Candida auris is an emerging pathogen associated with significant mortality and often multidrug resistance. VT-1598, a tetrazole-based fungal CYP51-specific inhibitor, was evaluated in vitro and in vivo against C. auris.
KEYWORDS: Candida auris, VT-1598, in vitro susceptibility, invasive candidiasis, murine model
ABSTRACT
Candida auris is an emerging pathogen associated with significant mortality and often multidrug resistance. VT-1598, a tetrazole-based fungal CYP51-specific inhibitor, was evaluated in vitro and in vivo against C. auris. Susceptibility testing was performed against 100 clinical isolates of C. auris by broth microdilution. Neutropenic mice were infected intravenously with C. auris, and treatment began 24 h postinoculation with a vehicle control, oral VT-1598 (5, 15, and 50 mg/kg of body weight once daily), oral fluconazole (20 mg/kg once daily), or intraperitoneal caspofungin (10 mg/kg once daily), which continued for 7 days. Fungal burden was assessed in the kidneys and brains on day 8 in the fungal burden arm and on the days the mice succumbed to infection or on day 21 in the survival arm. VT-1598 plasma trough concentrations were also assessed on day 8. VT-1598 demonstrated in vitro activity against C. auris, with a mode MIC of 0.25 μg/ml and MICs ranging from 0.03 to 8 μg/ml. Treatment with VT-1598 resulted in significant and dose-dependent improvements in survival (median survival, 15 and >21 days for VT-1598 at 15 and 50 mg/kg, respectively) and reductions in kidney and brain fungal burden (reductions of 1.88 to 3.61 log10 CFU/g) compared to the control (5 days). The reductions in fungal burden correlated with plasma trough concentrations. Treatment with caspofungin, but not fluconazole, also resulted in significant improvements in survival and reductions in fungal burden compared to those with the control. These results suggest that VT-1598 may be a future option for the treatment of invasive infections caused by C. auris.
INTRODUCTION
Candida auris is an emergingin pathogen that has become a significant clinical problem and has now been isolated in numerous countries worldwide (1, 2). In a recent retrospective review of the clinical history of 54 patients, most had multiple risk factors for invasive disease, and candidemia was observed in 61% (1). Strikingly, the mortality rate in this series of patients was 59%. In the United States, during the first 18 months of surveillance there were at least 98 documented cases of infection caused by this species (3). The number of cases in the United States has continued to increase, with 428 confirmed and 30 probable cases as of 28 September 2018 and almost 750 additional patients colonized (4). Unfortunately, antifungal therapy against invasive infections caused by this emerging pathogen may be limited, as up to 90% of isolates are resistant to fluconazole, and 50% have reduced susceptibility to voriconazole, as demonstrated by elevated MICs, which is due to point mutations in ERG11 and ERG3 (1, 2). Currently, the echinocandins are recommended for the treatment of C. auris infections. However, elevated MICs secondary to hot spot regions in the FKS genes (FKS1 and FKS2) known to cause resistance to the echinocandins in other Candida species and Aspergillus fumigatus have been found in some C. auris isolates (2, 5, 6). In addition, a small proportion of isolates have been documented to be resistant to all clinically available classes of antifungal agents. Thus, there is a clear need for the development of new therapeutic candidates and novel treatment strategies to combat infections caused by this emerging pathogen.
VT-1598 is an investigational tetrazole from Viamet Pharmaceuticals that selectively inhibits fungal Cyp51A compared to mammalian cytochrome P-450 enzymes (7, 8). Thus, the potential for clinically significant drug-drug interactions is reduced. This agent has demonstrated in vitro activity against various fungi (9, 10) and has also shown promising results in experimental models of invasive fungal infections, including cryptococcal meningitis and central nervous system (CNS) coccidioidomycosis (11, 12). The objective of this study was to evaluate the in vitro activity of VT-1598 against a collection of C. auris isolates and its in vivo efficacy in a neutropenic murine model of invasive candidiasis caused by this pathogen.
RESULTS
In vitro activity of VT-1598.
VT-1598 demonstrated in vitro activity against C. auris, as shown by the MIC parameters in Table 1 and the MIC distributions in Table 2. MICs ranged from 0.03 to 8 μg/ml against all isolates, with MIC50 and MIC90 values of 0.25 and 1 μg/ml, respectively. The MICs of VT-1598 were somewhat higher against the South Asian clade isolates (range, 0.125 to 8 μg/ml; MIC50 and MIC90, 0.5 and 8 μg/ml, respectively). In contrast, isolates in the other clades were inhibited by lower concentrations of VT-1598, as reflected by lower MIC ranges and MIC50 and MIC90 values. In these clades the MIC90 values were either 0.25 or 0.5 μg/ml. The MICs of VT-1598, fluconazole, and caspofungin against the isolate used in the murine model were 0.125, >64, and 0.25 μg/ml, respectively.
