochelatase that is a mitochondrial factor involved in protoheme biosynthesis. The latter is a vital process that also exists in F. hepatica and whose disruption may be lethal. Some known targets of the hit compounds identified here correspond to human proteins, which suggests that a level of toxicity may exist in humans. However, according to our results with cell lines, the compound concentrations needed to kill F. hepatica (IC50) were considerably less than those needed to cause cell death (GI50), which means that all these compounds except MMV690102 are nontoxic (Table 3). Although no F. hepatica target was recognized for our hit compounds, the demonstration of the inhibitory activity of such chemical agents against both the metacercaria and adult forms suggests that common targets may exist in both liver fluke stages. The identification of drug targets becomes an important step that drives the discovery of novel antiparasitic agents administered in various ways (34). For that reason, further studies to identify the potential F. hepatica targets of hit compounds are desirable. Such studies should consider the recognition of human homologs in F. hepatica, according to our results (Table 4).
Our study has some limitations. First, TCBZ metabolites (TCBZ sulfoxide and TCBZ sulfone) that are quickly released in vivo were not included in this pilot study. However, given that TCBZ has moderate in vivo and in vitro fasciocidal effects, it is suitable for use as a positive control in bioassays (48, 49). A second limitation is that live F. hepatica worms were collected from a local abattoir, where some animals may have been infected by various other pathogens or may have been treated with TCBZ. To guarantee the best quality of adult worms for bioassays, we performed a quality control on adult fasciolas before using these in the experiments. Thus, only worms that presented an intense brown or red color and that had active motility were selected. All the remaining worms were discarded. A third limitation is the low number of parasites used for the assays, which did not allow formal statistical comparisons of the activities between TCBZ and the test drugs to be performed. Obtaining MC and adults was a challenging task since both MC and adult worms were collected from natural reservoirs. Therefore, we had limited access to parasites for bioassays. However, our exploratory study aimed to identify fasciocidal compounds, and we found that the use of negative controls was enough for such purposes.
In conclusion, we identified three promising noncytotoxic drug-like compounds, MMV003270, MMV676380, and MMV1029203, that showed potent biological activity against F. hepatica metacercariae and adult worms. Such compounds represent new lead candidates to potentially become future anti-F. hepatica drugs. By acting both on the infective form and on adult worms, such agents may provide an appropriate treatment against fascioliasis.
MATERIALS AND METHODS
Study design. The study was conducted in three stages: (i) bioassays on metacercariae, (ii) bioassays on adult worms, and (iii) assays on cytotoxicity for cells (Fig. 1). The best fasciocidal compounds, based on in vitro biological activity, were selected at each stage to be tested in the next phase. To complement our knowledge on the active compounds obtained by the experimental assays, computational resources were consulted to describe the chemical properties as well as the in silico toxicology features and biological targets of these active compounds.
Drugs and media.
The Pathogen Box compounds were provided by MMV (Geneva, Switzerland) and manufactured by Evotec (USA). The 400 drug-like molecules were supplied in 96-well plates as stock solutions of 10 mM dissolved in dimethyl sulfoxide (DMSO). Full data on the Pathogen Box compounds is available at https://www.pathogenbox.org (50). TCBZ was purchased from Sigma-Aldrich (Buchs, Switzerland). All of the compounds in the Pathogen Box were dissolved in DMSO (Sigma-Aldrich, Irvine, UK) to make drug stock solutions of 200 μM. Additional vials of MMV063404, MMV003270, MMV085210, MMV676380, MMV687730, MMV687251, MMV1030799, MMV690102, MMV1029203, MMV676053, MMV688179, MMV023969, and MMV688921 were manufactured by Evotec (France). RPMI 1640 culture medium (Sigma-Aldrich, St. Louis, MO, USA) was used for both stages, metacercariae and adult worms, and was supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml) (Sigma-Aldrich, St. Louis, MO, USA).
Parasites.
