Toxoplasma gondii is one of the most widespread obligatory parasitic protozoa and infects nearly all warm-blooded animals, leading to toxoplasmosis. The therapeutic drugs currently administered, like the combination of pyrimethamine and sulfadiazine, show high rates of toxic side effects, and drug resistance is encountered in some cases.
KEYWORDS: Toxoplasma gondii, apicomplexan parasites, host-parasite relationship, intracellular parasites, resveratrol
ABSTRACT
Toxoplasma gondii is one of the most widespread obligatory parasitic protozoa and infects nearly all warm-blooded animals, leading to toxoplasmosis. The therapeutic drugs currently administered, like the combination of pyrimethamine and sulfadiazine, show high rates of toxic side effects, and drug resistance is encountered in some cases. Resveratrol is a natural plant extract with multiple functions, such as antibacterial, anticancer, and antiparasite activities. In this study, we evaluated the inhibitory effects of resveratrol on tachyzoites of the Toxoplasma gondii RH strain extracellularly and intracellularly. We demonstrate that resveratrol possesses direct antitoxoplasma activity by reducing the population of extracellularly grown tachyzoites, probably by disturbing the redox homeostasis of the parasites. Moreover, resveratrol was also able to release the burden of cellular stress, promote apoptosis, and maintain the autophagic status of macrophages, which turned out to be regulated by intracellular parasites, thereby functioning indirectly in eliminating T. gondii. In conclusion, resveratrol has both direct and indirect antitoxoplasma effects against RH tachyzoites and may possess the potential to be further evaluated and employed for toxoplasmosis treatment.
INTRODUCTION
Toxoplasma gondii is one of the obligatory parasitic protozoa which inhabits the nucleated cells of nearly all warm-blooded animals, including humans. Humans and other intermediate hosts become infected by ingestion of the sporulated oocysts shed by cats, the definitive hosts. At the same time, the cysts in raw or undercooked meat can also infect humans by mouth. It has been reported that approximately 1/3 of the world’s population is seropositive for toxoplasma infection (1). The disease caused by Toxoplasma gondii is called toxoplasmosis. Patients with competent immunity who become infected by this parasite show only mild clinical symptoms. However, severe clinical evidence of disease may occur if the immunity of the host, particularly AIDS patients, is compromised (2) and if infection is acquired for the first time by vertical transmission in women early during pregnancy (3). The typical manifestations include lesions of the central nervous system (CNS), toxoplasmic ophthalmopathy, and pneumonia (4). The pathogenesis of toxoplasmosis mainly occurs through the invasion, proliferation, and toxicity of the tachyzoites, which play a vital role in the morbidity and mortality from the disease. On the other hand, the parasites undergo a process of morphological transformation from tachyzoites into bradyzoites in hosts with a competent immune system, migrating into tissues, where they exist as cysts.
First-line therapy for toxoplasmosis consists of pyrimethamine and sulfadiazine (5). However, clinical trials of this combination have shown that it has high rates of toxic side effects (6), leading to the discontinuation of therapy. The well-known side effects of therapy including nausea, vomiting, allergy to sulfa drugs, and even abnormal liver function (5, 7). In addition, drug resistance is also suspected to be one of the causes of treatment failure (8, 9). Moreover, no specific therapeutic agent or regimen evaluated to date has been shown to be capable of clearing chronic infection in humans or in livestock animals (10, 11). Thus, the development and screening of new therapeutic drugs with less toxicity and better efficacy or an adjunctive therapy to reduce the toxicity and promote the curative effects of the current treatment are essential.
Resveratrol (3,4′,5-trihydroxystilbene) is a polyphenol naturally found in plants and fruits, including black grapes, mulberries, and also peanuts (12–15). Considered a powerful antioxidant, this biological compound has been reported to have antibacterial, antifungal, anticancer, and antiparasite activities (12, 15–18). Furthermore, it is well tolerated at a relatively high dose (19, 20). Previous studies indicated the potential activity of resveratrol against both promastigotes and amastigotes of Leishmania parasites (20). Moreover, resveratrol has also been shown to reduce oxidative damage and to prevent the behavior changes seen in T. gondii-infected mice when administered together with traditional therapeutic drugs (21–23). However, the mechanism behind the interactions between the compound, the parasites, and host cells remains unclear. This study aimed to assess the inhibitory effects of resveratrol on tachyzoites of a type I Toxoplasma gondii strain (the RH strain) under both extracellular and intracellular growth conditions by evaluating its impacts on the cell cycle, cell death, and oxidative stress of the parasite, as well as its synergistic role and mechanism of limiting the intracellular proliferation of T. gondii inside host macrophages, revealing the mechanism by which this plant extract eliminates T. gondii directly and indirectly.
RESULTS
Growth of tachyzoites extracellularly.
We first examined the inhibitory effects of resveratrol against RH tachyzoites at different concentrations by directly adding resveratrol into the extracellular cultivation system of tachyzoites for 24 h. Our results showed a dose-dependent inhibitory activity of resveratrol with a 50% inhibitory concentration (IC50) of 54.61 μM, whereas the IC50 of the positive control, pyrimethamine, was 17.78 μM. Moreover, at concentrations lower than 5 μM, both stimuli showed similar inhibitory abilities, while at concentrations higher than 5 μM, pyrimethamine possessed a greater inhibitory ability than resveratrol (Fig. 1). At the highest concentration tested of 200 μM, resveratrol was able to inhibit nearly 70% of the tachyzoite population, which was a rate just slightly lower than that for pyrimethamine, which had an inhibitory rate of approximately 80%.
FIG 1.
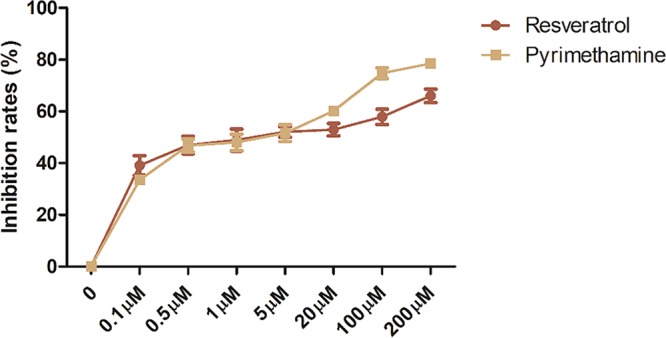
Rates of inhibition of RH tachyzoites by different stimuli. When incubated with different concentrations of resveratrol and pyrimethamine ranging from 0 to 200 μM, the extracellularly grown tachyzoites were terminated on an ascending trend. Resveratrol showed an inhibitory ability similar to that of pyrimethamine except at concentrations higher than 5 μM. The highest concentrations of resveratrol revealed a rate of inhibition of approximately 70%, while the same concentration of pyrimethamine showed a greater inhibitory ability by inhibiting approximately 80% of the growth of the tachyzoites. The results are shown as the means ± SEM from four independent experiments.
Cell death of tachyzoites.
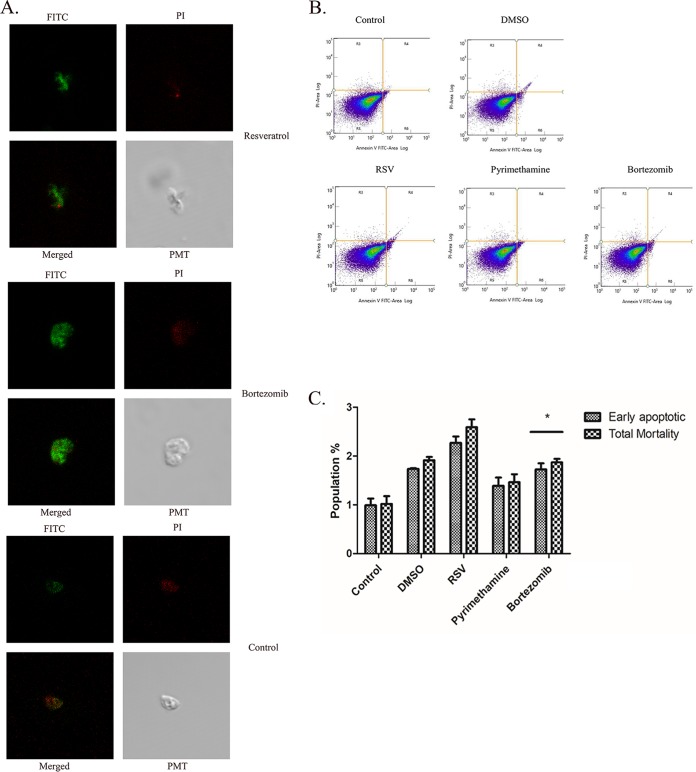
Since resveratrol has been considered to alter the signaling pathway for apoptosis in many tumor cells (24, 25) and we already saw its potential for RH tachyzoite inhibition, we were interested in examining whether it could promote phosphatidylserine (PS) exposure as a marker of the initiation of the early apoptotic process. We stimulated RH tachyzoites with resveratrol (50 μM) for 12 h, followed by PS detection through annexin V-fluorescein isothiocyanate (FITC) labeling. Resveratrol-treated tachyzoites showed visible PS exposure compared to the control group when the tachyzoites were examined under a confocal microscope (Fig. 2A). However, when subjected to flow cytometry for further counting, neither the early apoptotic mortality nor the total mortality (both early and late apoptotic/necrotic) ratio for the resveratrol-treated group was significantly different from that for the DMSO solvent control-treated group (Fig. 2B and C), although we did see a trend toward an increase in the value of each index for the parasites stimulated with resveratrol. In addition, the traditional therapeutic drug pyrimethamine also failed to induce a substantial increase in cell death. As a positive control for apoptosis, bortezomib (26) was able to induce the rate of death of RH tachyzoites compared to that for the control group, but only on a minor scale.
