Breastfeeding (BF) women are an important population for biomedical HIV prevention strategies, but they are rarely included in trials. The 25-mg dapivirine vaginal ring (VR) reduced women’s risk of sexually transmitted HIV infection in two phase 3 trials conducted in Africa.
KEYWORDS: HIV, breastfeeding, dapivirine, lactation, pharmacokinetics, vaginal
ABSTRACT
Breastfeeding (BF) women are an important population for biomedical HIV prevention strategies, but they are rarely included in trials. The 25-mg dapivirine vaginal ring (VR) reduced women’s risk of sexually transmitted HIV infection in two phase 3 trials conducted in Africa. We conducted a phase 1, open-label study (MTN-029/IPM 039) of dapivirine VR use among lactating women in Pittsburgh, PA, and Birmingham, AL, USA. MTN-029/IPM 039 enrolled 16 healthy adult women who had already weaned their infants but were still able to express breast milk. Women were instructed to use the VR continuously for 14 days and provided milk, plasma, and cervicovaginal fluid (CVF) samples for pharmacological analysis. No infants were exposed to the drug, but infant dosage was estimated according to FDA guidance. Adverse events (AEs) were collected at all contacts. The study was completed with 100% participant retention. Median dapivirine concentrations were 676 pg/ml in breast milk, 327 pg/ml in plasma (milk/plasma ratio ∼2.0), and 36.25 ng/mg in CVF. Six participants experienced 10 total AEs, none of which required VR discontinuation. The estimated mean daily infant dosage was 74.3 ng/kg/day. In this first study of dapivirine exposure during lactation, dapivirine VR use was associated with lower concentrations of detectable dapivirine in milk and plasma than in CVF samples and a favorable safety profile. Estimated daily levels of infant dapivirine exposure were also low. Additional studies are needed to evaluate longer periods of dapivirine VR use among BF mother-infant pairs living in regions with higher incidence of sexually transmitted HIV infection. (This study has been registered at ClinicalTrials.gov under registration no. NCT02808949.)
INTRODUCTION
Dapivirine is a nonnucleoside reverse transcriptase inhibitor (NNRTI) previously observed to have poor oral bioavailability (1). The 25-mg dapivirine vaginal ring (VR) has been shown to reduce women’s risk of acquiring sexually transmitted HIV infection in two phase 3 trials conducted in Kenya, Malawi, South Africa, Uganda, and Zimbabwe (2, 3). Low systemic dapivirine concentrations and a favorable safety profile have been observed in women using this product (2, 3).
Several factors support additional evaluation of the dapivirine VR in lactating women. Most importantly, a high total fertility rate and extended periods of breastfeeding (BF) are common in countries with high HIV incidence (WHO Global Health Observatory data repository; accessed 4 May 2018 [http://apps.who.int/gho/data/node.home]), and significant risk for HIV acquisition postpartum appears to continue during BF (4, 5). The World Health Organization (WHO) recommends exclusive BF for 6 months minimum and continued BF with appropriate complementary foods up to 2 years of age or beyond (6). However, normal physiologic changes during the postpartum period (e.g., hormonal, body fat, and muscle mass) may impact pharmacokinetics (PK) (7), so PK profiles may differ from those of nonlactating women. The United States Food and Drug Administration (FDA) advises clinical lactation studies when drug use is anticipated in women of reproductive age (8), and a recent FDA drug labeling rule includes provision for a lactation risk summary with actual or estimated infant dose (9). However, few studies have included lactating women or breastfeeding mother-infant pairs in evaluations of HIV chemoprevention products (10).
MTN-029/IPM 039 (ClinicalTrials registration no. NCT02808949) was a phase 1, open-label study of dapivirine VR use among lactating women. The primary study objective was to assess the PK of the dapivirine VR used for 14 consecutive days in lactating women who were not BF; MTN-029/IPM 039 also aimed to assess safety, tolerability, and adherence associated with 14-day use of the VR.
(Preliminary data were presented at the 9th International AIDS Society Conference on HIV Science and 2017 Infectious Diseases Society for Obstetrics and Gynecology Annual Meeting.)
