This study sought to characterize the impact of 3 types of variation on the Standardized Antimicrobial Administration Ratio (SAAR) utilizing local National Healthcare Safety Network (NHSN) data. SAAR and antimicrobial days per 1,000 days present (AD/1000DP) were compiled monthly for Northwestern Memorial Hospital from 2014 to 2016.
KEYWORDS: antibiotic consumption, Standardized Antimicrobial Utilization Ratio, SAAR
ABSTRACT
This study sought to characterize the impact of 3 types of variation on the Standardized Antimicrobial Administration Ratio (SAAR) utilizing local National Healthcare Safety Network (NHSN) data. SAAR and antimicrobial days per 1,000 days present (AD/1000DP) were compiled monthly for Northwestern Memorial Hospital from 2014 to 2016. Antimicrobial consumption was aggregated into agent categories (via NHSN criteria). Month-to-month changes in SAAR and AD/1000DP were evaluated. Azithromycin and oseltamivir AD/1000DP from 2012 through 2017 were explored for seasonal variation. A sensitivity analysis was performed to explore the effect of seasonality and altered consumption at other hypothetical hospitals on the SAAR. Across agent categories for both the intensive care unit (n = 4) and general wards (n = 4), the average matched-month percent change in AD/1000DP was correlated with the corresponding change in SAAR (coefficient of determination of 0.99). The monthly mean ± standard deviation (SD) AD/1000DP was 235 (range, 47.2 to 661.5), and the mean ± SD SAAR was 1.09 ± 0.26 (range, 0.79 to 1.09) across the NHSN agent categories. Five seasons exhibited seasonal variation in AD/1000DP for azithromycin with a mean percent change of 26.76% (range, 22.27 to 30.69). Eight seasons exhibited seasonal variation in AD/1000DP for oseltamivir with a mean percent change of 129.1% (range, 32.01 to 352.74). The sensitivity analyses confirm that antimicrobial usage at comparator hospitals does not impact the local SAAR, and seasonal variation of antibiotics has the potential to impact SAAR. Month-to-month changes in the SAAR mirror monthly changes in an institution’s AD/1000DP. Seasonal variation is an important variable for future SAAR consideration, and the variable antibiotic use at peer hospitals is not currently captured by the SAAR methodology.
INTRODUCTION
Infections caused by antibiotic-resistant organisms pose a major problem both clinically and financially, as they are responsible for more than 23,000 deaths in the United States annually (1). As resistance due to unnecessary antibiotic use continues to be a major issue worldwide, antimicrobial stewardship programs are increasingly relying on objective antibiotic consumption metrics to design local improvement strategies. Many hospitals now rely on reports that quantify antimicrobial use (AU) to implement appropriate antimicrobial stewardship interventions and guide judicious antimicrobial usage (2–7). The Standardized Antimicrobial Administration Ratio (SAAR) is a nationally reported metric used by antimicrobial stewardship programs to determine AU within an institution. Essentially, the SAAR compares the observed antibiotic use to a predicted ratio where a value of >1 indicates overuse at an institution after standardization.
The Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network (NHSN) has established a methodology to benchmark utilization across centers that participate in the antimicrobial use module. Antimicrobial days (AD) are calculated and quantified per 1,000 days present (AD/1000DP) at the level of a hospital ward (or compiled for the whole hospital) (7). A full list of antimicrobial agents collected in the NHSN AU option can be found in the supplemental appendix of the CDC AU module (8). The aggregate ADs are tallied monthly for locations and stratified (if applicable) by route of administration (i.e., intravenously or orally). The SAAR is calculated for each participating hospital, and the hospital’s consumption is compared to a national benchmark for antimicrobial usage (K. van Santen, unpublished data). The predictive models used to generate the SAAR were developed by the CDC and have been designed to risk adjust (i.e., indirect standardization of SAAR) antimicrobial utilization using multivariate negative binomial regression (van Santen, unpublished). The negative binomial regression model utilizes each hospital’s demographic variables to calculate the risk adjustment for each use category and for a facility-wide calculation (7). Thus, each hospital is classified with a risk adjustment based on demographic characteristics. This factor is then used to calculate the predicted antimicrobial consumption required to calculate the SAAR for each institution. For example, the model for broad-spectrum agents, predominantly used for community-acquired infections, incorporates teaching status, intensive care unit (ICU) location, and pediatric location in the model. By providing hospitals participating in the NHSN AU module with risk-adjusted consumption estimates, the CDC has created a pathway to establish robust national benchmarks. We sought to assess local month-to-month percent changes in AD/1000DP and the SAAR. We further explored potential seasonal variation with prototypical antibiotics (i.e., azithromycin and oseltamivir) and assessed how fluctuating antibiotic consumption at comparator hospitals impacts the local SAAR.