FIG 2.
The death of RH tachyzoites induced by different stimuli. (A) Fluorescent graphics showing the early apoptosis and the total mortality of RH tachyzoites incubated with 50 μM resveratrol for 12 h. Resveratrol was able to initiate PS exposure on the cell membrane of the tachyzoites, which was recognized as green fluorescence. At the same time, it also resulted in a mid to late stage of apoptosis or necrosis, as seen by the red fluorescence indicating the destruction of the cell membrane. FITC, green fluorescence; PI, red fluorescence; PMT (photomultiplier tube), light field with PMT as the detector and laser 488 nm as the light source. (B, C) However, when subjected to flow cytometry (B) and analyzed by use of a bar chart graph (C), it was revealed that resveratrol failed to induce a significant increase in either early apoptotic or total mortality of RH tachyzoites compared to that for the DMSO solvent control-treated group. Meanwhile, the therapeutic drug pyrimethamine (20 μM) showed an even weaker ability than resveratrol to induce either early apoptosis or total mortality. Although bortezomib (50 nM), the classical apoptosis inducer, was found to successfully induce early apoptosis and increase total mortality compared to the control, the percentage of dead tachyzoites was only about 2%. RSV, resveratrol. The results are shown as the means ± SEM from three independent experiments. *, P < 0.05.
Cell cycle of tachyzoites.
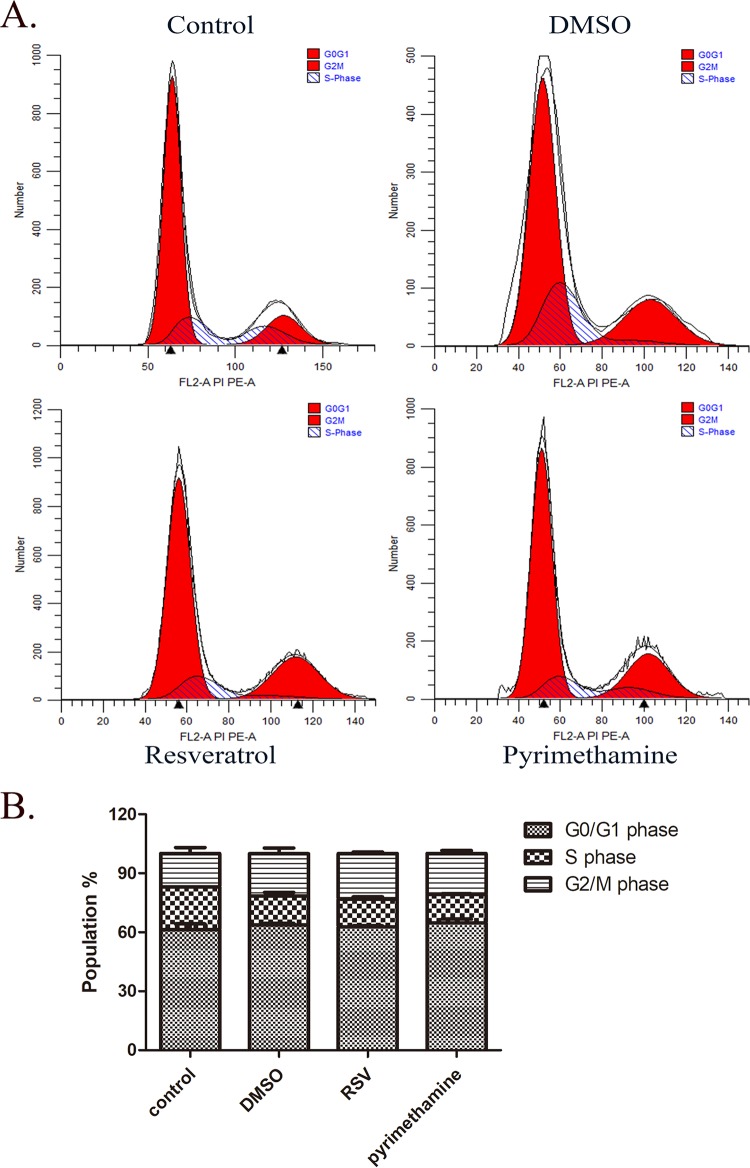
Next, we further examined whether the cell cycle of extracellularly growing tachyzoites could be changed by resveratrol. We stimulated RH tachyzoites with 50 μM resveratrol and pyrimethamine as the reference compound for 12 h, labeled the tachyzoites with propidium iodide (PI) in cell cycle solution, and analyzed the tachyzoites by flow cytometry. However, we could not see a significant arrest of the growth of treated tachyzoites at any stage of the cell cycle over 12 h (Fig. 3A and B; Table 1). Notably, 20 μM pyrimethamine also did not have an appreciable impact on the cell cycle of RH tachyzoites at the same time point.
FIG 3.
Cell cycle of RH tachyzoites stimulated by resveratrol. (A) RH tachyzoites were incubated with resveratrol (50 μM) for 12 h, followed by detection of alteration of the cell cycle by employing flow cytometry. (B) The corresponding bar chart shows that G0/G1 phase accounted for the largest proportion of all three stages, demonstrating no apparent alteration when tachyzoites were challenged with resveratrol, pyrimethamine, or DMSO. Although G2/M phase arrest was observed with stimulation with resveratrol, it was not significantly different from that for the DMSO control. Moreover, pyrimethamine (20 μM) also failed to change the ratio of cells in the three stages in the cell cycle compared to that for the DMSO control. RSV, resveratrol. These results represent the means ± SEM from two independent experiments performed in duplicate.
TABLE 1.
Cell cycle of RH tachyzoites incubated with stimulia
| Treatment | Mean ± SEM % of cells in: |
||
|---|---|---|---|
| G0/G1 phase | S phase | G2/M phase | |
| Control | 61.29 ± 2.94 | 21.8 ± 0.08 | 16.91 ± 3.02 |
| DMSO | 63.61 ± 0.98 | 14.74 ± 1.87 | 21.66 ± 2.84 |
| RSV | 62.73 ± 0.50 | 13.99 ± 1.28 | 23.29 ± 0.79 |
| Pyrimethamine | 64.78 ± 1.91 | 14.45 ± 0.36 | 20.78 ± 1.55 |
Values represent means ± SEM from two independent experiments performed in duplicate. RSV, resveratrol.
SOD activity and ROS production of tachyzoites.
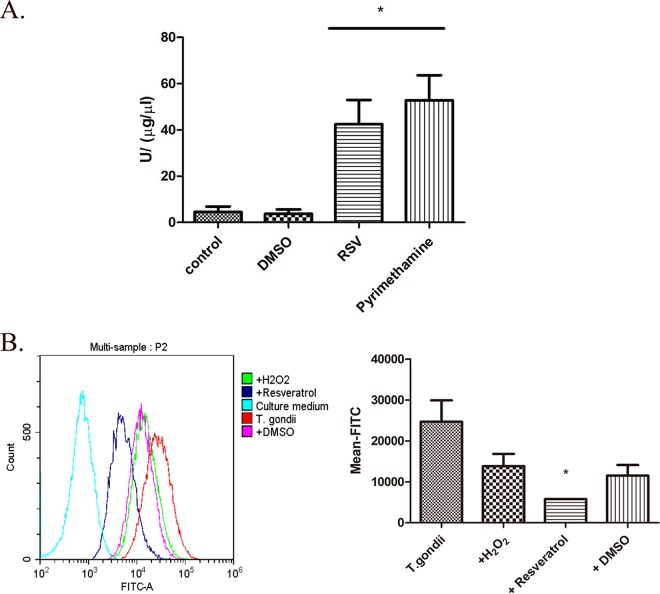
Since resveratrol incubated with RH tachyzoites revealed ideal inhibitory activity over the long term (24 h) but failed to change either the cell cycle or the apoptosis of the parasites over the shorter term (12 h), we attributed our observation to the possibility that RH tachyzoites might have a particular ability to withstand the stimuli, thereby delaying cellular damage. Superoxide dismutase (SOD) is one of the most important antioxidants produced by parasitic protozoa to maintain homeostasis and prevent themselves from being eliminated by host immune cells (27). Therefore, we assessed the SOD activity of RH tachyzoites challenged by resveratrol and pyrimethamine and discovered that RH tachyzoites upregulated their SOD activity significantly for their survival in the presence of both compounds (Fig. 4). On the other hand, we also investigated whether the level of production of reactive oxygen species (ROS) by tachyzoites stimulated by resveratrol could be elevated. Surprisingly, we discovered that 50 µM resveratrol was able to abate the ROS production of tachyzoites to a very low level in 12 h of incubation. This level was even lower than the baseline level of ROS produced by tachyzoites without any addition, and this activity was not due to dimethyl sulfoxide (DMSO) (since the solvent alone was not able to reduce ROS production). Moreover, the same trend toward a decrease in the level of ROS production was displayed with H2O2, which was employed as a positive control, though the decrease was not significant, indicating the instability of the intracellular redox balance. Taken together, the dramatically increased activity of SOD as well as the abnormally low level of production of ROS revealed that the tachyzoites were under a circumstance of an imbalanced redox system in the inner environment induced by resveratrol or at least by its solvent, DMSO.
