Infections caused by the coexistence of Candida glabrata echinocandin-resistant and echinocandin-susceptible cells may be possible, and the detection of FKS mutants when the proportions of FKS mutants are underrepresented poses a problem. We assessed the role of EUCAST and methods directly performed on positive blood cultures—Etest (ETDIR) and anidulafungin-containing agar plate assays—for detecting resistance in C. glabrata isolates containing different amounts of echinocandin-susceptible and -resistant Candida glabrata isolates.
KEYWORDS: Candida glabrata, EUCAST procedure, echinocandins, Etest, resistance
ABSTRACT
Infections caused by the coexistence of Candida glabrata echinocandin-resistant and echinocandin-susceptible cells may be possible, and the detection of FKS mutants when the proportions of FKS mutants are underrepresented poses a problem. We assessed the role of EUCAST and methods directly performed on positive blood cultures—Etest (ETDIR) and anidulafungin-containing agar plate assays—for detecting resistance in C. glabrata isolates containing different amounts of echinocandin-susceptible and -resistant Candida glabrata isolates. We studied 10 pairs of C. glabrata isolates involving parental echinocandin-susceptible isolates and isogenic echinocandin-resistant FKS mutant isolates. Three inocula per pair (1 × 103 to 5 × 103, 1 × 102 to 5 × 102, and 10 to 50 CFU/ml) spanning suspensions with different amounts of susceptible/resistant isolates (9/1, 5/5, and 1/9 proportions for each the three inocula) were prepared. The suspensions were spiked in Bactec bottles and incubated until they were positive, and the three methods were compared. The EUCAST method showed echinocandin resistance when the bottles were spiked with susceptible/resistant isolates at 5/5 and 1/9 proportions; the results for the suspensions with a 9/1 proportion of susceptible/resistant isolates were susceptible for three pairs. We observed with the ETDIR resistance to both echinocandins in all pairs (resistance to micafungin and anidulafungin; MICs, ≥0.064 mg/liter and ≥0.125 mg/liter, respectively) and a double ring of growth inhibition in two pairs. The anidulafungin-containing plates showed fungal growth in the 90 spiked blood cultures at 48 h. Testing of echinocandin susceptibility with the ETDIR directly on the positive blood culture bottles is a reliable and rapid method to detect echinocandin resistance in C. glabrata. On the other hand, resistance can be missed with the EUCAST method when resistant isolates are underrepresented.
INTRODUCTION
The incidence of invasive fungal infections is increasing, and mortality rises when the initiation of appropriate antifungal therapy is delayed (1–3). Candida glabrata is one of the main causes of invasive candidiasis, and its occurrence is growing (4, 5). Among the most important factors associated with invasive C. glabrata infections are the use of broad-spectrum antibiotics, catheters, and parenteral nutrition; the presence of immunosuppression; the disruption of mucosal barriers; and chemotherapy/radiotherapy (6).
Echinocandin resistance in C. glabrata poses a problem for the management of patients due to its intrinsic low level of susceptibility to azoles and the poor prognosis for patients infected by echinocandin-resistant isolates (4, 5). The risk factors for developing echinocandin-resistant C. glabrata candidemia are previous echinocandin exposure, solid organ transplantation, recent gastrointestinal surgery or a recent gastrointestinal disorder, and multiple episodes of C. glabrata bloodstream infections (4, 7). Moreover, recent studies have reported that the abdominal cavity and mucosal surfaces may serve as reservoirs for resistant isolates (8, 9). Echinocandins are indicated to be the first line of treatment in cases of invasive candidiasis (10), a recommendation supported by the low rate of echinocandin resistance (1, 11, 12). However, some studies have provided alerts on the increased rates of echinocandin resistance in C. glabrata strains causing infection in some geographic areas (4, 5). Echinocandin resistance in C. glabrata is associated with the presence of mutations in hot spots of the FKS1 and FKS2 genes (5).
The rapid detection of echinocandin resistance in C. glabrata in blood samples can contribute to the improvement of patient care. Molecular detection of resistance would speed up the results, although to date these techniques are pending on validation for their use with blood samples (5, 13). In a previous study, we showed that the Etest directly performed on positive blood cultures (ETDIR) is a reliable procedure to rapidly detect fluconazole- and echinocandin-resistant isolates (14–16). Moreover, anidulafungin-containing plates were useful to screen for the presence of echinocandin-resistant C. glabrata isolates directly from positive blood cultures (16).
Data on the antifungal susceptibility obtained using standardized testing procedures, such as the CLSI or EUCAST procedures, are mainly obtained from isolates recovered from automated blood culture systems, such as the Bactec FX system (Becton, Dickinson, Cockeysville, MD, USA) (17, 18). The scenario in which infections are caused by the coexistence of echinocandin-resistant cells and echinocandin-susceptible ones may be possible. In situations in which the proportion of C. glabrata FKS mutants in culture is underrepresented in comparison to the proportion of wild-type isolates, the reliability of detection of C. glabrata FKS mutants using standard methods and rapid methods (anidulafungin-containing agar plate assays or ETDIR) is unknown.