RESULTS
Antimicrobial days/SAAR.
Across the 24-month period, overall ADs (all locations) averaged 1,202.50 ± 97.71 (range, 149.92 to 5,650.6), where the predicted AD averaged 1,096.46 ± 1,627.67 (range, 189.81 to 5,167.31). Across the 24-month period, the overall DP (all locations) averaged 5,373.65 ± 3,325.65 (range, 1,420.88 to 8,718.2), and the overall AD/1000DP averaged 234.98 ± 219.65 (range, 47.18 to 661.49). The monthly SAAR (all locations) over the study period (24 months) averaged 1.09 ± 0.26 (range, 0.79-1.09). A summary of results can be found in Table 1.
TABLE 1.
Stewardship metrics summary in a 24-month period between 2014 and 2016 for 8 location groupings
| Location | Mean (±SD) AD | Mean (±SD) AD predicted | Mean (±SD) days present | Mean (±SD) AD/1000DP | Mean (±SD) SAAR | Overall R2 change in AD vs SAAR |
|---|---|---|---|---|---|---|
| All ICU wards | 5,650.6 (±321.65) | 5,167.31 (±152.81) | 8,718.2 (±267.22) | 661.49 (±29.27) | 1.12 (±0.05) | 0.97 |
| MDRI ICU | 668.63 (±56.79) | 423.98 (±24.72) | 1,420.88 (±83.15) | 470.66 (±29.57) | 1.58 (±0.10) | 0.99 |
| MDRI adults | 880.46 (±121.42) | 811.47 (±62.46) | 7,176.96 (±554.72) | 122.57 (±12.88) | 1.08 (±0.11) | 0.99 |
| Community ICU | 149.92 (±27.25) | 189.81 (±11.11) | 1,420.88 (±83.15) | 105.65 (±18.98) | 0.79 (±0.14) | 0.99 |
| Community adults | 827.71 (±90.55) | 848.64 (±65.59) | 7,176.96 (±554.72) | 115.29 (±8.75) | 0.98 (±0.07) | 0.99 |
| Anti-MRSA ICU | 376.38 (±39.63) | 281.83 (±16.38) | 1,420.88 (±83.15) | 264.87 (±23.02) | 1.34 (±0.11) | 0.99 |
| Anti-MRSA adults | 660.17 (±74.63) | 624.83 (±48.39) | 7,176.96 (±554.72) | 92.1 (±8.9) | 1.06 (±0.10) | 0.99 |
| Surgical PPXa adults | 406.13 (±49.73) | 507.72 (±38.25) | 8,597.83 (±602.12) | 47.18 (±3.91) | 0.80 (±0.06) | 0.96 |
| Overall mean (SD) | 1,202.50 (±1,813.98) | 1,096.46 (±1,627.67) | 5,373.65 (±3,325.65) | 234.98 (±219.65) | 1.09 (±0.26) | 0.99 (±0.02) |
PPX, prophylaxis.