FIG 4.
SOD activity and ROS production of RH tachyzoites incubated with different stimuli. (A) Extracellularly grown tachyzoites were incubated with or without 50 μM resveratrol, 20 μM pyrimethamine, or 30 μM H2O2 for 12 h and lysed or collected for detection of SOD activity and ROS production. Resveratrol-treated tachyzoites showed significant upregulated SOD activity compared to the negative and DMSO controls. In addition, pyrimethamine, employed as a therapeutic medication control, resulted in a slightly greater increase in SOD activity than that produced by resveratrol. (B) The bar chart derived from the mean FITC values for each experimental group by flow cytometry indicated that resveratrol (dissolved in DMSO) significantly decreased the level of ROS production by the tachyzoites compared to that for the negative control (tachyzoites without any addition), while neither the positive control (H2O2) nor the solvent control (DMSO) was able to reduce the ROS level of T. gondii. The columns in the graph refer to T. gondii without any addition or to which H2O2, resveratrol (dissolved in DMSO), or DMSO alone was added. The values shown are the means ± SEM from three independent experiments. RSV, resveratrol. *, P < 0.05.
Intracellular growth.
After we examined the extracellular inhibitory effects of resveratrol against RH tachyzoites, we further elucidated the role of resveratrol in the process of tachyzoite elimination by the innate immunity. Therefore, we infected mouse RAW 264.7 macrophages with RH tachyzoites, followed by challenge with resveratrol for 24 h. By counting the number of tachyzoites in each infected cell, we demonstrated that macrophages stimulated with resveratrol had a greater ability to kill the intracellular parasites (Fig. 5), similar to the result obtained for the pyrimethamine group.
FIG 5.
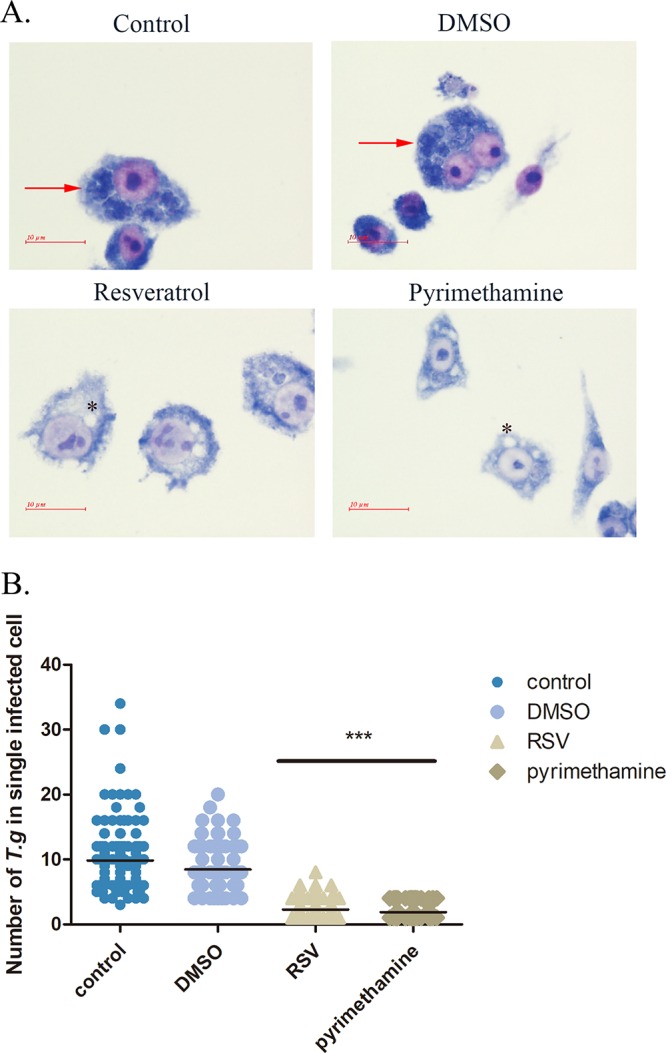
Intracellular growth of RH tachyzoites inside mouse macrophages with resveratrol in vitro. RH tachyzoites were harvested to infect RAW 264.7 mouse macrophages at an infection ratio of 5:1 (parasite/host cell) for 2 h, followed by the removal of excess parasites and stimulation with resveratrol (50 μM) and pyrimethamine (20 μM) for 24 h and further examination by staining with Wright’s stain. (A) The infected macrophages or the infected macrophages treated with DMSO had much heavier intracellular tachyzoite burdens than infected macrophages incubated with resveratrol and pyrimethamine. Arrows and asterisks, tachyzoites and empty vacuoles inside host cells, respectively. (B) A scattered distribution chart showing the means of the intracellular tachyzoite counts. Values represent actual numbers (scattered dots) and means (black line) from 2 independent experiments; in each case, at least 200 infected cells were counted. T.g, T. gondii tachyzoites; RSV, resveratrol. ***, P < 0.001.
Host cell autophagic status.
Autophagy has been considered one of the important strategies of the host to facilitate the intracellular elimination of parasites (28). After we obtained the results, presented abovem that indicated that resveratrol assists in eradicating the parasites from host cells, we had an interest to pursue the possible mechanism behind this effect. Since autophagy is a dynamic process of signal transduction, we decided to explore its initiation and execution by assessing autophagy at three different time points, representing the early response stage and the middle and late periods of autophagy of the infected macrophages, respectively. Surprisingly, we observed that the autophagic process initiated by infection of RH tachyzoites at the early time point was gradually neutralized with the growth of the parasites. The concurrent introduction of resveratrol and rapamycin not only was able to maintain a relatively high LC3 II level but also reduced the numbers of intracellular parasites, indicating that resveratrol plays a pivotal role in consistently promoting the execution of autophagy to benefit the elimination of host cells infected with parasites. Furthermore, we incubated uninfected macrophages with resveratrol for the same three times and also observed LC3 II produced by the lipidation of LC3 I, though it was very vague, during the early to late incubation stages, suggesting that host macrophages might be primed but not totally triggered for an autophagic program by resveratrol (Fig. 6).
FIG 6.