In this study, we aimed to examine the accuracy of the EUCAST EDef 7.3.1 standard procedure and the rapid techniques (ETDIR and anidulafungin-containing agar plates) for assessing susceptibility to echinocandin antifungals in C. glabrata isolates using inocula with different proportions of echinocandin-susceptible and echinocandin-resistant C. glabrata isolates.
RESULTS
Antifungal susceptibility of isolates spiked in bottles following the EUCAST standard procedure.
Ninety bottles were spiked with the nine possible combinations of inocula and different proportions of susceptible/resistant isolates. The antifungal susceptibility of the isolates was performed from the slime on the plates and is shown in Table 1. The isolates in cultures from bottles spiked with suspensions containing susceptible/resistant isolates in proportions of 5/5 and 1/9 were phenotypically resistant to both micafungin and anidulafungin. On the other hand, the isolates in cultures from 3 out of the 10 bottles (pairs 6, 7, and 9) spiked with suspensions of susceptible/resistant isolates in proportions of 9/1 were susceptible to both echinocandins (Table 1).
TABLE 1.
Micafungin and anidulafungin MICs against the isolates from bottles spiked with the different tested inocula and proportions
|
Inoculum (CFU/ml) |
Proportion | EUCAST micafungin/anidulafungin MIC (mg/liter) for the following paira: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 1 × 103–5 × 103 | 9S/1R | 1/2 | 2/1 | 4/2 | 0.25/0.5 | 0.064/0.125 | 0.015/0.064 | 0.015/0.064 | 0.5/2 | 0.015/0.064 | 0.125/0.25 |
| 5S/5R | 2/2 | 2/1 | 4/2 | 0.25/0.5 | 0.064/0.25 | 1/2 | 1/1 | 1/2 | 0.5/1 | 4/2 | |
| 1S/9R | 1/2 | 1/0.5 | 4/2 | 0.5/1 | 0.064/0.25 | 1/1 | 1/2 | 1/2 | 0.5/1 | 4/2 | |
| 1 × 102–5 × 102 | 9S/1R | 1/0.5 | 0.064/0.125 | 2/2 | 0.25/0.5 | 0.064/0.125 | 0.015/0.064 | 0.015/0.064 | 0.5/1 | 0.015/0.064 | 0.25/0.25 |
| 5S/5R | 0.5/0.5 | 0.5/0.25 | 4/2 | 0.25/0.5 | 0.064/0.125 | 1/1 | 2/1 | 0.5/2 | 0.5/0.5 | 4/2 | |
| 1S/9R | 0.25/0.125 | 0.25/0.125 | 4/2 | 0.25/0.5 | 0.064/0.25 | 1/2 | 1/2 | 1/2 | 0.5/1 | 4/2 | |
| 10–50 | 9S/1R | 1/0.5 | 0.25/0.125 | 1/0.5 | 0.25/0.5 | 0.064/0.125 | 0.015/0.064 | 0.015/0.064 | 1/2 | 0.032/0.064 | 0.125/0.25 |
| 5S/5R | 1/0.5 | 0.5/0.125 | 1/0.5 | 0.25/0.5 | 0.064/0.125 | 1/0.5 | 0.5/0.25 | 1/2 | 0.5/0.5 | 4/2 | |
| 1S/9R | 2/1 | 2/1 | 4/2 | 0.5/0.5 | 0.064/0.25 | 1/2 | 2/1 | 0.5/2 | 0.5/0.5 | 4/2 | |
Bold numbers indicate EUCAST MICs showing echinocandin susceptibility for the tested isolates after preparing the inoculum from slime.
Seven hundred fifty-two individual colonies from the 90 bottles (n = 266, n = 253, and n = 233 colonies from the 103-, 102-, and 10-CFU/ml inocula, respectively) were tested and determined to be susceptible (n = 393) or resistant (n = 359) to both echinocandins (Table 2). Overall differences in the number/percentage of echinocandin-resistant colonies (n = 124/46.6%, n = 120/47.4%, and n = 115/49.4% from the 103-, 102-, and 10-CFU/ml inocula, respectively) did not reach statistical significance (P > 0.05). However, the higher that the proportion of resistant isolates in the suspension used to spike the bottles was, the higher that the proportion of resistant colonies counted on the plates was, regardless of the inoculum used (P < 0.05) (see Fig. S2 in the supplemental material). This was consistently observed for every tested pair (Table 2). Colonies in cultures from bottles spiked with suspensions with susceptible/resistant isolates in 5/5 and 1/9 proportions were either susceptible and/or resistant to echinocandins. However, resistant colonies were missing from three pairs of cultures from bottles spiked with suspensions with susceptible/resistant isolates in a 9/1 proportion (pairs 1, 3, and 8; Table 2).
TABLE 2.
