LETTER
Ceftolozane-tazobactam (C/T) pharmacokinetics during extracorporeal membrane oxygenation (ECMO) has not been previously studied. In this work, we report on the C/T plasmatic levels in a lung transplant (LTX) recipient during ECMO who was treated with C/T (intravenous [i.v.], 2 g ceftolozane and 1 g tazobactam, every 8 h]) for a Pseudomonas aeruginosa pulmonary infection.
The patient was a 42-year-old female (weight, 65 kg; body surface area, 1.72 m2) with a history of sarcoidosis with pulmonary fibrosis (stage IV) and pulmonary emphysema with alpha-1 antitrypsin deficiency, admitted to the hospital due to acute respiratory failure. Upon admission, a chest X-ray showed bilateral infiltrates, and empirical antibiotic therapy was started with vancomycin and meropenem. Two days after admission, following worsening of respiratory conditions, a veno-venous ECMO (vvECMO) support was started according to the ELSO guidelines (www.elso.org). A permanent life support (PLS) circuit system (Maquet Holding B.V. & Co. KG., Rastatt, DE) with a preconnected PLS-i oxygenator and a centrifugal pump (Rotaflow) was used. Blood and gas flows were adapted to maintain a partial pressure of oxygen (PaO2) of >90 mm Hg, partial pressure of carbon dioxide (PCO2) of <40 mm Hg, and pH 7.4, under protective ventilation.
At the same time, the antimicrobial therapy was modified to vancomycin, amikacin, and ciprofloxacin, since the tracheal aspirate culture grew a carbapenem-resistant Pseudomonas aeruginosa strain (Table 1). On day 10, the culture of the tracheal aspirate was still positive for P. aeruginosa at >1 × 106 CFU/ml. On day 13, the patient was subjected to bilateral LTX. During surgery, the extracorporeal support was switched from veno-venous to veno-arterial ECMO (vaECMO).
TABLE 1.
Susceptibility profile of the Pseudomonas aeruginosa isolate obtained from tracheal aspirate culture
| Antibiotic | MIC (µg/ml) | Categorizationa |
|---|---|---|
| Amikacin | 4 | Susceptible |
| Aztreonam | 16 | Resistant |
| Ceftazidime | 2 | Susceptible |
| Ciprofloxacin | 0.5 | Susceptible |
| Colistin | 0.5 | Susceptible |
| Gentamicin | 1 | Susceptible |
| Imipenem | 16 | Resistant |
| Meropenem | 16 | Resistant |
| Piperacillin-tazobactamb | 32 | Resistant |
| Ceftolozane-tazobactamb | 4 | Susceptible |
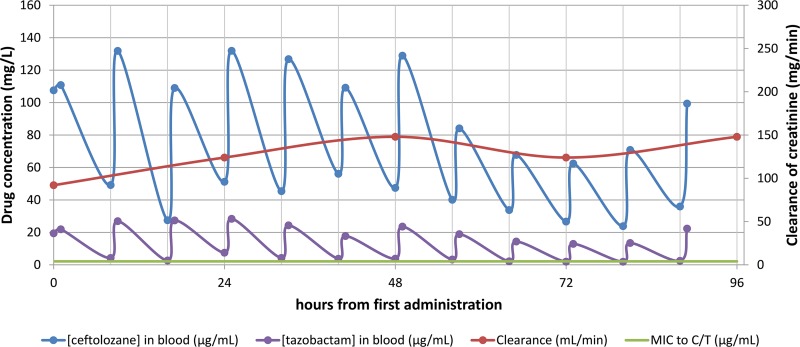
Immediately after surgery, the antibiotic therapy was modified to C/T (2 g/1 g) every 8 h over a 1-h infusion, 400 mg ciprofloxacin every 8 h, and vancomycin in continuous infusion (the vancomycin dose was adjusted to obtain target trough levels between 15 and 20 µg/ml). The patient had a normal renal function with creatinine clearance (estimated using the Cockcroft-Gault formula) (Fig. 1).
FIG 1.
Ceftolozane-tazobactam plasmatic concentrations in the 96 h following LTX.
C/T plasma concentrations obtained in the first 96 h after LTX were retrospectively determined using a validated high-performance liquid chromatography (HPLC) method (1) (maximum concentration of drug in serum [Cmax], ½ h after the end of infusion; minimum concentration of drug in serum [Cmin], immediately before the beginning of the following dose). Written patient consent was obtained for collection and publication of these data.