RESULTS
A total of 32 participants were screened, of whom 16 were enrolled and 16 not enrolled, for a ratio of 2.0 participants screened for every participant enrolled. The majority of women not enrolled were ineligible (13/16, 81%); 5 of 16 women (31%) had a milk supply of less than 1 oz per expression at screening or enrollment. Other reasons for failure to enroll were eligible but declined enrollment (2 women), and accrual closed prior to enrollment (1 woman). Enrollment occurred between 16 March 2016 and 15 February 2017; eight women were enrolled in Pittsburgh, PA, and eight in Birmingham, AL. All participants completed all scheduled visits.
Characteristics of study participants are included in Table 1.
TABLE 1.
Characteristics of enrolled participants
| Characteristic | Value (n = 16 participants) |
|---|---|
| Age (yrs) | |
| Mean (SD) | 29.9 (5.4) |
| Median | 29.5 |
| Range (min–maxa ) | 20–39 |
| No. (%) aged: | |
| 18 to <25 | 3 (18.8) |
| 25 to <30 | 5 (31.3) |
| 30 to <35 | 4 (25.0) |
| 35 to <40 | 4 (25.0) |
| No. (%) of indicated race | |
| American Indian or Alaska Native | 0 |
| Asian | 0 |
| Black or African American | 4 (25.0) |
| Native Hawaiian or Pacific Islander | 0 |
| White | 10 (62.5) |
| Other | 0 |
| Multiple | 2 (12.5) |
| No. (%) of indicated ethnicity | |
| Hispanic ethnicity | 0 |
| Weight (kg) at enrollment | |
| Mean (SD) | 74.2 (22.7) |
| Median | 68.0 |
| Range (min–max) | 45–127 |
| Liver function test results | |
| AST | |
| Mean | 17.7 |
| Median | 17.0 |
| Range (min–max) | 12–28 |
| ALT | |
| Mean | 16.6 |
| Median | 16.5 |
| Range (min–max) | 8–30 |
min, minimum; max, maximum; AST, aspartate transaminase; ALT, alanine transaminase.
Participants had a median body mass index of 23.6. Nine (56%) participants had resumed menses since delivery, four (25%) had not resumed menses, and three (19%) were unsure. Concomitant medications reported during study participation included contraceptives and other commonly used supplements and medications, e.g., allergy medications, vitamins, etc.
One (6%) participant reported temporary discontinuation of VR use. The first ring expulsion occurred on day 8 of the study, and the ring was outside the vagina for 1 min. A second ring expulsion for this same participant occurred on study day 9 and also lasted for 1 min. Both expulsions were due to bowel movements. All participants returned their VRs, which were tested for residual dapivirine. The mean quantity of dapivirine in the returned rings was 22.0 mg (standard deviation [SD], 0.731). Assuming each ring initially held 25 mg dapivirine, the mean amount of dapivirine released was 3.0 mg (SD, 0.731). The range of residual levels of dapivirine in the returned VRs was 20.6 to 23.3 mg.
Dapivirine concentrations in breast milk, blood plasma, and cervicovaginal fluid (CVF) samples were available at all seven time points for all 16 participants. After VR insertion, plasma dapivirine concentrations were detectable (greater than the lower limit of quantification [>LLOQ]) in all 16 participants at all time points, except for one participant with a plasma level <LLOQ at the 3-h-postinsertion time point. Dapivirine breast milk concentrations were >LLOQ in all 16 participants at all time points. After dapivirine VR insertion, dapivirine CVF concentrations were detectable in all participants at all time points during days 0, 1, 7, and 14; on day 16 (48 h after VR removal), CVF concentrations were <LLOQ in 5 (5/16, 31.25%) participants.
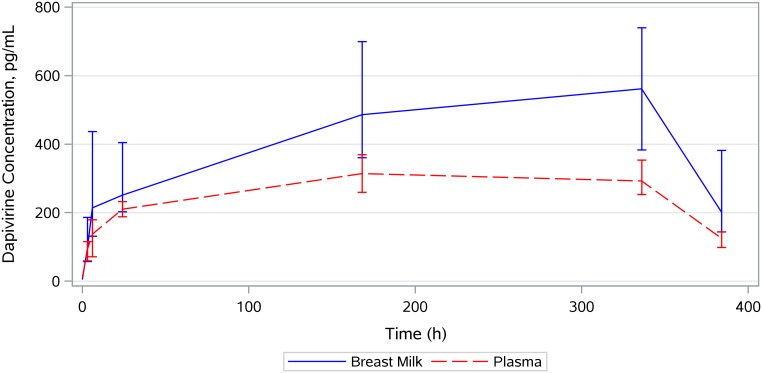
Table 2 provides a summary of PK parameters (maximum concentration [Cmax], time to maximum concentration [tmax], area under the concentration-time curve from 0 to 336 h [AUC0–336], and half-life [t1/2]) of dapivirine for plasma, milk, and CVF samples. Dapivirine breast milk concentrations increased rapidly during the 24 h following VR insertion. Peak dapivirine breast milk concentrations were achieved later than in plasma, at a median of 335.4 h (14 days) after ring insertion (tmax), with a median Cmax of 676.0 pg/ml, though not all peaks occurred on day 14. Following ring removal, dapivirine breast milk concentrations declined from a median concentration of 561.5 pg/ml on day 14 to 201.5 pg/ml on day 16 (i.e., 64% decline in 2 days).