Number of individual colonies obtained from each culture from the 90 spiked bottlesa
| Inoculum (CFU/ml) | Proportion | No. of colonies for the following pair: |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
||||||||||||
| S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | ||
| 1 × 103–5 × 103 (n = 266 colonies) | 9S/1R | 7 | 0 | 4 | 2 | 10 | 0 | 4 | 4 | 5 | 2 | 5 | 3 | 5 | 4 | 9 | 0 | 8 | 1 | 7 | 2 |
| 5S/5R | 7 | 3 | 5 | 5 | 4 | 6 | 6 | 4 | 5 | 4 | 4 | 5 | 6 | 4 | 6 | 4 | 4 | 4 | 6 | 3 | |
| 1S/9R | 2 | 6 | 3 | 7 | 2 | 5 | 2 | 8 | 3 | 5 | 2 | 8 | 4 | 5 | 0 | 8 | 4 | 6 | 3 | 6 | |
| 1 × 102–5 × 102 (n = 253 colonies) | 9S/1R | 5 | 1 | 6 | 3 | 4 | 1 | 8 | 2 | 6 | 2 | 4 | 5 | 6 | 4 | 8 | 2 | 8 | 1 | 6 | 2 |
| 5S/5R | 2 | 3 | 6 | 4 | 0 | 6 | 7 | 3 | 6 | 3 | 4 | 5 | 4 | 3 | 7 | 2 | 6 | 3 | 5 | 4 | |
| 1S/9R | 0 | 6 | 2 | 8 | 0 | 6 | 5 | 5 | 5 | 4 | 2 | 7 | 4 | 6 | 1 | 9 | 3 | 5 | 3 | 5 | |
| 10–50 (n = 233 colonies) | 9S/1R | 6 | 3 | 4 | 2 | 4 | 2 | 5 | 1 | 6 | 3 | 5 | 4 | 4 | 4 | 7 | 1 | 8 | 1 | 7 | 2 |
| 5S/5R | 4 | 4 | 3 | 2 | 0 | 6 | 5 | 3 | 4 | 5 | 4 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 7 | 2 | |
| 1S/9R | 0 | 6 | 3 | 5 | 0 | 4 | 4 | 6 | 3 | 5 | 3 | 5 | 2 | 5 | 2 | 5 | 3 | 5 | 6 | 3 | |
Colonies were classified as susceptible or resistant to both echinocandins according to the EUCAST clinical breakpoints. S, echinocandin-susceptible colonies; R, echinocandin-resistant colonies.
Antifungal susceptibility testing using ETDIR.
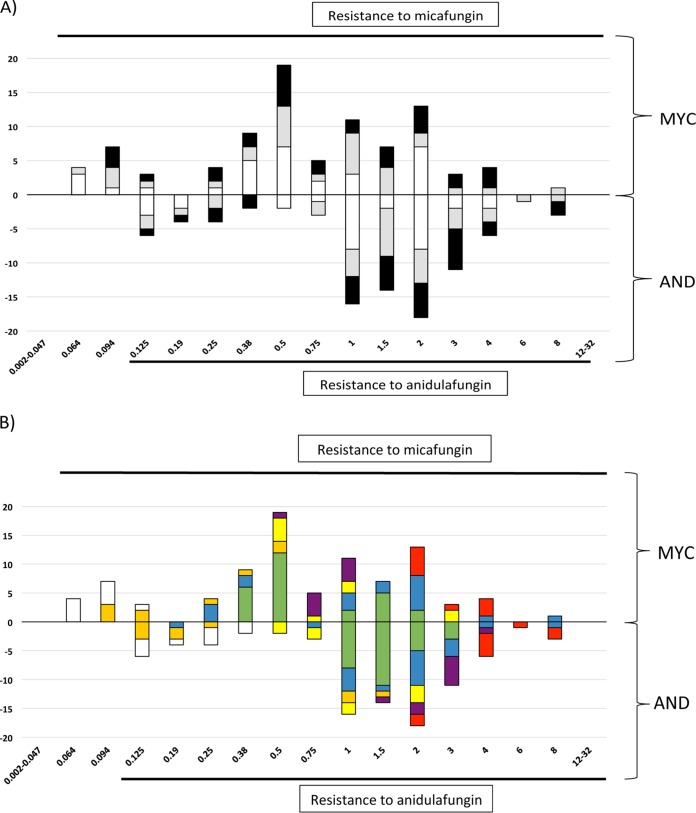
Ninety ETDIR tests to detect anidulafungin and micafungin susceptibility were performed. Using the breakpoints of EUCAST, ETDIR classified the isolates from the 90 bottles as resistant to both echinocandins. A wide distribution of MICs was observed, regardless of the proportion of susceptible/resistant isolates spiked in the blood culture (Fig. 1A). Conversely, the type of mutation was of great relevance regarding the MICs obtained by ETDIR; certain mutations leading to high MICs for both echinocandins by the EUCAST method resulted in elevated ETDIR MICs (Fig. 1B). The setting of the MIC was easy in most cases, but the ETDIR showed the presence a double ring of growth inhibition for pairs 7 and 8. The thickness of the inner halo (probably representing the resistant isolate) increased with higher proportions of the resistant isolate in the suspension used to spike the blood culture; inner halo growth was taken into account to set the MIC (Fig. 2).