TABLE 1.
MIC parameters for VT-1598 against 100 Candida auris isolatesa
| MIC parameter | MIC (μg/ml) |
||||
|---|---|---|---|---|---|
| All isolates (n = 100) | South Asian clade (n = 47) | African clade (n = 11) | South American clade (n = 39) | East Asian clade (n = 3) | |
| Range | 0.03–8 | 0.125–8 | 0.03–1 | 0.03–0.5 | 0.06–0.25 |
| Mode | 0.25 | 0.5 | 0.25 | 0.25 | 0.25 |
| MIC50 | 0.25 | 0.5 | 0.25 | 0.25 | 0.25 |
| MIC90 | 1 | 8 | 0.5 | 0.25 | 0.25 |
MICs were read visually after 24 h of incubation at 35°C as the lowest concentration that inhibited 50% of growth compared to the growth control.
TABLE 2.
MIC distributions of VT-1598 against 100 Candida auris isolatesa
| Isolate group | No. of isolates with indicated MIC (μg/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | ≥8 | |
| All isolates (n = 100) | 2 | 4 | 26 | 33 | 19 | 8 | 8 | |||
| South Asian clade (n = 47) | 12 | 4 | 16 | 7 | 8 | |||||
| African clade (n = 11) | 1 | 2 | 5 | 2 | 1 | |||||
| South American clade (n = 39) | 1 | 3 | 12 | 22 | 1 | |||||
| East Asian clade (n = 3) | 1 | 2 | ||||||||
The overall MIC distribution and the distributions for each C. auris clade are shown.
Survival.
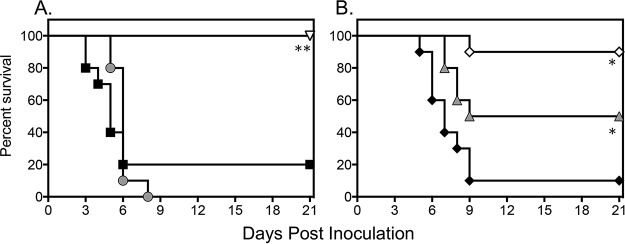
A significant and dose-dependent survival advantage was observed in mice treated with VT-1598. As shown in Fig. 1, treatment with this investigational tetrazole resulted in dose-dependent improvements in survival. Median survival in the VT-1598 15-mg/kg and 50-mg/kg groups (15 days and >21 days, respectively) were both significantly longer than that observed in the vehicle control group (5 days; P ≤ 0.01 for both comparisons), and percent survival at the predetermined study endpoint (day 21) was also higher in the VT-1598 50-mg/kg group (90%) than with the vehicle control (20%; P < 0.001). Similar results were also obtained with caspofungin (>21 days and 100%; P < 0.001). In contrast, no improvements in survival were observed with the lowest dose of VT-1598 (5 mg/kg) or with fluconazole.
FIG 1.
Survival curves in mice inoculated intravenously with C. auris and treated with a vehicle control, fluconazole at 20 mg/kg per os (p.o.) once daily (QD), or caspofungin at 10 mg/kg intraperitoneally (i.p.) QD (A) or VT-1598 at doses of 5 mg/kg, 15 mg/kg, and 50 mg/kg p.o. QD (B). Treatment started 1 day postinoculation and continued for 7 days. Mice were then followed off therapy until day 21 postinoculation (14 days after therapy stopped). Each group included 10 mice. Black squares, vehicle control; gray circles, fluconazole at 20 mg/kg; white inverted triangle, caspofungin at 10 mg/kg; black diamonds, VT-1598 at 5 mg/kg; gray triangles, VT-1598 at 15 mg/kg; white diamonds, VT-1598 at 50 mg/kg. *, P < 0.01 versus control; **, P < 0.001 versus control.
Fungal burden.