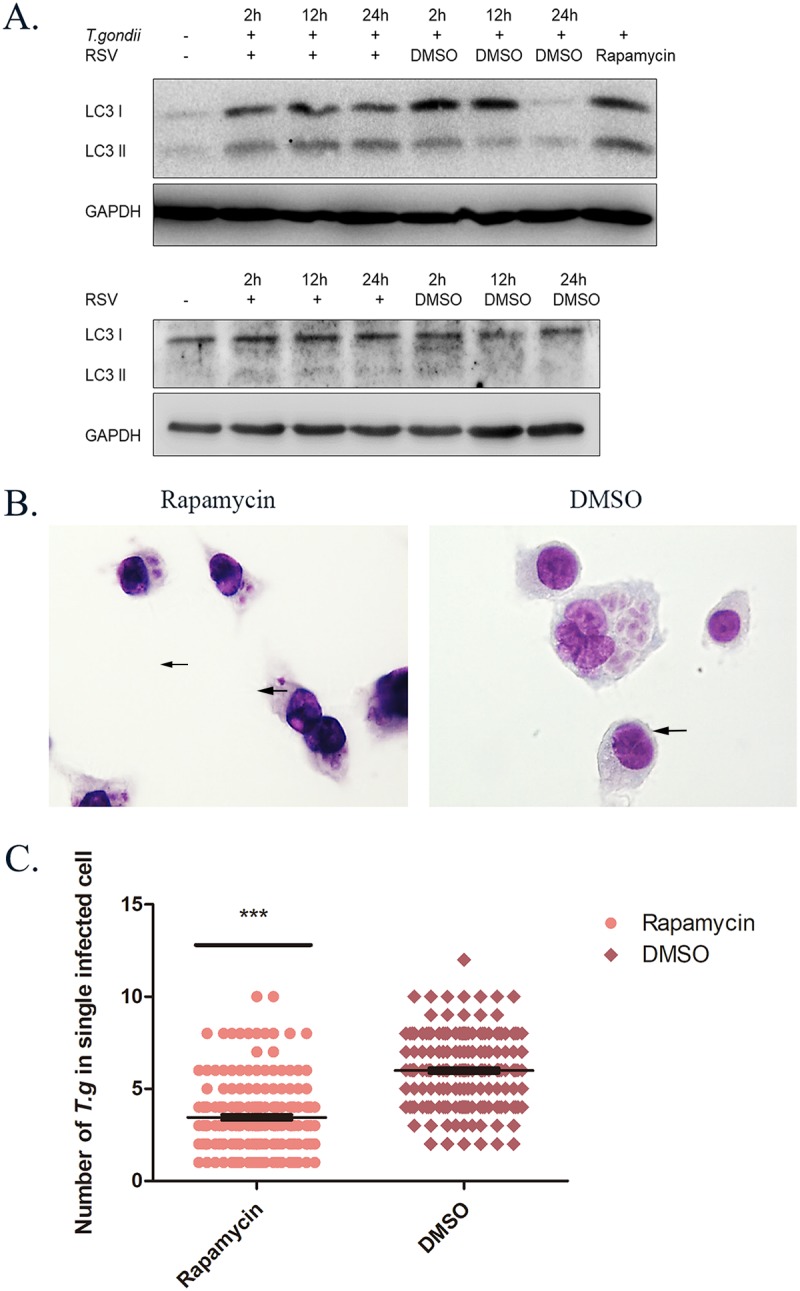
Intracellular autophagic protein-related killing effects of infected macrophages stimulated with resveratrol at different time points. RAW 264.7 macrophages were either infected with RH tachyzoites at a ratio of 5:1 (parasite/host cell) for 2 h or left in the culture plate without infection, followed by incubation with resveratrol (50 μM) or DMSO (1:2,000) for 2 h, 12 h, or 24 h and rapamycin (100 nM) or DMSO (1:10,000) for 24 h before collection of the cell lysates. (A) Infected macrophages treated with DMSO showed an upregulation of LC3 I at 2 h and 12 h but not at 24 h compared to that for the negative control. Meanwhile, LC3 II production was found to be increased the most at 2 h, followed by a gradual reduction of production from 12 h to 24 h. When incubated with resveratrol, both the LC3 I and the LC3 II production of the infected macrophages was consistently elevated compared to that for the negative control from 2 h to 24 h. Rapamycin was employed as an autophagy inducer and could also upregulate the production of both LC3 I and LC3 II. On the other hand, the uninfected macrophages incubated with resveratrol revealed a slight upregulation of LC3 II at 2 h and maintained its production until 24 h, while the DMSO solvent failed to maintain LC3 II production over time. At the same time, uninfected cells stimulated with resveratrol and the DMSO solvent showed comparable LC3 I levels. (B, C) Meanwhile, when the infected cells were stimulated with rapamycin for 24 h, the intracellular population of RH tachyzoites was reduced significantly compared to that for the DMSO control. These results were obtained from three independent experiments. The values shown are the means ± SEM from three independent experiments. The arrows indicate the tachyzoites inside host cells. RSV, resveratrol. ***, P < 0.001.
Cellular stress.
The metabolism and proliferation of the intracellular parasites may induce cellular stress in various dimensions. Since the survival, proliferation, and activities of certain kinases of the parasites rely on calcium (29, 30), host cells are very likely prone to alteration of their cellular calcium homeostasis. The endoplasmic reticulum (ER) is one of the organelles that are in great demand of intracellular calcium to fold proteins. Under the circumstance of abnormal intracellular calcium flow and the subsequent accumulation of unfolded proteins, a process named ER stress is initiated. In this case, a variety of chaperones and transmembrane signal regulators are upregulated to assist the ER in recovering from the abnormal conditions. However, the overloaded burden directly leads to the translocation of the ER overload response (EOR) signals to the nucleus, thus initiating apoptosis. C/EBP homologous protein (CHOP), also known as growth arrest and DNA damage-inducible gene 153 (GADD153), is one of the ER stress-apoptosis inducers that is able to promote apoptosis by inhibiting Bcl-2 or activating caspase-12 (31). We evaluated the production of CHOP from both infected and uninfected macrophages at 2 h, 12 h, and 24 h and found that resveratrol reversed the increase in the level of CHOP in macrophages infected by RH tachyzoites, indicating that resveratrol was able to alleviate the ER stress and retard the ER stress-induced apoptosis caused by growth of the parasites (Fig. 7). Interestingly, when we tried to investigate the apoptotic process by examining X-linked inhibitor of apoptosis protein (XIAP), the protein that most potently inhibits apoptosis (32), the result revealed that resveratrol reversed the upregulation of XIAP of host cells induced by parasites and even maintained its production at a relatively low level over time in the uninfected macrophages (Fig. 7). Considering the initial reduction in the level of XIAP production at the 2-h time point compared to that in uninfected cells, the early apoptosis-related eradication activity of host cells might be suppressed by the parasites to facilitate their growth.
FIG 7.
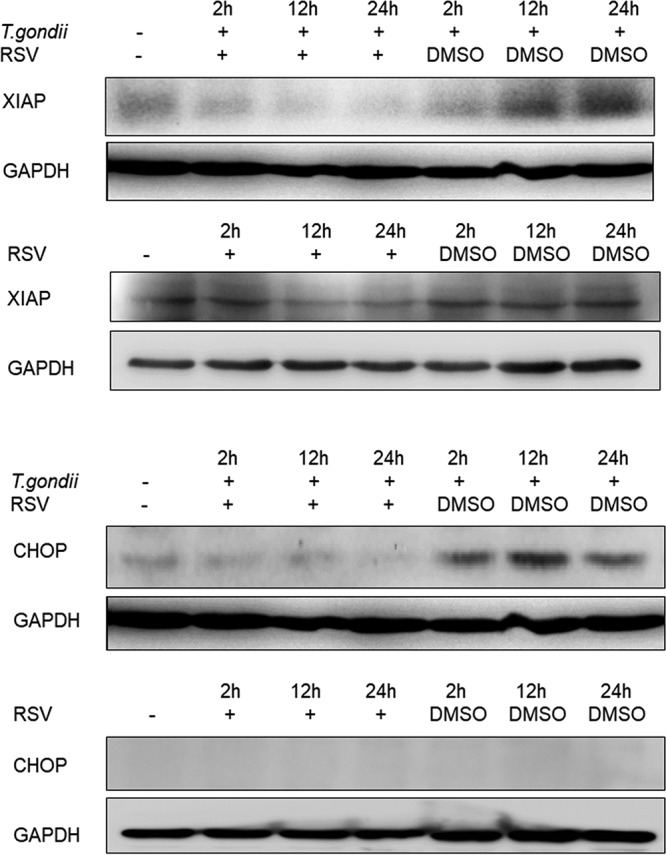
CHOP and XIAP production by infected macrophages stimulated with resveratrol. Infected RAW 264.7 mouse macrophages were stimulated with resveratrol for 2 h, 12 h, and 24 h before collection of the cell lysates. When infected by RH tachyzoites at the three different time points, the mouse macrophages displayed increased production of CHOP, while resveratrol could decrease the production of CHOP to the baseline level compared to that in uninfected cells. Meanwhile, even though a decrease in XIAP production was found in infected cells during the early infection time point (2 h) compared to that in uninfected cells, RH tachyzoites gradually increased the level of XIAP production in host cells over time. Resveratrol was discovered to turn over the upregulation by decreasing XIAP production in the infected cells to a level than even lower that in uninfected cells. Moreover, resveratrol was able to downregulate XIAP production over time even without infection. RSV, resveratrol.
Cytokines.
Cytokines play a vital role in mediating the killing of parasites by the host. We evaluated whether resveratrol was able to regulate the production of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10), which promote parasite killing and favor parasite survival, respectively. According to our data, resveratrol successfully induced TNF-α production by macrophages when they were infected by RH tachyzoites (Fig. 8). In addition, this induction ability was greater than that of pyrimethamine, indicating that, as a synergistic reagent, the parasite killing efficacy of resveratrol is better than that of pyrimethamine. On the other hand, resveratrol failed to interfere with the production of IL-10 by macrophages infected by RH tachyzoites at a significant level (Fig. 8).
FIG 8.
