Completed sequences of three plasmids from a carbapenem-resistant hypervirulent Klebsiella pneumoniae isolate, SH9, were obtained. In addition to the pLVPK-like virulence-conferring plasmid (pVir-CR-HvKP_SH9), the two multidrug-resistant plasmids (pKPC-CR-HvKP4_SH9 and pCTX-M-CR-HvKP4_SH9) were predicted to originate from a single pKPC-CR-HvKP4-like multireplicon plasmid through homologous recombination.
KEYWORDS: hypervirulent, Klebsiella pneumoniae, blaKPC-2, cointegration, tandem repeat
ABSTRACT
Completed sequences of three plasmids from a carbapenem-resistant hypervirulent Klebsiella pneumoniae isolate, SH9, were obtained. In addition to the pLVPK-like virulence-conferring plasmid (pVir-CR-HvKP_SH9), the two multidrug-resistant plasmids (pKPC-CR-HvKP4_SH9 and pCTX-M-CR-HvKP4_SH9) were predicted to originate from a single pKPC-CR-HvKP4-like multireplicon plasmid through homologous recombination. Interestingly, the blaKPC-2 gene was detectable in five tandem repeats exhibiting the format of an NTEKPC-Id-like transposon (IS26-ΔTn3-ISKpn8-blaKPC-2-ΔISKpn6-korC-orf-IS26). The data suggest an important role of DNA recombination in mediating active plasmid evolution.
INTRODUCTION
The notorious nosocomial pathogen Klebsiella pneumoniae has evolved into two clinically significant clades, namely, hypervirulent K. pneumoniae (hvKP) and carbapenem-resistant K. pneumoniae (CR-Kp), both of which can cause severe infections (1). Recently, convergence of genetic elements encoding hypervirulence and carbapenem resistance (i.e., CR-hvKP) in a single K. pneumoniae strain was reported, suggesting that such strains continue to evolve and pose a serious threat to public health (2–5). Emergence of CR-hvKP was due to acquisition of the virulence plasmid by a carbapenem-resistant strain or acquisition of a carbapenemase-producing plasmid by a hypervirulent strain (2, 3). The KPC-2-encoding gene blaKPC-2 normally exists as a single copy in plasmids. However, by using Oxford Nanopore sequencing technology, we detected five copies of blaKPC-2 on a single plasmid in an sequence type 11 (ST11) hvKP isolate. This study characterized the virulence potential and antimicrobial susceptibility of this phenotypically convergent superbug and unveiled the genetic basis of phenotypes conferred by the plasmids that this strain harbored.
K. pneumoniae SH9, a strain identified from a nationwide surveillance project, was subjected to antimicrobial susceptibility testing with the agar dilution method, and the results were interpreted according to CLSI guidelines (6). SH9 exhibited resistance to cefotaxime (MIC, >128 μg/ml), ceftazidime (MIC, 128 μg/ml), cefepime (MIC, 128 μg/ml), ertapenem (MIC, >128 μg/ml), imipenem (MIC, 16 μg/ml), meropenem (MIC, 128 μg/ml), amikacin (MIC, >256 μg/ml), and ciprofloxacin (MIC, 64 μg/ml). However, the strain remained susceptible to colistin (MIC, ≤0.25 μg/ml). Wax moth larvae were tested for virulence as described previously and SH9 was shown to be hypervirulent (data not shown) (7). MLST with Kleborate and capsular typing with Kaptive indicated that SH9 belonged to ST11 and serotype K47, a dominant clone of KPC-producing K. pneumoniae in China (8–11). These findings suggested that K. pneumoniae SH9 was an ST11 carbapenem-resistant hypervirulent strain that carried a pLVPK-like virulence-conferring plasmid and multidrug-resistant (MDR) plasmids resembling the previously reported ST11 CR-hvKP strains (2, 3).