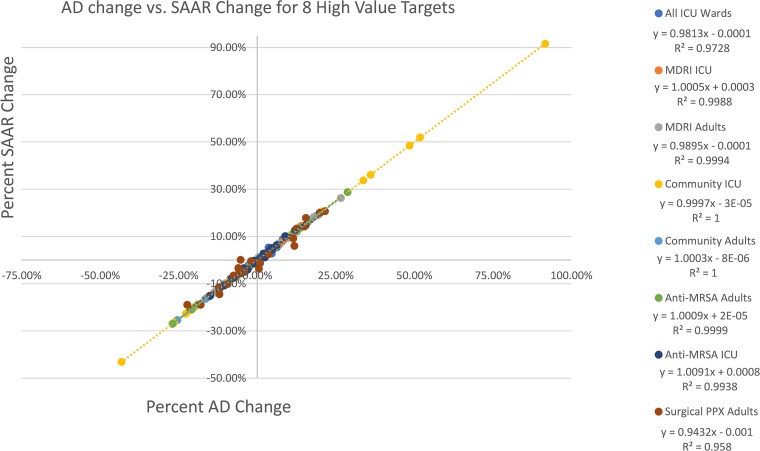
Across all antibiotic agent categories for both the ICU (n = 4) and general wards (n = 4), the matched-month percent change was closely correlated between ADs and the SAAR (Fig. 1), with an overall mean coefficient of determination (R2) of 0.99 ± 0.02. All 8 classifications demonstrated R2 of ≥0.96. Individual scatter plots with regression for each of the 8 classifications over the 24-month period can be found in Fig. S1 in the supplemental material.
FIG 1.
Overall matched-month percent change for all 8 classifications. PPX, prophylaxis.
Seasonality sensitivity analysis.
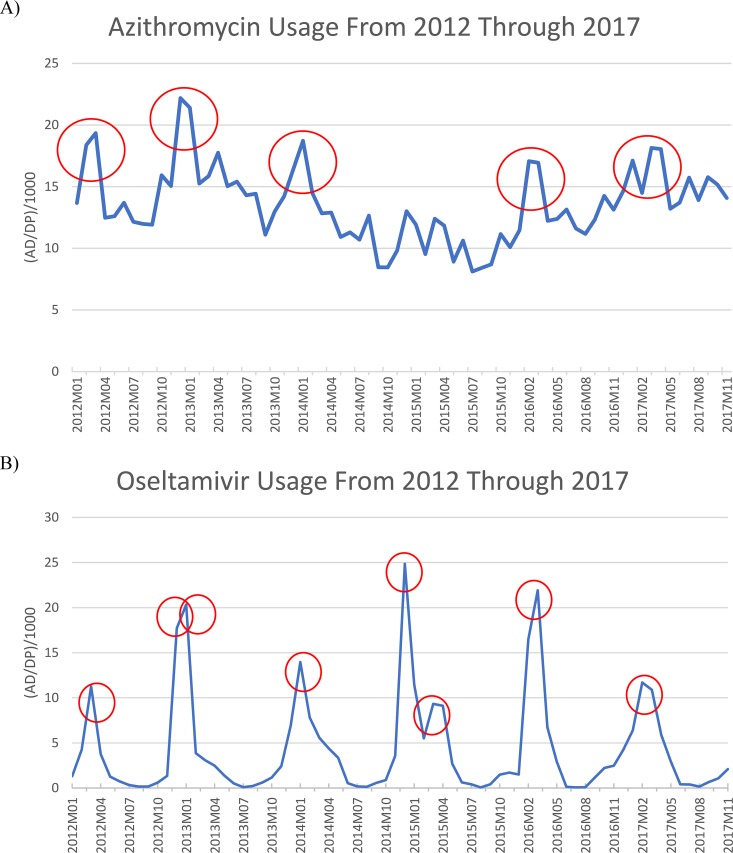
Azithromycin usage demonstrated cyclical increases and decreases across seasons. From 2012 through 2017 (24 seasons), five seasons exhibited variability with increased consumption according to the 20% threshold (Fig. 2A). The overall mean AD/1000DP for all months was 13.5 ± 3.04 (range, 10.42 to 17.72). The mean percent change for the five seasons was 26.76% (range, 22.27 to 30.69). Two seasons (season 1 of 2016 and season 4 of 2017) only comprised a 2-month period given missing azithromycin utilization reported to the NHSN.
FIG 2.