FIG 1.
Distribution of micafungin and anidulafungin MICs obtained using ETDIR of cultures from the 90 spiked bottles. Numbers of isolates are shown along the y axis and MICs (in mg/liter) are indicated along the x axis. (A) Micafungin (MYC) and anidulafungin (AND) MICs for isolates obtained from blood cultures spiked with suspensions containing different proportions of susceptible/resistant isolates (white bars, 9/1; gray bars, 5/5; black bars, 1/9). (B) The results for isolates with different FKS2 mutations, including the FKS wild-type isolate classified as resistant by the EUCAST method, are shown. White bars, FKS wild-type isolate classified as resistant by the EUCAST method; orange bars, isolate with the FKS2 E655A mutation; blue bars, isolate with the FKS2 S663P mutation; green bars, isolate with the FKS2 ΔF658 deletion; yellow bars, isolate with the FKS2 W715L mutation; purple bars, isolate with the FKS2 S663Y mutation; red bars, isolate with the FKS2 D666N mutation.
FIG 2.
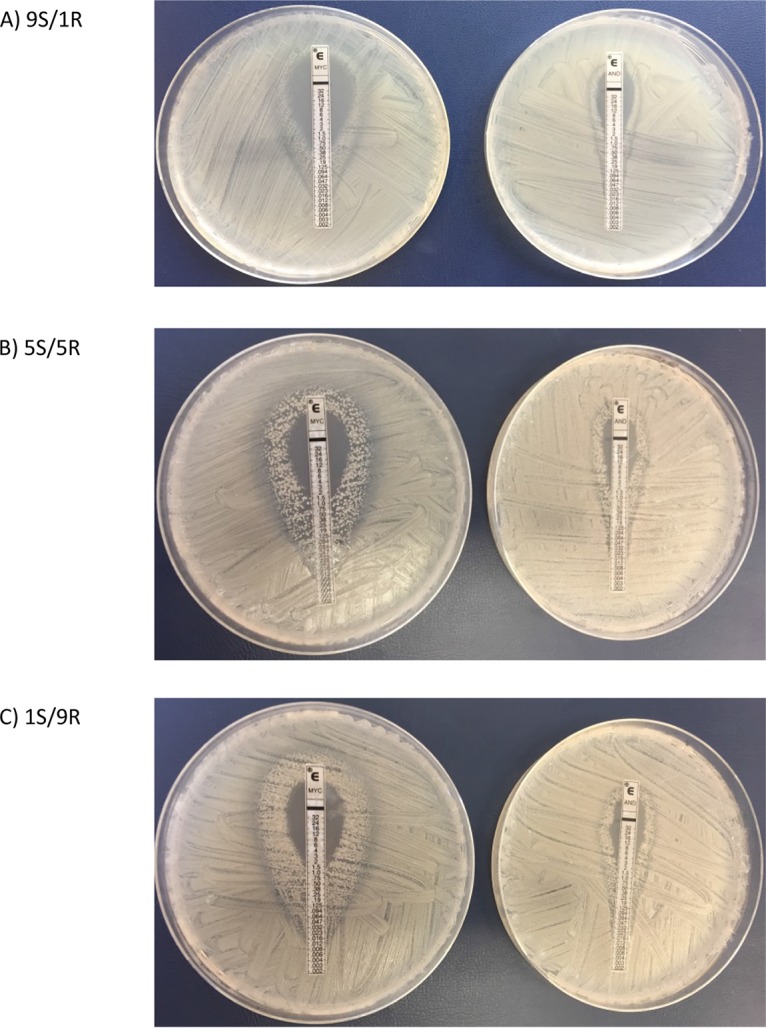
ETDIR of micafungin and anidulafungin showing a double ring of growth inhibition in the 1 × 103- to 5 × 103-CFU/ml inoculum in suspensions containing different proportions of susceptible/resistant isolates: 9/1 (A), 5/5 (B), or 1/9 (C). MYC, micafungin; AND, anidulafungin.
Screening of resistance on anidulafungin-containing agar plates.
Two fungal growth patterns were seen for the 90 spiked blood cultures in the plates incubated for 24 to 48 h. Slime-like growth was detected at 24 h of incubation, whereas single colonies were noticed in pairs 4, 5, and 9, which turned positive only when the incubation was extended to 48 h. Furthermore, the isolates producing single colonies were from blood cultures spiked with isogenic isolates with lower MICs of anidulafungin and micafungin by the EUCAST method (Table 3). As mentioned above for ETDIR, the results were not affected by the inoculum.
TABLE 3.