Whole-genome sequencing was conducted to study the complete sequence of plasmids in strain SH9 by using the Illumina NextSeq 500 platform and the long-read MinION sequencer (12). The rapid sequencing kit (SQK-RBK001) and flow cell type R9.4 were used for Nanopore MinION sequencing. Genome assembly was conducted using Illumina reads with SPAdes v.3.11.1 (13). Hybrid assembly of short Illumina reads and long MinION reads was constructed with Unicycler v.0.3.0 (14). The complete circular plasmid sequences were modified using Pilon v.1.22 for several rounds until no change was detected (15). Complete plasmid sequences were annotated by the RAST tool (16).
We recovered three circular plasmids of 188,648 bp (pVir-CR-HvKP_SH9; GenBank accession no. MH255828), 113,941 bp (pKPC-CR-HvKP4_SH9; GenBank accession no. MH255827), and 98,684 bp (pCTX-M-CR-HvKP4_SH9; GenBank accession no. MH255829). A BLASTN search against the NCBI nucleotide database indicated that plasmid pVir-CR-HvKP_SH9 was 99% identical to the pLVPK-like virulence plasmids pVir-CR-HvKP267 (GenBank accession no. MG053312) and pSGH10 (GenBank accession no. CP025081) recovered from clinical K. pneumoniae strains, with 97% coverages (17, 18). Plasmid pVir-CR-HvKP_SH9 contains the IncHI1B and IncFIB(K) replicons, exhibited a GC content of 49.9%, and comprised 225 predicted coding sequences. It also harbored virulence-associated genes rmpA, rmpA2, and iutAiucABCD (data not shown). However, a gene cluster encoding salmochelin (iroBCDN) in plasmid pLVPK was absent from pVir-CR-HvKP_SH9. By encoding siderophores (aerobactin and/or salmochelin) and regulators of the mucoid phenotype (RmpA and RmpA2), which were restricted to hvKP isolates, pLVPK-like virulence plasmids were found to play a pivotal role in K. pneumoniae hypervirulence (19).
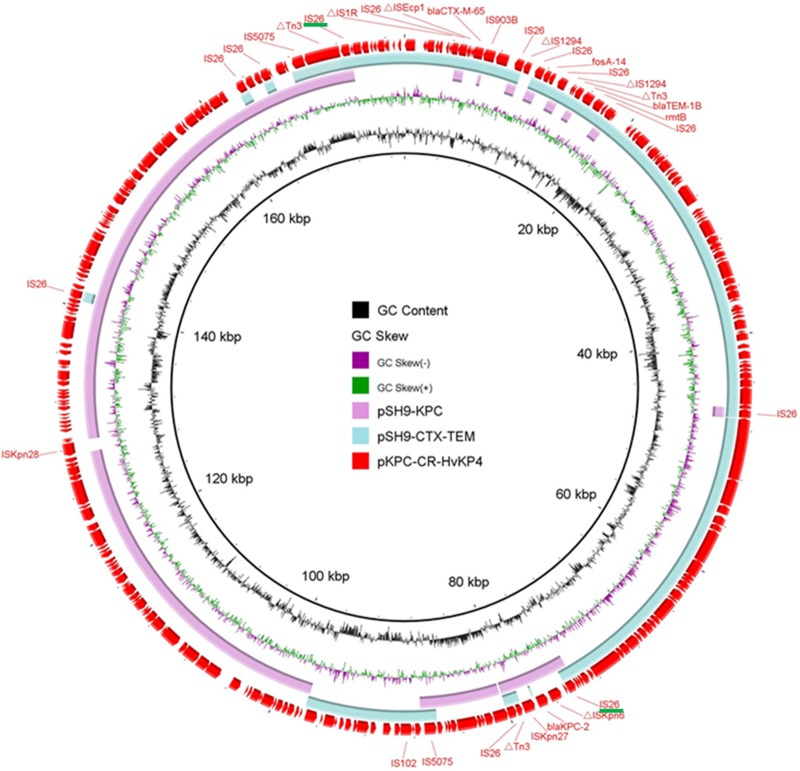
The carbapenem resistance-encoding plasmid (pKPC-CR-HvKP4_SH9) harbored by strain SH9 comprises the IncR replicon, exhibited a GC content of 55.0%, and comprised 159 predicted open reading frames (ORFs). This plasmid carried the catA2 gene and five copies of blaKPC-2, as confirmed by the Nanopore raw reads and assembled sequences. The third plasmid harbored