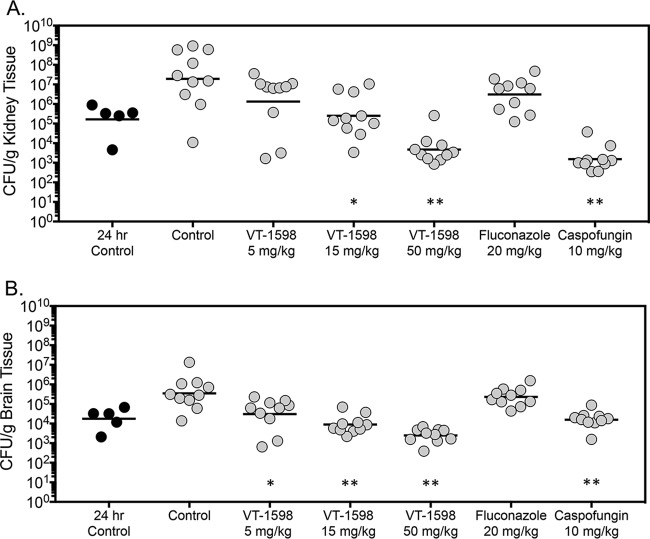
Dose-dependent reductions in fungal burden were also observed with VT-1598. On day 8 postinoculation, following 7 days of treatment, kidney fungal burden in mice treated with VT-1598 15-mg/kg and 50-mg/kg doses (mean log10 CFU/g, 5.40 and 3.67, respectively) was significantly lower than that observed in the vehicle control group (7.26 log10 CFU/g; P ≤ 0.005 for both comparisons) (Fig. 2A). Fungal burden was also lower with high-dose caspofungin (3.19 log10 CFU/g; P < 0.001). In contrast, kidney fungal burdens in mice treated with VT-1598 5 mg/kg and fluconazole were not significantly different than with the vehicle control. As determined by comparison to CFU measured just prior to the start of therapy (24 h postinoculation), both VT-1598 at 50 mg/kg and caspofungin resulted in fungicidal activity within the kidneys (−1.55 and −2.03 log10 CFU/g, respectively) (Table 3). In contrast, static activity was observed with the VT-1598 15-mg/kg dose (0.18 log10 CFU/g).
FIG 2.
Kidney (A) and brain (B) fungal burdens in mice with invasive candidiasis secondary to C. auris in the fungal burden arm. CFU were measured on day 8 postinoculation after 7 days of therapy. The vehicle control and treatment groups included 10 mice; the 24-h control group included 5 mice. *, P < 0.01 versus control; **, P < 0.0001 versus control.
TABLE 3.
Changes in kidney and brain CFU per gram of tissue for each treatment group compared to fungal burden measured prior to the start of therapy (24-h control) and in the vehicle control group on day 8 postinoculation
| Treatment group | Log10 change in: |
|||
|---|---|---|---|---|
| Kidney CFU/g vs. 24-h control (95% CIa ) | Kidney CFU/g vs. vehicle control (95% CI) | Brain CFU/g vs. 24-h control (95% CI) | Brain CFU/g vs. vehicle control (95% CI) | |
| Vehicle control | 2.06 (3.90 to 0.23) | 1.29 (0.31 to 2.29) | ||
| VT-1598, 5 mg/kg | 0.91 (−0.92 to 2.74) | −1.15 (−2.66 to 0.34) | 0.23 (−0.75 to 1.22) | −1.06 (−1.87 to −0.25) |
| VT-1598, 15 mg/kg | 0.18 (−1.65 to 2.02) | −1.88 (−3.38 to −0.39) | −0.3 (−1.29 to 0.69) | −1.59 (−2.40 to −0.78) |
| VT-1598, 50 mg/kg | −1.55 (−3.38 to 0.29) | −3.61 (−5.11 to −2.11) | −0.86 (−1.85 to 0.13) | −2.15 (−2.96 to − 1.34) |
| Fluconazole, 20 mg/kg | 1.26 (−0.56 to 3.10) | −0.8 (−2.30 to 0.70) | 1.11 (0.12 to 2.10) | −0.18 (−0.99 to 0.62) |
| Caspofungin, 10 mg/kg | −2.03 (−3.86 to −0.19) | −4.09 (−5.59 to −2.60) | −0.06 (−1.04 to 0.94) | −1.35 (−2.16 to −0.54) |
CI, confidence interval.
Dose-dependent reductions in fungal burden were also observed on day 8 postinoculation in the brains of mice that received VT-1598 (Fig. 2B). Fungal burden was significantly reduced in each VT-1598 dose group (mean range, 3.39 to 4.48 log10 CFU/g) compared to that with the vehicle control (5.54 log10 CFU/g; P < 0.01 for all comparisons). Similarly, brain fungal burden was also lower in mice treated with caspofungin (4.19 log10 CFU/g; P < 0.001), but not in mice that received fluconazole (5.36 log10 CFU/g). No treatment resulted in fungicidal activity, as the changes in brain fungal burden compared to that measured prior to the start of therapy were less than 1 log10 CFU/g (Table 2).