Micafungin and anidulafungin MICs for the isolates in each used pair to prepare the spiked suspensions in Bactec bottles and FKS2 gene sequence of the tested isolatesa
| Pair | Isolate | EUCAST MYC/AND MIC (mg/liter) | FKS2 gene sequence |
|---|---|---|---|
| 1 | Parental | 0.015/0.032 | WT |
| Isogenic | 4/2 | ΔF658 | |
| 2 | Parental | 0.015/0.032 | WT |
| Isogenic | 4/2 | ΔF658 | |
| 3 | Parental | 0.015/0.032 | WT |
| Isogenic | 4/2 | S663P | |
| 4 | Parental | 0.015/0.032 | WT |
| Isogenic | 0.25/0.5 | E655A | |
| 5 | Parental | 0.015/0.032 | WT |
| Isogenic | 0.064/0.25 | WT | |
| 6 | Parental | 0.015/0.015 | WT |
| Isogenic | 0.5/0.5 | W715L | |
| 7 | Parental | 0.015/0.015 | WT |
| Isogenic | 2/1 | ΔF658 | |
| 8 | Parental | 0.015/0.064 | WT |
| Isogenic | 1/2 | S663Y | |
| 9 | Parental | 0.015/0.032 | WT |
| Isogenic | 0.064/0.5 | D666N | |
| 10 | Parental | 0.015/0.032 | WT |
| Isogenic | 2/1 | S663P |
MYC, micafungin; AND, anidulafungin. Parental isolates were phenotypically echinocandin susceptible, and isogenic ones were phenotypically echinocandin resistant. Pairs 1 to 8 came from a previous study and involved susceptible isolates from blood samples exposed in vitro to either micafungin or anidulafungin and the corresponding resistant ones generated (27, 28). Pairs 9 and 10 originated in two patients with candidemia who developed concomitant echinocandin-resistant endocarditis. The parental and isogenic isolates in each pair proved to be genotypically identical. WT, wild type.
DISCUSSION
To the best of our knowledge, in this study we detected for the first time, using a Bactec FX automated blood culture system, echinocandin-resistant C. glabrata isolates present in low proportions, regardless of the type of FKS2 gene mutation or echinocandin MIC. ETDIR and assays with anidulafungin-containing agar plates performed directly with spiked positive blood cultures proved to be reliable procedures to detect echinocandin resistance in all the tested scenarios.
Current Infectious Diseases Society of America (IDSA) guidelines recommend echinocandin susceptibility testing on isolates causing fungemia, particularly in patients who had previously been exposed to echinocandins or infected by C. glabrata (10). The screening for echinocandin-resistant C. glabrata is a must, given the emergence of resistance in some institutions (4, 19, 20). The reasons for the differences in the rates of echinocandin resistance between institutions is unclear. These may be due to conditions of the blood culture systems that prevent resistant isolates from thriving or missed resistance detection when standard antifungal susceptibility testing methods, such as the EUCAST method, are used. Different proportions of susceptible/resistant isolates were spiked into the blood cultures. Thus, we performed antifungal susceptibility testing by preparing different inocula for the EUCAST method. When we tested a loopful from the slime, resistance was missed in 3 out of the 10 pairs with the lowest proportion of the resistant isolate. Not being able to detect resistance by the EUCAST method was not related to a FKS2 mutation or to the MIC (Table 3). Likewise, resistance was also missed in blood cultures with the lowest proportion of resistant isolates after picking up single colonies from the plates in three pairs. This implies that the preparation of inoculum suspensions following the EUCAST EDef 7.3.1 method (21) (selecting 4 to 5 colonies from the plate) does not ensure the detection of resistance, as shown by pairs 1, 3 and 8, from which only susceptible colonies were obtained from the bottles spiked with the lowest proportion of resistant isolates (Table 2).
Since the EUCAST procedure does not ensure the detection of resistant isolates, we studied alternative methods, such as ETDIR and assays with anidulafungin-containing plates. We had previously shown that ETDIR performed directly with positive blood cultures allowed detection of resistance to fluconazole and echinocandins (14–16). Furthermore, we studied ETDIR using cultures from bottles spiked with different proportions of echinocandin-susceptible/echinocandin-resistant C. glabrata isolates. ETDIR showed micafungin and anidulafungin MICs of ≥0.064 mg/liter and ≥0.125 mg/liter, respectively; the MIC values depended on the type of FKS2 mutation rather than on the proportion of resistant isolates and the inoculum spiked in the bottles. We did not spike the bottles with inocula containing only susceptible isolate in the pairs, because a previous study carried out by our group showed MICs of anidulafungin and micafungin of ≤0.047 mg/liter against the same isolates by ETDIR (16). A double ring of growth inhibition was observed in some cases with ETDIR; this phenomenon has previously been reported in other species, such as Candida lusitaniae with amphotericin B and Aspergillus fumigatus with caspofungin (22, 23). The wider that the inner halo is, the higher that the proportion of spiked resistant isolates is (Fig. 2). These results suggest that ETDIR can rapidly (24 h) determine the presence of heteroresistance in the blood cultures, which can be missed using the EUCAST standard procedure.