by this strain, pCTX-M-CR-HvKP4_SH9, was found to exhibit a GC content of 51.5% and comprise 139 predicted coding sequences, among which were the IncFII replicon and the blaCTX-M-65, blaTEM-1B, rmtB, and fosA_14 genes bound by various insertion sequences. Interestingly, both pKPC-CR-HvKP4_SH9C and pCTX-M-CR-HvKP4_SH9 exhibited >99% identities with the IncFII/R-type conjugative MDR plasmid pKPC-CR-HvKP4 (GenBank accession no. MF437312), previously recovered from an ST11 CR-hvKP isolate (2), at 100% and 92% coverage, respectively (Fig. 1), suggesting that genetic recombination events might be responsible for generation of these plasmids. As many as 12 copies of IS26 were scattered across the complete sequence of plasmid pKPC-CR-HvKP4; however, target site duplications flanking IS26 were not observed, indicating that homologous recombination events mediated by two IS26 elements in plasmids of different replicons rather than replicative transposition among the three plasmids occurred (Fig. 2c) (20, 21). The conjugation experiment was performed using an azide-resistant Escherichia coli J53 (Azr) strain as the recipient, and MacConkey agar supplemented with 100 mg/ml sodium azide and 2 mg/ml meropenem was used to select transconjugants (22). A previous study indicated that IncR plasmids did not possess conjugational transfer genes but may be mobilizable, thus broadening their host spectrum by forming multireplicon cointegrates with plasmids of other incompatible types, such as IncA/C and IncN (23). In this study, plasmid pKPC-CR-HvKP4_SH9 was not transferrable to the recipient E. coli strain J53 via conjugation. Also, no pKPC-CR-HvKP4-like cointegrate was detected after conjugation, leading us to hypothesize that homologous recombination occurs at a relatively low frequency, thereby limiting the rate of transmission of blaKPC-2 genes located in IncR plasmids.
FIG 1.
Circular maps of two MDR plasmids recovered from K. pneumoniae SH9 and a similar plasmid pKPC-CR-HvKP4 recorded in the NCBI database. The two IS26 elements responsible for the homologous recombination are underlined in green. This figure was constructed with BRIG (33).
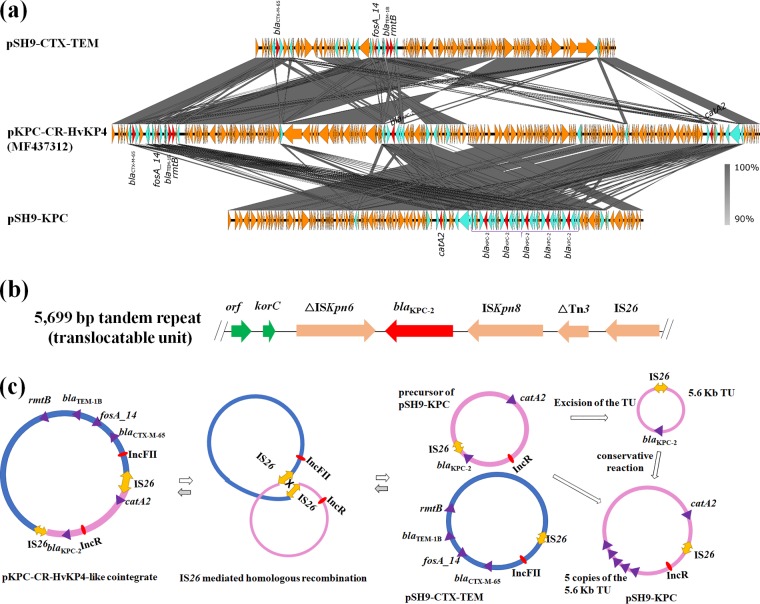
FIG 2.