Facility-wide, seasonal variation of azithromycin (A) and oseltamivir (B) between 2012 and 2017. Each circle represents a season found to have seasonal variation. Seasonal variation was defined as seasonal change >20% above the overall mean for all months (10).
Oseltamivir usage also demonstrated cyclical increases and decreases across seasons, where eight seasons were found to exhibit increased consumption according to the 20% threshold (Fig. 2B). The overall mean AD/1000DP for all months was 4.24 ± 1.62 (range, 0.21 to 19.2). The mean percent change for the five seasons was 129.1% (range, 32.01 to 352.74). Similar to azithromycin, two seasons (season 1 of 2016 and season 4 of 2017) only comprised a 2-month period given missing utilization reported to NHSN.
Fluctuating antimicrobial utilization sensitivity analysis.
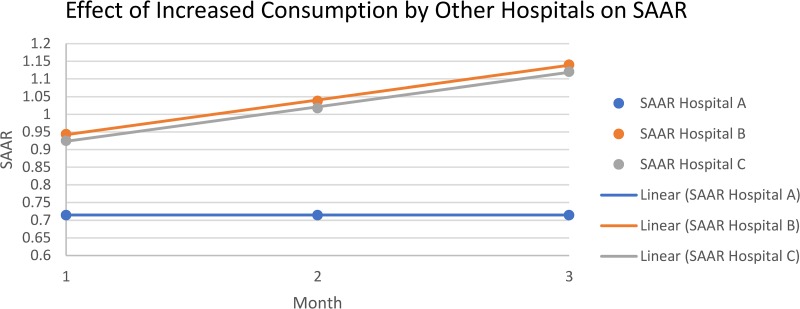
The sensitivity analysis for impact of fluctuating antimicrobial utilization on a single institution’s SAAR for antimicrobials used for community-acquired infections in adult ICUs can be found in Fig. 3. Hospital A’s (NMH) antimicrobial consumption and SAAR value stayed constant/fixed. Hospitals B and C displayed an increasing SAAR according to the sensitivity analysis method. The sensitivity analysis showed that the increase in antimicrobial usage (via SAAR) by the other hospitals (hospitals B and C) does not currently impact hospital A’s (NMH) SAAR.
FIG 3.
Sensitivity analysis graphical results (corresponding to Tables 2 and 3). The consumption of hospitals B and C are not incorporated into the monthly SAAR calculation for hospital A. The SAAR denominator is established by NHSN and, for this example, hospital A’s consumption is held constant.
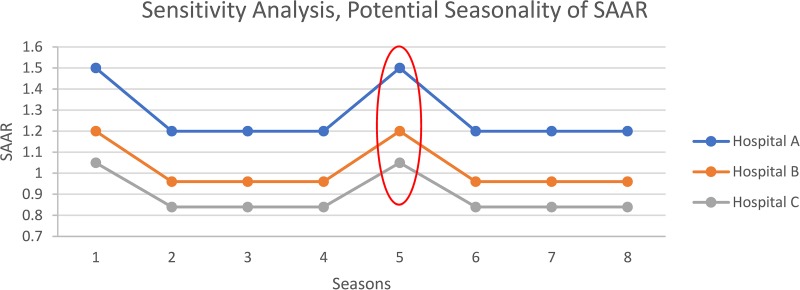
The results of the sensitivity analysis (8 seasons total) for impact on potential seasonality of the SAAR can be found in Fig. 4. Per the sensitivity analysis method, all three hospital’s SAAR values were elevated (25% increase) for seasons 1 and 5. The sensitivity analysis demonstrates that while each hospital’s individual SAAR rises for seasonal variation, each hospital rises identically according to percent rise.
FIG 4.
Sensitivity analysis graphical results (corresponding to Table 4). The SAARs for all three hospitals rise and fall together due to potential seasonality, which is currently not adjusted for in the SAAR calculation. NMH, Northwestern Memorial Hospital.