The assay with antifungal-containing plates, an inexpensive and easy procedure to rule out the presence of resistance, has recently been tested to screen antifungal resistance in Candida and Aspergillus (16, 24). In this study, we found that all cultures from anidulafungin-containing plates were positive, regardless of the proportion of resistant isolates or the inoculum used. However, the two detected growth patterns mirrored the MICs of the isolates: isolates with high MICs were easily detected after 24 h of incubation, whereas the other isolates, including the FKS wild-type, phenotypically resistant isolate, required up to 48 h of incubation. Likewise, in our previous study we showed that phenotypically susceptible isolates failed to grow on the plates (16).
The median number of Candida spp. circulating in the bloodstream has been estimated to be ≤1 CFU/ml (range, 0.1 and >1,000 CFU/ml), and the number for C. glabrata is lower than that for other species (25). Our experimental conditions simulated real-life candidemia (assuming that 10 ml of blood from venipuncture was inoculated in the bottles and that the lowest inoculum spiked [10 to 50 CFU/ml] mimicked a load of 1 to 5 CFU/ml circulating in the blood). Given that the inoculum did not seem to have a great impact on the results, our experimental conditions can be extrapolated to clinical samples.
There are certain limitations in this study. First, we studied only C. glabrata isolates; however, the emergence of resistance to echinocandins and/or to multiple antifungals mainly affects this species (4, 19, 20). Second, not all C. glabrata FKS1 and FKS2 gene mutations have been studied, although the most commonly reported substitution, S663, was included among the six mutations tested in this study (5). Third, studies should be carried out with automatic systems other than the Bactec system. Fourth, the reliability of our procedure for the detection of mutants in cases of candidemia episodes caused by Candida blood loads below 1 CFU/ml is unknown. Finally, although the procedure worked well in our hospital, future interlaboratory studies to validate the role of ETDIR are warranted.
In conclusion, the Bactec automatic system allows the detection of echinocandin-resistant C. glabrata isolates from blood cultures. However, when resistant isolates are underrepresented, their detection can be missed with the EUCAST standard procedure. ETDIR is a reliable and a rapid method to detect resistance to micafungin and anidulafungin, ensuring detection in potential situations of increasing echinocandin resistance.
MATERIALS AND METHODS
Isolates.
We studied 10 pairs of molecularly identified C. glabrata isolates (26) involving parental echinocandin-susceptible isolates causing candidemia and isogenic echinocandin-resistant ones either generated in vitro (n = 8) (27, 28) or recovered from the heart valves of patients with concomitant endocarditis (n = 2). Microsatellite markers showed that the parental and isogenic isolates had the same genotype (29). The characteristics of the isolates are shown in Table 3.
Inocula used to spike blood culture bottles.
McFarland 0.5 suspensions (corresponding to 1 × 106 to 5 × 106 CFU/ml) of each pair of susceptible and resistant isolates were prepared. The suspensions were diluted to 1 × 103 to 5 × 103, 1 × 102 to 5 × 102, and 10 to 50 CFU/ml. Finally, different proportions of susceptible/resistant isolates (9/1, 5/5, and 1/9) for each pair of each of the three tested inocula were prepared. The concentrations of the inocula and the proportions were confirmed through colony counting on Sabouraud dextrose agar plates (data not shown). Cultures from bottles previously inoculated with blood from patients that remained negative after 7 days of incubation were subsequently used for the experiments. The bottles were reincubated at 35°C under continuous agitation in a Bactec FX system until they were flagged as positive (range, 23.5 h to 65.5 h). One milliliter of each suspension was spiked in nonfungemic/bacteremic Bactec bottles (Bactec Plus Aerobic/F; Becton, Dickinson, Cockeysville, MD, USA) (9 bottles per pair) (see Fig. S1 in the supplemental material).
Antifungal susceptibility testing and screening for resistance.
Antifungal susceptibility was determined following the EUCAST standard procedure and procedures performed directly on blood cultures (ETDIR and resistance screening on anidulafungin-containing agar plates).
Five to 6 drops of the broth medium from the bottles flagged as positive were stroked onto Sabouraud dextrose agar plates, and the plates were incubated at 35°C for 24 h. A loopful of the slime growth was collected and suspended in water to examine susceptibility to micafungin and anidulafungin per the EUCAST EDef 7.3.1 method (21). Isolates were considered resistant to micafungin or anidulafungin when the MICs were above 0.032 mg/liter and 0.064 mg/liter, respectively. Additionally, to assess the proportion of echinocandin-susceptible and echinocandin-resistant colonies in each of the 9 bottles, the following volumes were stroked onto Sabouraud plates in triplicate, depending on the spiked inoculum: 10 µl (a 1:10 dilution of the 1 × 103- to 5 × 103-CFU/ml inoculum was prepared to obtain single colonies), 10 µl (1 × 102 to 5 × 102 CFU/ml), and 100 µl (10 to 50 CFU/ml). The plates were then incubated at 35°C for 48 h. We performed the EUCAST EDef 7.3.1 antifungal susceptibility test on single colonies (up to 10 colonies per bottle) for determination of susceptibility to micafungin and anidulafungin (21).