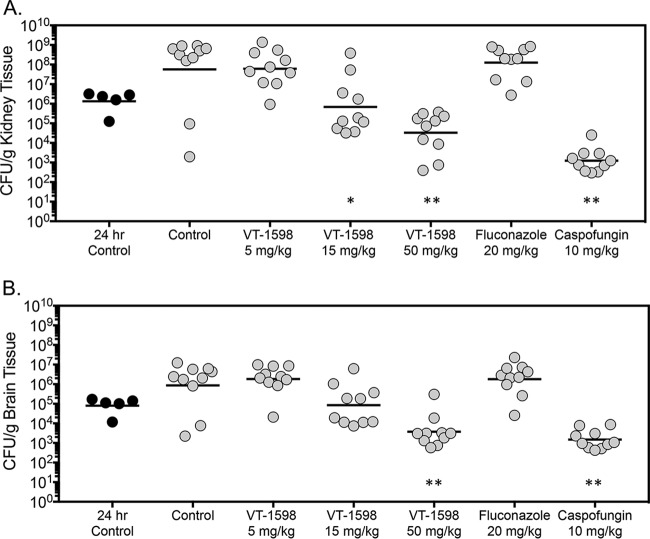
Fungal burden was also assessed in mice in the survival arm on day 21 postinoculation, 14 days after therapy stopped, or as the mice succumbed to infection. Similar to the results obtained in the fungal burden arm, fungal burden in the survival arm in mice treated with VT-1598 was reduced in a dose-dependent fashion. Kidney fungal burden in the VT-1598 15-mg/kg and 50-mg/kg groups (5.83 and 4.52 log10 CFU/g, respectively) was significantly lower than that observed in the vehicle control group (7.75 log10 CFU/g; P < 0.01 for both comparisons) (Fig. 3A). Kidney fungal burden was also significantly lower in mice treated with caspofungin (3.09 log10 CFU/g; P < 0.001). No reductions in fungal burden were observed in mice treated with VT-1598 at 5 mg/kg (7.79 log10 CFU/g) or fluconazole (8.10 log10 CFU/g).
FIG 3.
Kidney (A) and brain (B) fungal burdens in mice with invasive candidiasis secondary to C. auris in the survival arm. CFU were measured at the time of moribundity or at the prespecified study endpoint (day 21 postinoculation). The vehicle control and treatment groups included 10 mice; the 24-h control group included 5 mice. *, P < 0.01 versus control; **, P < 0.0001 versus control.
Dose-dependent reductions in brain fungal burden were also observed in mice treated with VT-1598 in the survival arm (Fig. 3B). However, only treatment with VT-1598 at 50 mg/kg resulted in brain fungal burden that was significantly lower than with the vehicle control (3.57 versus 5.93 log10 CFU/g; P < 0.001). As observed in the kidneys, brain fungal burden was significantly lower in mice treated with caspofungin (3.17 log10 CFU/g; P < 0.001) but not with VT-1598 at 5 mg/kg (6.26 log10 CFU/g) or fluconazole (6.26 log10 CFU/g). There was a numerical decrease of brain fungal burden with VT-1598 at 15 mg/kg (4.92 log10 CFU/g) that did not reach statistical significance (P = 0.147).
VT-1598 plasma concentrations.
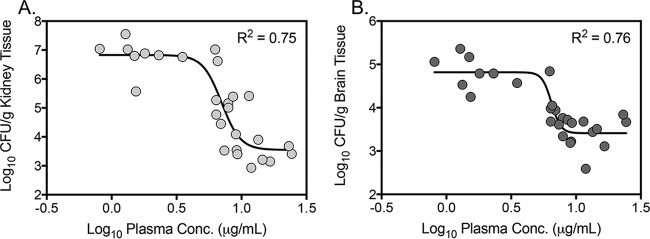
VT-1598 mean trough concentrations after 7 days of therapy were 1.55 μg/ml (±0.48) in the 5-mg/kg group, 6.78 μg/ml (±1.40) in the 15-mg/kg group, and 14.2 μg/ml (±5.56) in the 50-mg/kg group. Trough concentrations at each dose level remained well above the MIC against the C. auris strain used to infect the mice in this model (0.125 μg/ml). The mean trough achieved with the VT-1598 15-mg/kg dose, which was associated with a significant improvement in survival and fungistatic activity in this model, was more than 10 times higher than the MIC50 values observed against each clade and greater than 10-times the MIC90 values for three of the four clades (with the exception of the South Asian clade). In addition, a clear concentration-response relationship was observed between the VT-1598 plasma concentrations and the reductions observed both within the kidneys and brains of mice with invasive candidiasis due to C. auris, with coefficients of determination (R2) values of 0.75 and 0.76, respectively (Fig. 4).
FIG 4.
VT-1598 plasma concentration response curves as measured by changes in fungal burden in kidneys (A) and brains (B) on day 8 postinoculation after 7 days of therapy.