Mechanisms of plasmid recombination. (a) Structure alignment of the three plasmids using EasyFig (34). Yellow, blue, and red triangles indicate ORFs, insertion sequences, and antimicrobial resistance genes, respectively. (b) Genetic composition of the 5,699-bp blaKPC-2-bearing tandem repeat region that formed the translocatable unit (TU) that was excised from the precursor of plasmid pKPC-CR-HvKP4_SH9. (c) Mechanisms of plasmid recombination. IS26 mediated the homologous recombination leads to generation of plasmids pKPC-CR-HvKP4-like cointegrate, pKPC-CR-HvKP4_SH9, and pCTX-M-CR-HvKP4_SH9. Five tandem repeats of blaKPC-2-bearing fragments were generated via Tnp26-catalyzed conservative reaction, which incorporated the 5.6-kb TU next to a preexisting IS26. pSH9-KPC represents pKPC-CR-HvKP4_SH9, and pSH9-CTX-TEM represents pCTX-M-CR-HvKP4_SH9 in the figure.
Five copies of blaKPC-2 genes were identified on pKPC-CR-HvKP4_SH9, each in one of five identical 5,699-bp regions linked to each other in tandem, suggesting that this region exhibits a high degree of mobility and has been heavily duplicated (Fig. 2). The 5,699-bp region was located in a non-Tn4401 element homologous to the NTEKPC-Id fragment in plasmid pKPC-LKEc (GenBank accession no. KC788405), with the structure of IS26-ΔTn3-ISKpn8-blaKPC-2-ΔISKpn6-korC-orf (Fig. 2) (24, 25). The first copy of the 5,699-bp NTEKPC-Id-like region was located directly upstream of an IS26 element, generating a 6.5-kb fragment bordered by two IS26s. Such a 6.5-kb element may effectively act as a composite transposon that can mobilize the intervening genetic components. Detailed sequence analysis of the blaKPC-2 region enabled us to predict the duplication mechanism that creates the tandem repeats. First, the 5.6-kb translocatable unit (TU), a circular form of the NTEKPC-Id-like fragment, was generated via excision from a preexisting IS26-bound transposon via homologous recombination. The TU was then incorporated into an existing IS26 by using the conservative Tnp26-catalyzed mechanism or homologous recombination (less frequently). Last, repetition of the incorporation process with the same TU should lead to formation of the NTEKPC-Id-like tandem repeats in pKPC-CR-HvKP4_SH9 (21). To our knowledge, coexistence of five blaKPC-2 genes with the NTEKPC-Id structure within a single plasmid was not reported previously, but carriage of multiple copies of blaKPC on Tn4401 has been described (26–32). Whether the carriage of multiple copies of blaKPC-2 genes further enhances carbapenem resistance level in the host strain needs further investigation.
Here, we reported an ST11 CR-hvKP isolate carrying three plasmids, one pLVPK-like virulence plasmid and two MDR plasmids, predicted to originate from a single pKPC-CR-HvKP4-like multireplicon plasmid through homologous recombination. Five copies of blaKPC-2 genes were tandemly located on a nonconjugative plasmid with the NTEKPC-Id-like structure (IS26-ΔTn3-ISKpn8-blaKPC-2-ΔISKpn6-korC-orf-IS26), which was presumably created as a result of Tnp26-catalyzed conservative reaction through activity of TUs. Findings in this study indicate that plasmids in the ST11 CR-hvKP clone can undergo active genetic recombination events, the evolution trends of which should be closely monitored.
ACKNOWLEDGMENTS
This study was funded by the Collaborative Research Fund from the Research Grant Council (C5026-16G) and from the Health and Medical Research Fund (15141322).