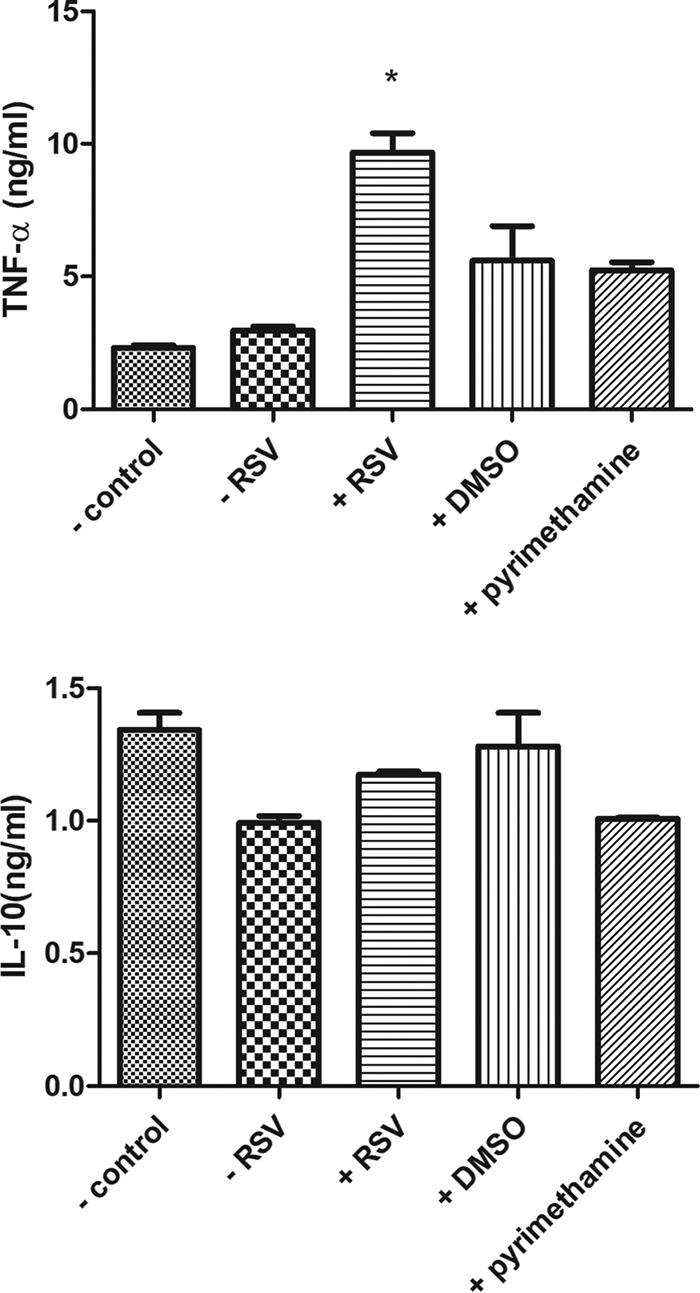
Cytokines produced by host cells infected by RH tachyzoites with different stimuli. When infected by RH tachyzoites for 24h, mouse macrophages failed to produce significantly more TNF-α or less IL-10 than uninfected cells. Incubation of resveratrol with the infected cells could elevate the production of TNF-α but had no impact on IL-10 production in these cells. Meanwhile, pyrimethamine had an ignorable effect on the production of the two cytokines. −, uninfected by RH tachyzoites with or without reagents; +, infected by RH tachyzoites with reagents; RSV, resveratrol. The values shown are the means ± SEM from three independent experiments. *, P < 0.05.
DISCUSSION
The polyphenol resveratrol has been widely studied for its potential for the treatment of multiple different diseases. The biological features of this compound enable it to act against several medically important protozoa, including leishmania and amoeba (12, 20). It has been demonstrated that resveratrol has antitoxoplasma activity as well as a synergistic therapeutic effect that is able to alleviate liver damage, regulate oxidant and antioxidant levels, and prevent the alteration of the behavior of infected mice in vivo as a result of its antioxidant and anti-inflammatory functions (21). Recently, it has also been considered to be a therapy for congenital toxoplasmosis because of its ability to restore the neurogliogenesis of neural progenitor cells infected by T. gondii (33). However, whether resveratrol has an inhibitory potential on T. gondii and the integrated mechanism of its synergistic role in eliminating intracellular parasites remain blurred. Therefore, we expected to further characterize the antitoxoplasma specialty of resveratrol by evaluating the interaction of this compound with extracellularly and intracellularly growing tachyzoites of the RH strain of T. gondii. The RH strain belongs to type I T. gondii, which represents the most virulent of the three main clonal lineages in its population structure (34). Mice infected by the RH strain develop severe acute clinical symptoms, leading to death in a very short time. We initially profiled the inhibitory effect of resveratrol against RH tachyzoites by drawing an inhibitory line suggesting a 50% inhibitory concentration (IC50) of about 50 μM, whereas pyrimethamine, one of the first-line drugs used clinically, has an IC50 of about 20 μM. In addition, although the highest concentration that we chose for use in the experiment (200 μM for both resveratrol and pyrimethamine) could lead to high rates of inhibition, the solvent DMSO also contributed to the inhibitory effect to some degree, revealing that the actual inhibition rates should be about or less than 80% for both compounds at 200 μM. Interestingly, at low concentrations, such as 1 μM or less, resveratrol showed an inhibitory ability comparable to that of pyrimethamine. Hence, resveratrol could be a slightly milder inhibiting reagent than pyrimethamine but nevertheless a significant inhibitor. Consequently, the IC50 of each compound (approximately 50 μM and 20 μM for resveratrol and pyrimethamine, respectively) was chosen for further stimulation and examination.
To illustrate the mechanism by which resveratrol acts against RH tachyzoites, we carried out a series of experiments to evaluate the proliferation and death of the parasites. In order to depict the early apoptotic and mid-to-late apoptotic/necrotic signals, we shortened the incubation time from 24 h to 12 h and, surprisingly, failed to observe any significant increase of both the early and mid-to-late apoptotic/necrotic signal ratio of parasites stimulated by resveratrol and pyrimethamine. Moreover, although bortezomib could induce apoptosis of the tachyzoites, the extent of apoptosis was minor. The impact on the proliferation of the tachyzoites by resveratrol and pyrimethamine was determined by alteration of the cell cycle. Similar to the results obtained from the cell death experiment, we found out that neither resveratrol nor pyrimethamine was able to cause cell cycle arrest of the tachyzoites in 12 h. Here we noticed that Ferreira and colleagues incubated amastigotes/promastigotes of Leishmania amazonensis with resveratrol at 27 μM and 100 μM for 48 h and demonstrated a distinct increase in the sub-G0/G1 population along with a decrease in the G0/G1 population (20). Based on the results for cell death and the cell cycle, we attributed the outcome to two possibilities: the time that we used for challenge was not long enough to acquire differentiated data, and some other process prevented the initiation of apoptosis and alteration of the cell cycle.
Superoxide dismutase (SOD) is one of the most important reductases, existing in a wide range of organisms. SOD promotes the conversion of extra superoxide (O2−) anion into hydrogen peroxide and oxygen (27). In T. gondii, SOD has been considered to protect cells from oxidative damage and also participate in the process of growth of both bradyzoites and tachyzoites (35). To verify the second assumption that we made, we evaluated the SOD activities of the tachyzoites incubated with resveratrol and also pyrimethamine, as the reference therapeutic compound, for 12 h. We saw a striking increase in the SOD activity of tachyzoites stimulated by resveratrol, indicating that the activation of SOD might contribute greatly to neutralization of the harm induced by resveratrol. At the same time, as expected, pyrimethamine could also induce the activation of SOD significantly, thereby failing to cause evident damage affecting cell death and the cell cycle of RH tachyzoites within the short time that we described earlier. Additionally, since the dramatic activation of this enzyme might indicate the alteration of the oxidative status brought about by external damage or internal stress, we further assessed the reactive oxygen species (ROS) production of tachyzoites with different stimuli, including resveratrol, to illustrate the relationship between the killing by resveratrol and the survival of the parasites. ROS, including the superoxide anion, hydrogen peroxide, and hydroxyl radicals, are by-products of aerobic metabolism and are of great importance in a variety of biological processes, such as cell proliferation, differentiation, and migration (36). Since the dismutase activity of SOD could catalyze the transformation of the superoxide anion into hydrogen peroxide and oxygen (37), we expected that the pronounced upregulated SOD activity of infected host cells might be due to the increased ROS production induced by resveratrol. Contrary to our expectation, resveratrol, together with its solvent, DMSO, brought down the level of ROS production in 12 h to a level even lower than that of ROS produced by the parasites physiologically. Another toxin, H2O2, used as the positive control, also failed to increase the level of ROS production but instead produced a slight decrease. Although the harm of superabundant ROS production to the parasites has largely been documented, it was nonnegligible that a moderate ROS level was also a promoter of their propagation, as argued by some researchers in reports of studies of Trypanosoma cruzi (38, 39). Therefore, we conceived of the idea that the extremely low level of ROS production might be a result of the significantly elevated SOD activity induced by resveratrol in 12 h, and it undermined the physiological redox biological signaling, thereby interfering with the metabolism or proliferation ability of the parasites. At the same time, we cannot rule out other possible factors that were involved in the relationship between the reagent and the parasites, including cross talk between ROS and reactive nitrogen species (RNS) and alterations of other reductases of the parasites. Anyhow, resveratrol was considered to destroy the oxidant-antioxidant homeostasis of tachyzoites by upregulating the SOD activity in 12 h, thus gradually inhibiting the growth of extracellular tachyzoites in 24 h.
In addition to the direct inhibitory effects of resveratrol against RH tachyzoites, we were also interested in the indirect inhibitory effects of it. Macrophages, which are part of innate immunity, play a pivotal role in eliminating invading pathogens. As one of the types of phagocytic nucleated cells, they serve as both an active and a passive source of entry of the tachyzoites, leading to a battle between the immune elimination process of host cells and the immune evasion strategy of the parasites. To illustrate the impact on the growth of the parasites by resveratrol, we infected RAW 264.7 mouse macrophages at a parasite/host cell ratio of 5:1 for a short period of time before addition of resveratrol for further incubation for 24 h. We observed that the mean population of intracellular parasites in each infected macrophage was about 10 for the control and DMSO-treated groups, while this number dropped dramatically in the resveratrol- and pyrimethamine-treated groups. Meanwhile, we noticed that the infected macrophages stimulated by resveratrol and pyrimethamine showed an accumulation of empty vacuoles in the cytoplasm, indicating a more activated status of these macrophages.