DISCUSSION
To our knowledge, this is the first exploration of the relationship between month-to-month changes of the SAAR and AD/1000DP at the institutional level. We found that the percent change in the month-to-month SAAR over a 24-month period was predictable and correlated well with percent change in AD/1000DP. We also demonstrated that seasonal variation exists for azithromycin, and this may have important implications for future SAAR calculations. Seasonal variation is a form of potentially explainable variability which could be interpreted as consumption greater than that of peers with the current SAAR. Finally, we demonstrated that variable antibiotic use by peer hospitals does not impact one’s local SAAR. SAAR calculations are presently static and do not track with changing use of the SAAR reporting community.
Our data illustrate the relationship between SAAR and AD/1000DP, i.e., SAAR changes are due to local consumption changes only after a one-time adjustment for demographics is made (7). As demonstrated by the sensitivity analyses, if antibiotic consumption at an institution remains constant but usage at other institutions increases, the SAAR of the institution with unchanged use will not be affected. A similar relevant example exists in financial markets. If an annual rate of return is 3%, then gain is 3% above the principal. The annual rate of return is independent of how well others performed in the market. If others average an annual rate of return of 7%, the 3% indicates underperformance relative to the market. Future SAAR values would benefit from dynamic and real-time assessments of peer performance. The rate of recalibration for adjustment factor directly impacts how the SAAR should be interpreted. If adjustment factors are dynamically made monthly, outside use would impact the local SAAR even for isometric local use. Currently, recalibrations are performed at staggered intervals. Thus, those utilizing the SAAR should be cognizant of the timeline and recurrence rate for SAAR baseline calibrations by the CDC.
We have also demonstrated that the SAAR does not currently control for seasonal variation in consumption. Seasonality is a form of potentially explainable variability that could be interpreted as inappropriate use with the current SAAR. In a study by Patrick and colleagues, an association was seen between antimicrobial consumption and seasonality, with peak consumption of intravenous administration of antimicrobial agents (which included macrolides, tetracyclines, cephalosporins, fluoroquinolones, lincosamides, trimethoprim/derivatives, and combinations of sulfonamides and trimethoprim) between the months of January and March (10). We demonstrated that azithromycin and oseltamivir exhibit seasonality, with multiple instances of seasonality between January and March. While azithromycin and oseltamivir are not used in current SAAR calculations, they were selected as prototypic agents for demonstration purposes. Notably, fluoroquinolones can also follow this seasonality pattern, often coinciding with respiratory virus/bacterial superinfection seasons (11). In our example, seasonality of the SAAR results in a higher SAAR for any of the given hospitals during seasons 1 and 5; however, every hospital’s usage was relatively increased during these two seasons. The percent change from hospital A to hospital B and C was equal during all 8 seasons (20% versus 30%). The sensitivity analysis demonstrates that the SAAR does not currently capture seasonal variation that can be caused by seasonality. As antibiotics for community-acquired infections are being explored for future iterations of the SAAR calculation, it will be important to explore seasonality for the various SAAR categories to properly create benchmarking metrics. Furthermore, seasonal infection rates and patterns can vary by region or country where increased antimicrobial utilization due to seasonality will occur in different months (i.e., not seasons 1 and 4) (12). These differences will also impact the SAAR if not accounted for appropriately in specific regions. This observation warrants further study to appropriately account for the indirect standardization of SAAR by different regions.
Limitations to this study exist. First, this is a single-center study, and the SAAR values for NMH were not compared to those of other hospitals within the Northwestern Hospital System. Second, our seasonality analysis excluded fluoroquinolones because of formulary changes that occurred in 2014. We felt that utilizing ciprofloxacin as a surrogate fluoroquinolone would be misleading, as ciprofloxacin is not generally utilized for upper respiratory symptoms. Nevertheless, both azithromycin and oseltamivir demonstrated seasonality in AU. Lastly, we were unable to examine potential seasonality in SAAR given the limited time frame of 2014 to 2016 for available SAAR data during the time of analysis.