TABLE 2.
Pharmacokinetic parameters for dapivirine in breast milk, blood plasma, and cervicovaginal fluid samples
| Parametera | Value for: |
|||
|---|---|---|---|---|
| Breast milk | Blood plasma | Breast milk/blood plasma ratio | Cervicovaginal fluid | |
| Cmax (pg/ml) | ||||
| Mean (SD) | 723.1 (317.1) | 330.8 (85.5) | 2.2 (1.0) | 49,566.9 (47,034.1) |
| Median | 676.0 | 327.0 | 2.0 | 36,245.0 |
| IQR | 443.0, 924.5 | 274.5, 378.0 | 1.5, 2.5 | 22,170.0, 58,435.0 |
| Min, max | 297.0, 1,420.0 | 199.0, 559.0 | 1.3, 5.1 | 4,540, 200,000 |
| tmax (h) | ||||
| Mean (SD) | 266.2 (105.8) | 212.8 (95.1) | 83.4 (102.5) | |
| Median | 335.4 | 172.0 | 24.8 | |
| IQR | 171.1, 339.0 | 169.0, 333.8 | 6.0, 168.6 | |
| Min, Max | 6.0, 358.3 | 6.0, 338.1 | 3.0, 340.2 | |
| AUC0–336 (pg · h/ml) | ||||
| Mean (SD) | 168,283.5 (68,318.0) | 95,778.6 (29,030.7) | 1.8 (0.6) | 8,227,682.7 (8,764,783.6) |
| Median | 152,604.9 | 93,717.7 | 1.7 | 5,555,367.1 |
| IQR | 119,122.5, 191,806.4 | 77,318.8, 106,607.9 | 1.4, 1.9 | 2,426,211.9, 9,331,004.4 |
| Min, max | 88,625.8, 324,138.0 | 59,402.3, 181,492.4 | 1.1, 3.8 | 1,145,204.1, 36,357,657.9 |
| t1/2 (h) | ||||
| Mean (SD) | 45.8 (28.9) | 42.2 (19.0) | 9.5 (4.7) | |
| Median | 39.0 | 35.2 | 7.8 | |
| IQR | 27.1, 53.4 | 29.8, 46.4 | 6.3, 12.3 | |
| Min, max | 18.9, 141.1 | 22.7, 93.4 | 3.6, 22.7 | |
IQR, interquartile range; min, minimum; max, maximum.
Median dapivirine CVF concentrations increased rapidly in the first 24 h following VR insertion. Peak dapivirine CVF concentrations were achieved at a median of 24.8 h after VR insertion (tmax), with a median Cmax of 36.25 ng/mg. Following VR removal, dapivirine CVF concentrations declined from a median concentration of 5.78 ng/mg on day 14 to 0.13 ng/mg on day 16 (i.e., 98% in 2 days).
The median breast milk-to-plasma ratio of dapivirine concentrations was 2-fold based on Cmax and 1.7-fold based on AUC0–336. The median dapivirine concentration-time profile for breast milk overlaid with the profile for plasma is provided in Fig. 1. Mean dapivirine intake for a BF infant was estimated at 74.3 ng/kg of body weight/day, or for an 8-kg infant (median weight for male infant at ∼6 months old [11]), approximately 594.4 ng/day (<1 μg/day).
FIG 1.
Median concentration-time profile for breast milk overlaid with the profile for plasma. Vertical bars show the interquartile ranges.