DISCUSSION
In the United States, the majority of proven C. auris infections have occurred due to isolates from the South Asian clade, primarily in New York State (13), and this clade is one of the four separate clades that have been observed by phylogenetic analysis following whole-genome sequencing (1). The echinocandins are currently recommended as the treatment of choice in patients with invasive infections caused by C. auris (14). However, the duration of therapy and the clinical effectiveness of these antifungals in the treatment of C. auris infections are unknown. Prolonged treatment may be needed, and this may be problematic with the echinocandins given the need for daily intravenous administration. Other azoles, including posaconazole and isavuconazole, appear to retain in vitro activity against most C. auris isolates; however, it is unclear if they are truly effective options in the setting of resistance to other azoles (1, 15). Additionally, these agents are associated with drug-drug interactions that may complicate therapy in patients with multiple comorbidities who are receiving polypharmacy. Several investigational agents that may alleviate the need for daily intravenous administration have shown promising in vitro activity against C. auris and in vivo efficacy in animal models of invasive infections caused by this species. These include the echinocandin rezafungin (formerly CD101) (16–18), and APX001, the prodrug of APX001A, which inhibits inositol acyltransferase, thereby preventing glycosylphosphatidylinositol-anchored protein maturation (19–22). In addition, the glucan synthase inhibitor ibrexafungerp (formerly SCY-078) has demonstrated promising in vitro activity against planktonic cells and biofilms of C. auris (23, 24).
In the current study, the orally administered tetrazole VT-1598, which inhibits the production of ergosterol by acting on the fungal Cyp51 enzyme in a highly selective manner compared to the other azoles, demonstrated in vitro activity against a large collection of C. auris isolates, including strains from each of the four known clades. This in vitro activity also translated into in vivo efficacy in a neutropenic model of invasive candidiasis. Dose-dependent reductions in fungal burden within the kidneys and brains were observed in mice treated with VT-1598. The effectiveness in reducing fungal burden was maintained in the survival arm 14 days after therapy stopped. Although drug plasma levels were not measured in the survival study, based on other in vivo fungal infection studies using the same or similar doses of VT-1598 (9, 11, 12), 14 days should be long enough for VT-1598 to fall below levels of detection (10 ng/ml). A survival advantage was also observed in mice treated with VT-1598, as both median survival and percent survival were improved with the middle and high doses of this tetrazole. The reductions in fungal burden within the kidneys and brains also correlated with plasma trough concentrations of VT-1598. The improvements in survival and reductions in brain fungal burden observed in this neutropenic murine model are consistent with the in vivo efficacy that has been observed in experimental models of central nervous system coccidioidomycosis and cryptococcal meningitis (11, 12). Interestingly, in the kidneys the static 15-mg/kg dose resulted in a trough concentration that was ∼50 times higher than the MIC against the isolate used to establish infection, while the fungicidal 50-mg/kg dose was over 100 times higher than the MIC. Although a formal pharmacodynamic/pharmacokinetic analysis was not performed, the need for high trough concentrations of VT-1598 may be related to the high degree of plasma protein binding observed with this agent (>99%) (11). This may also reflect the difficulty of treating C. auris infections due to the ability of these organisms to form biofilms and persist both within the host and the environment (24). Others have also reported that higher exposures for other antifungal classes are needed to optimize the treatment of C. auris infections (25).
Further studies are needed to determine how this investigational tetrazole may be used in the treatment of C. auris infections. These includes additional in vivo studies, including pharmacokinetic/pharmacodynamic evaluations and experimental models using isolates with different resistance profiles, in order to evaluate the utility of VT-1598 against these infections and optimize potential dosing regimens that may be used in humans. As further evidence that VT-1598 has antifungal activity against resistant Candida species, a previous study has reported that the in vitro and in vivo activity of VT-1598 was maintained against fluconazole-resistant C. albicans isolates (9). In addition, while the results of the current study and other preclinical studies have demonstrated that efficacious concentrations of VT-1598 can be reached, clinical studies are needed to explore if such levels are safely achieved. Overall, the results of the current study, coupled with the reduced potential for significant drug-drug interactions, suggest that VT-1598 may be a future option for the treatment of C. auris infections.
MATERIALS AND METHODS
Antifungal agents.
For in vitro studies, VT-1598 powder was provided by Viamet Pharmaceuticals, Inc. (Durham, NC), and fluconazole and caspofungin were obtained from Sigma (St. Louis, MO). Antifungal stocks were prepared in dimethyl sulfoxide (DMSO), with further dilutions in made RPMI 1640 (0.165 M morpholinepropanesulfonic acid [MOPS], pH 7.0, without bicarbonate) following the standards in CLSI document M27-A3 (26). The final DMSO concentration was 1% (vol/vol). Broth microdilution plates were stored at −70°C and were used within 1 week of preparation. For in vivo studies, VT-1598 was provided by Viamet Pharmaceuticals as a salt with a conversion factor from the active component to the salt of 1.3. VT-1598 for oral dosing was prepared using Cremophor EL (20% [vol/vol]). The clinically available intravenous formulations of fluconazole and caspofungin were used.