Metacercariae of F. hepatica were obtained, following the protocol described by Ortiz et al. (16), at the Immunology and Research Laboratory of the Faculty of Veterinary Sciences of the Universidad Nacional de Cajamarca in Peru. Eggs of F. hepatica were collected directly from the gallbladder of sheep slaughtered in a popular abattoir in the city of Cajamarca, Peru (an area of endemicity for fascioliasis where TCBZ resistance has been seen). Miracidia were collected from Fasciola eggs that had been incubated for 15 days at 25°C. Afterwards, they were used to infect Lymnaea sp. snails (5 to 6 mm) in a proportion of two miracidia per snail. The infected snails were kept in plastic containers for 45 to 60 days at room temperature. After this time, the snails were stimulated by direct solar exposure and with water at 4 to 8°C to produce metacercaria. Approximately 20,000 metacercariae were obtained for this study and stored in distilled water in cryovials at 4 to 8°C. Adult worms were collected from the bile ducts of infected cattle from a slaughterhouse in Lima, Peru, and maintained at 37°C until usage (within 2h). Before incubation, three washes with phosphate-buffered saline (PBS; HiMedia, India) and one additional wash with supplemented RPMI 1640 medium were performed to remove host debris. All the incubations for both metacercariae and adults were carried out at 37°C with 5% CO2.
In vitro screening of activity against metacercariae.
The activities of the 400 compounds against F. hepatica metacercariae were initially tested at 33 μM. Drug stock solutions were diluted in 96-well plates (BD Falcon, USA) with RPMI 1640 medium supplemented with antibiotic up to a final volume of 180 μl. In all in vitro assays, positive and negative controls were run in parallel for each assay batch. A range of 7 to 10 metacercariae that had previously been analyzed microscopically to confirm their viability (microscopic features intact) were added to each well. Some physical properties of the parasite, determined by microscopy, as described previously (51, 52), were considered to determine the viability of the metacercariae. MC viability was surveyed as a function of both damage to the membrane and fluke color (translucence). Therefore, low viability corresponded to heavy damage and high translucence. The viability scale was scored as follows: +++, total damage (dead parasite, shattered membrane, and mostly translucent); ++, partial damage (partial membrane damage and highly translucent); +, mild damage (partial membrane damage, poorly translucent); and no damage (intact membrane, dark metacercariae, a lack of translucence).
Positive-control wells contained TCBZ at 10 μM, whereas F. hepatica metacercariae incubated in the presence of the highest concentration of DMSO tested served as negative controls. Each test was performed in triplicate. Culture plates were incubated at 37°C in a humidified 5% CO2 atmosphere for 72 h. First, metacercariae were evaluated by inverted microscopy (PhotoZoom microscope; Cambridge Instruments) at magnifications of ×10 and ×20 at 24, 48, and 72 h after drug exposure to determine their viability. Only the compounds that caused, on average, at least 25% metacercaria mortality at 72 h were considered for 50% inhibitory concentration (IC50) determination. Experiments were run in sets of triplicates. The mean percent mortality caused by the study compounds was compared to that caused by DMSO. A standard deviation (SD) was also estimated.
In the second part, we determined the IC50s of the selected compounds chosen in the previous bioassay. Drugs were tested at concentrations of 2.1, 4.2, 8.4, and 33 μM using supplemented culture medium. The incubation was done under the conditions described above, in triplicate and by considering TCBZ and DMSO as controls. Antiparasite activity was evaluated at 24, 48, and 72 h postexposure, using the above-mentioned metacercaria viability scale. Viability (the mean percentage of viable parasites) at 72 h was considered for the estimation of the IC50. The IC50 values of the test compounds were determined by linear regression analysis using CompuSyn software (version 3.0.1, 2007; ComboSyn Inc., USA). The linear correlation coefficient (r) was obtained.
Assessment of in vitro activity against adult Fasciola worms.