Ten AEs were reported from six participants. Of the 10 AEs, 8 (80%) were grade 1 and two (20%) were grade 2. No AEs occurred with severity greater than grade 2. Only two AEs were deemed related to the study product (grade 1 vaginal discharge and vaginal odor), and they were experienced by the same participant. All participants tested negative for pregnancy at screening, enrollment, and day 16. Two abnormal pelvic exam findings occurred during study participation (vaginal spotting at day 7 and vaginal abrasions at day 1).
DISCUSSION
In this first study of dapivirine exposure during lactation, dapivirine VR use was associated with low concentrations of detectable dapivirine in milk and plasma and a favorable safety profile in lactating women. Pharmacokinetic parameters for both plasma and vaginal fluid (i.e., Cmax, tmax, and AUC) measured in lactating women in this study were highly similar to those observed for healthy, nonlactating women using the same VR for 14 days in a phase 1 study (12). Some dapivirine accumulation was observed in milk over the 14-day exposure, but the absolute levels were very low. Approximately half of the drug was eliminated from blood and breast milk in less than 2 days after VR removal. The estimated potential daily levels of infant exposure to dapivirine were significantly lower (∼75 ng/kg/day) than the maximum tolerated dose for multiple oral dapivirine doses in adults of 300 mg twice daily (equivalent to 12 mg/kg/day for a 50-kg woman). The daily vaginal dose observed here in lactating women is approximately 25-fold greater than the estimated oral infant dose on a per-kilogram basis; however, relative exposure may be impacted by potential differences in bioavailability between the maternal vaginal tissue and the infant gut.
Oral formulations of dapivirine have been evaluated in adults but not infants or children. In adults, the plasma dapivirine concentration observed at the maximum tolerated dose for oral treatment (300 mg twice daily for 14 days, with a mean Cmax of 2,286 ng/ml) is significantly higher than the level of dapivirine we observed here in breast milk (12). While orally administered dapivirine in healthy term infants may have similarly low bioavailability, the lipophilic nature of dapivirine and intermittently high lipid content of breast milk could impact infant drug exposure, which will be important to consider in future studies that include breastfeeding infants. In vitro metabolism studies have shown the main metabolic pathways for dapivirine to be slow oxidation and glucuronidation, processes that are immature in infants (12, 13). Additionally, pharmacokinetics in adults and infants are known to differ, including for NNRTIs (14). However, given the very low levels of dapivirine measured in breast milk in this study, it is unlikely that such exposure would be clinically significant.
This study had several notable strengths, including its high participant retention and procedure completion and use of sensitive validated assays for measurement of dapivirine. Both phase 3 trials of the dapivirine VR predefined as adherent those participants with plasma DPV concentrations of ≥95 pg/ml and residual levels of ≤23.5 mg of dapivirine in used rings (i.e., with >1.5 mg released) and found that the majority of participants met these criteria (2, 3). Thus, the blood dapivirine concentrations and analyses of residual drug in VR reported here suggest that the product was used consistently during the 14-day product use period for all 16 participants and that the amount of dapivirine released was consistent with that observed in recent efficacy trials.
Several limitations of this study should be acknowledged. Of note, t1/2 was based on only two sample concentrations, and therefore, the values were not expected to provide a robust estimate of the t1/2 but, rather, to provide a quantitative estimate of rate of change in dapivirine concentrations. Thus, the t1/2 values should be interpreted with caution. Additionally, pharmacokinetic profiles may differ in women who are still BF versus those who recently completed weaning, due to lower total milk volume and potentially a longer interval since the last breastfeeding, resulting in lower milk lipid levels (15). Limited data are available for the dapivirine VR on potential drug-drug interactions; however, no clinically significant drug-drug interactions have been identified, including for hormonal contraceptives, which were used by some women during study participation (16). The study was limited by its lack of a placebo control, which would have been preferable in an evaluation of drug safety; however, very few safety events were observed among the study participants. Finally, a thorough assessment of risk during breastfeeding includes available information on the likelihood and seriousness of known or predicted effects on the breastfed child from exposure to a drug and/or its active metabolite(s) through human breast milk (9). However, this first study of dapivirine exposure in lactation did not include breastfed-infant exposure to drug or measurement of metabolites in breast milk.
Breastfeeding women are a critically important population for HIV prevention, but they have frequently been excluded from HIV chemoprevention research (10), creating gaps in understanding PK and drug safety. The World Health Organization has recommended that oral preexposure prophylaxis containing tenofovir disoproxil fumarate be offered as an additional HIV prevention choice for BF women at significant risk for HIV infection (18). However, a range of methods should be offered to women to address their different product preferences and maximize the potential for method adherence, a factor critically related to efficacy in antiretroviral-based HIV chemoprevention (18).