Isolates.
For in vitro susceptibility testing, 100 Candida auris isolates collected from throughout the world and covering each of the four known C. auris clades were used. This included isolates known to have reduced susceptibility to fluconazole, the echinocandins, and/or amphotericin B. For the in vivo study, a clinical isolate of C. auris (UTHSCSA DI17-46), originally cultured in 2016 from the bloodstream of a patient with invasive candidiasis from New York State, was used. The isolate was received by the Fungus Testing Laboratory at UT Health San Antonio, and the species identification was confirmed by DNA sequence analysis of the internal transcribed spacer (ITS) and D1/D2 domains of rRNA (27–29). The isolate was subcultured twice at 37°C for 48 h on Sabouraud dextrose agar. Prior to inoculation, colonies were taken from the second subculture, placed into brain heart infusion broth, and grown overnight at 37°C with shaking at 200 rpm; cells were then collected by centrifugation and washed three times in sterile saline with 0.1% Tween 20. The starting inoculum was determined by counting Candida cells using a hemocytometer and adjusting to the target number of cells. Following the preparation of the inocula, viability was assessed by serially diluting an aliquot and plating on Sabouraud dextrose agar to determine the number of colonies after incubation at 37°C.
In vitro susceptibility testing.
In vitro susceptibility testing of VT-1598 was performed according to the standards of the Clinical and Laboratory Standards Institute reference methodology M27-A3 against the 100 C. auris isolates described above (26). MIC results were read visually after 24 h of incubation at the lowest drug concentration at which there was a 50% decrease in growth compared to the growth control. This endpoint and time point were chosen because they are the ones by the CLSI for the triazoles against Candida species, which also prevent ergosterol biosynthesis through the inhibition of the 14α-demethylase enzyme (26). The MICs of VT-1598, fluconazole, and caspofungin were also determined against the isolate used in the murine model as described above. The concentration ranges tested were 0.015 to 8 μg/ml for VT-1598, 0.125 to 64 μg/ml for fluconazole, and 0.015 to 8 μg/ml for caspofungin.
Murine model of invasive candidiasis.
Mice were rendered neutropenic with a single dose of pharmaceutical-grade 5-fluorouracil at 5 mg/mouse administered intravenously 1 day prior to intravenous inoculation (day −1). To prevent bacterial superinfection and deaths in the immunosuppressed mice, mice received antibacterial prophylaxis consisting of enrofloxacin at 50 ppm in their drinking water beginning 1 day prior to infection. On the day of inoculation (day 0), mice were infected intravenously via the lateral tail vein with 0.2 ml of C. auris (target inocula of 1 × 107 cells/mouse and 5 × 106 cells/mouse in the survival and fungal burden arms, respectively). Therapy began 24 h following inoculation and continued through day 7. Treatment groups consisted of a vehicle control (20% Cremaphor EL), VT-1598 at 5 mg/kg, 15 mg/kg, or 50 mg/kg administered by oral gavage once daily, fluconazole at 20 mg/kg by oral gavage once daily, and caspofungin at 10 mg/kg once daily by intraperitoneal injection. Treatment studies consisted of both survival and fungal burden arms. Throughout the studies, mice were observed multiple times per day to prevent and minimize unnecessary pain and distress that may have occurred with infection. Any animal that appeared moribund prior to the scheduled endpoint was euthanized. This animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio, and all animals were maintained in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
Survival.
In the survival arm, mice received treatment for 7 days and were then followed off therapy for 14 days until day 21 postinoculation. Mice were monitored multiple times per day, and any animal that appeared moribund prior to the designated endpoint was humanely euthanized and death was recorded as occurring the next day.
Fungal burden.
In the fungal burden arm, mice were treated for 7 days. On day 8 postinoculation, mice were humanely euthanized and the kidneys and brains were aseptically collected for fungal burden analysis by quantification of CFU. The organs were homogenized in sterile saline, serial dilutions of the homogenate were prepared in sterile saline to control for antifungal carryover, and the dilutions were plated onto Sabouraud dextrose agar. Following 48 h of incubation, colonies were counted and the number of CFU per gram of tissue was calculated for each animal.
VT-1598 plasma concentrations.
Blood samples were also collected in anesthetized mice by cardiac puncture on day 8 in the fungal burden arm in order to measure VT-1598 trough concentrations. Plasma was separated and immediately frozen. The samples were then sent frozen to a designated laboratory (OpAns, Durham, NC) for the measurement of VT-1598 concentrations by an established liquid chromatography-tandem mass spectrometry (LC/MS-MS) assay that has previously been reported for VT-1598 and a related compound, VT-1161 (12, 30).