The SAAR is a new and useful antibiotic consumption benchmarking tool; nuanced understanding of the drivers of increased and decreased SAAR values is critical for guiding antibiotic stewardship efforts. Month-to-month changes in the SAAR mirror those of local AD/1000DP. Thus, the SAAR can be used for tracking monthly antibiotic use at the local level and provides insight into month-to-month changes in local antibiotic use. However, the SAAR employs a static adjustment for hospital demography, and antibiotic consumption variations at peer hospitals do not affect local SAAR numbers. Only antibiotic use of the individual institution affects the rise and fall of its SAAR after the NHSN applies the one-time adjustment for demographics. Seasonality is not presently assessed within the SAAR, and antibiotics that follow seasonal usage patterns should not be assumed to be outside the range (low or high) based solely on the season. These observations warrant further study.
MATERIALS AND METHODS
Design and data sources.
A retrospective, epidemiologic analysis was performed on 24 months of NHSN antibiotic consumption data from January 2014 through December 2015 at Northwestern Memorial Hospital (NMH). ADs and DPs were obtained from TheraDoc (Premier, Salt Lake City, Utah) and uploaded to the NHSN AU module (13, 14). The SAAR was calculated and obtained through the NHSN individual facility report. The sum of antimicrobials used was calculated for 4 different CDC specific antibiotic categories in both ICU and general wards: multidrug-resistant infections (MDRI), community-acquired infections, anti-methicillin-resistant Staphylococcus aureus (MRSA) agents, and surgical site infection prophylaxis (7). Pediatric antibiotic classifications were not examined, as they are not available in our data set.
Definitions.
The NHSN AU definitions and methods were used for all calculations, standardizing AD to 1,000 DPs (7, 8). Briefly, AD is an aggregate sum of days of therapy whereby an antimicrobial agent is administered to patients as documented in the electronic medical record (EMR).
Data analysis.
The facility-wide AD/1000DP and SAAR were calculated monthly for 24 months (i.e., between January 2014 and December 2015). The coefficient of determination (R2) was calculated from the simple linear regression model and used to explore the relationships between percent change of AD/1000DP and SAAR from the previous month for each consecutive month in the 24-month period (equations 1 and 2). All calculations were performed and all plots generated using Microsoft Excel 2016 (Microsoft, Redmond, WA).
| (1) |
| (2) |
Seasonal variation.
The potential impact of seasonal variation was assessed by measuring the AD/1000DP of azithromycin and oseltamivir. These agents were selected because they are archetypal drugs predicted to exhibit seasonality. As moxifloxacin was removed and replaced by levofloxacin in 2014 as the hospital formulary fluoroquinolone, fluoroquinolones were not utilized to assess seasonality because of variable usage. Seasonality was evaluated from 2012 through 2017. Years were divided into 4 seasons (definition of season equivalent to one-quarter of a year), where each season comprised either a 2- or 3-month period (i.e., January to March, etc.), consistent with previous definitions (1, 10). A 2-month season was used if there were missing data for any month between 2012 and 2017 (data not submitted and adjudicated by the NHSN). The AD/1000DP for each period was used to calculate the mean value for each season. Similar to previous reports, seasonal variation was defined as a >20% increase in the AD/1000DP of each season to the overall mean AD/1000DP for all months (10). However, our study was more conservative in that a >20% increase in any season was defined as a seasonal effect (as opposed to comparing means of seasons 1 and 4 to those of seasons 2 and 3) (10). Seasons not found to exhibit seasonality defined the baseline (or decreased utilization periods).
Sensitivity analysis methodology.