Additional analyses will examine the vaginal microenvironment associated with dapivirine ring use in lactating women. Given emerging evidence of increased risk of HIV infection during the postnatal period (19), the favorable safety profile and low level of drug transfer to milk observed in this study, and the ethical imperative to include BF women in drug safety studies (10), future research (i.e., MTN-043) is planned to evaluate longer periods of dapivirine VR use among BF mother-infant pairs living in regions with higher incidence of sexually transmitted HIV infection (20).
MATERIALS AND METHODS
Participants.
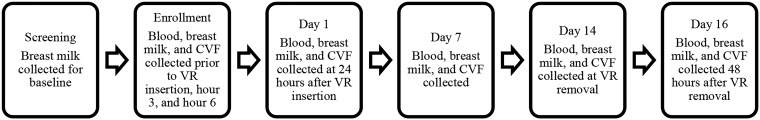
Participants were healthy women aged 18 or older, at least 6 weeks postpartum, HIV-1/-2 uninfected, no longer BF but able to express breast milk at least twice daily, using an effective method of contraception, and with negative screening for chlamydia, gonorrhea, trichomonas, and urinary tract infection. Women over age 21 were required to have had a negative Pap test in the past 3 years. The study excluded women with mastitis or other complications of lactation and those unable to express at least 1 oz of milk at baseline. Women were discouraged from discontinuing BF to become eligible for study participation. Participants were enrolled from two clinical research sites in Pittsburgh, PA, and Birmingham, AL. The study schedule included the following six visits: screening, enrollment (day 0), day 1, day 7, day 14, and day 16 (exit).
Study product regimen.
The International Partnership for Microbicides developed and provided the study product, a silicone elastomer vaginal ring (VR), containing 25 mg of dapivirine dispersed in a platinum-cured silicone matrix, designed to provide sustained release of dapivirine over a 28-day period (dapivirine vaginal ring-004). Each participant received a VR to wear continuously for approximately 14 days. An authorized clinician inserted the VR for the participant. Participants wishing to practice insertion and removal did so immediately before final VR insertion, which was done by the clinician. The clinician performed a digital exam to check placement after reviewing instructions. VRs were collected for analysis of residual levels of dapivirine in used rings.
Six participant visits occurred over 16 days, according to the schedule noted in Fig. 2.
FIG 2.
Participant visits.
Specimen collection and analytical assays.
Home-based breast milk collection occurred on days 2 through 6 inclusive and on day 15; women were advised to collect breast milk at least twice daily, but no maximum was specified. All breast milk specimens were collected into commercially available polypropylene bottles especially designed for breast milk collection and storage and then transferred to polypropylene cryovials. Dapivirine concentrations in plasma and CVF were measured using validated, previously described, liquid chromatographic-tandem mass spectrometric (LC-MS/MS) methods (21, 22). Dapivirine metabolites were not measured. Dapivirine quantification in breast milk was performed via LC-MS/MS analysis following both liquid-liquid extraction and solid-phase extraction to isolate dapivirine from the breast milk matrix with a primary linearity of 10 to 1,000 pg/ml. The assay’s lower limits of quantification were 10 pg/ml for breast milk, 20 pg/ml for plasma, and 0.25 ng/swab for CVF samples. For result reporting, CVF results (initially captured as ng/swab) were normalized to fluid weight by dividing the concentration yielded by the weight of the swab (mg); the median (interquartile range [IQR]) LLOQ for CVF samples normalized to swab weight (mg) was 0.00068 (0.0042, 0.0132) ng/mg. This approach allows normalization of results and takes into account variability in the amount of fluid collected on each swab. All assays met the acceptability criteria of the FDA’s Bioanalytical Method Validation: Guidance for Industry recommendations (23) and were peer reviewed by the DAIDS-sponsored Clinical Pharmacology Quality Assurance (CPQA) program. CVF collection was performed by inserting a Dacron swab into the upper vagina near the cervix at the location nearest to where the VR resides, without touching the VR. The swab was rotated for 10 to 20 seconds in a circular motion touching all vaginal walls to absorb as much fluid as possible and placed into a cryovial immediately after sampling. Residual levels of dapivirine were determined in all used VRs. Concentrations were measured by Parexel International using high-performance liquid chromatography–UV testing (24).