During this period, ceftolozane levels remained above the ceftolozane MIC (4 µg/ml). In the first 48 h, the ceftolozane Cmax was above 100 µg/ml. Later, from 48 to 96 h, there was a decline in Cmax and Cmin, which, however, remained above 60 and 20 µg/ml, respectively. Tazobactam concentrations followed the same trend, remaining at above 1.9 µg/ml for the whole period (Fig. 1).
vaECMO was halted on day 8 after LTX. The patient’s condition eventually improved, and on day 15 after LTX, the antimicrobial therapy was discontinued. Four consecutive tracheal aspirate cultural exams, performed in the last day of treatment and 3, 4, and 7 days after the discontinuation of antibiotics, did not grow any pathogen.
In conclusion, we report the success of C/T treatment (in association with ciprofloxacin) for a case of a carbapenem-resistant P. aeruginosa low respiratory tract infection in an LTX recipient subjected to vaECMO support. Retrospective assessment of C/T plasmatic levels revealed that good drug exposure was achieved with a free drug concentration that exceeds the MIC (fT>MIC) of 100% (60% fT>MIC is commonly considered predictive of cephalosporin microbiological success in pneumonia and CFU reduction against Gram-negative bacteria) (2–4).
During the monitored period, we observed a decrease in C/T Cmin and Cmax that could be probably attributed to the increase in the patient’s creatinine clearance. In fact, it has been previously demonstrated that C/T clearance is highly correlated with renal function, with creatinine clearance significantly influencing exposure. However, alterations in the volume of distribution (V) (very frequent in critical patients) and the possible increase in V related to ECMO can cause variations in C/T concentrations (5–7). In our patient, in the period 48 to 96 h after LTX, a positive body fluid balance was recorded (≈2,000 ml input excess). This could have also partially contributed to the observed decrease in C/T plasmatic levels in this period.
Our findings suggest that optimal ceftolozane-tazobactam C/T PK parameters can be achieved in severely ill patients with normal renal function requiring ECMO, without the need for dose or infusion time adjustment.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
A.N. has provided consultancies/advisory services to and received research funding from Merck Sharp & Dohme Corp. G.M.R. has provided speaker bureau and consultancies and received research grants from Merck Sharp & Dohme Corp.
REFERENCES
- 1.Sutherland CA, Nicolau DP. 2016. Development of an HPLC method for the determination of ceftolozane/tazobactam in biological and aqueous matrixes. J Chromatogr Sci 54:1037–1040. doi: 10.1093/chromsci/bmw047. [DOI] [PubMed] [Google Scholar]
- 2.Crandon JL, Bulik CC, Kuti JL, Nicolau DP. 2010. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother 54:1111–1116. doi: 10.1128/AAC.01183-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacVane SH, Kuti JL, Nicolau DP. 2014. Clinical pharmacodynamics of antipseudomonal cephalosporins in patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 58:1359–1364. doi: 10.1128/AAC.01463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller AE, Punt N, Mouton JW. 2013. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J Antimicrob Chemother 68:900–906. doi: 10.1093/jac/dks468. [DOI] [PubMed] [Google Scholar]
- 5.Wooley M, Miller B, Krishna G, Hershberger E, Chandorkar G. 2014. Impact of renal function on the pharmacokinetics and safety of ceftolozane-tazobactam. Antimicrob Agents Chemother 58:2249–2255. doi: 10.1128/AAC.02151-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandorkar G, Xiao A, Mouksassi M-S, Hershberger E, Krishna G. 2015. Population pharmacokinetics of ceftolozane/tazobactam in healthy volunteers, subjects with varying degrees of renal function and patients with bacterial infections. J Clin Pharmacol 55:230–239. doi: 10.1002/jcph.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natesan S, Pai MP, Lodise TP. 2017. Determination of alternative ceftolozane/tazobactam dosing regimens for patients with infections due to Pseudomonas aeruginosa with MIC values between 4 and 32 mg/L. J Antimicrob Chemother 72:2813–2816. doi: 10.1093/jac/dkx221. [DOI] [PubMed] [Google Scholar]
- 8.European Committee on Antimicrobial Susceptibility Testing. 2018. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.1. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute (CLSI). 2018. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; approved standard, 11th ed. CLSI M07Ed11. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]