Autophagy is an evolutionarily conserved, multistep lysosomal degradation process for the removal of damaged or redundant proteins and organelles. Since autophagy is a process linked to the energy metabolism of the cell (40), conventionally, the depletion of nutrients from host cells by the inhabitation of the intracellular parasites is highly likely to pull the trigger for autophagy. Several researchers aimed at discovering the interaction between parasites and hosts argued that autophagy might play an essential role in the host process of elimination of parasites. Muniz-Feliciano and colleagues declared that fusion of the parasitic vacuole with the lysosome might rely on the accumulation of some key autophagic proteins and is interrupted by T. gondii parasites, thereby protecting themselves from intracellular degradation by host cells (28). Moreover, a study focusing on Salmonella enterica serovar Typhimurium further supported that assumption by demonstrating the suppression of host autophagy by bacterial infection through the degradation of SIRT1 of host cells (41). In this study, we found out that the infection by RH tachyzoites induced the upregulation of LC3 production as well as a shift from LC3 I to LC3 II in host macrophages at 2 h. However, both the upregulation and the shift were gradually attenuated afterwards until they dropped to a baseline level that was identical to that for uninfected cells at 24 h. Therefore, at the very beginning of the infection, the host cells were assumed to respond to the parasites by initiation of autophagy. Nevertheless, when adapted to the intracellular environment, the RH tachyzoites began to precisely regulate the clearance process and, finally, suppressed it totally. Conversely, the addition of resveratrol and rapamycin maintained a relatively high level of LC3 II production from 2 h to 24 h and significantly cut down the average numbers of intracellular tachyzoites at the same time. In this case, we argue that autophagy plays a pivotal role in eliminating intracellular tachyzoites and could be suppressed in favor of their survival. Furthermore, resveratrol was able to induce LC3 II production by uninfected macrophages, though very mildly, indicating its contribution to autophagic activities. Therefore, by upregulating autophagy or the autophagic elimination process, resveratrol was able to assist host immune cells to terminate RH tachyzoites.
Along with the expansion of the parasite population, we assumed that the infected macrophages were in a stressed condition. C/EBP homologous protein (CHOP), also known as growth arrest and DNA damage-inducible protein 153 (GADD153), plays a key role during endoplasmic reticulum (ER) stress and amino acid limitation (42). As a stress-responsive transcription factor, CHOP expression is induced to control numerous genes involved in multiple cellular processes, including inflammation, differentiation, autophagy, and apoptosis (42, 43). The sustained CHOP activation has been considered a pivotal trigger for ER stress-related apoptosis. We found that the production of CHOP by host cells was elevated with time by infection with RH tachyzoites. Meanwhile, resveratrol reversed the elevation by maintaining the level of CHOP production at the baseline level, indicating that resveratrol could save the host cell from the risk of suicide that ER stress brought about. Surprisingly, when we tried to evaluate the protein level of XIAP, which has been considered one of the most powerful apoptotic inhibitors, it turned out that resveratrol restrained the production of XIAP, which was found to be upregulated in infected macrophages and also in uninfected macrophages over time, revealing the induction of apoptosis by resveratrol through suppression of the production of its inhibitor. The conflicting results from our experiments might suggest the possibility that resveratrol is able to restore homeostasis within the ER and prevent ER stress-related apoptosis and also simultaneously promotes the initiation of classical apoptosis. We already pointed out that resveratrol enhances the autophagic clearance of the host cells in response to infection with RH tachyzoites, thus alleviating cellular stress by degradation and recycling of the harmful components. Therefore, we believe that the process of autophagy could help the ER to maintain homeostasis, thereby reducing the production of CHOP to delay the overload reaction. On the other hand, resveratrol might initiate the classical apoptosis irrelevant to the ER function to favor the recognition of infected cells to be terminated by other phagocytes, thus limiting the proliferation of the tachyzoites to a single cell.
Since macrophages are the main source of inflammatory cytokines under circumstances of infection, we sought to examine whether resveratrol has a potent influence on inflammatory cytokine production by infected host cells. TNF-α is one of the proinflammatory cytokines secreted by macrophages to mediate parasite killing. It was noteworthy that resveratrol had an ability to induce the production of TNF-α by infected macrophages compared to that of DMSO and pyrimethamine. In addition to TNF-α, we also evaluated the level of IL-10, one of the anti-inflammatory cytokines that favors the inhabitation of pathogens. However, neither resveratrol nor pyrimethamine was able to regulate the production of this cytokine significantly, despite the trend toward a slight decrease that we observed. Resveratrol has been considered a powerful anti-inflammatory compound that is expected to reduce the production of inflammatory cytokines, such as TNF-α and IL-1β, in most of the cases examined by many researchers (44, 45). However, it is noteworthy that a few studies reported that resveratrol could also regulate this cytokine in the opposite way. It was shown to reverse the downregulation of several proinflammatory cytokines, including TNF-α, in a model consisting of rats with chemically induced hepatic cancer (46). Furthermore, oral administration of resveratrol was found to enhance the TNF-α level in healthy human males as well as primary peripheral blood monocytes upon bacterial stimulation (47). In addition to its well-known role in mediating the inflammatory process, TNF-α also participates in the signaling pathway of apoptosis in a variety of cell types (48) through binding to the corresponding cell surface death receptor, TNFR. Moreover, we have already shown that resveratrol induces apoptosis by suppressing the XIAP level in infected cells. Therefore, a possible explanation for our observation is that resveratrol triggers apoptosis through the induction of TNF-α; hence, the IL-10 alteration is missing upon treatment with resveratrol. On the other hand, macrophages are essential in innate immunity and function by reacting to pathogens by secreting cytokines, such as TNF-α. Therefore, resveratrol may contribute to elimination of the parasites by enhancing TNF-α production in these cells.
In conclusion, resveratrol can successfully inhibit the growth of RH tachyzoites presumably through the deprivation of redox homeostasis of the parasites, alleviate the stress status of host macrophages caused by parasite infection, and finally, assist macrophages with the elimination of intracellular tachyzoites by facilitating both the autophagic and the apoptotic processes.
MATERIALS AND METHODS
Cell cultivation.
A murine macrophage-stable cell line (RAW 264.7), a murine hepatocyte cell line (AML-12), and a human foreskin fibroblast (HFF) cell line (purchased from Jennio Biotech Co., Ltd., Guangzhou, China) were cultured at 37°C in a 5% CO2−95% air mixture in RPMI 1640 medium (Gibco, USA) containing 2.05 mM l-glutamine, Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA), or DMEM/F-12 medium (Gibco, USA), respectively, supplemented with 10% fetal bovine serum (PAN, Germany) and 100 μg/ml of antibiotics (penicillin and streptomycin; Ameresco, USA), with passage taking place every 3 to 4 days. When cells were over 80% confluent, 5 ml of trypsin with 0.25% EDTA and phenol red (Solarbio, China) was employed to digest the cells before they were washed off with fresh medium by pipetting for further seeding procedures.
Parasite culture.
The type I virulent RH strain of Toxoplasma gondii (generously donated by Guizhou Medical University, China) was stored in phosphate-buffered saline (PBS) solution containing 20% glycerin in liquid nitrogen. The RH strain was removed from liquid nitrogen and recovered in a water bath of 37°C for 30 min and then maintained in vitro by serial passage on human foreskin fibroblasts (HFF) or mouse hepatocytes (AML-12 cells). When over 90% of the infected cells were lysed, the parasites were obtained together with the attached cells with a gentle scrape by a cell scraper, followed by centrifugation at 100 × g for 10 min at 4°C before the supernatants were collected. Next, the mixture was passed through a 25-gauge syringe needle several times, centrifuged at 2,000 × g for 10 min to remove the supernatants, and resuspended in either PBS (for infection) or RPMI 1640 medium supplemented with 5% fetal bovine serum (Endo buffer) at 37°C in 5% CO2 (for treatment).
Parasite survival.
Parasites were collected and loaded in a 96-well cell culture plate at 5 × 105 cells/per well and challenged with resveratrol (J&K Scientific, China) and pyrimethamine (J&K Scientific, China) at different concentrations ranging from 0 to 200 μM for 24 h (for each well, 90 μl of parasites in Endo buffer and 10 μl of diluted chemicals were added to acquire the corresponding concentrations of the chemicals) before the determination of parasite inhibition rates. Parasite survival was performed after stimulation by a standard trypan blue dye exclusion test to determine the number of viable cells. In these tests, medium alone and dimethyl sulfoxide (DMSO; the solvent used to dissolve resveratrol and pyrimethamine) diluted with culture medium were used as controls. All of the cultures were performed four independent times, and the results are expressed as the inhibition rate (in percentage), which is equal to [(number of parasites in culture medium with DMSO − number of parasites in medium with resveratrol or pyrimethamine)/number of parasites in culture medium with DMSO] × 100.
Phosphatidylserine (PS) exposure and cell death.