Clinical procedures.
Medical and menstrual histories were assessed at screening and were reviewed at enrollment and all follow-up visits. Concomitant medications were recorded at screening and reviewed/updated at subsequent visits. Physical examinations were conducted at screening and enrollment, pelvic examinations were conducted at screening, enrollment, and at all follow-up visits, and breast examinations were conducted at screening, enrollment, and the day 16 visit. Adverse events (AEs) were recorded at all participant contacts.
Pharmacokinetic analysis.
Cmax (peak concentration) and tmax (time at peak concentration) were determined for breast milk, blood plasma, and CVF. Area under the curve (AUC) was determined using the trapezoidal method, using SAS for Linux/Unix (version 9.4; SAS Institute, Inc., Cary, NC, USA). Concentrations at days 14 and 16 were used to determine the terminal concentration half-life (t1/2) of dapivirine in breast milk, blood plasma, and CVF after VR removal using the following formula:
No infant exposure or PK sampling occurred (only women who did not plan to provide an infant with breast milk produced after VR use were enrolled). Therefore, the systemic dapivirine exposure of breastfed infants was not measured. However, infant dapivirine intake (ng/kg/day) was estimated using the following FDA-recommended formula: milk-to-plasma ratio (M/P) × average maternal serum concentration × 150 ml/kg/day, where M/P = ratio of AUCmilk to AUCplasma (8).
Ethics statement.
All participants provided written informed consent for the study, which was approved by Institutional Review Boards in Pittsburgh and Birmingham. MTN-029/IPM 039 was not subject to Data and Safety Monitoring Board review.
ACKNOWLEDGMENTS
The study was designed and implemented by the Microbicide Trials Network (MTN) and funded by the National Institute of Allergy and Infectious Diseases through individual grants (grants number UM1AI068633, UM1AI068615, and UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health (NIH).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The International Partnership for Microbicides manufactured and provided the dapivirine VR (Vaginal Ring-004).
We gratefully acknowledge the contributions of all study team members and participants.
REFERENCES
- 1.Jespers V, Van Roey J, Beets G, Buvé A. 2007. Dose-ranging phase 1 study of TMC120, a promising vaginal microbicide, in HIV-negative and HIV-positive female volunteers. J Acquir Immune Defic Syndr 44:154–158. doi: 10.1097/QAI.0b013e31802bb35f. [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres L, Govender V, Mgodi NM, Matovu F, Nair G, Mhlanga F, Siva S, Bekker L, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery E, Mensch B, Torjesen K, Grossman C, Chakhtoura N, Nel A, Rosenberg Z, McGowan I, Hillier S, for The MTN-020–Aspire Study Team. 2016. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 375:2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nel A, van Niekerk N, Kapiga S, Bekker L, Gama C, Gill K, Kamali A, Kotze P, Louw C, Mabude Z, Miti N, Kusemererwa S, Tempelman H, Carstens H, Devlin B, Isaacs M, Malherbe M, Mans W, Nuttall J, Russell M, Ntshele S, Smit M, Solai L, Spence P, Steytler J, Windle K, Borremans M, Resseler S, Van Roey J, Parys W, Vangeneugden T, Van Baelen B, Rosenberg Z, for the Ring Study Team. 2016. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 375:2133–2143. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 4.De Schacht C, Mabunda N, Ferreira OC, Ismael N, Calú N, Santos I, Hoffman HJ, Alons C, Guay L, Jani IV. 2014. High HIV incidence in the postpartum period sustains vertical transmission in settings with generalized epidemics: a cohort study in Southern Mozambique. J Int AIDS Soc 17:18808. doi: 10.7448/IAS.17.1.18808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson KA, Hughes J, Baeten JM, John-Stewart G, Celum C, Cohen CR, Ngure K, Kiarie J , Mugo N , Heffron R, Partners in Prevention HSV/HIV Transmission Study and Partners PrEP Study Teams. 2018. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis 218:16–25. doi: 10.1093/infdis/jiy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. 2018. WHO health topics: breastfeeding. WHO, Geneva, Switzerland. http://www.who.int/topics/breastfeeding/en/. Accessed 4 May 2018.