Five to 6 drops of the broth medium were stroked onto RPMI 1640 agar plates, and after placing the Etest strips for anidulafungin and micafungin, the plates were incubated at 35°C for 24 h (ETDIR). Isolates were classified as echinocandin resistant per the ETDIR MICs using the same clinical breakpoints of the EUCAST microdilution method (30). Five or 6 drops of the broth medium were stroked on Sabouraud agar plates containing 2 mg/liter of anidulafungin that were incubated at 35°C for 24 h. In the absence of growth at 24 h, the plates were incubated at 35°C for 48 h. Isolates growing on anidulafungin-containing agar plates were considered echinocandin resistant, as reported elsewhere (16).
Statistical analysis.
We calculated the total number of pooled resistant colonies from the bottles spiked with a given inoculum and compared the proportions of resistant colonies found in the three groups of bottles spiked with the different inocula (103, 102, and 10 CFU/ml). The comparison of proportions was done using a standard binomial method (95% confidence interval) (Epidat [version 3.1] software; Servicio de Información sobre Saúde Pública de la Dirección Xeral de Saúde Pública de la Consellería de Sanidade, Xunta de Galicia, Spain).
Ethical considerations.
This study was approved by the Ethics Committee of the Hospital Gregorio Marañón (CEIC-A1; study no. 208/16).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dainora Jaloveckas (Ciencia Traducida) for editing and proofreading assistance.
This work was supported by grants PI14/00740, PI16/01012, and MSI15/00115 from the Fondo de Investigación Sanitaria (FIS; Instituto de Salud Carlos III, Plan Nacional de I+D+I 2013-2016) and cofunded by the European Regional Development Fund (FEDER), A way of making Europe. P.E. (CPI15/00115) and J.G. (CPII15/00006) are the recipients of a Miguel Servet contract supported by the FIS; M.A.B.-C. received a predoctoral grant from the Instituto de Investigación Sanitaria Gregorio Marañón (II-Predoc-2016-IISGM).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02004-18.
REFERENCES
- 1.Guinea J, Zaragoza Ó, Escribano P, Martín-Mazuelos E, Pemán J, Sánchez-Reus F, Cuenca-Estrella M. 2014. Molecular identification and antifungal susceptibility of yeast isolates causing fungemia collected in a population-based study in Spain in 2010 and 2011. Antimicrob Agents Chemother 58:1529–1537. doi: 10.1128/AAC.02155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 3.Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arendrup MC, Perlin DS. 2014. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigues CF, Silva S, Henriques M. 2014. Candida glabrata: a review of its features and resistance. Eur J Clin Microbiol Infect Dis 33:673–688. doi: 10.1007/s10096-013-2009-3. [DOI] [PubMed] [Google Scholar]
- 7.Shields RK, Nguyen MH, Press EG, Kwa AL, Cheng S, Du C, Clancy CJ. 2012. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob Agents Chemother 56:4862–4869. doi: 10.1128/AAC.00027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields RK, Nguyen MH, Press EG, Clancy CJ. 2014. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob Agents Chemother 58:7601–7605. doi: 10.1128/AAC.04134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen RH, Johansen HK, Soes LM, Lemming LE, Rosenvinge FS, Nielsen L, Olesen B, Kristensen L, Dzajic E, Astvad KM, Arendrup MC. 2015. Posttreatment antifungal resistance among colonizing Candida isolates in candidemia patients: results from a systematic multicenter study. Antimicrob Agents Chemother 60:1500–1508. doi: 10.1128/AAC.01763-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Matta DA, Souza ACR, Colombo AL. 2017. Revisiting species distribution and antifungal susceptibility of Candida bloodstream isolates from Latin American medical centers. J Fungi (Basel) 3:E24. doi: 10.3390/jof3020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A, Alvarez-Fernandez M, Cantón E, Carver PL, Chen SC-A, Eschenauer G, Getsinger DL, Gonzalez GM, Govender NP, Grancini A, Hanson KE, Kidd SE, Klinker K, Kubin CJ, Kus JV, Lockhart SR, Meletiadis J, Morris AJ, Pelaez T, Quindós G, Rodriguez-Iglesias M, Sánchez-Reus F, Shoham S, Wengenack NL, Borrell Solé N, Echeverria J, Esperalba J, Gómez-G de la Pedrosa E, García García I, Linares MJ, Marco F, Merino P, Pemán J, Pérez del Molino L, Roselló Mayans E, Rubio Calvo C, Ruiz Pérez de Pipaon M, Yagüe G, Garcia-Effron G, Guinea J, Perlin DS, Sanguinetti M, Shields R, Turnidge J. 2015. Multicenter study of epidemiological cutoff values and detection of resistance in Candida spp. to anidulafungin, caspofungin, and micafungin using the Sensititre YeastOne colorimetric method. Antimicrob Agents Chemother 59:6725–6732. doi: 10.1128/AAC.01250-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlin DS. 2015. Mechanisms of echinocandin antifungal drug resistance. Ann N Y Acad Sci 1354:1–11. doi: 10.1111/nyas.