Approximately 1 × 106 tachyzoites were employed for coculture with resveratrol, pyrimethamine, and bortezomib, followed by collection and centrifugation at 1,300 × g for 15 min at 4°C to remove the supernatants before the addition of annexin V-FITC dye in the dark for 10 min. After the addition of PI dye for another 5 min followed by the addition of the ligation buffer, the mixture was subjected to flow cytometry (ZE5 flow cytometer; Bio-Rad) or confocal microscopy (LSM 880 microscope; Zeiss) for further detection, as required by the protocol.
Cell cycle detection.
Approximately 1 × 106 tachyzoites were collected and centrifuged at 1,300 × g for 15 min at 4°C after incubation with resveratrol and pyrimethamine, followed by three washes with cold PBS. Five hundred microliters of PI-RNase was added to the tachyzoites away from the light for 30 min. After addition of an extra volume of 1 ml PBS, the mixture was centrifuged and resuspended in 500 μl of PBS, followed by detection by flow cytometry (ZE5 flow cytometer; Bio-Rad).
SOD activity.
Approximately 1 × 107 tachyzoites were collected and lysed after incubation with resveratrol and pyrimethamine by Western-PI lysis buffer (without enzyme) (Beyotime, China), followed by detection of the total protein in each sample. SOD activity was measured by a total SOD assay kit (the wst-8 method; Beyotime, China). According to the instruction manual, sample wells together with 3 blank control wells in a 96-well plate were loaded with cell lysates and the corresponding buffer before incubation at 37°C away from the light for 30 min. The absorbance of each sample was measured at 450 nm, followed by homogenization of the data through division of the value by the total amount of protein in each sample.
ROS production.
Approximately 1 × 106 tachyzoites in Endo buffer were seeded into each well of a 24-well cell culture plate, followed by addition of 50 µM resveratrol, 30 µM H2O2, and DMSO for 12 h. DCFH-DA (2′,7′-dichlorofluorescein diacetate; 10 µM; Njjcbio, China) was then added to the culture medium, and the tachyzoites were incubated for an extra 60 min before collection by centrifugation at 1,300 × g for 10 min and washing with sterile PBS. Pellets were resuspended in PBS and detected by use of the FITC channel of a flow cytometer (Cytoflex S; Beckman Coulter, USA).
Phagocytosis.
RAW 264.7 mouse macrophages were maintained at 37°C in 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum and 100 μg/ml of antibiotics (penicillin and streptomycin). Cells (1 × 105) were seeded into each well of a 24-well cell culture plate with sterile glass coverslips for attachment. The macrophages were infected with RH tachyzoites at a 5:1 parasite-to-cell ratio for 2 h, followed by three washes with PBS to remove excess parasites in the medium. After the replacement of the medium with new culture medium, resveratrol, pyrimethamine, rapamycin (Sigma), or DMSO was added for an extra 24 h. Infected and stimulated cells were then observed with an optical microscope and Wright’s stain. The average number of tachyzoites in each cell was determined by randomly counting 200 infected cells in each of the duplicate coverslips.
Detection and analysis of protein production.
The macrophages (2.5 × 106) in each well of a 12-well tissue culture plate were lysed with Western-PI lysis buffer supplemented with phenylmethylsulfonyl fluoride, followed by detection of total protein in each sample. A Western blot analysis of prepared protein samples was performed following standard protocols. Approximately 20 to 30 μg of protein was loaded in each lane of a 12% acrylamide gel. Proteins were separated at 120 V until the dye front reached the bottom of the gel, followed by transferring the gels to polyvinylidene fluoride (PVDF) membranes (Whatman) in transfer buffer (25 mM Tris-HCl, 192 mM glycine, 20% methanol, 0.02% SDS, pH 8.3), where they were held at 200 mA for 60 min. The membranes were then soaked in a blocking solution consisting of PBS-Tween 20 buffer (1× PBS, 0.1% Tween 20) supplemented with 1% bovine serum albumin (BSA; Sigma) for 2 h, before incubation overnight at 4°C individually with XIAP, LC3, and CHOP antibodies (Cell Signaling Technology, USA) and GAPDH antibody (glyceraldehyde-3-phosphate dehydrogenase; ZSGB-Bio, China) at the corresponding dilutions. All primary antibodies were used at a 1:1,000 dilution, and horseradish peroxidase-conjugated anti-rabbit/anti-mouse immunoglobulin secondary antibodies (ZSGB-Bio) were used at a 1:4,000 dilution. The membranes were then visualized with an enhanced chemiluminescence Western blotting detection kit (Beyotime).
Cytokine production.
Macrophages (2.5 × 106) were seeded into each well of a 12-well plate and incubated with the corresponding stimuli for 24 h before collection of the cultivation medium for enzyme-linked immunosorbent assay detection of TNF-α and IL-10 following a standard protocol.
Statistics.
IC50s were calculated according to a nonlinear regression using a log inhibitor-versus-response equation with 95% confidence intervals by GraphPad Prism (version 5) software. The data were analyzed by Student's t test for comparison of two groups or by one-way analysis of variance (ANOVA) for comparison of three or more groups using GraphPad Prism (version 5) software. P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant numbers 81672048, 31572240, 81171607, and 31872959).
We declare that we have no conflict of interest.
REFERENCES
- 1.Hide G. 2016. Role of vertical transmission of Toxoplasma gondii in prevalence of infection. Expert Rev Anti Infect Ther 14:335–344. doi: 10.1586/14787210.2016.1146131. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z-D, Wang S-C, Liu H-H, Ma H-Y, Li Z-Y, Wei F, Zhu X-Q, Liu Q. 2017. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV 4:e177. doi: 10.1016/S2352-3018(17)30005-X. [DOI] [PubMed] [Google Scholar]
- 3.Opsteegh M, Kortbeek TM, Havelaar AH, van der Giessen JWB. 2015. Intervention strategies to reduce human Toxoplasma gondii disease burden. Clin Infect Dis 60:101–107. doi: 10.1093/cid/ciu721. [DOI] [PubMed] [Google Scholar]
- 4.Cuomo G, D’Abrosca V, Rizzo V, Nardiello S, La Montagna G, Gaeta GB, Valentini G. 2013. Severe polymyositis due to Toxoplasma gondii in an adult immunocompetent patient: a case report and review of the literature. Infection 41:859–862. doi: 10.1007/s15010-013-0427-x. [DOI] [PubMed] [Google Scholar]
- 5.Alday PH, Doggett JS. 2017. Drugs in development for toxoplasmosis: advances, challenges, and current status. Drug Des Devel Ther 11:273–293. doi: 10.2147/DDDT.S60973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter SB, Sande MA. 1992. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 7.Chirgwin K, Hafner R, Leport C, Remington J, Andersen J, Bosler EM, Roque C, Rajicic N, McAuliffe V, Morlat P, Jayaweera DT, Vilde J-L, Luft BJ, AIDS Clinical Trials Group 237/Agence Nationale de Recherche sur le SIDA Essai 039 Study Team. 2002. Randomized phase II trial of atovaquone with pyrimethamine or sulfadiazine for treatment of toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome: ACTG 237/ANRS 039 study. Clin Infect Dis 34:1243–1250. doi: 10.1086/339551. [DOI] [PubMed] [Google Scholar]
- 8.Baatz H, Mirshahi A, Puchta J, Gümbel H, Hattenbach L-O. 2006. Reactivation of toxoplasma retinochoroiditis under atovaquone therapy in an immunocompetent patient. Ocul Immunol Inflamm 14:185–187. doi: 10.1080/09273940600659740. [DOI] [PubMed] [Google Scholar]
- 9.Aspinall TV, Joynson DHM, Guy E, Hyde JE, Sims PFG. 2002. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J Infect Dis 185:1637–1643. doi: 10.1086/340577. [DOI] [PubMed] [Google Scholar]
- 10.Di Cristina M, Marocco D, Galizi R, Proietti C, Spaccapelo R, Crisanti A. 2008. Temporal and spatial distribution of Toxoplasma gondii differentiation into bradyzoites and tissue cyst formation in vivo. Infect Immun 76:3491–3501. doi: 10.1128/IAI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, Bordón C, Charman SA, Katneni K, Schultz T, Burrows JN, Hinrichs DJ, Meunier B, Carruthers VB, Riscoe MK. 2012. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Natl Acad Sci U S A 109:15936. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pais-Morales J, Betanzos A, García-Rivera G, Chávez-Munguía B, Shibayama M, Orozco E. 2016. Resveratrol induces apoptosis-like death and prevents in vitro and in vivo virulence of Entamoeba histolytica. PLoS One 11:e0146287. doi: 10.1371/journal.pone.0146287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonard SS, Xia C, Jiang B-H, Stinefelt B, Klandorf H, Harris GK, Shi X. 2003. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun 309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 14.Pirola L, Fröjdö S. 2008. Resveratrol: one molecule, many targets. IUBMB Life 60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 15.Chan MM-Y. 2002. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem Pharmacol 63:99–104. doi: 10.1016/S0006-2952(01)00886-3. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes GFS, Silva GDB, Pavan AR, Chiba DE, Chin CM, Dos Santos JL. 2017. Epigenetic regulatory mechanisms induced by resveratrol. Nutrients 9:E1201. doi: 10.3390/nu9111201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM, Tsao JW, Juan YN, Chiu HY, Yang JS, Wang CC. 2017. Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: a key role of AMPK and Akt/mTOR signaling. Int J Oncol 50:873–882. doi: 10.3892/ijo.2017.3866. [DOI] [PubMed] [Google Scholar]