Those compounds that showed activity with an IC50 of ≤10 μM for metacercariae were subsequently tested for their activity against the adult stage of F. hepatica. In all in vitro assays, positive and negative controls were run in parallel for each assay batch. First, the selected compounds were tested at 33 μM in triplicate, using drug stock solutions diluted in supplemented RPMI 1640 medium on a 6-well plate to a final volume of 4 ml. Adult worms were thoroughly washed with PBS to remove host debris, and then three worms were placed in each well. The incubation was done under the same conditions applied in bioassays with metacercariae. The positive control consisted of 50 μM TCBZ, and the negative control was DMSO at the highest concentration tested. The viability of the adult flukes was scored after 24 and 48 h using a motility criterion described previously (48) and also the color and rigidity criteria previously applied by our team (data not published). Motility was assessed only in adults and not in MC because the latter has no movements. Rigidity was a parameter used to confirm the damage caused by the drug once the incubation time finished. In general, a low motility level corresponded to transparent and rigid worms. Those changes were attributed to the damage caused by a drug. The viability scale was determined as follows: (i) for worm motility, a score of 3 was assigned for normal movements, a score of 2 was assigned for reduced movements, a score of 1 was assigned for very weak movements, and a score of 0 was assigned for the absence of movements (i.e., death of worm); (ii) for worm color, +++ was assigned for dark red, ++ was assigned for pink, + was assigned for slightly transparent, and − was assigned for totally transparent; and (iii) for worm rigidity, − was assigned for no rigidity, + was assigned for rigidity, and ++ was assigned for cell breakage when the cell was touched. Assessments were not done at 72 h after drug exposure because the death of the worms always occurred at ≤48 h. Experiments were run in triplicate. The mean percent mortality and SD of the study compounds were estimated. The selected compounds were those that caused an average mortality of >50% in adult parasites. Then, IC50 assays were conducted by testing the selected compounds at five different concentrations of 0.31, 0.93, 2.78, 8.33, and 25.0 μg/ml. DMSO and TCBZ were used as negative and positive controls, respectively. Parasite viability at 24 h was estimated on the basis of survival in DMSO. The IC50s and 95% confidence intervals (CI) were estimated, using GraphPad Prism (version 7.0) software, using the variable slope of the sigmoidal curve from the normalized percent activity values and log10-transformed concentrations. The top and bottom values were constrained to 100 and 0, respectively. The fasciocidal activity was determined by considering the adult viability scale described above.
Computational analysis.
(i) Evaluation of biological targets of small compounds. To learn about biological targets, those compounds that showed promising anti-Fasciola activity in the adult stage as well as TCBZ were entered into the ChEMBL database (https://www.ebi.ac.uk/chembl/) (53). First, the SMILES (simplified molecular-input line-entry system) notation of each of the selected compounds was obtained from the supplemental material provided by MMV (also available at www.mmv.org). Then, the SMILES notations were entered into ChEMBL, and known targets of each compound were retrieved. ChEMBL compares the query compound to a large database of compounds and their targets available from multiple sources, including the projects funded by MMV (54). The target name, organism, and protein target classification were collected.
(ii) In silico cytotoxicity prediction.
lazar (lazy structure-activity relationships), a modular framework for predictive toxicology, was consulted to predict the toxic effects of the selected compounds that showed activity on the metacercariae (55–57). lazar was accessed through https://lazar.in-silico.de/predict, and the SMILES notation of each compound was entered. Relevant data, including carcinogenicity in rodents, mutagenicity in Salmonella enterica serovar Typhi, acute toxicity for the fathead minnow, blood-brain barrier penetration, and the maximum recommended daily dose in humans, were predicted.
Cell growth inhibition bioassay.
The cytotoxicity of the compounds was evaluated in tumor and nontumor cell lines using the sulforhodamine B (SRB) assay method (58, 59). The cell lines tested included BALB/3T3 (nontumorigenic, BALB/c mouse embryo cells), H460 (human lung large cell carcinoma), DU145 (human prostate carcinoma), and HT-29 (human colon adenocarcinoma).