We have no conflicts of interest to declare.
REFERENCES
- 1.Paczosa MK, Mecsas J. 2016. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu D, Dong N, Zheng Z, Lin D, Huang M, Wang L, Chan EW-C, Shu L, Yu J, Zhang R, Chen S. 2018. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis 18:37–46. doi: 10.1016/s1473-3099(17)30489-9. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Kreiswirth BN. 2017. Convergence of carbapenem-resistance and hypervirulence in Klebsiella pneumoniae. Lancet Infect Dis 18:2–3. doi: 10.1016/S1473-3099(17)30517-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Zeng J, Liu W, Zhao F, Hu Z, Zhao C, Wang Q, Wang X, Chen H, Li H, Zhang F, Li S, Cao B, Wang H. 2015. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect 71:553–560. doi: 10.1016/j.jinf.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Zhang R, Lin D, Chan EW, Gu D, Chen GX, Chen S. 2016. Emergence of carbapenem-resistant serotype K1 hypervirulent Klebsiella pneumoniae strains in China. Antimicrob Agents Chemother 60:709–711. doi: 10.1128/AAC.02173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Liu L, Zhou H, Chan EW, Li J, Fang Y, Li Y, Liao K, Chen S. 2017. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine 19:98–106. doi: 10.1016/j.ebiom.2017.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wand ME, Müller CM, Titball RW, Michell SL. 2011. Macrophage and Galleria mellonella infection models reflect the virulence of naturally occurring isolates of B. pseudomallei, B. thailandensis and B. oklahomensis. BMC Microbiol 11:11. doi: 10.1186/1471-2180-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. 2011. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother 66:307–312. doi: 10.1093/jac/dkq431. [DOI] [PubMed] [Google Scholar]
- 9.Dong N, Zhang R, Liu L, Li R, Lin D, Chan EW-C, Chen S. 2018. Genome analysis of clinical multilocus sequence type 11 Klebsiella pneumoniae from China. Microbial Genom 4. doi: 10.1099/mgen.0.000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyres KL, Wick RR, Gorrie C, Jenney A, Follador R, Thomson NR, Holt KE. 2016. Identification of Klebsiella capsule synthesis loci from whole genome data. Microbial Genom 2:e000102. doi: 10.1099/mgen.0.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam MM, Wick RR, Wyres KL, Gorrie CL, Judd LM, Jenney AW, Brisse S, Holt KE. 2018. Genetic diversity, mobilisation and spread of the yersiniabactin-encoding mobile element ICEKp in Klebsiella pneumoniae populations. Microbial Genom 4. doi: 10.1099/mgen.0.000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R, Xie M, Dong N, Lin D, Yang X, Wong MHY, Chan EW-C, Chen S. 2018. Efficient generation of complete sequences of MDR-encoding plasmids by rapid assembly of MinION barcoding sequencing data. GigaScience 7:1–9. doi: 10.1093/gigascience/gix132:gix132-gix132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, Earl AM. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao H, Qin S, Chen S, Shen J, Du X-D. 2018. Emergence of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Lancet Infect Dis 18:25. doi: 10.1016/S1473-3099(17)30628-X. [DOI] [PubMed] [Google Scholar]
- 18.Lam MM, Wyres KL, Duchene S, Wick RR, Judd LM, Gan Y-H, Hoh C-H, Achuleta S, Molton J, Kalimuddin S, Koh TH, Passet V, Brisse S, Holt KE. 2018. Population genomics of hypervirulent Klebsiella pneumoniae clonal group 23 reveals early emergence and rapid global dissemination. Nat Commun 9:2703. doi: 10.1038/s41467-018-05114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Struve C, Roe CC, Stegger M, Stahlhut SG, Hansen DS, Engelthaler DM, Andersen PS, Driebe EM, Keim P, Krogfelt KA. 2015. Mapping the evolution of hypervirulent Klebsiella pneumoniae. mBio 6:e00630. doi: 10.1128/mBio.00630-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmer CJ, Moran RA, Hall RM. 2014. Movement of IS26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio 5:e01801-14. doi: 10.1128/mBio.01801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmer CJ, Hall RM. 2016. IS26-mediated formation of transposons carrying antibiotic resistance genes. mSphere 1:e00038-16. doi: 10.1128/mSphere.00038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Lin D, Chen K, Wong MHY, Chen S. 2015. First detection of AmpC β-lactamase blaCMY-2 on a conjugative IncA/C plasmid in a Vibrio parahaemolyticus isolate of food origin. Antimicrob Agents Chemother 59:4106–4111. doi: 10.1128/AAC.05008-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rozwandowicz M, Brouwer M, Fischer J, Wagenaar J, Gonzalez-Zorn B, Guerra B, Mevius D, Hordijk J. 2018. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother 73:1121–1137. doi: 10.1093/jac/dkx488. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. 2014. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol 22:686–696. doi: 10.1016/j.tim.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YT, Lin JC, Fung CP, Lu PL, Chuang YC, Wu TL, Siu LK. 2014. KPC-2-encoding plasmids from Escherichia coli and Klebsiella pneumoniae in Taiwan. J Antimicrob Chemother 69:628–631. doi: 10.1093/jac/dkt409. [DOI] [PubMed] [Google Scholar]
- 26.Fortini D, Villa L, Feudi C, Pires J, Bonura C, Mammina C, Endimiani A, Carattoli A. 2016. Double copies of blaKPC-3::Tn4401a on an IncX3 plasmid in Klebsiella pneumoniae successful clone ST512 from Italy. Antimicrob Agents Chemother 60:646–649. doi: 10.1128/AAC.01886-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitchel B, Rasheed JK, Endimiani A, Hujer AM, Anderson KF, Bonomo RA, Patel JB. 2010. Genetic factors associated with elevated carbapenem resistance in KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 54:4201–4207. doi: 10.1128/AAC.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gootz TD, Lescoe MK, Dib-Hajj F, Dougherty BA, He W, Della-Latta P, Huard RC. 2009. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob Agents Chemother 53:1998–2004. doi: 10.1128/AAC.01355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheppard AE, Stoesser N, Wilson DJ, Sebra R, Kasarskis A, Anson LW, Giess A, Pankhurst LJ, Vaughan A, Grim CJ. 2016. Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob Agents Chemother 60:e00464-16. doi: 10.1128/AAC.00464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathers AJ, Stoesser N, Chai W, Carroll J, Barry K, Cherunvanky A, Sebra R, Kasarskis A, Peto TE, Walker AS. 2017. Chromosomal integration of the Klebsiella pneumoniae carbapenemase gene, blaKPC, in Klebsiella species is elusive but not rare. Antimicrob Agents Chemother 61:e01823-16. doi: 10.1128/AAC.01823-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weingarten RA, Johnson RC, Conlan S, Ramsburg AM, Dekker JP, Lau AF, Khil P, Odom RT, Deming C, Park M. 2018. Genomic analysis of hospital plumbing reveals diverse reservoir of bacterial plasmids conferring carbapenem resistance. mBio 9:e02011-17. doi: 10.1128/mBio.02011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conlan S, Thomas PJ, Deming C, Park M, Lau AF, Dekker JP, Snitkin ES, Clark TA, Luong K, Song Y, Tsai Y-C, Boitano M, Dayal J, Brooks SY, Schmidt B, Young AC, Thomas JW, Bouffard GG, Blakesley RW, Mullikin JC, Korlach J, Henderson DK, Frank KM, Palmore TN, Segre JA. 2014. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci Transl Med 6:254ra126. doi: 10.1126/scitranslmed.3009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alikhan N-F, Petty NK, Zakour NLB, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:1. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan MJ, Petty NK, Beatson SA. 2011. EasyFig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]