Data analysis.
Descriptive statistics were used to evaluate the in vitro susceptibility of VT-1598, including MIC ranges, modal MICs, and MIC50 and MIC90 values. Survival was plotted by Kaplan-Meier analysis, and differences in the median survival and percent survival were analyzed by the log rank test and Fisher’s exact test, respectively. Analysis of variance (ANOVA) with Tukey’s posttest for multiple comparisons was used to assess for differences in fungal burden. In vivo fungicidal activity was defined as a 1-log10 CFU/g reduction in fungal burden compared to that measured 24 h postinoculation just prior to the start of therapy. To assess the relationships between VT-1598 plasma concentrations and kidney and brain fungal burden, nonlinear regression was used, and the data were fit to a four-parameter inhibitory sigmoid model (modified Hill equation) using curve fitting software (Prism 7.0d; GraphPad Software, Inc., La Jolla, CA). The goodness of fit was assessed by the coefficient of determination (R2).
ACKNOWLEDGMENTS
We thank OpAns, LLC, for bioanalysis of plasma concentrations of VT-1598.
This project utilized preclinical services funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Department of Health and Human Services, under contract no. HHS272201100018I and HHSN272201700039I—Task Orders A28 and A01, respectively, to the University of Texas Health Science Center at San Antonio.
N.P.W. has received research support to the UT Health San Antonio from Astellas, bioMérieux, Cidara, F2G, Merck, Pfizer, and Viamet and has served on advisory boards for Astellas and Mayne Pharma and as a speaker for Gilead. T.F.P. has received research grants to UT Health San Antonio from Astellas, Merck, and Revolution Medicines and has served as a consultant for Astellas, Gilead, Merck, Pfizer, Revolution Medicines, Toyama, Viamet, and Scynexis. L.K.N. has received travel support from Viamet Pharmaceuticals, Inc. E.P.G., C.M.Y., and R.J.S. are employees of Viamet Pharmaceuticals, Inc.
The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhary A, Sharma C, Meis JF. 2017. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13:e1006290. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsay S, Welsh RM, Adams EH, Chow NA, Gade L, Berkow EL, Poirot E, Lutterloh E, Quinn M, Chaturvedi S, Kerins J, Black SR, Kemble SK, Barrett PM, Barton K, Shannon DJ, Bradley K, Lockhart SR, Litvintseva AP, Moulton-Meissner H, Shugart A, Kallen A, Vallabhaneni S, Chiller TM, Jackson BR. 2017. Notes from the field: ongoing transmission of Candida auris in health care facilities—United States, June 2016–May 2017. MMWR Morb Mortal Wkly Rep 66:514–515. doi: 10.15585/mmwr.mm6619a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. 2018. Tracking Candida auris. https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html. Accessed 10 October 2018.
- 5.Kathuria S, Singh PK, Sharma C, Prakash A, Masih A, Kumar A, Meis JF, Chowdhary A. 2015. Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization–time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J Clin Microbiol 53:1823–1830. doi: 10.1128/JCM.00367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma C, Kumar N, Pandey R, Meis JF, Chowdhary A. 2016. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect 13:77–82. doi: 10.1016/j.nmni.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yates CM, Garvey EP, Shaver SR, Schotzinger RJ, Hoekstra WJ. 2017. Design and optimization of highly-selective, broad spectrum fungal CYP51 inhibitors. Bioorg Med Chem Lett 27:3243–3248. doi: 10.1016/j.bmcl.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Hargrove TY, Garvey EP, Hoekstra WJ, Yates CM, Wawrzak Z, Rachakonda G, Villalta F, Lepesheva GI. 2017. Crystal structure of the new investigational drug candidate VT-1598 in complex with Aspergillus fumigatus sterol 14alpha-demethylase provides insights into its broad-spectrum antifungal activity. Antimicrob Agents Chemother 61:e00570-17. doi: 10.1128/AAC.00570-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Break TJ, Desai JV, Healey KR, Natarajan M, Ferre EMN, Henderson C, Zelazny A, Siebenlist U, Yates CM, Cohen OJ, Schotzinger RJ, Perlin DS, Garvey EP, Lionakis MS. 2018. VT-1598 inhibits the in vitro growth of mucosal Candida strains and protects against fluconazole-susceptible and -resistant oral candidiasis in IL-17 signalling-deficient mice. J Antimicrob Chemother 73:2089–2094. doi: 10.1093/jac/dky170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiederhold NP, Patterson HP, Tran BH, Yates CM, Schotzinger RJ, Garvey EP. 2018. Fungal-specific Cyp51 inhibitor VT-1598 demonstrates in vitro activity against Candida and Cryptococcus species, endemic fungi, including Coccidioides species, Aspergillus species and Rhizopus arrhizus. J Antimicrob Chemother 73:404–408. doi: 10.1093/jac/dkx410. [DOI] [PubMed] [Google Scholar]