To determine the cytotoxicity of the compounds, cells were plated into 96-well tissue culture plates and in their corresponding growth medium, Dulbecco’s modified Eagle medium (DMEM), at approximately 10% confluence (BALB/3T3 cells at 3,500 cells/well, H460 cells at 1,500 cells/well, DU145 cells at 3,500 cells/well, and HT-29 cells at 3,000 cells/well) and incubated at 37°C in a 5% CO2 and 95% air humidified atmosphere for 24 h to allow the cells to attach. A plate containing each of these cells was fixed in situ with trichloroacetic acid (TCA) in order to obtain the cell values at zero time before adding the compounds. The rest of the plates containing the different cell lines received serial dilutions of the compound to be tested at the following final concentrations: 4, 1, 0.25, and 0.0625 μg/ml. The plates were then incubated at 37°C in a 5% CO2 and 95% air humidified atmosphere for 48 h. The assay was terminated by the addition of cold TCA. TCA-treated plates were incubated at 4°C for 1 h and then washed five times with tap water to remove TCA and air dried. Background optical densities were measured in wells incubated with growth medium without cells. TCA-fixed cells were stained for 20 min with 0.4% (wt/vol) SRB dissolved in 1% acetic acid. At the end of the staining period, unbound dye was removed by washing four times with 1% acetic acid. After air drying the plates, bound dye was solubilized with 10 mM Tris base (pH 10.5) and the absorbance was read on an automated plate reader at a wavelength of 550 nm. The GI50 value was defined as the concentration of test sample resulting in a 50% reduction of the absorbance compared with that for the untreated controls that received a serial dilution of the solvent in which the test samples were dissolved and was determined by linear regression analysis. The optical density values obtained were used to determine the cell growth and cytotoxicity from each compound.
Ethics.
This study was approved by the Animal Ethics Committee of the Universidad Peruana Cayetano Heredia (approval identification code 41-07-16).
Supplementary Material
ACKNOWLEDGMENTS
We thank the Medicines Malaria Ventures foundation for having funded this study and providing the Pathogen Box.
We acknowledge Jennifer Keiser for her invaluable advice regarding the preparation of bioassays.
We state that we have no conflict of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02373-18.
REFERENCES
- 1.Dalton JP. 1999. Fasciolosis. CAB International, Wallingford, United Kingdom. [Google Scholar]
- 2.Marcos LA, Yi P, Machicado A, Andrade R, Samalvides F, Sánchez J, Terashima A. 2007. Hepatic fibrosis and Fasciola hepatica infection in cattle. J Helminthol 81:381–386. doi: 10.1017/S0022149X07850231. [DOI] [PubMed] [Google Scholar]
- 3.Keiser J, Utzinger J. 2009. Food-borne trematodiases. Clin Microbiol Rev 22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fürst T, Keiser J, Utzinger J. 2012. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis 12:210–221. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- 5.Mas-Coma S, Bargues MD, Valero MA. 2005. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol 35:1255–1278. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization 2007. Fact sheet on fascioliasis. Action against worms. p 1–8. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Espinoza J, Maco V, Marcos L, Saez S, Neyra V, Terashima A, Samalvides F, Gotuzzo E, Chavarry E, Huaman MC, Bargues MD, Valero A, Mas-Coma S. 2007. Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am J Trop Med Hyg 76:977–982. doi: 10.4269/ajtmh.2007.76.977. [DOI] [PubMed] [Google Scholar]
- 8.Carmona C, Tort JF. 2017. Fasciolosis in South America: epidemiology and control challenges. J Helminthol 91:99–109. doi: 10.1017/S0022149X16000560. [DOI] [PubMed] [Google Scholar]
- 9.Bennett J, Köhler P. 1987. Fasciola hepatica: action in vitro of triclabendazole on immature and adult stage. Exp Parasitol 63:49–57. doi: 10.1016/0014-4894(87)90077-4. [DOI] [PubMed] [Google Scholar]