- 7.Fleishaker JC, Desai N, McNamara PJ. 1989. Possible effect of lactational period on the milk-to-plasma drug concentration ratio in lactating women: results of an in vitro evaluation. J Pharm Sci 78:137–141. doi: 10.1002/jps.2600780213. [DOI] [PubMed] [Google Scholar]
- 8.FDA. 2005. Guidance for industry: clinical lactation studies—study design, data analysis, and recommendations for labeling. Food and Drug Administration, Department of Health and Human Services, Rockville, MD. https://www.fda.gov/downloads/regulatoryinformation/guidances/ucm127505.pdf.
- 9.Federal Register. 2014. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Regist 79:72063–72103. https://www.federalregister.gov/documents/2014/12/04/2014-28241/content-and-format-of-labeling-for-human-prescription-drug-and-biological-products-requirements-for. [PubMed] [Google Scholar]
- 10.Beigi RH, Noguchi LM, Brown G, Piper JM, Watts DH. 2016. Performing drug safety research during pregnancy and lactation: biomedical HIV prevention research as a template. J Womens Health 25:761–766. doi: 10.1089/jwh.2013.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. 2018. WHO child growth standards: weight-for-age boys. WHO, Geneva, Switzerland. http://www.who.int/childgrowth/standards/cht_wfa_boys_p_0_6.pdf?ua=1.
- 12.International Partnership for Microbicides. 2018. Dapivirine investigator’s brochure, version 12.0.
- 13.Fernandez E, Perez R, Hernandez A, Tejada P, Arteta M, Ramos JT. 2011. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics 3:53–72. doi: 10.3390/pharmaceutics3010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu H, Rosenbaum S. 2014. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther 19:262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koletzko B, Rodriguez-Palmero M, Demmelmair H, Fidler N, Jensen R, Sauerwald T. 2001. Physiological aspects of human milk lipids. Early Hum Dev 65:S3. doi: 10.1016/S0378-3782(01)00204-3. [DOI] [PubMed] [Google Scholar]
- 16.Balkus JE, Palanee-Phillips T, Reddy K, Siva S, Harkoo I, Nakabiito C, Kintu K, Nair G, Chappell C, Kiweewa FM, Kabwigu S, Naidoo L, Jeenarain N, Marzinke M, Soto-Torres L, Brown ER, Baeten JM. 2017. Brief report: dapivirine vaginal ring use does not diminish the effectiveness. of hormonal contraception. J Acquir Immune Defic Syndr 76:e47–e51. doi: 10.1097/QAI.0000000000001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.WHO. 2017. WHO technical brief: preventing HIV during pregnancy and breastfeeding in the context of pre-exposure prophylaxis (PrEP). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 19.Thomson KA, Hughes J, Baeten JM, John-Stewart G, Celum C, Cohen CR, Ngure K, Kiarie J, Mugo N, Heffron R, Partners in Prevention HSV/HIV Transmission Study and Partners PrEP Study Teams. 2018. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis 5:16–25. doi: 10.1093/infdis/jiy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Microbicide Trials Network. 2018. A time to deliver: preventing HIV in pregnant and breastfeeding women. https://mtnstopshiv.org/news/time-deliver-preventing-hiv-pregnant-and-breastfeeding-women. Accessed 23 November 2018.
- 21.Seserko LA, Emory JF, Hendrix CW, Marzinke MA. 2013. The development and validation of an UHPLC–MS/MS method for the rapid quantification of the antiretroviral agent dapivirine in human plasma. Bioanalysis 5:2771–2783. doi: 10.4155/bio.13.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons TL, Emory JF, Seserko LA, Aung WS, Marzinke MA. 2014. Dual quantification of dapivirine and maraviroc in cervicovaginal secretions from ophthalmic tear strips and polyester-based swabs via liquid chromatographic-tandem mass spectrometric (LC-MS/MS) analysis. J Pharm Biomed Anal 98:407–416. doi: 10.1016/j.jpba.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FDA. 2001. Bioanalytical method validation: guidance for industry. Food and Drug Administration, Department of Health and Human Services, Rockville, MD. [Google Scholar]
- 24.Lyndgaard LB, Spångberg R, Gilmour C, Lyndgaard CB, van den Berg F. 2014. A process analytical approach for quality control of dapivirine in HIV preventive vaginal rings by Raman spectroscopy. J Raman Spectrosc 45:149–156. doi: 10.1002/jrs.4433. [DOI] [Google Scholar]