The hospital-specific SAAR risk adjustment for antimicrobials used for community-acquired infections in adult ICUs was calculated as a sensitivity analysis/demonstration example (see Example S1 in the supplemental material) (7). The parameter and estimates in the model included the intercept (−1.759), teaching status (−0.376), ICU location (0.122), and pediatric location (−0.202). The impact of fluctuating antimicrobial utilization on a single institution’s SAAR was explored by plotting antimicrobials used for community-acquired infections in adult ICUs for a period of 12 months (i.e., January to December). To allow for comparison, a sensitivity analysis was created for two additional hypothetical hospitals (hospitals B and C) with different demographic covariates (Tables 2 and 3). Using the same parameter estimates (intercept = −1.759, teaching status = −0.376, ICU location = 0.122, and pediatric location = −0.202), different risk adjustments were calculated using different characteristics for the hypothetical hospitals (having ICU location/pediatric location/teaching status versus not having ICU location/pediatric location/teaching status). For example, hospital B was a nonteaching hospital with both an ICU (0.122) and pediatric location (−0.202), whereas hospital C was a nonteaching hospital with only an ICU (0.122). The complete calculation for the risk adjustment factor for each hospital can be found in Example S2. The assignment of hospital demographics (from the model) (9) for the two hypothetical hospitals (hospitals B and C) was selected to demonstrate SAAR values between disparate hospitals. Two total sensitivity analyses were performed.
TABLE 2.
Sensitivity analysis for antimicrobials used for community-acquired infections in adult ICU
| Parameter (7) | Parameter estimate | Valuea |
|---|---|---|
| Intercept | −1.759 | 0.1722 |
| B1 (teaching status) | −0.376 | 0.6866 |
| B2 (ICU location) | 0.122 | 1.1298 |
| B3 (pediatric location) | −0.202 | 0.8171 |
Transformed value [exp(parameter estimate)]. The risk adjustment binomial regression was determined as exp(intercept) × exp(B1) × exp(B2) × exp(B3), where B1, B2, and B3 = parameter estimate or 0 depending on yes/no status. Risk adjustments: hospital A (NMH), 0.134; hospital B, 0.159; hospital C, 0.195.
TABLE 3.
Sensitivity analysis resultsa
| Hospital and parameterb | Value in 2014 |
||
|---|---|---|---|
| January | February | March | |
| DP | |||
| A (NMH) | 1,330 | 1,330 | 1,330 |
| B | 800 | 800 | 800 |
| C | 500 | 500 | 500 |
| P AD | |||
| A (NMH) | 177.67 | 177.67 | 177.67 |
| B | 127.18 | 127.18 | 127.18 |
| C | 97.28 | 97.28 | 97.28 |
| AD | |||
| A (NMH) | 127 | 127 | 127 |
| B | 120 | 132a | 145a |
| C | 90 | 99a | 109a |
| SAAR 2014 | |||
| A (NMH) | 0.71 | 0.71 | 0.71 |
| B | 0.94 | 1.04 | 1.14 |
| C | 0.93 | 1.02 | 1.12 |
Sensitivity analysis with 10% increase from previous month.
P AD, predicted antimicrobial days.
First, to assess the impact of increasing use at other hospitals while use stayed constant at NMH (i.e., hospital A), the DP was held constant for all hospitals (i.e., hospital A and the comparator hospitals, B and C). A 10% increase in AD was assumed for hospitals B and C, while use at hospital A was held constant. Predicted AD was calculated using the SAAR risk adjustments (predicted AD = DP × risk adjustment) for each hospital and was constant over the 3 months, given DP was held constant for this sensitivity analysis. The SAAR for each hospital’s month was calculated (SAAR = AD/predicted AD) and plotted over the 3 months using the scatter plot function in Microsoft Excel 2013 (Redmond, WA).
Second, the impact of seasonal variation on the SAAR was explored through a sensitivity analysis by plotting SAAR for 2014 through 2015 (8 seasons). Antibiotic use was created for all three hospitals (hospitals A, B, and C). To show seasonality, SAAR values for the hospitals were held constant, except for seasons 1 and 5, where a 25% increase in SAAR was applied, as these seasons have been previously shown to express seasonality (10). Baseline SAAR values for all three hospitals were arbitrarily chosen for sensitivity analysis/demonstration purposes (hospital A, 1.2; hospital B, 0.96; hospital C, 0.84) (Table 4). The SAAR values for each of the three hospitals were then plotted over the 8 seasons using the scatter plot function in Microsoft Excel 2013 (Redmond, WA).
TABLE 4.