- 11.Garvey EP, Sharp AD, Warn PA, Yates CM, Schotzinger RJ. 2018. The novel fungal CYP51 inhibitor VT-1598 is efficacious alone and in combination with liposomal amphotericin B in a murine model of cryptococcal meningitis. J Antimicrob Chemother 73:2815–2822. doi: 10.1093/jac/dky242. [DOI] [PubMed] [Google Scholar]
- 12.Wiederhold NP, Shubitz LF, Najvar LK, Jaramillo R, Olivo M, Catano G, Trinh HT, Yates CM, Schotzinger RJ, Garvey EP, Patterson TF. 2018. The novel fungal Cyp51 inhibitor VT-1598 is efficacious in experimental models of central nervous system coccidioidomycosis caused by Coccidioides posadasii and Coccidioides immitis. Antimicrob Agents Chemother 62:e02258-17. doi: 10.1128/AAC.02258-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow NA, Gade L, Tsay SV, Forsberg K, Greenko JA, Southwick KL, Barrett PM, Kerins JL, Lockhart SR, Chiller TM, Litvintseva AP, US Candida auris Investigation Team. 2018. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: a molecular epidemiological survey. Lancet Infect Dis 18:P1377–P1384. doi: 10.1016/S1473-3099(18)30597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC. 2018. Recommendations for treatment of Candida auris. https://www.cdc.gov/fungal/candida-auris/c-auris-treatment.html. Accessed 6 October 2018.
- 15.Arendrup MC, Prakash A, Meletiadis J, Sharma C, Chowdhary A. 2017. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother 61:e00485-17. doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hager CL, Larkin EL, Long LA, Ghannoum MA. 2018. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother 73:2085–2088. doi: 10.1093/jac/dky153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepak AJ, Zhao M, Andes DR. 2018. Pharmacodynamic evaluation of rezafungin (CD101) against Candida auris in the neutropenic mouse invasive candidiasis model. Antimicrob Agents Chemother 62:e01572-18. doi: 10.1128/AAC.01572-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berkow EL, Lockhart SR. 2018. Activity of CD101, a long-acting echinocandin, against clinical isolates of Candida auris. Diagn Microbiol Infect Dis 90:196–197. doi: 10.1016/j.diagmicrobio.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Arendrup MC, Chowdhary A, Astvad KMT, Jorgensen KM. 2018. APX001A in vitro activity against contemporary blood isolates and Candida auris determined by the EUCAST reference method. Antimicrob Agents Chemother 62:e01225-18. doi: 10.1128/AAC.01225-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkow EL, Lockhart SR. 2018. Activity of novel antifungal compound APX001A against a large collection of Candida auris. J Antimicrob Chemother 73:3060–3062. doi: 10.1093/jac/dky302. [DOI] [PubMed] [Google Scholar]
- 21.Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. 2018. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao M, Lepak AJ, VanScoy B, Bader JC, Marchillo K, Vanhecker J, Ambrose PG, Andes DR. 2018. In vivo pharmacokinetics and pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob Agents Chemother 62:e02542-17. doi: 10.1128/AAC.02542-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkow EL, Angulo D, Lockhart SR. 2017. In vitro activity of a novel glucan synthase inhibitor, SCY-078, against clinical isolates of Candida auris. Antimicrob Agents Chemother 61:e00435-17. doi: 10.1128/AAC.00435-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin E, Hager C, Chandra J, Mukherjee PK, Retuerto M, Salem I, Long L, Isham N, Kovanda L, Borroto-Esoda K, Wring S, Angulo D, Ghannoum M. 2017. The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother 61:e02396-16. doi: 10.1128/AAC.02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepak AJ, Zhao M, Berkow EL, Lockhart SR, Andes DR. 2017. Pharmacodynamic optimization for treatment of invasive Candida auris infection. Antimicrob Agents Chemother 61:e00791-17. doi: 10.1128/AAC.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeast: approved standard, 2nd ed. Document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 27.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 28.Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J Clin Microbiol 48:741–752. doi: 10.1128/JCM.01948-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtzman CP, Robnett CJ. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shubitz LF, Trinh HT, Galgiani JN, Lewis ML, Fothergill AW, Wiederhold NP, Barker BM, Lewis ER, Doyle AL, Hoekstra WJ, Schotzinger RJ, Garvey EP. 2015. Evaluation of VT-1161 for treatment of coccidioidomycosis in murine infection models. Antimicrob Agents Chemother 59:7249–7254. doi: 10.1128/AAC.00593-15. [DOI] [PMC free article] [PubMed] [Google Scholar]