- 10.Apt W, Aguilera X, Vega F, Miranda C, Zulantay I, Perez C, Gabor M, Apt P. 1995. Treatment of human chronic fascioliasis with triclabendazole: drug efficacy and serologic response. Am J Trop Med Hyg 52:532–535. doi: 10.4269/ajtmh.1995.52.532. [DOI] [PubMed] [Google Scholar]
- 11.Fairweather I. 2005. Triclabendazole: new skills to unravel an old(ish) enigma. J Helminthol 79:227–234. doi: 10.1079/JOH2005298. [DOI] [PubMed] [Google Scholar]
- 12.Overend DJ, Bowen FL. 1995. Resistance of Fasciola hepatica to triclabendazole. Aust Vet J 72:275–276. doi: 10.1111/j.1751-0813.1995.tb03546.x. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell GB, Maris L, Bonniwel MA. 1998. Triclabendazole-resistant liver fluke in Scottish sheep. Vet Rec 143:399. [PubMed] [Google Scholar]
- 14.Moll L, Gaasenbeek CP, Vellema P, Borgsteede FH. 2000. Resistance of Fasciola hepatica against triclabendazole in cattle and sheep in the Netherlands. Vet Parasitol 91:153–158. doi: 10.1016/S0304-4017(00)00267-3. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira DR, Ferreira DM, Stival CC, Romero F, Cavagnolli F, Kloss A, Araújo FB, Molento MB. 2008. Triclabendazole resistance involving Fasciola hepatica in sheep and goats during an outbreak in Almirante Tamandare, Paraná, Brazil. Rev Bras Parasitol Vet 17(Suppl 1):149–153. [PubMed] [Google Scholar]
- 16.Ortiz P, Scarcella S, Cerna C, Rosales C, Cabrera M, Guzman M, Lamenza P, Solana H. 2013. Resistance of Fasciola hepatica against triclabendazole in cattle in Cajamarca (Peru): a clinical trial and an in vivo efficacy test in sheep. Vet Parasitol 195:118–121. doi: 10.1016/j.vetpar.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Winkelhagen AJ, Mank T, de Vries PJ, Soetekouw R. 2012. Apparent triclabendazole-resistant human Fasciola hepatica infection, the Netherlands. Emerg Infect Dis 18:1028–1029. doi: 10.3201/eid1806.120302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabada MM, Lopez M, Cruz M, Delgado JR, Hill V, White AC Jr. 2016. Treatment failure after multiple courses of triclabendazole among patients with fascioliasis in Cusco, Peru: a case series. PLoS Negl Trop Dis 10:e0004361. doi: 10.1371/journal.pntd.0004361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekele M, Tesfay H, Getachew Y. 2010. Bovine fasciolosis: prevalence and its economic loss due to liver condemnation at Adwa municipal abattoir. Ejast 1:39–47. [Google Scholar]
- 20.Patrick DM, Isaac-Renton J. 1992. Praziquantel failure in the treatment of Fasciola hepatica. Can J Infect Dis 3:33–36. doi: 10.1155/1992/864093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Urbina MT, Garcia HH, Gonzalez AE, Gomez-Puerta LA, Gavidia C. 2012. Efficacy of a single oral dose of oxfendazole against Fasciola hepatica in naturally infected sheep. Am J Trop Med Hyg 86:486–488. doi: 10.4269/ajtmh.2012.11-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bessoff K, Spangenberg T, Foderaro JE, Jumani RS, Ward GE, Huston CD. 2014. Identification of Cryptosporidium parvum active chemical series by repurposing the open access Malaria Box. Antimicrob Agents Chemother 58:2731–2739. doi: 10.1128/AAC.02641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paiardini A, Bamert RS, Kannan-Sivaraman K, Drinkwater N, Mistry SN, Scammells PJ, McGowan S. 2015. Screening the Medicines for Malaria Venture “Malaria Box” against the Plasmodium falciparum aminopeptidases, M1, M17 and M18. PLoS One 10:e0115859. doi: 10.1371/journal.pone.0115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman JD, Merino EF, Brooks CF, Striepen B, Carlier PR, Cassera MB. 2014. Antiapicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the Malaria Box. Antimicrob Agents Chemother 58:811–819. doi: 10.1128/AAC.