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guinea J, Recio S, Escribano P, Torres-Narbona M, Pelaez T, Sanchez-Carrillo C, Rodriguez-Creixems M, Bouza E. 2010. Rapid antifungal susceptibility determination for yeast isolates by use of Etest performed directly on blood samples from patients with fungemia. J Clin Microbiol 48:2205–2212. doi: 10.1128/JCM.02321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escribano P, Marcos-Zambrano LJ, Gomez A, Sanchez C, Martinez-Jimenez MC, Bouza E, Guinea J. 2017. The Etest performed directly on blood culture bottles is a reliable tool for detection of fluconazole-resistant Candida albicans isolates. Antimicrob Agents Chemother 61:e00400-17. doi: 10.1128/AAC.00400-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordallo-Cardona MA, Marcos-Zambrano LJ, Sanchez-Carrillo C, Bouza E, Munoz P, Escribano P, Guinea J. 2018. Resistance to echinocandins in Candida can be detected by performing the Etest directly on blood culture samples. Antimicrob Agents Chemother 62:e00162-18. doi: 10.1128/AAC.00162-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jekarl DW, Lee SY, Lee S, Park YJ, Lee J, Baek SM, An YJ, Ock SM, Lee MK. 2012. Comparison of the Bactec Fx Plus, Mycosis IC/F, Mycosis/F Lytic blood culture media and the BacT/Alert 3D FA media for detection of Candida species in seeded blood culture specimens containing therapeutic peak levels of fluconazole. J Clin Lab Anal 26:412–419. doi: 10.1002/jcla.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riedel S, Eisinger SW, Dam L, Stamper PD, Carroll KC. 2011. Comparison of BD Bactec Plus Aerobic/F medium to VersaTREK Redox 1 blood culture medium for detection of Candida spp. in seeded blood culture specimens containing therapeutic levels of antifungal agents. J Clin Microbiol 49:1524–1529. doi: 10.1128/JCM.02260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, Schaffner W, Beldavs ZG, Chiller TM, Park BJ, Cleveland AA, Lockhart SR. 2014. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother 58:4690–4696. doi: 10.1128/AAC.03255-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Astvad KMT, Johansen HK, Roder BL, Rosenvinge FS, Knudsen JD, Lemming L, Schonheyder HC, Hare RK, Kristensen L, Nielsen L, Gertsen JB, Dzajic E, Pedersen M, Ostergard C, Olesen B, Sondergaard TS, Arendrup MC. 2017. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol 56:e01564-17. doi: 10.1128/JCM.01564-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J. 2017. EUCAST definitive document E.Def 7.3.1 method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. EUCAST. http://www.eucast.org.
- 22.Peyron F, Favel A, Michel-Nguyen A, Gilly M, Regli P, Bolmström A. 2001. Improved detection of amphotericin B-resistant isolates of Candida lusitaniae by Etest. J Clin Microbiol 39:339–342. doi: 10.1128/JCM.39.1.339-342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arendrup MC, Perkhofer S, Howard SJ, Garcia-Effron G, Vishukumar A, Perlin D, Lass FC. 2008. Establishing in vitro-in vivo correlations for Aspergillus fumigatus: the challenge of azoles versus echinocandins. Antimicrob Agents Chemother 52:3504–3511. doi: 10.1128/AAC.00190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arendrup MC, Verweij PE, Mouton JW, Lagrou K, Meletiadis J. 2017. Multicentre validation of 4-well azole agar plates as a screening method for detection of clinically relevant azole-resistant Aspergillus fumigatus. J Antimicrob Chemother 72:3325–3333. doi: 10.1093/jac/dkx319. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer CD, Samsa GP, Schell WA, Reller LB, Perfect JR, Alexander BD. 2011. Quantitation of Candida CFU in initial positive blood cultures. J Clin Microbiol 49:2879–2883. doi: 10.1128/JCM.00609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 27.Bordallo-Cardona MA, Escribano P, de la Pedrosa EG, Marcos-Zambrano LJ, Canton R, Bouza E, Guinea J. 2017. In vitro exposure to increasing micafungin concentrations easily promotes echinocandin resistance in Candida glabrata isolates. Antimicrob Agents Chemother 61:e01542-16. doi: 10.1128/AAC.01542-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bordallo-Cardona MA, Marcos-Zambrano LJ, Sanchez-Carrillo C, de la Pedrosa EGG, Canton R, Bouza E, Escribano P, Guinea J. 2018. Mutant prevention concentration and mutant selection window of micafungin and anidulafungin in clinical Candida glabrata isolates. Antimicrob Agents Chemother 62:e01982-17. doi: 10.1128/AAC.01982-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordallo-Cardona M, Agnelli C, Gómez-Nunez A, Sánchez-Carrillo C, Bouza E, Muñoz P, Escribano P, Guinea J. 2019. MSH2 gene point mutations are not antifungal resistance markers in Candida glabrata. Antimicrob Agents Chemother 63:e01876-18. doi: 10.1128/AAC.01876-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Committee on Antimicrobial Susceptibility Testing. 2018. Antifungal agents. Breakpoint tables for interpretation of MICs. http://www.eucast.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.