Sensitivity analysis for the potential of seasonal effect on SAAR
| Season | SAAR for hospital: |
Percent change between hospitals |
|||
|---|---|---|---|---|---|
| A (NMH) | B | C | B relative to A | C relative to A | |
| 1a | 1.5 | 1.2 | 1.05 | 20 | 30 |
| 2 | 1.2 | 0.96 | 0.84 | 20 | 30 |
| 3 | 1.2 | 0.96 | 0.84 | 20 | 30 |
| 4 | 1.2 | 0.96 | 0.84 | 20 | 30 |
| 5a | 1.5 | 1.2 | 1.05 | 20 | 30 |
| 6 | 1.2 | 0.96 | 0.84 | 20 | 30 |
| 7 | 1.2 | 0.96 | 0.84 | 20 | 30 |
| 8 | 1.2 | 0.96 | 0.84 | 20 | 30 |
Twenty-five percent increase in SAAR to show seasonality.
Supplementary Material
ACKNOWLEDGMENTS
No financial support for the present study was received. The project was completed as part of our normal work.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01780-18.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States available. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Accessed 22 March 2018. [Google Scholar]
- 2.Ansari F, Gray K, Nathwani D, Phillips G, Ogston S, Ramsay C, Davey P. 2003. Outcomes of an intervention to improve hospital antibiotic prescribing: interrupted time series with segmented regression analysis. J Antimicrob Chemother 52:842–848. doi: 10.1093/jac/dkg459. [DOI] [PubMed] [Google Scholar]
- 3.Solomon DH, Van Houten L, Glynn RJ, Baden L, Curtis K, Schrager H, Avorn J. 2001. Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Arch Intern Med 161:1897–1902. doi: 10.1001/archinte.161.15.1897. [DOI] [PubMed] [Google Scholar]
- 4.Joint Commission on Hospital Accreditation. 2016. Approved: new antimicrobial stewardship standard. Jt Comm Perspect 36:1–8. [PubMed] [Google Scholar]
- 5.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. 2016. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Healthcare Safety Network (NHSN). 2015. Surveillance for antimicrobial use and antimicrobial resistance options. Protocols: antimicrobial use and resistance (AUR) module. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nhsn/acute-care-hospital/aur/index.html Accessed 14 August 2018. [Google Scholar]
- 7.van Santen KL, Edwards JR, Webb AK, Pollack LA, O'Leary E, Neuhauser MM, Srinivasan A, Pollock DA. 2018. The standardized antimicrobial administration ratio: a new metric for measuring and comparing antibiotic use. Clin Infect Dis 67:179–185. doi: 10.1093/cid/ciy075. [DOI] [PubMed] [Google Scholar]
- 8.Center of Diseases Control and Prevention. 2017. Antimicrobial use and resistance (AUR) module. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/nhsn/PDFs/pscManual/11pscAURcurrent.pdf Accessed 22 March 2018. [Google Scholar]
- 9.Reference deleted.
- 10.Patrick DM, Marra F, Hutchinson J, Monnet DL, Ng H, Bowie WR. 2004. Per capita antibiotic consumption: how does a North American jurisdiction compare with Europe? Clin Infect Dis 39:11–17. doi: 10.1086/420825. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, Klein EY, Laxminarayan R. 2012. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin Infect Dis 55:687–694. doi: 10.1093/cid/cis509. [DOI] [PubMed] [Google Scholar]
- 12.Fisman DN. 2007. Seasonality of infectious diseases. Annu Rev Public Health 28:127–143. doi: 10.1146/annurev.publhealth.28.021406.144128. [DOI] [PubMed] [Google Scholar]
- 13.Miglis C, Rhodes NJ, Avedissian SN, Zembower TR, Postelnick M, Wunderink RG, Sutton SH, Scheetz MH. 2017. A simple Microsoft Excel method to predict antibiotic outbreaks and underutilization. Infect Control Hosp Epidemiol 38:860–862. doi: 10.1017/ice.2017.72. [DOI] [PubMed] [Google Scholar]
- 14.National Healthcare Safety Network (NHSN). 2016. Antimicrobial use and resistance (AUR) module. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nhsn/PDFs/pscManual/11pscAURcurrent.pdf Accessed 11 April 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.