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasche V, Laleu B, Keiser J. 2018. Screening a repurposing library, the Medicines for Malaria Venture Stasis Box, against Schistosoma mansoni. Parasit Vectors 11:298. doi: 10.1186/s13071-018-2855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingram-Sieber K, Cowan N, Panic G, Vargas M, Mansour NR, Bickle QD, Wells TN, Spangenberg T, Keiser J. 2014. Orally active antischistosomal early leads identified from the open access Malaria Box. PLoS Negl Trop Dis 8:e2610. doi: 10.1371/journal.pntd.0002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyom FF, Fokou PV, Tchokouaha LR, Spangenberg T, Mfopa AN, Kouipou RM, Mbouna CJ, Donfack VF, Zollo PH. 2014. Repurposing the open access Malaria Box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob Agents Chemother 58:5848–5854. doi: 10.1128/AAC.02541-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Voorhis WC, Adams JH, Adelfio R, Ahyong V, Akabas MH, Alano P, Alday A, Alemán Resto Y, Alsibaee A, Alzualde A, Andrews KT, Avery SV, Avery VM, Ayong L, Baker M, Baker S, Ben Mamoun C, Bhatia S, Bickle Q, Bounaadja L, Bowling T, Bosch J, Boucher LE, Boyom FF, Brea J, Brennan M, Burton A, Caffrey CR, Camarda G, Carrasquilla M, Carter D, Belen Cassera M, Chih-Chien Cheng K, Chindaudomsate W, Chubb A, Colon BL, Colón-López DD, Corbett Y, Crowther GJ, Cowan N, D'Alessandro S, Le Dang N, Delves M, DeRisi JL, Du AY, Duffy S, Abd El-Salam El-Sayed S, Ferdig MT, Fernández Robledo J, Fidock DA, et al. 2016. Open source drug discovery with the Malaria Box compound collection for neglected diseases and beyond. PLoS Pathog 12:e1005763. doi: 10.1371/journal.ppat.1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low JL, Wu ML, Aziz DB, Laleu B, Dick T. 2017. Screening of TB actives for activity against nontuberculous mycobacteria delivers high hit rates. Front Microbiol 8:1539. doi: 10.3389/fmicb.2017.01539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preston S, Jiao Y, Jabbar A, McGee SL, Laleu SB, Willis P, Wells TNC, Gasser RB. 2016. Screening of the ‘Pathogen Box’ identifies an approved pesticide with major anthelmintic activity against the barber’s pole worm. Int J Parasitol Drugs Drug Resist 6:329–334. doi: 10.1016/j.ijpddr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spalenka J, Escotte-Binet S, Bakiri A, Hubert J, Renault JH, Velard F, Duchateau S, Aubert D, Huguenin A, Villena I. 2018. Discovery of new inhibitors of Toxoplasma gondii via the Pathogen Box. Antimicrob Agents Chemother 62:e01640-17. doi: 10.1128/AAC.01640-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong J, Kim G, Moon C, Kim HJ, Kim TH, Jang J. 2018. Pathogen Box screening for hit identification against Mycobacterium abscessus. PLoS One 13:e0195595. doi: 10.1371/journal.pone.0195595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller J, Aguado A, Laleu B, Balmer V, Ritler D, Hemphill A. 2017. In vitro screening of the open source Pathogen Box identifies novel compounds with profound activities against Neospora caninum. J Parasitol 47:801–809. doi: 10.1016/j.ijpara.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Meier A, Erler H, Beitz E. 2018. Targeting channels and transporters in protozoan parasite infections. Front Chem 6:88. doi: 10.3389/fchem.2018.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spangenberg T, Burrows JN, Kowalczyk P, McDonald S, Wells TN, Willis P. 2013. The open access Malaria Box: a drug discovery catalyst for neglected diseases. PLoS One 8:e62906. doi: 10.1371/journal.pone.0062906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffy S, Sykes ML, Jones AJ, Shelper TB, Simpson M, Lang R, Poulsen SA, Sleebs BE, Avery VM. 2017. Screening the Medicines for Malaria Venture Pathogen Box across multiple pathogens reclassifies starting points for open-source drug discovery. Antimicrob Agents Chemother 61:e00379-17. doi: 10.1128/AAC.00379-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vila T, Lopez-Ribot JL. 2016. Screening the Pathogen Box for identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother 61:e02006-16. doi: 10.1128/AAC.02006-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer FL, Kronstad JW. 2017. Discovery of a novel antifungal agent in the Pathogen Box. mSphere 2(2):e00120-17. doi: 10.1128/mSphere.00120-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy MW, Walsh TJ. 2017. Drugs currently under investigation for the treatment of invasive candidiasis. Expert Opin Investig Drugs 26:825–831. doi: 10.1080/13543784.2017.1341488. [DOI] [PubMed] [Google Scholar]