- 18.Leischner C, Burkard M, Pfeiffer MM, Lauer UM, Busch C, Venturelli S. 2016. Nutritional immunology: function of natural killer cells and their modulation by resveratrol for cancer prevention and treatment. Nutr J 15:47. doi: 10.1186/s12937-016-0167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passos CLA, Ferreira C, Soares DC, Saraiva EM. 2015. Leishmanicidal effect of synthetic trans-resveratrol analogs. PLoS One 10:e0141778. doi: 10.1371/journal.pone.0141778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira C, Soares DC, do Nascimento MTC, Pinto-da-Silva LH, Sarzedas CG, Tinoco LW, Saraiva EM. 2014. Resveratrol is active against Leishmania amazonensis: in vitro effect of its association with amphotericin B. Antimicrob Agents Chemother 58:6197–6208. doi: 10.1128/AAC.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottari NB, Baldissera MD, Tonin AA, Rech VC, Alves CB, D'Avila F, Thomé GR, Guarda NS, Moresco RN, Camillo G, Vogel FF, Luchese C, Schetinger MRC, Morsch VM, Tochetto C, Fighera R, Nishihira VSK, Da Silva AS. 2016. Synergistic effects of resveratrol (free and inclusion complex) and sulfamethoxazole-trimetropim treatment on pathology, oxidant/antioxidant status and behavior of mice infected with Toxoplasma gondii. Microb Pathog 95:166–174. doi: 10.1016/j.micpath.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Bottari NB, Baldissera MD, Tonin AA, Rech VC, Nishihira VSK, Thomé GR, Camillo G, Vogel FF, Duarte MMMF, Schetinger MRC, Morsch VM, Tochetto C, Fighera R, Da Silva AS. 2015. Effects of sulfamethoxazole-trimethoprim associated to resveratrol on its free form and complexed with 2-hydroxypropyl-β-cyclodextrin on cytokines levels of mice infected by Toxoplasma gondii. Microb Pathog 87:40–44. doi: 10.1016/j.micpath.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Bottari NB, Baldissera MD, Tonin AA, Rech VC, Nishihira VSK, Thomé GR, Schetinger MRC, Morsch VM, Camillo G, Vogel FF, Tochetto C, Fighera R, Machado G, Stefani LM, Da Silva AS. 2015. Sulfamethoxazole-trimethoprim associated with resveratrol for the treatment of toxoplasmosis in mice: influence on the activity of enzymes involved in brain neurotransmission. Microb Pathog 79:17–23. doi: 10.1016/j.micpath.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Stokes J, Singh UP, Scissum-Gunn K, Singh R, Manne U, Mishra MK. 2017. Prolonged exposure of resveratrol induces reactive superoxide species–independent apoptosis in murine prostate cells. Tumour Biol 39:1010428317715039. doi: 10.1177/1010428317715039. [DOI] [PubMed] [Google Scholar]
- 25.Carlson A, Alderete KS, Grant MKO, Seelig DM, Sharkey LC, Zordoky BNM. 2018. Anticancer effects of resveratrol in canine hemangiosarcoma cell lines. Vet Comp Oncol 16:253–261. doi: 10.1111/vco.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Liang C, Zhang B, Ma J, He X, Chen S, Zhang X, Chen W. 2015. Bortezomib enhances the therapeutic efficacy of dasatinib by promoting c-KIT internalization-induced apoptosis in gastrointestinal stromal tumor cells. Cancer Lett 361:137–146. doi: 10.1016/j.canlet.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 27.Miller A-F. 2012. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett 586:585–595. doi: 10.1016/j.febslet.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muniz-Feliciano L, Van Grol J, Portillo JA, Liew L, Liu B, Carlin CR, Carruthers VB, Matthews S, Subauste CS. 2013. Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite. PLoS Pathog 9:e1003809. doi: 10.1371/journal.ppat.1003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morlon-Guyot J, Berry L, Chen C-T, Gubbels M-J, Lebrun M, Daher W. 2014. The Toxoplasma gondii calcium dependent protein kinase 7 is involved in early steps of parasite division and is crucial for parasite survival. Cell Microbiol 16:95–114. doi: 10.1111/cmi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. 2004. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117:503–514. doi: 10.1016/S0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury S, Sinha K, Banerjee S, Sil PC. 2016. Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. Biofactors 42:647–664. doi: 10.1002/biof.1301. [DOI] [PubMed] [Google Scholar]
- 32.Eckelman BP, Salvesen GS, Scott FL. 2006. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bottari NBA-O, Schetinger MRC, Pillat MM, Palma TV, Ulrich H, Alves MS, Morsch VM, Melazzo C, de Barros LD, Garcia JL, Da Silva AS. 19 July 2018. Resveratrol as a therapy to restore neurogliogenesis of neural progenitor cells infected by Toxoplasma gondii. Mol Neurobiol doi: 10.1007/s12035-018-1180-z. [DOI] [PubMed] [Google Scholar]
- 34.Montazeri M, Sharif M, Sarvi S, Mehrzadi S, Ahmadpour E, Daryani A. 2017. A systematic review of in vitro and in vivo activities of anti-toxoplasma drugs and compounds (2006–2016). Front Microbiol 8:25. doi: 10.3389/fmicb.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ödberg-Ferragut C, Philippe Renault J, Viscogliosi E, Toursel C, Briche I, Engels A, Lepage G, Morgenstern-Badarau I, Camus D, Tomavo S, Dive D. 2000. Molecular cloning, expression analysis and iron metal cofactor characterisation of a superoxide dismutase from Toxoplasma gondii. Mol Biochem Parasitol 106:121–129. doi: 10.1016/S0166-6851(99)00211-X. [DOI] [PubMed] [Google Scholar]
- 36.Schieber M, Chandel NS. 2014. ROS function in redox signaling and oxidative stress. Curr Biol 24:R453. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Branicky R, Noe A, Hekimi SA-O. 2018. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paiva CN, Medei E, Bozza MT. 2018. ROS and Trypanosoma cruzi: fuel to infection, poison to the heart. PLoS Pathog 14:e1006928. doi: 10.1371/journal.ppat.1006928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dias PP, Capila RF, do Couto NF, Estrada D, Gadelha FR, Radi R, Piacenza L, Andrade LO. 2017. Cardiomyocyte oxidants production may signal to T. cruzi intracellular development. PLoS Negl Trop Dis 11:e0005852. doi: 10.1371/journal.pntd.0005852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vincent G. 2018. Canonical signaling and nuclear activity of mTOR—a teamwork effort to regulate metabolism and cell growth. FEBS J 285:1572–1588. doi: 10.1111/febs.14384. [DOI] [PubMed] [Google Scholar]
- 41.Ganesan R, Hos NJ, Gutierrez S, Fischer J, Stepek JM, Daglidu E, Krönke M, Robinson N. 2017. Salmonella Typhimurium disrupts Sirt1/AMPK checkpoint control of mTOR to impair autophagy. PLoS Pathog 13:e1006227. doi: 10.1371/journal.ppat.1006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. 2016. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med 16:533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Qu X, Jiang L. 2017. An oasis in the desert of cancer chemotherapeutic resistance: the enlightenment from reciprocal crosstalk between signaling pathways of UPR and autophagy in cancers. Biomed Pharmacother 92:972–981. doi: 10.1016/j.biopha.2017.05.132. [DOI] [PubMed] [Google Scholar]
- 44.Chu H, Li H, Guan X, Yan H, Zhang X, Cui X, Li X, Cheng M. 2018. Resveratrol protects late endothelial progenitor cells from TNF-α-induced inflammatory damage by upregulating Krüppel-like factor-2. Mol Med Rep 17:5708–5715. doi: 10.3892/mmr.2018.8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Wang Y, Xiao C, Wei Z, Wang J, Yang Z, Fu Y. 2017. Resveratrol inhibits LPS-induced mice mastitis through attenuating the MAPK and NF-κB signaling pathway. Microb Pathog 107:462–467. doi: 10.1016/j.micpath.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Mbimba T, Awale P, Bhatia D, Geldenhuys WJ, Darvesh AS, Carroll RT, Bishayee A. 2012. Alteration of hepatic proinflammatory cytokines is involved in the resveratrol-mediated chemoprevention of chemically-induced hepatocarcinogenesis. Curr Pharm Biotechnol 13:229–234. doi: 10.2174/138920112798868575. [DOI] [PubMed] [Google Scholar]
- 47.Gualdoni GA, Kovarik JJ, Hofer J, Dose F, Pignitter M, Doberer D, Steinberger P, Somoza V, Wolzt M, Zlabinger GJ. 2014. Resveratrol enhances TNF-α production in human monocytes upon bacterial stimulation. Biochim Biophys Acta 1840:95–105. doi: 10.1016/j.bbagen.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Gaur U, Aggarwal BB. 2003. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol 66:1403–1408. doi: 10.1016/S0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]