- 40.Brennan GP, Fairweather I, Trudgett A, Hoey E, McCoy, McConville M, Meaney M, Robinson M, McFerran N, Ryan L, Lanusse C, Mottier L, Alvarez L, Solana H, Virkel G, Brophy PM. 2007. Understanding triclabendazole resistance. Exp Mol Pathol 82:104–109. doi: 10.1016/j.yexmp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Sargison ND, Scott PR. 2011. Diagnosis and economic consequences of triclabendazole resistance in Fasciola hepatica in a sheep flock in south-east Scotland. Vet Rec 168:159. doi: 10.1136/vr.c5332. [DOI] [PubMed] [Google Scholar]
- 42.Sargison N. 2012. Diagnosis of triclabendazole resistance in Fasciola hepatica. Vet Rec 171:151–152. doi: 10.1136/vr.e5357. [DOI] [PubMed] [Google Scholar]
- 43.Novobilský A, Averpil HB, Höglund J. 2012. The field evaluation of albendazole and triclabendazole efficacy against Fasciola hepatica by coproantigen ELISA in naturally infected sheep. Vet Parasitol 190:272–276. doi: 10.1016/j.vetpar.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Novobilský A, Höglund J. 2015. First report of closantel treatment failure against Fasciola hepatica in cattle. Int J Parasitol Drugs Drug Resist 5:172–177. doi: 10.1016/j.ijpddr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong JX, Chandramohanadas R, Tan KS. 2018. High-content screening of the Medicines for Malaria Venture Pathogen Box for Plasmodium falciparum digestive vacuole-disrupting molecules reveals valuable starting points for drug discovery. Antimicrob Agents Chemother 62:e02031-17. doi: 10.1128/AAC.02031-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fairweather I, Boray JC. 1999. Fasciolicides: efficacy, actions, resistance and its management. Vet J 158:81–112. doi: 10.1053/tvjl.1999.0377. [DOI] [PubMed] [Google Scholar]
- 47.Lacey E. 1988. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int J Parasitol 18:885–936. doi: 10.1016/0020-7519(88)90175-0. [DOI] [PubMed] [Google Scholar]
- 48.Duthaler U, Smith T, Keiser J. 2010. In vivo and in vitro sensitivity of Fasciola hepatica to triclabendazole combined with artesunate, artemether, or OZ78. Antimicrob Agents Chemother 54:4596–4604. doi: 10.1128/AAC.00828-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farahnak A, Golmohamdi T, Eshraghian M. 2012. In vitro effects of triclabendazole (TCBZ) on the excretory-secretory products (ESP) of Fasciola spp parasites. Acta Med Iran 50:164–168. [PubMed] [Google Scholar]
- 50.The Pathogen Box. https://www.pathogenbox.org. Accessed April 2017.
- 51.Boray JC. 1969. Experimental fascioliasis in Australia. Adv Parasitol 8:95–210. [DOI] [PubMed] [Google Scholar]
- 52.Valero MA, Mas-Coma S. 2000. Comparative infectivity of Fasciola hepatica metacercariae from isolates of the main and secondary reservoir animal host species in the Bolivian Altiplano high human endemic region. Folia Parasitol (Praha) 47:17–22. doi: 10.14411/fp.2000.004. [DOI] [PubMed] [Google Scholar]
- 53.ChEMBL. https://www.ebi.ac.uk/chembl/. Accessed September 2017.
- 54.Bento AP, Gaulton A, Hersey A, Bellis LJ, Chambers J, Davies M, Krüger FA, Light Y, Mak L, McGlinchey S, Nowotka M, Papadatos G, Santos R, Overington JP. 2014. The ChEMBL bioactivity database: an update. Nucleic Acids Res 42:D1083–D1090. doi: 10.1093/nar/gkt1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.lazar. https://lazar.in-silico.de/predict. Accessed October 2017.
- 56.Wexler P. 2004. The U.S. National Library of Medicine's Toxicology and Environmental Health Information Program. Toxicology 198:161–168. doi: 10.1016/j.tox.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 57.Maunz A, Gütlein M, Rautenberg M, Vorgrimmler D, Gebele D, Helma C. 2013. lazar: a modular predictive toxicology framework. Front Pharmacol 4:38. doi: 10.3389/fphar.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. 1990. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 59.Boyd MR, Paull KD. 1995. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res 34:91–109. doi: 10.1002/ddr.430340203. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.